3628ff2603a6bbae12b3b74ee4d27abc.ppt
- Количество слайдов: 20

Plant-cell-expressed recombinant glucocerebrosidase: taliglucerase-alfa a therapy for Gaucher disease in patients previously treated with imiglucerase. Gregory M. Pastores 1; Jeffrey Szer 2; Milan Petakov 3; Tim Cox 4; Pilar Giraldo 5; Hanna Rosenbaum 6; Dominick Amato 7; Eugen Mengel 8; Raul Chertkoff 9 ; Einat Almon-Brill 9; Ari Zimran 10 NYU School of Medicine, New York USA; 2 Royal Melbourne Hospital, Australia; 3 Clinical Center of Serbia, Belgrade, Serbia; 4 Addenbrooke’s Hospital, Cambridge, UK; 5 Hospital Universitario Miguel Servet Zaragoza, Spain; 6 Rambam Medical center, Haifa, Israel; 7 Mount Sinai Hospital, Toronto, Canada; 8 University Mainz, Germany; 9 Protalix Biotherapeutics, Carmiel, Israel; 10 Shaare Zedek Medical Center, Jerusalem, Israel. 1 10 th EWGGD Meeting Paris June 2012

Disclosure GM PastoresNYU Neurogenetics is the recipient of research grantssupport from Actelion, AmicusGSK, Biomarin, GenzymeSanofi, ProtalixPfizer, Shire HGT, Synegeva and Ultragenics; pharmaceuticalbiotechnology companies engaged in drug development programs for the Lysosomal storage disorders

Taliglucerase alfa* Plant cell expressed human recombinant Glucocerebrosidase Produced in disposable bioreactors Expression process is free of any mammalian components As of May 1, 2012, approved by the US Food and Drug Administration as an ERT for Gaucher disease * Y. Shaaltiel el al; , Plant Biotechnology J. , September 2007, volume 5, Issue 5 * D. Aviezer et al; , 1 March 2009 Volume 4, Issue 3

A Phase 3 Multicenter, Open-label, Switchover Trial to Assess the Safety and Efficacy of taliglucerase alfa in Patients with Gaucher Disease Treated with Imiglucerase (Cerezyme®) Enzyme Replacement Therapy PB-06 -002

Objective § Assess the safety and efficacy of taliglucerase alfa in patients with Gaucher disease who are currently being treated with imiglucerase enzyme replacement therapy (ERT).
Sites Investigator Institution Ari Zimran Shaare Zedek Medical Center, Jerusalem, Israel Dominick Amato Mount Sinai Hospital, Toronto, Canada Eugen Mengel University Mainz, Germany Gregory Pastores NYU School of Medicine, New York USA Hanna Rosenbaum Rambam Medical center, Haifa, Israel Jeffrey Szer Royal Melbourne Hospital, Australia Milan Petakov Clinical Center of Serbia, Belgrade, Serbia Paul Fernhoff Emory University School of Medicine, GA USA Pilar Giraldo Hospital Universitario Miguel Servet, Zaragoza, Spain Tim Cox Addenbrooke’s Hospital, Cambridge, UK
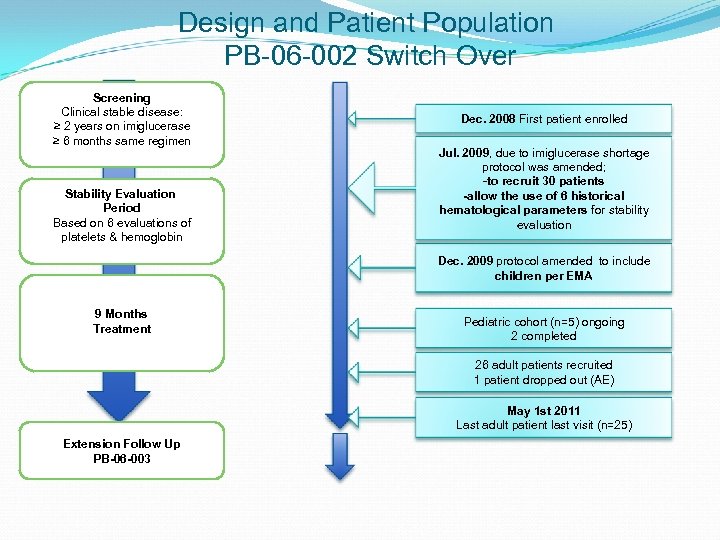
Design and Patient Population PB-06 -002 Switch Over Screening Clinical stable disease: ≥ 2 years on imiglucerase ≥ 6 months same regimen Stability Evaluation Period Based on 6 evaluations of platelets & hemoglobin Dec. 2008 First patient enrolled Jul. 2009, due to imiglucerase shortage protocol was amended; -to recruit 30 patients -allow the use of 6 historical hematological parameters for stability evaluation Dec. 2009 protocol amended to include children per EMA 9 Months Treatment Pediatric cohort (n=5) ongoing 2 completed 26 adult patients recruited 1 patient dropped out (AE) May 1 st 2011 Last adult patient last visit (n=25) Extension Follow Up PB-06 -003

Disease Stability Evaluation Hematological Stability Evaluation Parameters: 12 weeks while on imiglucerase or 6 historical values for those patients impacted by ERT shortage No major surgery in the last year “Clinically Relevant Deterioration” Criteria Protocol Defined Criteria Sustained reduction of platelet count: A decrease of >20% from the mean value of the Stability Evaluation Period values of ≤ 120, 000 or a decrease of >40% from the mean value of the Stability Evaluation Period values of >120, 000 No blood transfusion or major bleeding episode in the last year Sustained reduction of hemoglobin: No acute avascular necrosis event in the last year Increase in spleen volume: No evidence of spleen or liver increasing enlargement Increase in liver volume: A decrease of >20% from the mean value of the Stability Evaluation Period A 20% increase in spleen volume by MRI from Baseline to Month 9 A 10% increase in liver volume by MRI from Baseline to Month 9
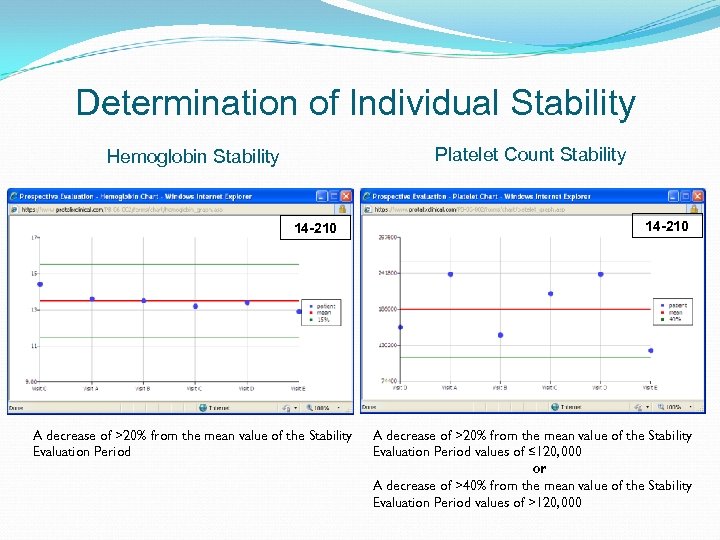
Determination of Individual Stability Platelet Count Stability Hemoglobin Stability 14 -210 A decrease of >20% from the mean value of the Stability Evaluation Period values of ≤ 120, 000 or A decrease of >40% from the mean value of the Stability Evaluation Period values of >120, 000
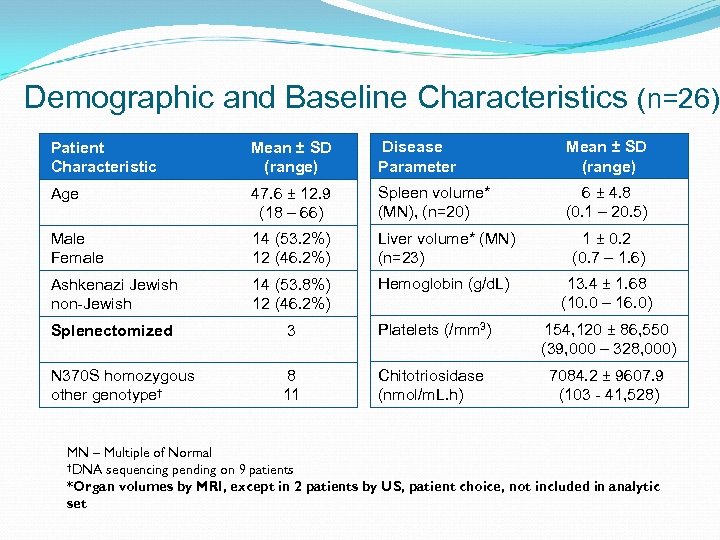
Demographic and Baseline Characteristics (n=26) Patient Characteristic Mean ± SD (range) Disease Parameter Mean ± SD (range) Age 47. 6 ± 12. 9 (18 – 66) Spleen volume* (MN), (n=20) 6 ± 4. 8 (0. 1 – 20. 5) Male Female 14 (53. 2%) 12 (46. 2%) Liver volume* (MN) (n=23) 1 ± 0. 2 (0. 7 – 1. 6) Ashkenazi Jewish non-Jewish 14 (53. 8%) 12 (46. 2%) Hemoglobin (g/d. L) 13. 4 ± 1. 68 (10. 0 – 16. 0) Splenectomized 3 Platelets (/mm 3) 154, 120 ± 86, 550 (39, 000 – 328, 000) N 370 S homozygous other genotype† 8 11 Chitotriosidase (nmol/m. L. h) 7084. 2 ± 9607. 9 (103 - 41, 528) MN – Multiple of Normal †DNA sequencing pending on 9 patients *Organ volumes by MRI, except in 2 patients by US, patient choice, not included in analytic set
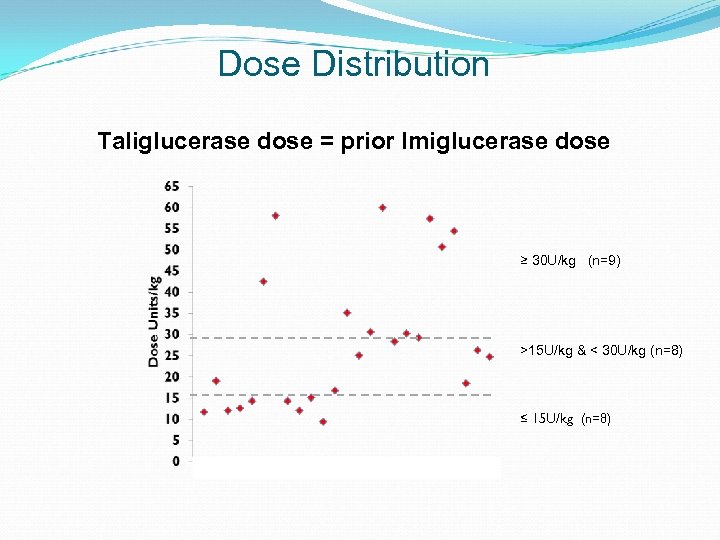
Dose Distribution Taliglucerase dose = prior Imiglucerase dose ≥ 30 U/kg (n=9) >15 U/kg & < 30 U/kg (n=8) ≤ 15 U/kg (n=8)
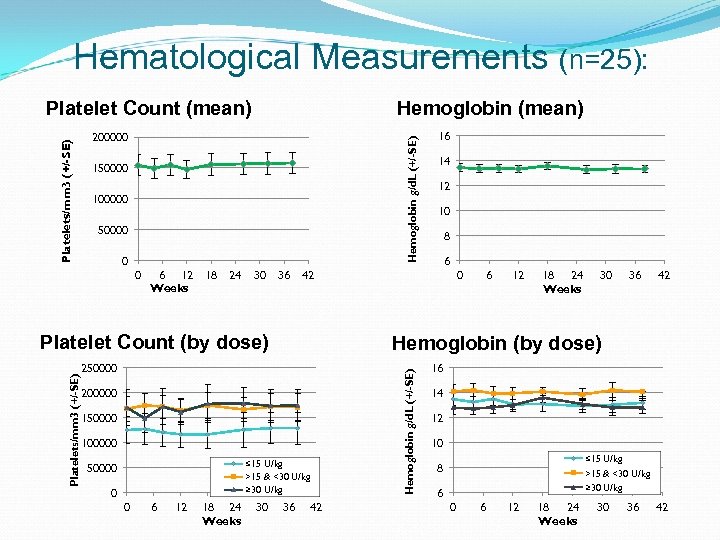
Hematological Measurements (n=25): Hemoglobin (mean) Hemoglobin g/d. L (+/-SE) 200000 150000 100000 50000 0 0 6 12 Weeks 18 24 30 36 Platelets/mm 3 (+/-SE) Platelet Count (by dose) 200000 150000 100000 ≤ 15 U/kg >15 & <30 U/kg ≥ 30 U/kg 0 0 6 12 18 24 Weeks 30 14 12 10 8 6 0 6 12 18 24 Weeks 30 36 42 Hemoglobin (by dose) 250000 16 42 36 42 Hemoglobin g/d. L (+/-SE) Platelets/mm 3 (+/-SE) Platelet Count (mean) 16 14 12 10 ≤ 15 U/kg >15 & <30 U/kg ≥ 30 U/kg 8 6 0 6 12 18 24 Weeks 30 36 42
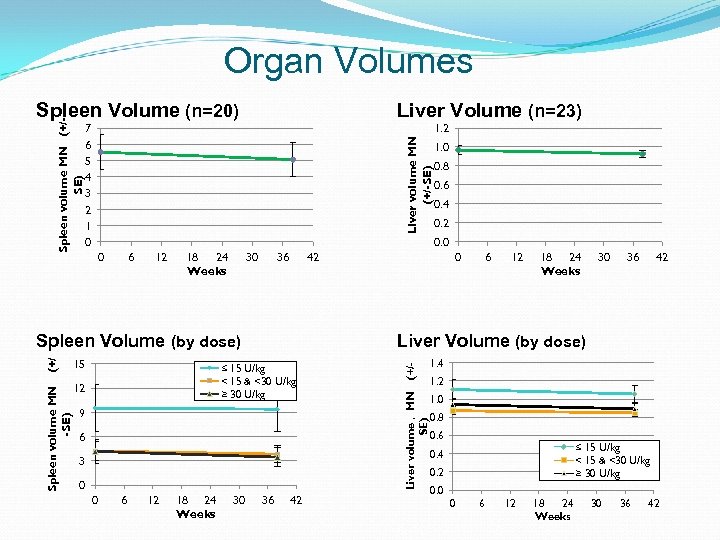
Organ Volumes Liver Volume (n=23) 1. 2 Liver volume MN (+/-SE) 7 6 5 4 3 2 1 0 6 12 18 24 Weeks 30 36 (+/ 0. 8 0. 6 0. 4 0. 2 42 15 12 9 6 3 0 6 12 18 24 Weeks 30 36 42 Liver Volume (by dose) ≤ 15 U/kg < 15 & <30 U/kg ≥ 30 U/kg 0 0 36 42 (+/- 0 Spleen Volume (by dose) Spleen volume MN -SE) 1. 0 0. 0 Liver volume , MN SE) Spleen volume MN (+/SE) Spleen Volume (n=20) 1. 4 1. 2 1. 0 0. 8 0. 6 ≤ 15 U/kg < 15 & <30 U/kg ≥ 30 U/kg 0. 4 0. 2 0. 0 0 6 12 18 24 Weeks 30 36 42
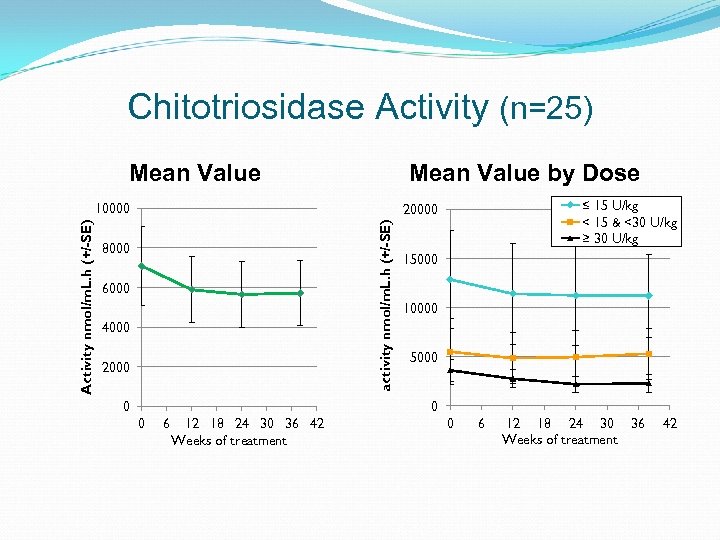
Chitotriosidase Activity (n=25) Mean Value by Dose ≤ 15 U/kg < 15 & <30 U/kg ≥ 30 U/kg 20000 activity nmol/m. L. h (+/-SE) Activity nmol/m. L. h (+/-SE) 10000 8000 6000 4000 2000 15000 10000 5000 0 6 12 18 24 30 36 42 Weeks of treatment 0 6 12 18 24 30 36 Weeks of treatment 42
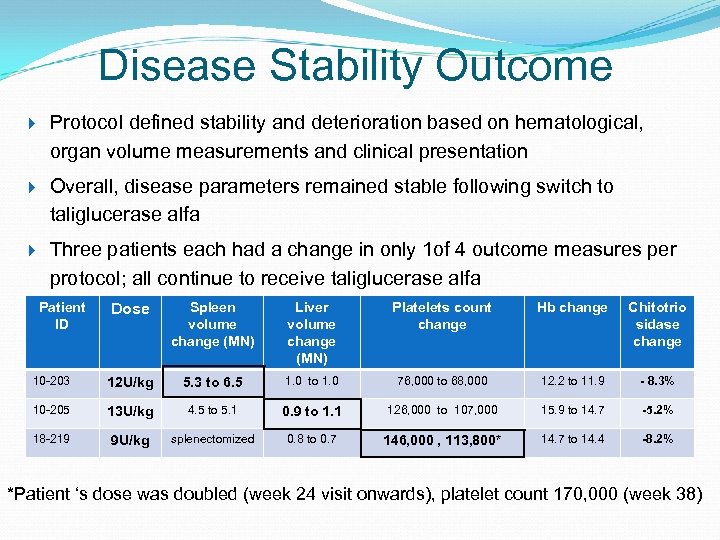
Disease Stability Outcome Protocol defined stability and deterioration based on hematological, organ volume measurements and clinical presentation Overall, disease parameters remained stable following switch to taliglucerase alfa Three patients each had a change in only 1 of 4 outcome measures per protocol; all continue to receive taliglucerase alfa Patient ID Dose Spleen volume change (MN) Liver volume change (MN) Platelets count change Hb change Chitotrio sidase change 10 -203 12 U/kg 5. 3 to 6. 5 1. 0 to 1. 0 76, 000 to 68, 000 12. 2 to 11. 9 - 8. 3% 10 -205 13 U/kg 4. 5 to 5. 1 0. 9 to 1. 1 126, 000 to 107, 000 15. 9 to 14. 7 -5. 2% 18 -219 9 U/kg splenectomized 0. 8 to 0. 7 146, 000 , 113, 800* 14. 7 to 14. 4 -8. 2% *Patient ‘s dose was doubled (week 24 visit onwards), platelet count 170, 000 (week 38)
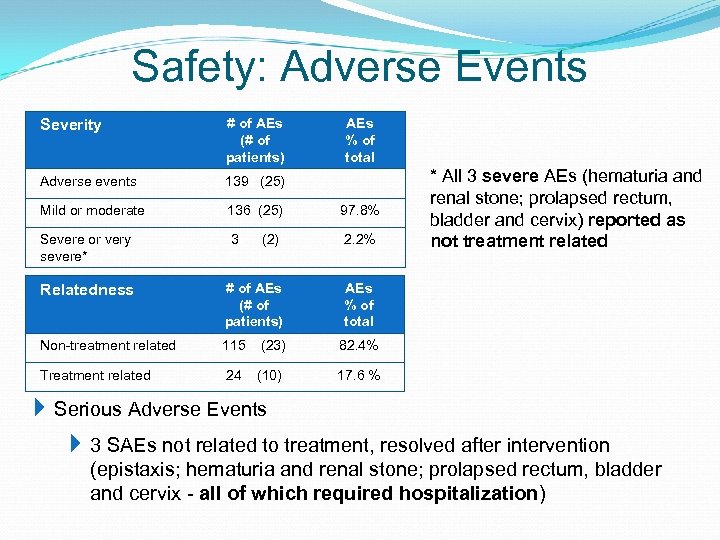
Safety: Adverse Events Severity # of AEs (# of patients) Adverse events 139 (25) Mild or moderate 136 (25) 97. 8% Severe or very severe* 3 (2) 2. 2% Relatedness # of AEs (# of patients) AEs % of total Non-treatment related 115 (23) 82. 4% Treatment related 24 (10) 17. 6 % AEs % of total * All 3 severe AEs (hematuria and renal stone; prolapsed rectum, bladder and cervix) reported as not treatment related Serious Adverse Events 3 SAEs not related to treatment, resolved after intervention (epistaxis; hematuria and renal stone; prolapsed rectum, bladder and cervix - all of which required hospitalization)
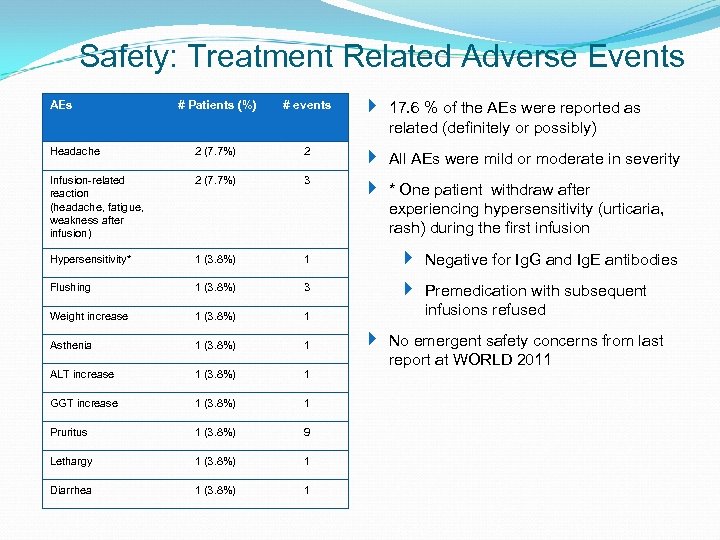
Safety: Treatment Related Adverse Events # Patients (%) # events 17. 6 % of the AEs were reported as related (definitely or possibly) Headache 2 (7. 7%) 2 All AEs were mild or moderate in severity Infusion-related reaction (headache, fatigue, weakness after infusion) 2 (7. 7%) 3 * One patient withdraw after experiencing hypersensitivity (urticaria, rash) during the first infusion Hypersensitivity* 1 (3. 8%) 1 Negative for Ig. G and Ig. E antibodies Flushing 1 (3. 8%) 3 Weight increase 1 (3. 8%) 1 Premedication with subsequent infusions refused Asthenia 1 (3. 8%) 1 ALT increase 1 (3. 8%) 1 GGT increase 1 (3. 8%) 1 Pruritus 1 (3. 8%) 9 Lethargy 1 (3. 8%) 1 Diarrhea 1 (3. 8%) 1 AEs No emergent safety concerns from last report at WORLD 2011
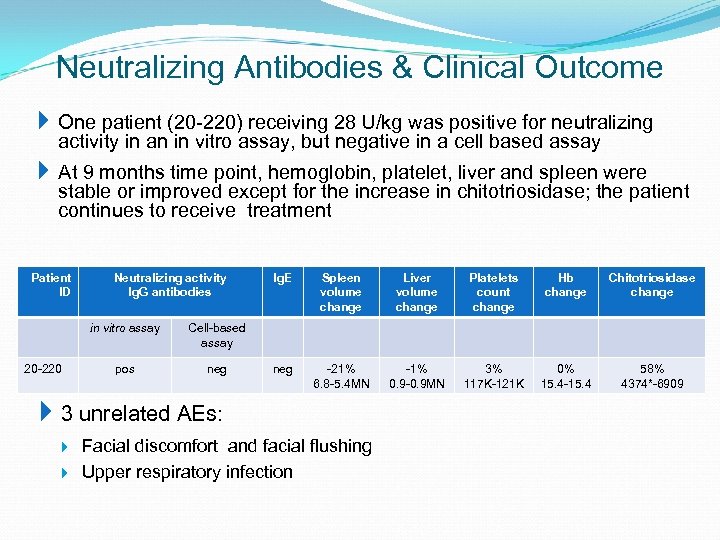
Neutralizing Antibodies & Clinical Outcome One patient (20 -220) receiving 28 U/kg was positive for neutralizing activity in an in vitro assay, but negative in a cell based assay At 9 months time point, hemoglobin, platelet, liver and spleen were stable or improved except for the increase in chitotriosidase; the patient continues to receive treatment Patient ID Neutralizing activity Ig. G antibodies in vitro assay 20 -220 neg Spleen volume change Liver volume change Platelets count change Hb change Chitotriosidase change neg -21% 6. 8 -5. 4 MN -1% 0. 9 -0. 9 MN 3% 117 K-121 K 0% 15. 4 -15. 4 58% 4374*-6909 Cell-based assay pos Ig. E 3 unrelated AEs: Facial discomfort and facial flushing Upper respiratory infection
Summary – Switched Patients Efficacy Overall, patients remained stable with regards to the main disease parameters (hemoglobin, platelet count, spleen and liver volume and chitotriosidase) after switching from imiglucerase to taliglucerase alfa Safety All AEs were mild or moderate and transient in nature, none of the patients are receiving premedication All patients continue to receive taliglucerase except the one patient with hypersensitivity who declined to continue infusions with premedication This study demonstrates that taliglucerase alfa has the potential to be an alternative treatment for Gaucher disease

Thank You
3628ff2603a6bbae12b3b74ee4d27abc.ppt