791b86e5129dcffdc0b8682277941373.ppt
- Количество слайдов: 34
 Please note: Due to technical difficulties the medical contrast images will not be in motion, the image will appear as a still picture. For medical images please refer to the transcript, Part 1 beginning on page 64, for the description of the images that were provided by the presenter. 1 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Please note: Due to technical difficulties the medical contrast images will not be in motion, the image will appear as a still picture. For medical images please refer to the transcript, Part 1 beginning on page 64, for the description of the images that were provided by the presenter. 1 R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Clinical Experience and Safety Considerations Professor R. Senior Consultant Cardiologist and Director of Cardiac Research Northwick Park Hospital, Harrow, UK 2 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Clinical Experience and Safety Considerations Professor R. Senior Consultant Cardiologist and Director of Cardiac Research Northwick Park Hospital, Harrow, UK 2 R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Contrast Echocardiography with Sono. Vue • Clinical Utility: Assessment of LV structure and function (global and regional) and assessment of microcirculation (cardiac and extra-cardiac) • Side-effect Profile • Risk-Benefit 3 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Contrast Echocardiography with Sono. Vue • Clinical Utility: Assessment of LV structure and function (global and regional) and assessment of microcirculation (cardiac and extra-cardiac) • Side-effect Profile • Risk-Benefit 3 R. Senior – FDA Advisory Board – June 24, 2008 * * *
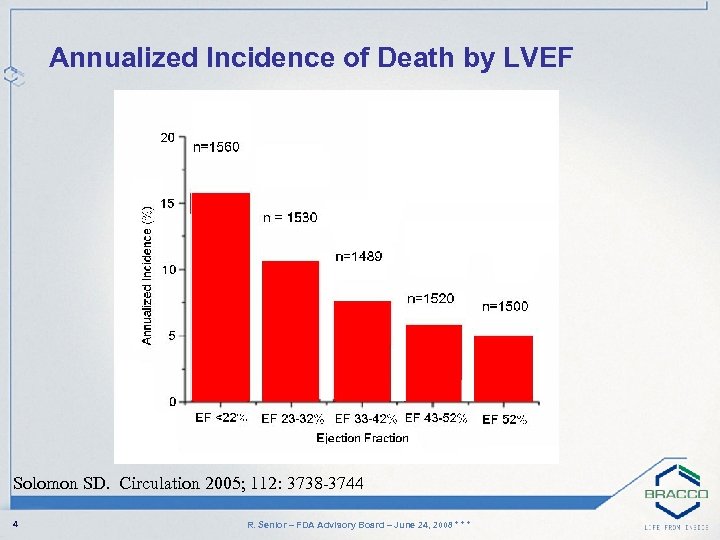 Annualized Incidence of Death by LVEF Solomon SD. Circulation 2005; 112: 3738 -3744 4 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Annualized Incidence of Death by LVEF Solomon SD. Circulation 2005; 112: 3738 -3744 4 R. Senior – FDA Advisory Board – June 24, 2008 * * *
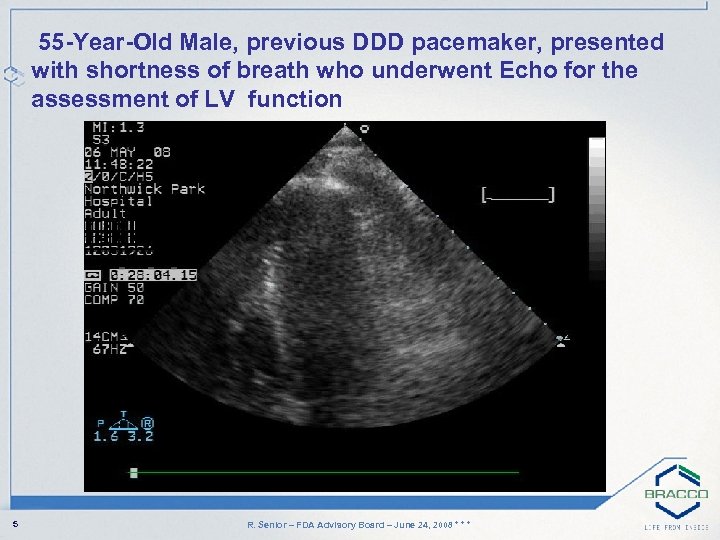 55 -Year-Old Male, previous DDD pacemaker, presented with shortness of breath who underwent Echo for the assessment of LV function 5 R. Senior – FDA Advisory Board – June 24, 2008 * * *
55 -Year-Old Male, previous DDD pacemaker, presented with shortness of breath who underwent Echo for the assessment of LV function 5 R. Senior – FDA Advisory Board – June 24, 2008 * * *
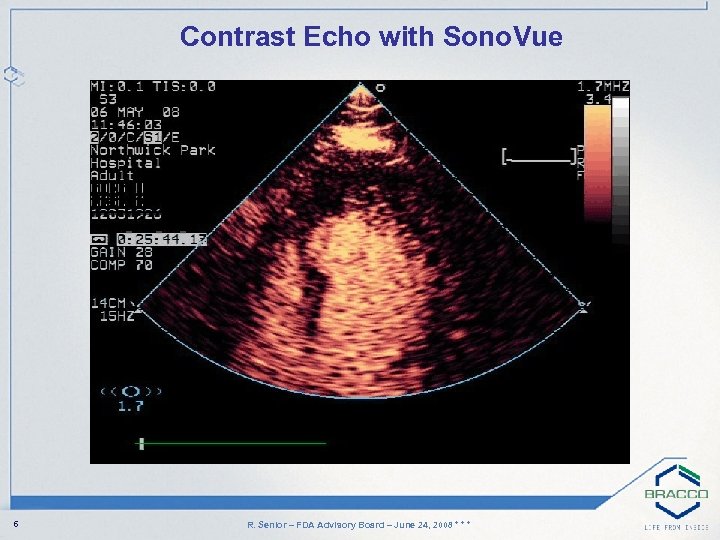 Contrast Echo with Sono. Vue 6 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Contrast Echo with Sono. Vue 6 R. Senior – FDA Advisory Board – June 24, 2008 * * *
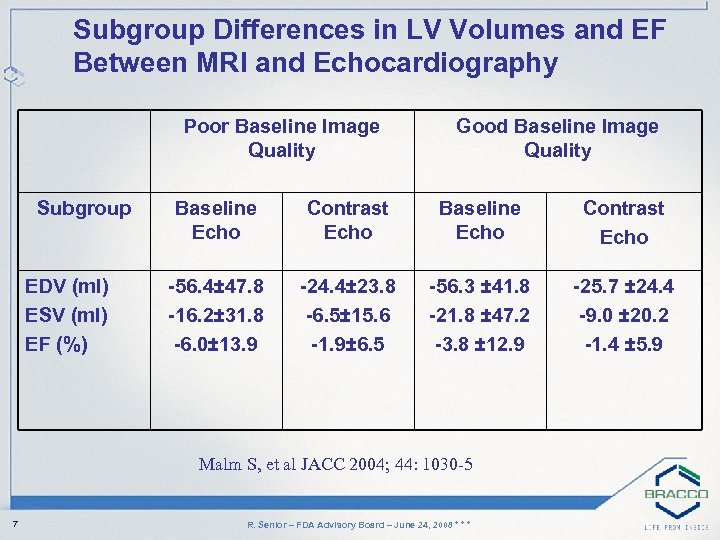 Subgroup Differences in LV Volumes and EF Between MRI and Echocardiography Poor Baseline Image Quality Subgroup EDV (ml) ESV (ml) EF (%) Good Baseline Image Quality Baseline Echo Contrast Echo -56. 4± 47. 8 -16. 2± 31. 8 -6. 0± 13. 9 -24. 4± 23. 8 -6. 5± 15. 6 -1. 9± 6. 5 -56. 3 ± 41. 8 -21. 8 ± 47. 2 -3. 8 ± 12. 9 -25. 7 ± 24. 4 -9. 0 ± 20. 2 -1. 4 ± 5. 9 Malm S, et al JACC 2004; 44: 1030 -5 7 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Subgroup Differences in LV Volumes and EF Between MRI and Echocardiography Poor Baseline Image Quality Subgroup EDV (ml) ESV (ml) EF (%) Good Baseline Image Quality Baseline Echo Contrast Echo -56. 4± 47. 8 -16. 2± 31. 8 -6. 0± 13. 9 -24. 4± 23. 8 -6. 5± 15. 6 -1. 9± 6. 5 -56. 3 ± 41. 8 -21. 8 ± 47. 2 -3. 8 ± 12. 9 -25. 7 ± 24. 4 -9. 0 ± 20. 2 -1. 4 ± 5. 9 Malm S, et al JACC 2004; 44: 1030 -5 7 R. Senior – FDA Advisory Board – June 24, 2008 * * *
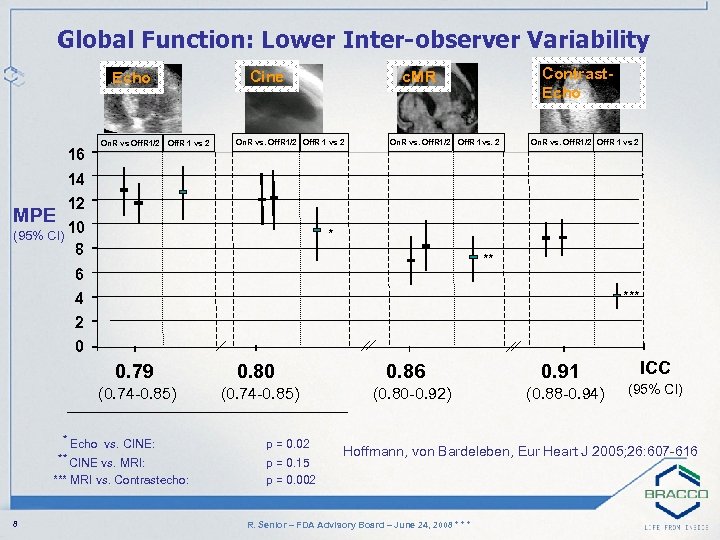 Global Function: Lower Inter-observer Variability Cine Echo 16 On. R vs Off. R 1/2 Off. R 1 vs 2 Contrast. Echo c. MR On. R vs. Off. R 1/2 Off. R 1 vs 2 On. R vs. Off. R 1/2 Off. R 1 vs. 2 On. R vs. Off. R 1/2 Off. R 1 vs 2 14 12 10 (95% CI) 8 MPE * ** 6 *** 4 2 0 0. 79 0. 80 (0. 74 -0. 85) * Echo vs. CINE: ** CINE vs. MRI: *** MRI vs. Contrastecho: 8 p = 0. 02 p = 0. 15 p = 0. 002 0. 86 0. 91 ICC (0. 80 -0. 92) (0. 88 -0. 94) (95% CI) Hoffmann, von Bardeleben, Eur Heart J 2005; 26: 607 -616 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Global Function: Lower Inter-observer Variability Cine Echo 16 On. R vs Off. R 1/2 Off. R 1 vs 2 Contrast. Echo c. MR On. R vs. Off. R 1/2 Off. R 1 vs 2 On. R vs. Off. R 1/2 Off. R 1 vs. 2 On. R vs. Off. R 1/2 Off. R 1 vs 2 14 12 10 (95% CI) 8 MPE * ** 6 *** 4 2 0 0. 79 0. 80 (0. 74 -0. 85) * Echo vs. CINE: ** CINE vs. MRI: *** MRI vs. Contrastecho: 8 p = 0. 02 p = 0. 15 p = 0. 002 0. 86 0. 91 ICC (0. 80 -0. 92) (0. 88 -0. 94) (95% CI) Hoffmann, von Bardeleben, Eur Heart J 2005; 26: 607 -616 R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Case Study: History • 29 -year-old obese Asian female • Right shoulder pain and short of breath two months post delivery when pushing pram • Diabetic for 2 years • Normal resting ECG • Non-diagnostic Exercise ECG • Advised to have Stress Echo 9 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Case Study: History • 29 -year-old obese Asian female • Right shoulder pain and short of breath two months post delivery when pushing pram • Diabetic for 2 years • Normal resting ECG • Non-diagnostic Exercise ECG • Advised to have Stress Echo 9 R. Senior – FDA Advisory Board – June 24, 2008 * * *
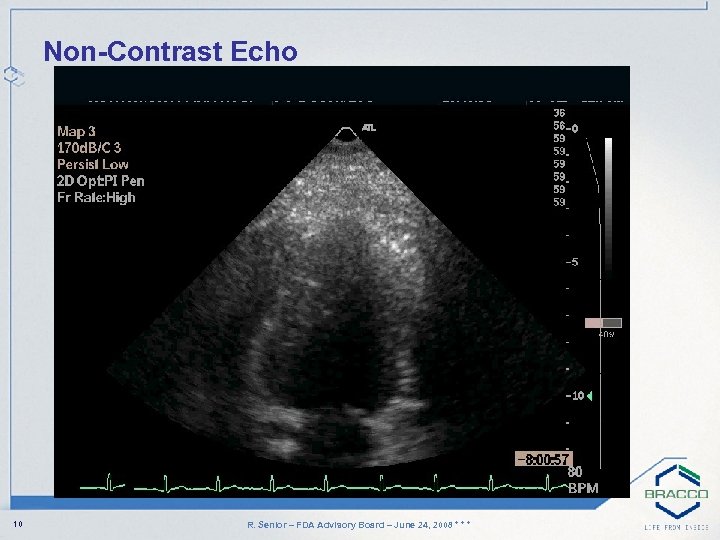 Non-Contrast Echo 10 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Non-Contrast Echo 10 R. Senior – FDA Advisory Board – June 24, 2008 * * *
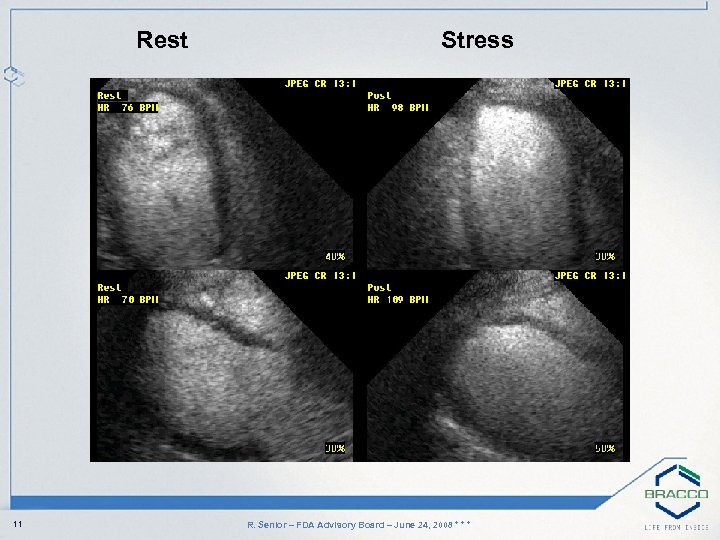 Rest 11 Stress R. Senior – FDA Advisory Board – June 24, 2008 * * *
Rest 11 Stress R. Senior – FDA Advisory Board – June 24, 2008 * * *
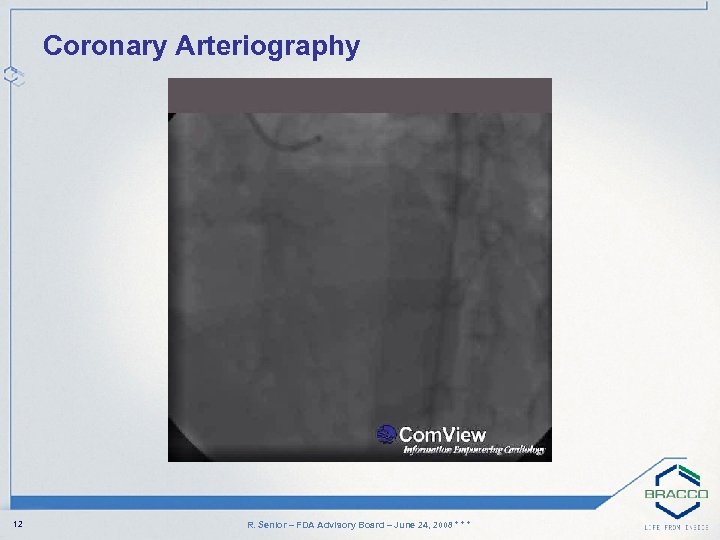 Coronary Arteriography 12 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Coronary Arteriography 12 R. Senior – FDA Advisory Board – June 24, 2008 * * *
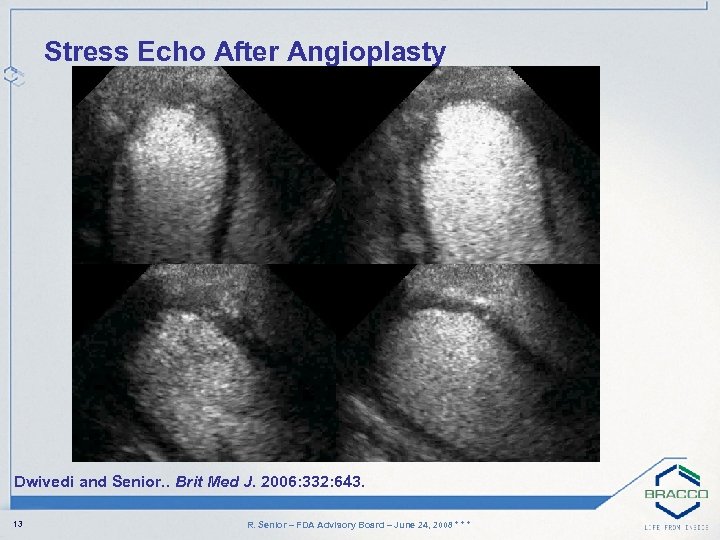 Stress Echo After Angioplasty Dwivedi and Senior. . Brit Med J. 2006: 332: 643. 13 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Stress Echo After Angioplasty Dwivedi and Senior. . Brit Med J. 2006: 332: 643. 13 R. Senior – FDA Advisory Board – June 24, 2008 * * *
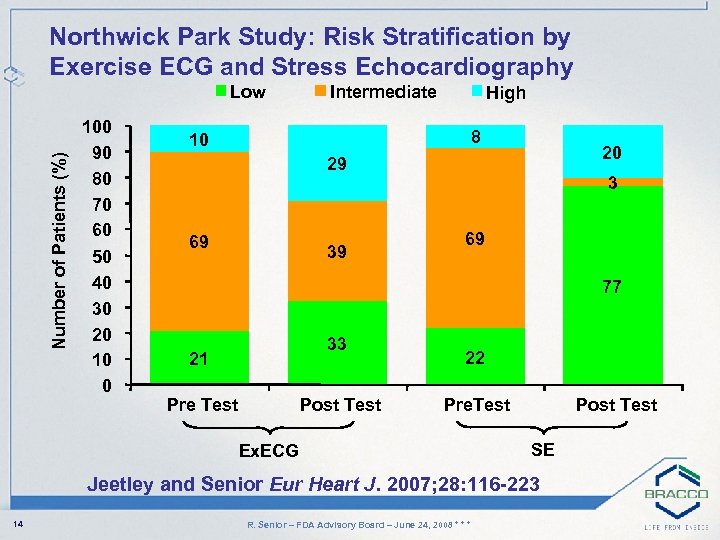 Northwick Park Study: Risk Stratification by Exercise ECG and Stress Echocardiography Number of Patients (%) Low 100 90 80 70 60 50 40 30 20 10 0 Intermediate High 8 10 20 29 69 39 3 69 77 33 21 Pre Test Post Test 22 Pre. Test Ex. ECG Post Test SE Jeetley and Senior Eur Heart J. 2007; 28: 116 -223 14 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Northwick Park Study: Risk Stratification by Exercise ECG and Stress Echocardiography Number of Patients (%) Low 100 90 80 70 60 50 40 30 20 10 0 Intermediate High 8 10 20 29 69 39 3 69 77 33 21 Pre Test Post Test 22 Pre. Test Ex. ECG Post Test SE Jeetley and Senior Eur Heart J. 2007; 28: 116 -223 14 R. Senior – FDA Advisory Board – June 24, 2008 * * *
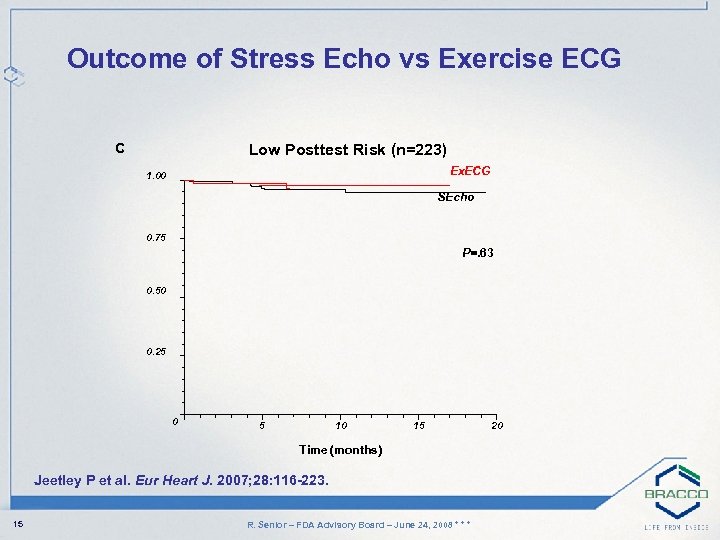 Outcome of Stress Echo vs Exercise ECG C Low Posttest Risk (n=223) Ex. ECG 1. 00 SEcho 0. 75 P=. 63 0. 50 0. 25 0 5 10 15 Time (months) Jeetley P et al. Eur Heart J. 2007; 28: 116 -223. 15 R. Senior – FDA Advisory Board – June 24, 2008 * * * 20
Outcome of Stress Echo vs Exercise ECG C Low Posttest Risk (n=223) Ex. ECG 1. 00 SEcho 0. 75 P=. 63 0. 50 0. 25 0 5 10 15 Time (months) Jeetley P et al. Eur Heart J. 2007; 28: 116 -223. 15 R. Senior – FDA Advisory Board – June 24, 2008 * * * 20
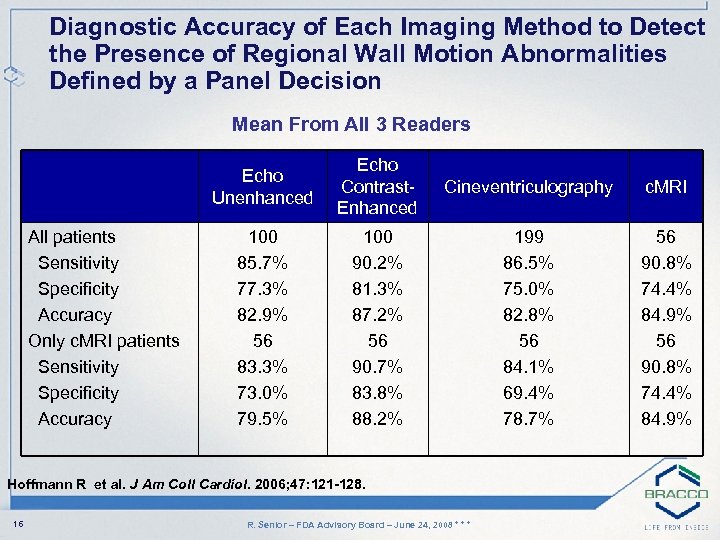 Diagnostic Accuracy of Each Imaging Method to Detect the Presence of Regional Wall Motion Abnormalities Defined by a Panel Decision Mean From All 3 Readers Echo Unenhanced All patients Sensitivity Specificity Accuracy Only c. MRI patients Sensitivity Specificity Accuracy Echo Contrast. Enhanced Cineventriculography c. MRI 100 85. 7% 77. 3% 82. 9% 56 83. 3% 73. 0% 79. 5% 100 90. 2% 81. 3% 87. 2% 56 90. 7% 83. 8% 88. 2% 199 86. 5% 75. 0% 82. 8% 56 84. 1% 69. 4% 78. 7% 56 90. 8% 74. 4% 84. 9% Hoffmann R et al. J Am Coll Cardiol. 2006; 47: 121 -128. 16 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Diagnostic Accuracy of Each Imaging Method to Detect the Presence of Regional Wall Motion Abnormalities Defined by a Panel Decision Mean From All 3 Readers Echo Unenhanced All patients Sensitivity Specificity Accuracy Only c. MRI patients Sensitivity Specificity Accuracy Echo Contrast. Enhanced Cineventriculography c. MRI 100 85. 7% 77. 3% 82. 9% 56 83. 3% 73. 0% 79. 5% 100 90. 2% 81. 3% 87. 2% 56 90. 7% 83. 8% 88. 2% 199 86. 5% 75. 0% 82. 8% 56 84. 1% 69. 4% 78. 7% 56 90. 8% 74. 4% 84. 9% Hoffmann R et al. J Am Coll Cardiol. 2006; 47: 121 -128. 16 R. Senior – FDA Advisory Board – June 24, 2008 * * *
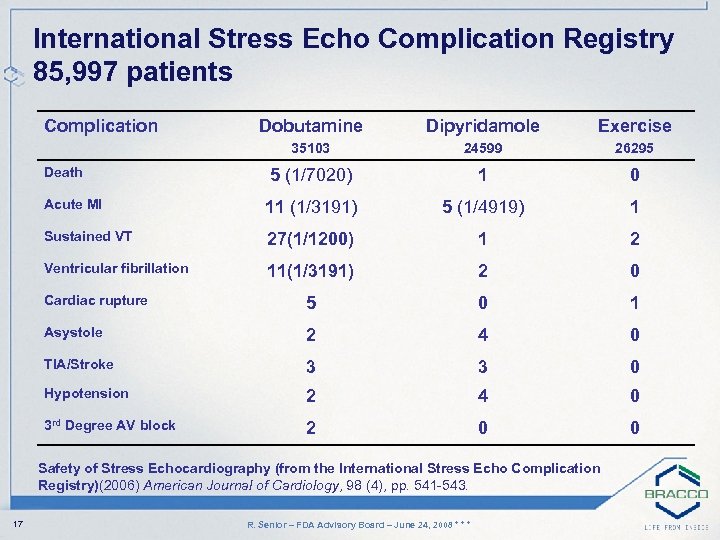 International Stress Echo Complication Registry 85, 997 patients Complication Dobutamine Dipyridamole Exercise 35103 24599 26295 5 (1/7020) 1 0 Acute MI 11 (1/3191) 5 (1/4919) 1 Sustained VT 27(1/1200) 1 2 Ventricular fibrillation 11(1/3191) 2 0 Cardiac rupture 5 0 1 Asystole 2 4 0 TIA/Stroke 3 3 0 Hypotension 2 4 0 3 rd Degree AV block 2 0 0 Death Safety of Stress Echocardiography (from the International Stress Echo Complication Registry)(2006) American Journal of Cardiology, 98 (4), pp. 541 -543. 17 R. Senior – FDA Advisory Board – June 24, 2008 * * *
International Stress Echo Complication Registry 85, 997 patients Complication Dobutamine Dipyridamole Exercise 35103 24599 26295 5 (1/7020) 1 0 Acute MI 11 (1/3191) 5 (1/4919) 1 Sustained VT 27(1/1200) 1 2 Ventricular fibrillation 11(1/3191) 2 0 Cardiac rupture 5 0 1 Asystole 2 4 0 TIA/Stroke 3 3 0 Hypotension 2 4 0 3 rd Degree AV block 2 0 0 Death Safety of Stress Echocardiography (from the International Stress Echo Complication Registry)(2006) American Journal of Cardiology, 98 (4), pp. 541 -543. 17 R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Safety of Sono. Vue Enhanced Stress Echocardiography 5250 patients undergoing dobutamine stress test* • No deaths, No AMI, No life threatening allergic reactions • 2 life threatening events (Ventricular tachycardia/Ventricular fibrillation)(0. 04%) • Patients with EF<0. 40 (N=1153, 22. 0%) showed higher frequency of cardiac arrhythmias of any kind (9. 5%) versus patients with EF>0. 40 (5. 4%, p<0. 001). • 5 vasovagal reactions (0. 1%) • 3 elevation of troponin I (0. 04%) • Dry mouth most frequent symptom (19. 8%) *Aggeli et al. Heart 2008 18 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Safety of Sono. Vue Enhanced Stress Echocardiography 5250 patients undergoing dobutamine stress test* • No deaths, No AMI, No life threatening allergic reactions • 2 life threatening events (Ventricular tachycardia/Ventricular fibrillation)(0. 04%) • Patients with EF<0. 40 (N=1153, 22. 0%) showed higher frequency of cardiac arrhythmias of any kind (9. 5%) versus patients with EF>0. 40 (5. 4%, p<0. 001). • 5 vasovagal reactions (0. 1%) • 3 elevation of troponin I (0. 04%) • Dry mouth most frequent symptom (19. 8%) *Aggeli et al. Heart 2008 18 R. Senior – FDA Advisory Board – June 24, 2008 * * *
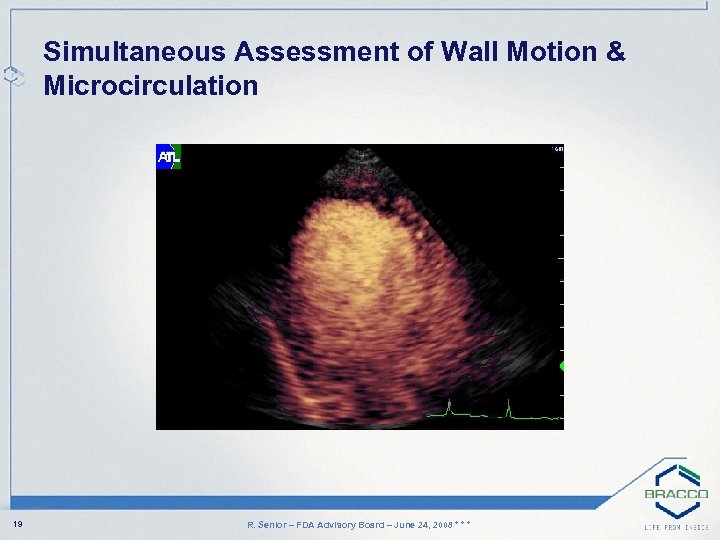 Simultaneous Assessment of Wall Motion & Microcirculation 19 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Simultaneous Assessment of Wall Motion & Microcirculation 19 R. Senior – FDA Advisory Board – June 24, 2008 * * *
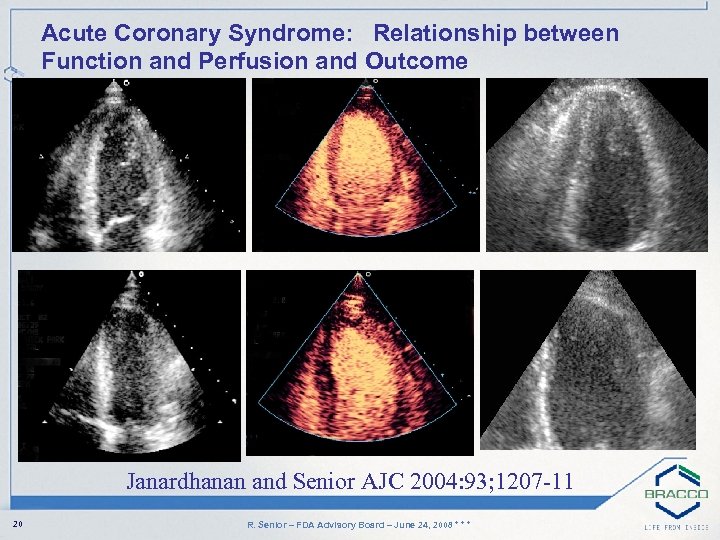 Acute Coronary Syndrome: Relationship between Function and Perfusion and Outcome Janardhanan and Senior AJC 2004: 93; 1207 -11 20 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Acute Coronary Syndrome: Relationship between Function and Perfusion and Outcome Janardhanan and Senior AJC 2004: 93; 1207 -11 20 R. Senior – FDA Advisory Board – June 24, 2008 * * *
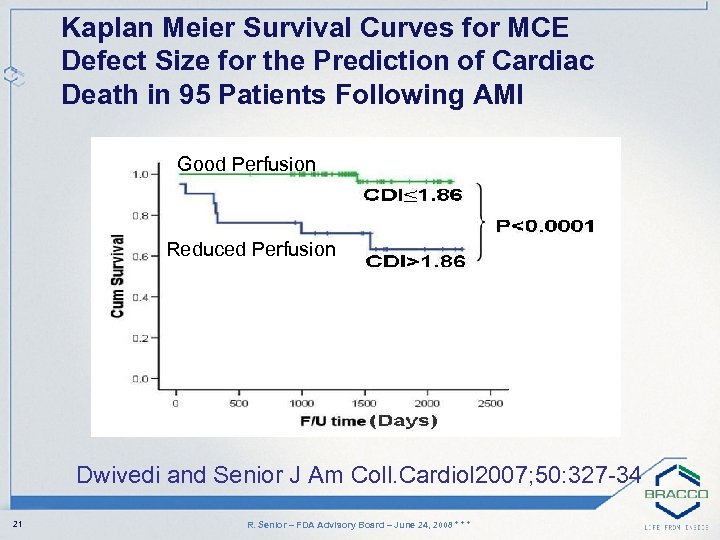 Kaplan Meier Survival Curves for MCE Defect Size for the Prediction of Cardiac Death in 95 Patients Following AMI Good Perfusion Reduced Perfusion Dwivedi and Senior J Am Coll. Cardiol 2007; 50: 327 -34 21 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Kaplan Meier Survival Curves for MCE Defect Size for the Prediction of Cardiac Death in 95 Patients Following AMI Good Perfusion Reduced Perfusion Dwivedi and Senior J Am Coll. Cardiol 2007; 50: 327 -34 21 R. Senior – FDA Advisory Board – June 24, 2008 * * *
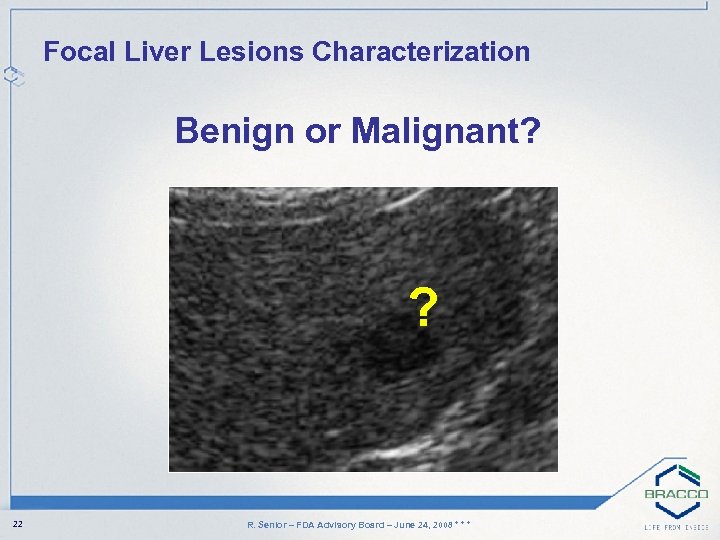 Focal Liver Lesions Characterization Benign or Malignant? ? 22 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Focal Liver Lesions Characterization Benign or Malignant? ? 22 R. Senior – FDA Advisory Board – June 24, 2008 * * *
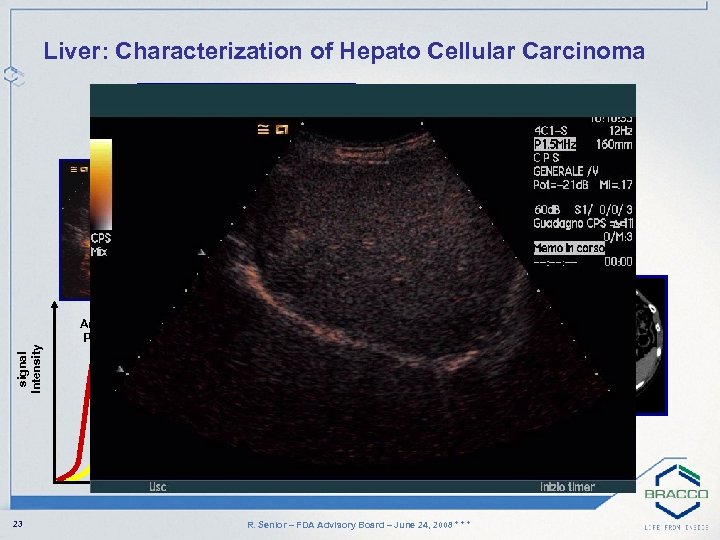 signal Intensity Liver: Characterization of Hepato Cellular Carcinoma Arterial Phase Portal Phase Late Phase time 23 R. Senior – FDA Advisory Board – June 24, 2008 * * *
signal Intensity Liver: Characterization of Hepato Cellular Carcinoma Arterial Phase Portal Phase Late Phase time 23 R. Senior – FDA Advisory Board – June 24, 2008 * * *
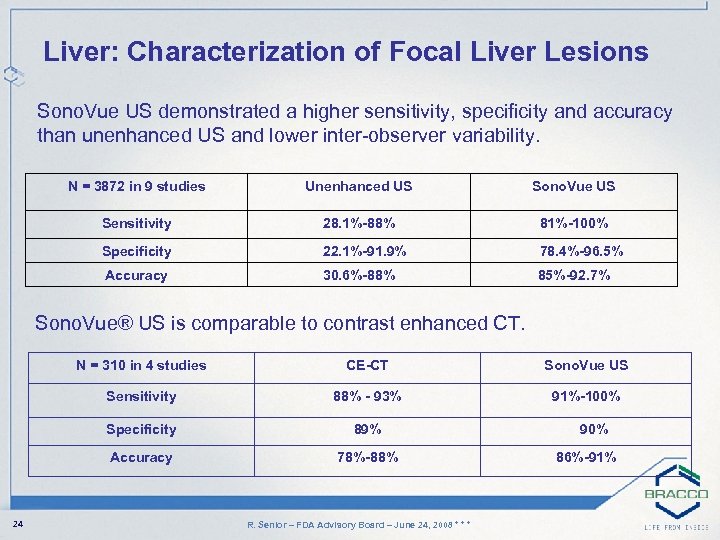 Liver: Characterization of Focal Liver Lesions Sono. Vue US demonstrated a higher sensitivity, specificity and accuracy than unenhanced US and lower inter-observer variability. N = 3872 in 9 studies Unenhanced US Sono. Vue US Sensitivity 28. 1%-88% 81%-100% Specificity 22. 1%-91. 9% 78. 4%-96. 5% Accuracy 30. 6%-88% 85%-92. 7% Sono. Vue® US is comparable to contrast enhanced CT. N = 310 in 4 studies Sono. Vue US Sensitivity 88% - 93% 91%-100% Specificity 89% Accuracy 24 CE-CT 78%-88% R. Senior – FDA Advisory Board – June 24, 2008 * * * 90% 86%-91%
Liver: Characterization of Focal Liver Lesions Sono. Vue US demonstrated a higher sensitivity, specificity and accuracy than unenhanced US and lower inter-observer variability. N = 3872 in 9 studies Unenhanced US Sono. Vue US Sensitivity 28. 1%-88% 81%-100% Specificity 22. 1%-91. 9% 78. 4%-96. 5% Accuracy 30. 6%-88% 85%-92. 7% Sono. Vue® US is comparable to contrast enhanced CT. N = 310 in 4 studies Sono. Vue US Sensitivity 88% - 93% 91%-100% Specificity 89% Accuracy 24 CE-CT 78%-88% R. Senior – FDA Advisory Board – June 24, 2008 * * * 90% 86%-91%
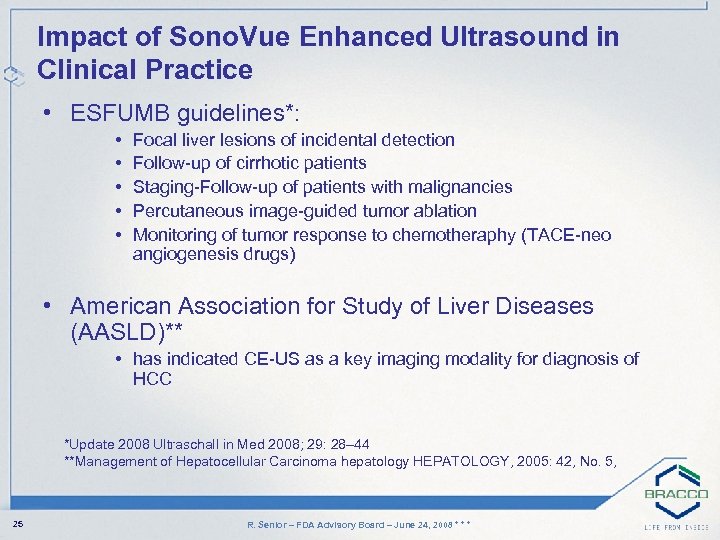 Impact of Sono. Vue Enhanced Ultrasound in Clinical Practice • ESFUMB guidelines*: • • • Focal liver lesions of incidental detection Follow-up of cirrhotic patients Staging-Follow-up of patients with malignancies Percutaneous image-guided tumor ablation Monitoring of tumor response to chemotheraphy (TACE-neo angiogenesis drugs) • American Association for Study of Liver Diseases (AASLD)** • has indicated CE-US as a key imaging modality for diagnosis of HCC *Update 2008 Ultraschall in Med 2008; 29: 28– 44 **Management of Hepatocellular Carcinoma hepatology HEPATOLOGY, 2005: 42, No. 5, 25 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Impact of Sono. Vue Enhanced Ultrasound in Clinical Practice • ESFUMB guidelines*: • • • Focal liver lesions of incidental detection Follow-up of cirrhotic patients Staging-Follow-up of patients with malignancies Percutaneous image-guided tumor ablation Monitoring of tumor response to chemotheraphy (TACE-neo angiogenesis drugs) • American Association for Study of Liver Diseases (AASLD)** • has indicated CE-US as a key imaging modality for diagnosis of HCC *Update 2008 Ultraschall in Med 2008; 29: 28– 44 **Management of Hepatocellular Carcinoma hepatology HEPATOLOGY, 2005: 42, No. 5, 25 R. Senior – FDA Advisory Board – June 24, 2008 * * *
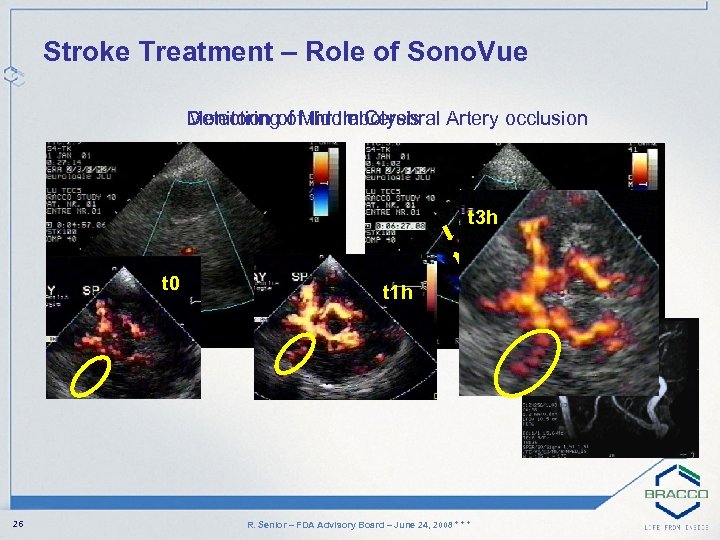 Stroke Treatment – Role of Sono. Vue Monitoring of thrombolysis Detection of Middle Cerebral Artery occlusion t 3 h t 0 native 26 t 1 h contrast R. Senior – FDA Advisory Board – June 24, 2008 * * *
Stroke Treatment – Role of Sono. Vue Monitoring of thrombolysis Detection of Middle Cerebral Artery occlusion t 3 h t 0 native 26 t 1 h contrast R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Safety in Worldwide Bracco Clinical Trials • • • Completed clinical trials: Patients enrolled: Cardiac studies: Microvascular studies: Macrovascular studies: 57 4730 1840 2133 555 Low incidence of adverse events: All: 11. 9%, of which 6. 1% drug related The most frequent were headache (1. 2%) and nausea (0. 7%) Serious: 0. 5% of which 0. 02% drug related. 7 special population studies did not demonstrate any significant clinical signs after Sono. Vue administration 27 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Safety in Worldwide Bracco Clinical Trials • • • Completed clinical trials: Patients enrolled: Cardiac studies: Microvascular studies: Macrovascular studies: 57 4730 1840 2133 555 Low incidence of adverse events: All: 11. 9%, of which 6. 1% drug related The most frequent were headache (1. 2%) and nausea (0. 7%) Serious: 0. 5% of which 0. 02% drug related. 7 special population studies did not demonstrate any significant clinical signs after Sono. Vue administration 27 R. Senior – FDA Advisory Board – June 24, 2008 * * *
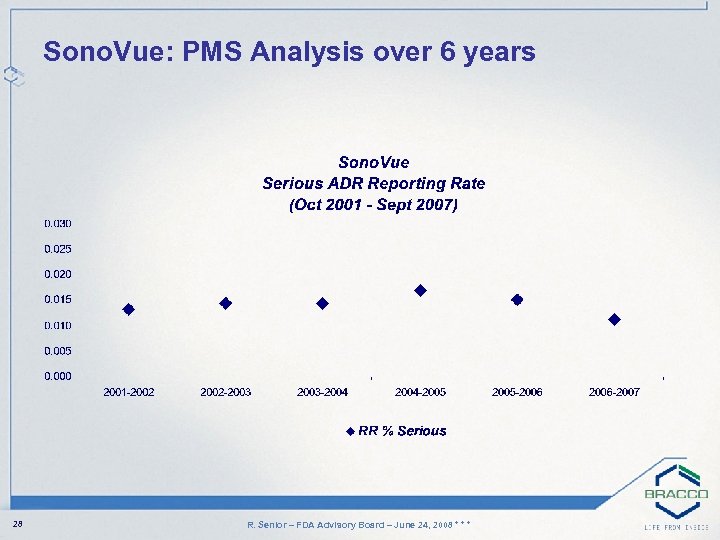 Sono. Vue: PMS Analysis over 6 years 28 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Sono. Vue: PMS Analysis over 6 years 28 R. Senior – FDA Advisory Board – June 24, 2008 * * *
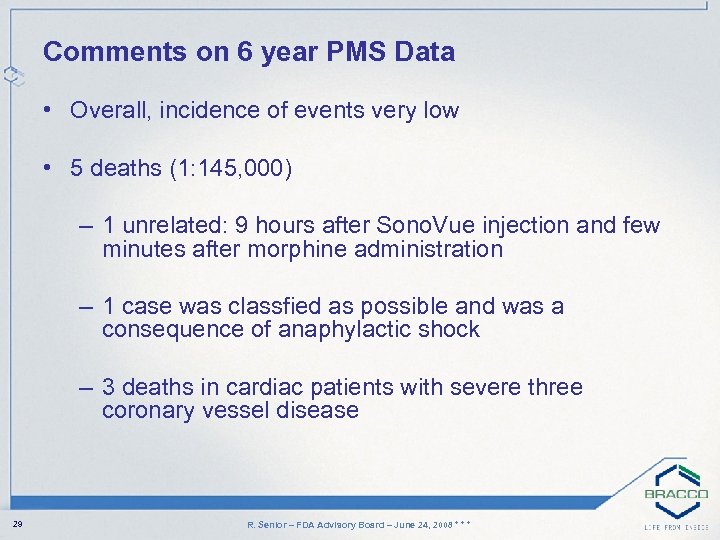 Comments on 6 year PMS Data • Overall, incidence of events very low • 5 deaths (1: 145, 000) – 1 unrelated: 9 hours after Sono. Vue injection and few minutes after morphine administration – 1 case was classfied as possible and was a consequence of anaphylactic shock – 3 deaths in cardiac patients with severe three coronary vessel disease 29 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Comments on 6 year PMS Data • Overall, incidence of events very low • 5 deaths (1: 145, 000) – 1 unrelated: 9 hours after Sono. Vue injection and few minutes after morphine administration – 1 case was classfied as possible and was a consequence of anaphylactic shock – 3 deaths in cardiac patients with severe three coronary vessel disease 29 R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Event Cascade in Majority (76%) of Serious Sono. Vue ADRs • Classified as allergic-like reactions • Independent of dose • Onset: within 1 -2 minutes from injection • First symptom: Hypotension, vasovagal syncope. • Resolve after administration of Adrenaline and/or Cortisone 30 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Event Cascade in Majority (76%) of Serious Sono. Vue ADRs • Classified as allergic-like reactions • Independent of dose • Onset: within 1 -2 minutes from injection • First symptom: Hypotension, vasovagal syncope. • Resolve after administration of Adrenaline and/or Cortisone 30 R. Senior – FDA Advisory Board – June 24, 2008 * * *
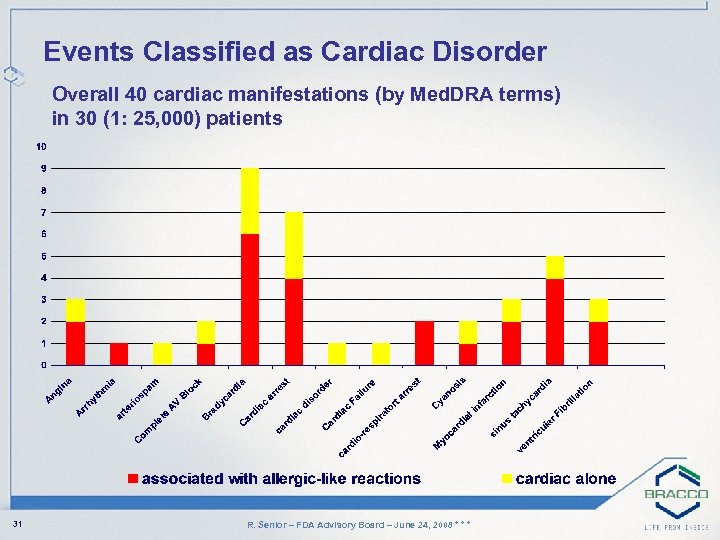 Events Classified as Cardiac Disorder Overall 40 cardiac manifestations (by Med. DRA terms) in 30 (1: 25, 000) patients 31 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Events Classified as Cardiac Disorder Overall 40 cardiac manifestations (by Med. DRA terms) in 30 (1: 25, 000) patients 31 R. Senior – FDA Advisory Board – June 24, 2008 * * *
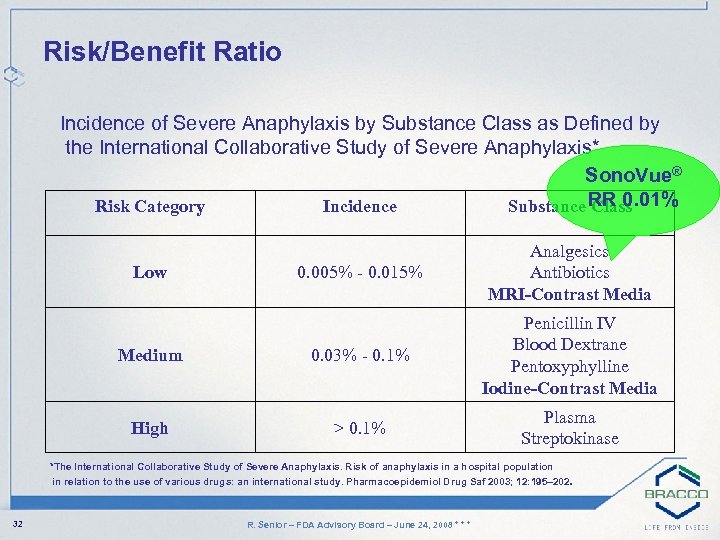 Risk/Benefit Ratio Incidence of Severe Anaphylaxis by Substance Class as Defined by the International Collaborative Study of Severe Anaphylaxis* Sono. Vue® Risk Category Incidence Substance RR 0. 01% Class 0. 005% - 0. 015% Analgesics Antibiotics MRI-Contrast Media Medium 0. 03% - 0. 1% Penicillin IV Blood Dextrane Pentoxyphylline Iodine-Contrast Media High > 0. 1% Plasma Streptokinase Low *The International Collaborative Study of Severe Anaphylaxis. Risk of anaphylaxis in a hospital population in relation to the use of various drugs: an international study. Pharmacoepidemiol Drug Saf 2003; 12: 195– 202. 32 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Risk/Benefit Ratio Incidence of Severe Anaphylaxis by Substance Class as Defined by the International Collaborative Study of Severe Anaphylaxis* Sono. Vue® Risk Category Incidence Substance RR 0. 01% Class 0. 005% - 0. 015% Analgesics Antibiotics MRI-Contrast Media Medium 0. 03% - 0. 1% Penicillin IV Blood Dextrane Pentoxyphylline Iodine-Contrast Media High > 0. 1% Plasma Streptokinase Low *The International Collaborative Study of Severe Anaphylaxis. Risk of anaphylaxis in a hospital population in relation to the use of various drugs: an international study. Pharmacoepidemiol Drug Saf 2003; 12: 195– 202. 32 R. Senior – FDA Advisory Board – June 24, 2008 * * *
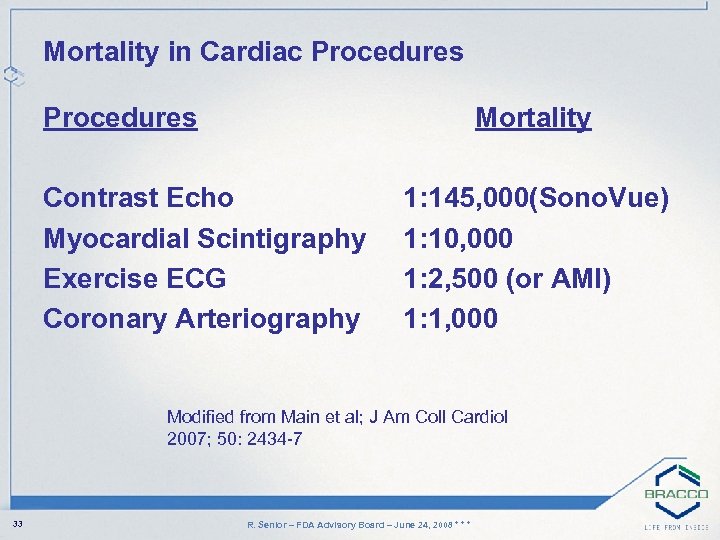 Mortality in Cardiac Procedures Mortality Contrast Echo Myocardial Scintigraphy Exercise ECG Coronary Arteriography 1: 145, 000(Sono. Vue) 1: 10, 000 1: 2, 500 (or AMI) 1: 1, 000 Modified from Main et al; J Am Coll Cardiol 2007; 50: 2434 -7 33 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Mortality in Cardiac Procedures Mortality Contrast Echo Myocardial Scintigraphy Exercise ECG Coronary Arteriography 1: 145, 000(Sono. Vue) 1: 10, 000 1: 2, 500 (or AMI) 1: 1, 000 Modified from Main et al; J Am Coll Cardiol 2007; 50: 2434 -7 33 R. Senior – FDA Advisory Board – June 24, 2008 * * *
 Conclusions • Contrast Enhanced Ultrasound provides incremental diagnostic and prognostic information over standard clinical criteria and may be superior to competing techniques • Sono. Vue safety analysis of Bracco-sponsored clinical trials and 6 years of PMS suggests Sono. Vue falls in the low-risk category • Sono. Vue has excellent risk benefit profile 34 R. Senior – FDA Advisory Board – June 24, 2008 * * *
Conclusions • Contrast Enhanced Ultrasound provides incremental diagnostic and prognostic information over standard clinical criteria and may be superior to competing techniques • Sono. Vue safety analysis of Bracco-sponsored clinical trials and 6 years of PMS suggests Sono. Vue falls in the low-risk category • Sono. Vue has excellent risk benefit profile 34 R. Senior – FDA Advisory Board – June 24, 2008 * * *


