2b0de06ede3e94eb31180744f4c7321c.ppt
- Количество слайдов: 20
 Plant steroids Anna Drew with grateful acknowledgement for inspirational teaching received at The School of Pharmacy, University of London
Plant steroids Anna Drew with grateful acknowledgement for inspirational teaching received at The School of Pharmacy, University of London
 PLANT STEROIDS • Used for: – – – replacement therapy (male +female) athletes (glucocorticoids) skin conditions (hydrocortisone) antifertility pill (oestrogens + progesterones) cancer (breast, testes, prostrate) rheumatoid arthritis • Industrial demand may be met by plant sources or replaced by synthetic sources (expensive)
PLANT STEROIDS • Used for: – – – replacement therapy (male +female) athletes (glucocorticoids) skin conditions (hydrocortisone) antifertility pill (oestrogens + progesterones) cancer (breast, testes, prostrate) rheumatoid arthritis • Industrial demand may be met by plant sources or replaced by synthetic sources (expensive)
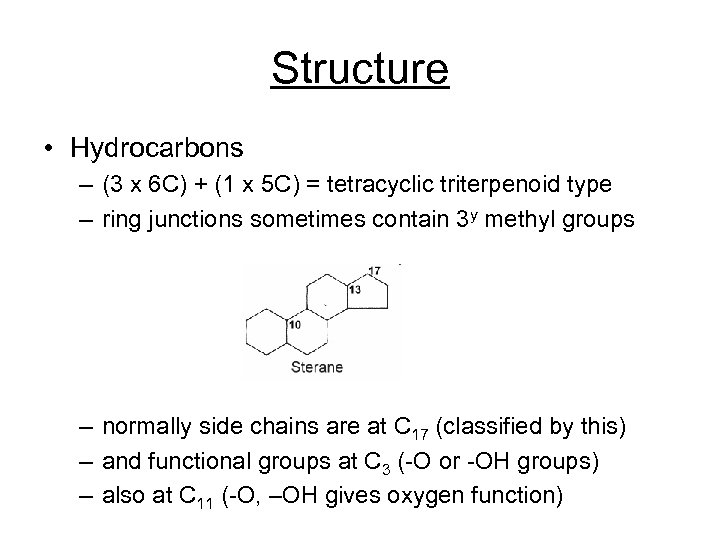 Structure • Hydrocarbons – (3 x 6 C) + (1 x 5 C) = tetracyclic triterpenoid type – ring junctions sometimes contain 3 y methyl groups – normally side chains are at C 17 (classified by this) – and functional groups at C 3 (-O or -OH groups) – also at C 11 (-O, –OH gives oxygen function)
Structure • Hydrocarbons – (3 x 6 C) + (1 x 5 C) = tetracyclic triterpenoid type – ring junctions sometimes contain 3 y methyl groups – normally side chains are at C 17 (classified by this) – and functional groups at C 3 (-O or -OH groups) – also at C 11 (-O, –OH gives oxygen function)
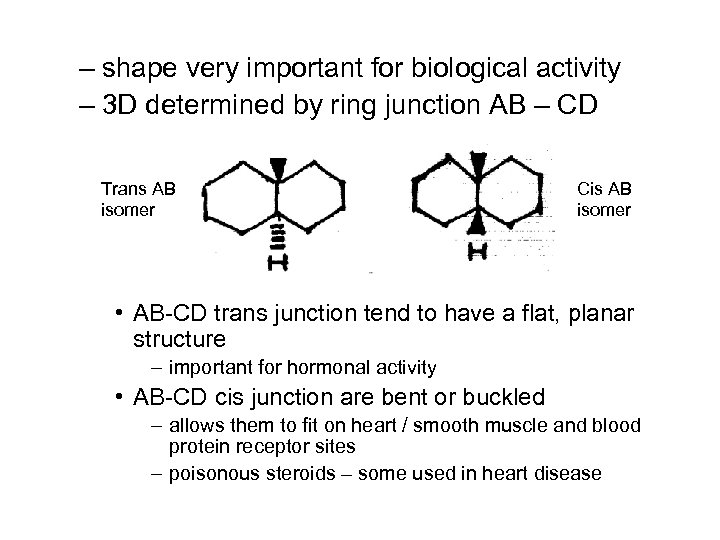 – shape very important for biological activity – 3 D determined by ring junction AB – CD Trans AB isomer Cis AB isomer • AB-CD trans junction tend to have a flat, planar structure – important for hormonal activity • AB-CD cis junction are bent or buckled – allows them to fit on heart / smooth muscle and blood protein receptor sites – poisonous steroids – some used in heart disease
– shape very important for biological activity – 3 D determined by ring junction AB – CD Trans AB isomer Cis AB isomer • AB-CD trans junction tend to have a flat, planar structure – important for hormonal activity • AB-CD cis junction are bent or buckled – allows them to fit on heart / smooth muscle and blood protein receptor sites – poisonous steroids – some used in heart disease
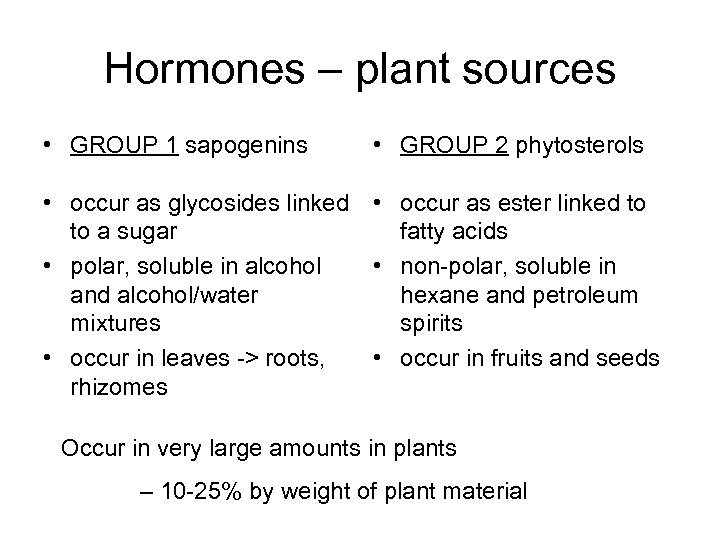 Hormones – plant sources • GROUP 1 sapogenins • GROUP 2 phytosterols • occur as glycosides linked • occur as ester linked to to a sugar fatty acids • polar, soluble in alcohol • non-polar, soluble in and alcohol/water hexane and petroleum mixtures spirits • occur in leaves -> roots, • occur in fruits and seeds rhizomes Occur in very large amounts in plants – 10 -25% by weight of plant material
Hormones – plant sources • GROUP 1 sapogenins • GROUP 2 phytosterols • occur as glycosides linked • occur as ester linked to to a sugar fatty acids • polar, soluble in alcohol • non-polar, soluble in and alcohol/water hexane and petroleum mixtures spirits • occur in leaves -> roots, • occur in fruits and seeds rhizomes Occur in very large amounts in plants – 10 -25% by weight of plant material
 Sapogenins • Sapogenin = steroid nucleus • Saponin = glycosides + sugars – ‘soap-like’ in nature – have been used to poison fish • accumulates in gills preventing O 2 transfer – also frogs and toads • breathe through skin and hence are killed • not poisonous to mammals when eaten – not absorbed in intestine or stomach – may irritate bowel causing diarrhoea – few effects
Sapogenins • Sapogenin = steroid nucleus • Saponin = glycosides + sugars – ‘soap-like’ in nature – have been used to poison fish • accumulates in gills preventing O 2 transfer – also frogs and toads • breathe through skin and hence are killed • not poisonous to mammals when eaten – not absorbed in intestine or stomach – may irritate bowel causing diarrhoea – few effects
 • if injected different – used in arrow poisons – cause haemolysis of red blood cells • breaks down red blood cell membrane • haemoglobinuria • some used as emulsifying agents • interested in the aglycone from a saponin • Saponins occur widely in plants – some economically important ones: Sources: [1] Dioscoreaceae (yam family) • Dioscorea genus – dicots – vines – sweet yam – food source, very low steroid content – bitter yam – Mexico, South America – high content
• if injected different – used in arrow poisons – cause haemolysis of red blood cells • breaks down red blood cell membrane • haemoglobinuria • some used as emulsifying agents • interested in the aglycone from a saponin • Saponins occur widely in plants – some economically important ones: Sources: [1] Dioscoreaceae (yam family) • Dioscorea genus – dicots – vines – sweet yam – food source, very low steroid content – bitter yam – Mexico, South America – high content
![[2] Liliaceae family • monocots – Far East, Phillipines – Smilax or Yucca • [2] Liliaceae family • monocots – Far East, Phillipines – Smilax or Yucca •](https://present5.com/presentation/2b0de06ede3e94eb31180744f4c7321c/image-8.jpg) [2] Liliaceae family • monocots – Far East, Phillipines – Smilax or Yucca • very important since these provide sapogenins for manufacture of corticosteroids [3] Amaryllidaceae • Agave sisalana sisal leaf, East Africa [4] Solanaceae • can be used when supply of [1] and [2] short or too expensive • Solanum sp. contain steroidal saponins – as well as tropane alkaloids, atropine, etc – eg tomato, potato, woody nightshade [5] Scrophulariaceae • Digitalis seeds full of steroids, rich source [6] Leguminosae • Trigonella-foeum-graecum fengreek seed
[2] Liliaceae family • monocots – Far East, Phillipines – Smilax or Yucca • very important since these provide sapogenins for manufacture of corticosteroids [3] Amaryllidaceae • Agave sisalana sisal leaf, East Africa [4] Solanaceae • can be used when supply of [1] and [2] short or too expensive • Solanum sp. contain steroidal saponins – as well as tropane alkaloids, atropine, etc – eg tomato, potato, woody nightshade [5] Scrophulariaceae • Digitalis seeds full of steroids, rich source [6] Leguminosae • Trigonella-foeum-graecum fengreek seed
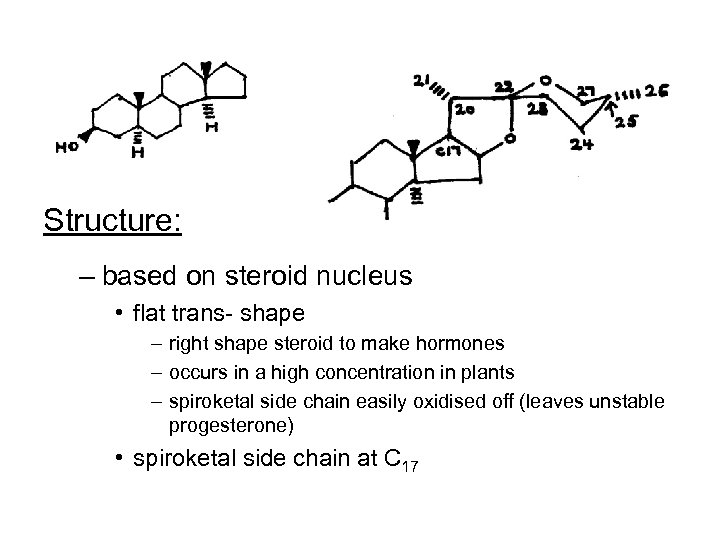 Structure: – based on steroid nucleus • flat trans- shape – right shape steroid to make hormones – occurs in a high concentration in plants – spiroketal side chain easily oxidised off (leaves unstable progesterone) • spiroketal side chain at C 17
Structure: – based on steroid nucleus • flat trans- shape – right shape steroid to make hormones – occurs in a high concentration in plants – spiroketal side chain easily oxidised off (leaves unstable progesterone) • spiroketal side chain at C 17
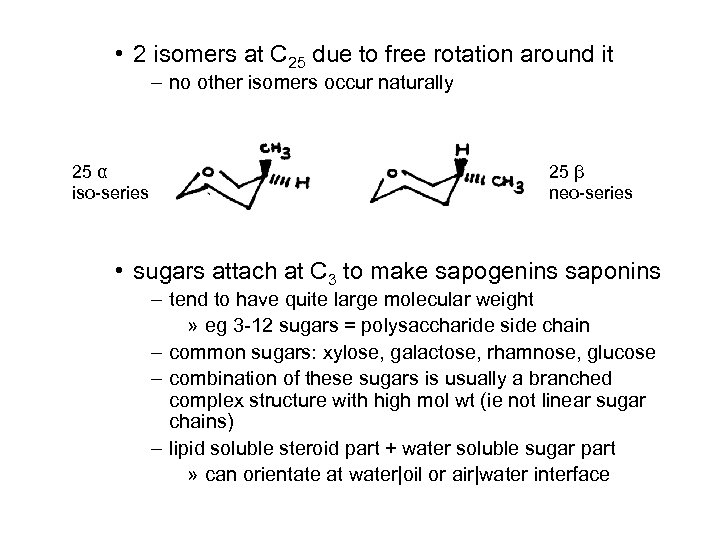 • 2 isomers at C 25 due to free rotation around it – no other isomers occur naturally 25 α iso-series 25 β neo-series • sugars attach at C 3 to make sapogenins saponins – tend to have quite large molecular weight » eg 3 -12 sugars = polysaccharide side chain – common sugars: xylose, galactose, rhamnose, glucose – combination of these sugars is usually a branched complex structure with high mol wt (ie not linear sugar chains) – lipid soluble steroid part + water soluble sugar part » can orientate at water|oil or air|water interface
• 2 isomers at C 25 due to free rotation around it – no other isomers occur naturally 25 α iso-series 25 β neo-series • sugars attach at C 3 to make sapogenins saponins – tend to have quite large molecular weight » eg 3 -12 sugars = polysaccharide side chain – common sugars: xylose, galactose, rhamnose, glucose – combination of these sugars is usually a branched complex structure with high mol wt (ie not linear sugar chains) – lipid soluble steroid part + water soluble sugar part » can orientate at water|oil or air|water interface
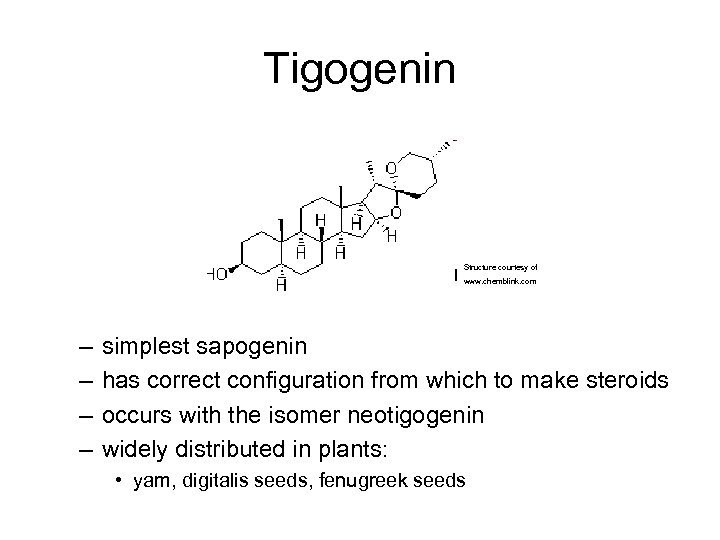 Tigogenin Structure courtesy of www. chemblink. com – – simplest sapogenin has correct configuration from which to make steroids occurs with the isomer neotigogenin widely distributed in plants: • yam, digitalis seeds, fenugreek seeds
Tigogenin Structure courtesy of www. chemblink. com – – simplest sapogenin has correct configuration from which to make steroids occurs with the isomer neotigogenin widely distributed in plants: • yam, digitalis seeds, fenugreek seeds
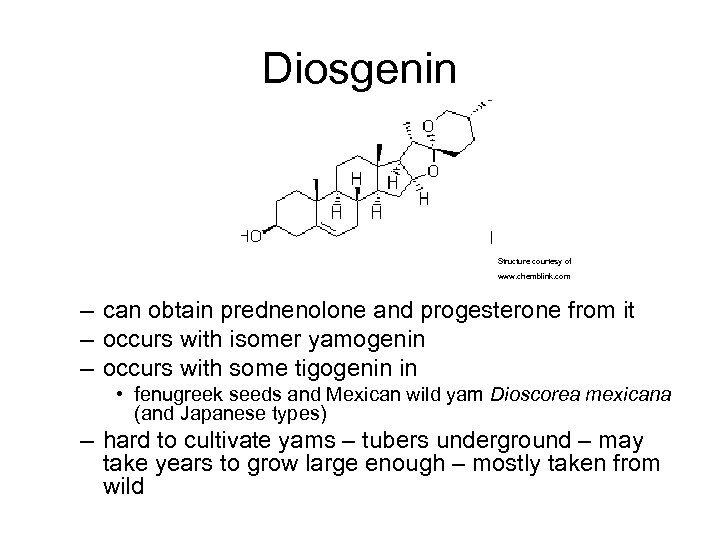 Diosgenin Structure courtesy of www. chemblink. com – can obtain prednenolone and progesterone from it – occurs with isomer yamogenin – occurs with some tigogenin in • fenugreek seeds and Mexican wild yam Dioscorea mexicana (and Japanese types) – hard to cultivate yams – tubers underground – may take years to grow large enough – mostly taken from wild
Diosgenin Structure courtesy of www. chemblink. com – can obtain prednenolone and progesterone from it – occurs with isomer yamogenin – occurs with some tigogenin in • fenugreek seeds and Mexican wild yam Dioscorea mexicana (and Japanese types) – hard to cultivate yams – tubers underground – may take years to grow large enough – mostly taken from wild
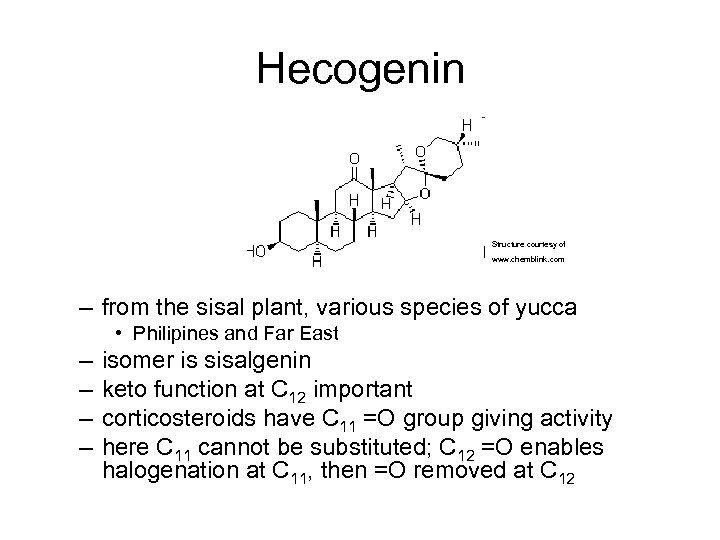 Hecogenin Structure courtesy of www. chemblink. com – from the sisal plant, various species of yucca • Philipines and Far East – – isomer is sisalgenin keto function at C 12 important corticosteroids have C 11 =O group giving activity here C 11 cannot be substituted; C 12 =O enables halogenation at C 11, then =O removed at C 12
Hecogenin Structure courtesy of www. chemblink. com – from the sisal plant, various species of yucca • Philipines and Far East – – isomer is sisalgenin keto function at C 12 important corticosteroids have C 11 =O group giving activity here C 11 cannot be substituted; C 12 =O enables halogenation at C 11, then =O removed at C 12
 Commercial extraction • sources crushed – tubers – yams; seeds – fenugreek, digitalis; leaves – sisal • fermentation – add excess water in fermenting vat and leave 24 -48 hour – saponins are covalently bonded into cellulose wall – own enzymes act on the polysaccharides in the cell wall to liberate them • filtration to collect plant powder • acid hydrolysis to split off saponins from sapogenins – equal HCL, Me. OH, H 2 O
Commercial extraction • sources crushed – tubers – yams; seeds – fenugreek, digitalis; leaves – sisal • fermentation – add excess water in fermenting vat and leave 24 -48 hour – saponins are covalently bonded into cellulose wall – own enzymes act on the polysaccharides in the cell wall to liberate them • filtration to collect plant powder • acid hydrolysis to split off saponins from sapogenins – equal HCL, Me. OH, H 2 O
 • plant material dried in an oven • Soxhlet extraction with petroleum spirit – to distill over saponins – crystallise out in receiver – 10 g/100 g yam tuber – high yield – economic • recrystallise – using various solvents depending on desired compound – can be carried out on a large scale – cheap • no chromatographic process • materials cheap (H 2 O, HCl) – petroleum spirit can be recovered • recrystallisation expensive but gives a high yield • p’ceutical companies will buy compounds in pure
• plant material dried in an oven • Soxhlet extraction with petroleum spirit – to distill over saponins – crystallise out in receiver – 10 g/100 g yam tuber – high yield – economic • recrystallise – using various solvents depending on desired compound – can be carried out on a large scale – cheap • no chromatographic process • materials cheap (H 2 O, HCl) – petroleum spirit can be recovered • recrystallisation expensive but gives a high yield • p’ceutical companies will buy compounds in pure
 Analysis of plant material • Qualitative: – TLC on sulphuric acid to indicate spot position (chloroform solvent) • Quantitative: – i) colorimetric assay – sulphuric acid produces orange colour with steroids – ii) IR spectrometry – 960 cm-1 – need a lot of plant material – iii) GLC micromethod – draw up assay with suitable standard and do many samples in one day – quickest – qualitative and quantitative
Analysis of plant material • Qualitative: – TLC on sulphuric acid to indicate spot position (chloroform solvent) • Quantitative: – i) colorimetric assay – sulphuric acid produces orange colour with steroids – ii) IR spectrometry – 960 cm-1 – need a lot of plant material – iii) GLC micromethod – draw up assay with suitable standard and do many samples in one day – quickest – qualitative and quantitative
 Commerical use • production of steroids from diosgenin • before 1940 isolate from animal glands or urine – expensive • 1940 Marker (USA) discovered a process – essentially same process is still used • diosgenin extracted from Mexican yam • then spiroketone chain is opened up. .
Commerical use • production of steroids from diosgenin • before 1940 isolate from animal glands or urine – expensive • 1940 Marker (USA) discovered a process – essentially same process is still used • diosgenin extracted from Mexican yam • then spiroketone chain is opened up. .
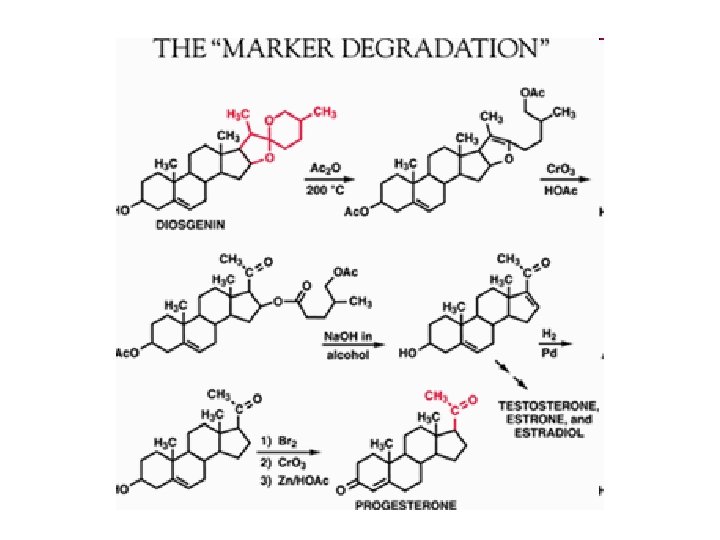
 • in theory process gave 100% yield • progesterone known to prevent ovulation – tried to produce ‘the pill’ (1950) • now have combination pills • 1950 -1960 corticosteroids needed – antiinflammatory, anticancer, antirheumatoid • hydrocortisone and cortisone (which can be fluorinated) couldn’t be produced from progesterone [1] Fermentation – – arose by accident when making antibiotics from Rhizopus needed steroids in medium to grow produced 11 keto progesterone analysed fermentation to confirm pregnenolone / progesterone were producing 11 keto progesterone (from which hydrocortisone can be made) – biotechnology expensive
• in theory process gave 100% yield • progesterone known to prevent ovulation – tried to produce ‘the pill’ (1950) • now have combination pills • 1950 -1960 corticosteroids needed – antiinflammatory, anticancer, antirheumatoid • hydrocortisone and cortisone (which can be fluorinated) couldn’t be produced from progesterone [1] Fermentation – – arose by accident when making antibiotics from Rhizopus needed steroids in medium to grow produced 11 keto progesterone analysed fermentation to confirm pregnenolone / progesterone were producing 11 keto progesterone (from which hydrocortisone can be made) – biotechnology expensive
![[2] Hecogenins – more economical 11 keto progesterone hydrocortisone Marker 11 keto diosgenin tautomers [2] Hecogenins – more economical 11 keto progesterone hydrocortisone Marker 11 keto diosgenin tautomers](https://present5.com/presentation/2b0de06ede3e94eb31180744f4c7321c/image-20.jpg) [2] Hecogenins – more economical 11 keto progesterone hydrocortisone Marker 11 keto diosgenin tautomers – NB Diosgenin can be used to produce hydrocortisone but a fermentation stage is needed to introduce O- at C 11 keto α position of pregnane nucleus
[2] Hecogenins – more economical 11 keto progesterone hydrocortisone Marker 11 keto diosgenin tautomers – NB Diosgenin can be used to produce hydrocortisone but a fermentation stage is needed to introduce O- at C 11 keto α position of pregnane nucleus


