cb5c493c64064475e359e36e8fcb5420.ppt
- Количество слайдов: 10
 Plans for International Standard for HIV-2 RNA and 2 nd Second International Reference Panel for HIV-1 RNA Genotypes Harvey Holmes, Clare Morris and Neil Berry Division of Retrovirology NIBSC, UK (In collaboration with Indira Hewlett, CBER/FDA)
Plans for International Standard for HIV-2 RNA and 2 nd Second International Reference Panel for HIV-1 RNA Genotypes Harvey Holmes, Clare Morris and Neil Berry Division of Retrovirology NIBSC, UK (In collaboration with Indira Hewlett, CBER/FDA)
 Current HIV Reference Reagents Available from NIBSC • 2 nd International Standard for HIV-1 RNA. (code 97/650) – Unitage 5. 56 IU Log 10/ml • International Reference Panel for HIV-1 RNA Genotypes (code 01/466) established in 2003 – No unitage assigned • Working reagents for NAT – Working Reagent 1 – medium copy number (3. 56 log 10 IU/ml) – Working Reagent 2 – high copy number (4. 56 log 10 IU/ml) – Working Reagent 3 – low copy number (2. 56 log 10 IU/ml) • Working Reagents and Proficiency Samples can also be made at NIBSC under contract to a pre-defined concentration and using various HIV-1 strains and genotypes
Current HIV Reference Reagents Available from NIBSC • 2 nd International Standard for HIV-1 RNA. (code 97/650) – Unitage 5. 56 IU Log 10/ml • International Reference Panel for HIV-1 RNA Genotypes (code 01/466) established in 2003 – No unitage assigned • Working reagents for NAT – Working Reagent 1 – medium copy number (3. 56 log 10 IU/ml) – Working Reagent 2 – high copy number (4. 56 log 10 IU/ml) – Working Reagent 3 – low copy number (2. 56 log 10 IU/ml) • Working Reagents and Proficiency Samples can also be made at NIBSC under contract to a pre-defined concentration and using various HIV-1 strains and genotypes
 • Rationale International Standard for HIV-2 RNA – Global HIV pandemic due to HIV-1 – Most NAT assays aimed at HIV-1 detection and quantification – HIV-2 mainly found in West Africa and European countries with close links – eg Portugal, France, Belgium. – Assays capable of detecting HIV-2 RNA or both HIV-1/HIV-2 are either available (eg Roche Cobas Taqscreen MPX) or in development – International Standard for the HIV-2 RNA component would be valuable once assays in wider use • Anticipated need/usage – Currently limited demand – Once commercial assays that can detect HIV-2 more available, demand may be greater • WHO Status – New project – endorsed by ECBS in October 2006 – Discussed at meeting of WHO Collaborating Centres in Jan 07
• Rationale International Standard for HIV-2 RNA – Global HIV pandemic due to HIV-1 – Most NAT assays aimed at HIV-1 detection and quantification – HIV-2 mainly found in West Africa and European countries with close links – eg Portugal, France, Belgium. – Assays capable of detecting HIV-2 RNA or both HIV-1/HIV-2 are either available (eg Roche Cobas Taqscreen MPX) or in development – International Standard for the HIV-2 RNA component would be valuable once assays in wider use • Anticipated need/usage – Currently limited demand – Once commercial assays that can detect HIV-2 more available, demand may be greater • WHO Status – New project – endorsed by ECBS in October 2006 – Discussed at meeting of WHO Collaborating Centres in Jan 07
 Key Scientific issues • Few commercial assays available that can detect/quantitate HIV-2 RNA • At NIBSC, in house real-time PCR assay based on LTR sequence used for HIV-2 quantification • Source/Type of Material – To be based on HIV-2 grown in culture and diluted in negative plasma – Currently assessing representatives of HIV-2 subtype A and subtype B – Suitable strain(s) to be selected and stock laid down • • Virus stock to be characterised and sequence confirmed Heat inactivation to be evaluated Batch to be freeze-dried International collaborative study to be organised
Key Scientific issues • Few commercial assays available that can detect/quantitate HIV-2 RNA • At NIBSC, in house real-time PCR assay based on LTR sequence used for HIV-2 quantification • Source/Type of Material – To be based on HIV-2 grown in culture and diluted in negative plasma – Currently assessing representatives of HIV-2 subtype A and subtype B – Suitable strain(s) to be selected and stock laid down • • Virus stock to be characterised and sequence confirmed Heat inactivation to be evaluated Batch to be freeze-dried International collaborative study to be organised
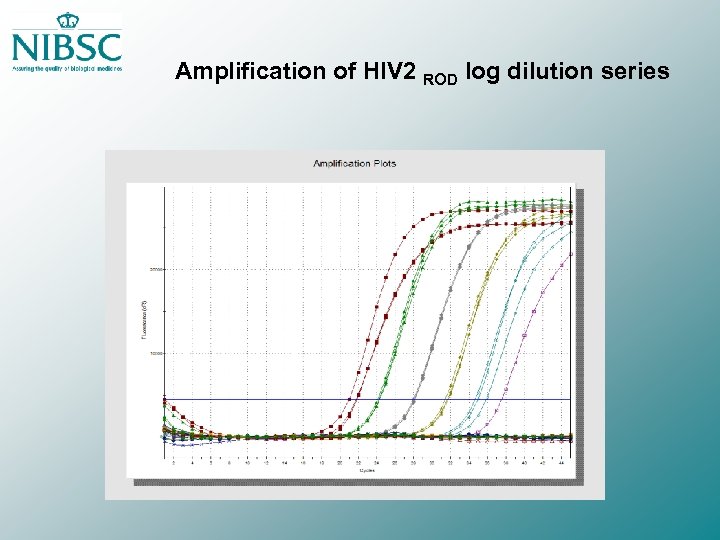 Amplification of HIV 2 ROD log dilution series
Amplification of HIV 2 ROD log dilution series
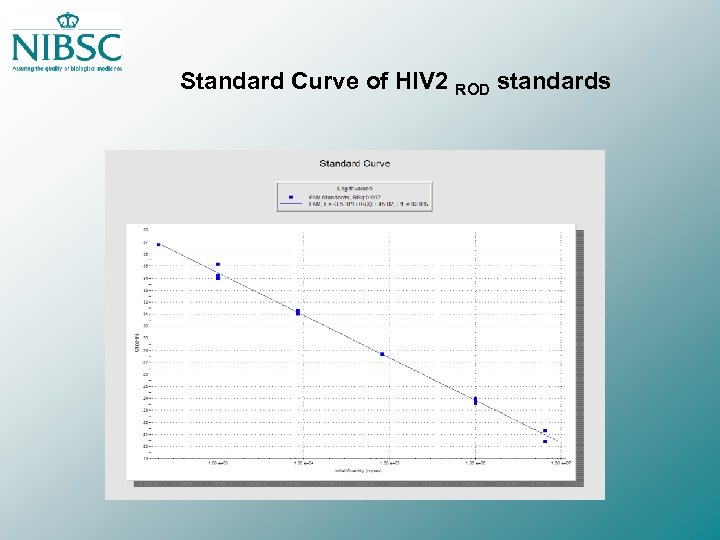 Standard Curve of HIV 2 ROD standards
Standard Curve of HIV 2 ROD standards
 1 st International Reference Panel for HIV-1 RNA Genotypes (code 01/466) • Established by ECBS in 2003 • 500 panels prepared and frozen at -80 C • Contains representatives of HIV-1: – Subtypes A, B, C, D, AE, F, G, AGH – Group N and O • ~120 panels remain • Stability at -80 C very good over 5 years • Proposal for 2 nd IRP
1 st International Reference Panel for HIV-1 RNA Genotypes (code 01/466) • Established by ECBS in 2003 • 500 panels prepared and frozen at -80 C • Contains representatives of HIV-1: – Subtypes A, B, C, D, AE, F, G, AGH – Group N and O • ~120 panels remain • Stability at -80 C very good over 5 years • Proposal for 2 nd IRP
 • • • Second Genotype Panel for HIV-1 RNA Rationale – Many inter-subtype recombinant forms now circulating (CRF 01 – CRF 32) – Increasingly being encountered – important pandemic strains – Less common subtypes may be difficult to detect – Proposal to prepare extended panel containing: • Subtypes G, H, J and K, group N and O • Range of CRFs • Will give kit manufacturers and others access to rare and challenging strains of HIV to enable them to assess their ability to detect these viruses Anticipated need/usage – Discussed at So. GAT working group meeting – Limited need – Provides a source of well characterised diverse CRFs and isolates WHO Status – Project endorsed by ECBS October 2006 – Discussed at meeting of WHO Collaborating Centres in Jan 07
• • • Second Genotype Panel for HIV-1 RNA Rationale – Many inter-subtype recombinant forms now circulating (CRF 01 – CRF 32) – Increasingly being encountered – important pandemic strains – Less common subtypes may be difficult to detect – Proposal to prepare extended panel containing: • Subtypes G, H, J and K, group N and O • Range of CRFs • Will give kit manufacturers and others access to rare and challenging strains of HIV to enable them to assess their ability to detect these viruses Anticipated need/usage – Discussed at So. GAT working group meeting – Limited need – Provides a source of well characterised diverse CRFs and isolates WHO Status – Project endorsed by ECBS October 2006 – Discussed at meeting of WHO Collaborating Centres in Jan 07
 Second Genotype Panel for HIV-1 RNA • Source/Type of Material – Only relatively small number of characterised viruses belonging to uncommon subtypes or CRFs available as infectious virus • many only available as cloned DNA – Will source viruses from CFAR/NIBSC, CBER and NIH ARRRP and from scientists who have described them in the literature – Viruses to be grown in PBMC culture and seed stocks stored down – Viruses will be characterised including sequence confirmation – Viruses will be spiked into HIV-negative plasma – Heat inactivation and freeze-drying to be evaluated – International collaborative study to be organised
Second Genotype Panel for HIV-1 RNA • Source/Type of Material – Only relatively small number of characterised viruses belonging to uncommon subtypes or CRFs available as infectious virus • many only available as cloned DNA – Will source viruses from CFAR/NIBSC, CBER and NIH ARRRP and from scientists who have described them in the literature – Viruses to be grown in PBMC culture and seed stocks stored down – Viruses will be characterised including sequence confirmation – Viruses will be spiked into HIV-negative plasma – Heat inactivation and freeze-drying to be evaluated – International collaborative study to be organised
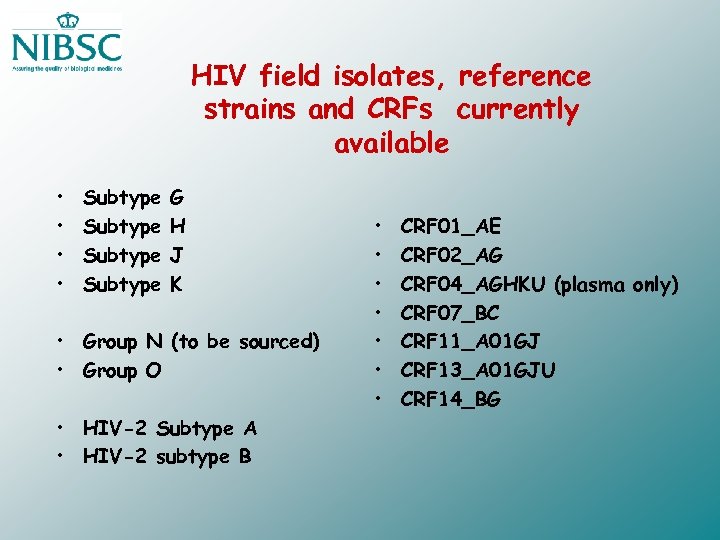 HIV field isolates, reference strains and CRFs currently available • • Subtype G H J K • Group N (to be sourced) • Group O • HIV-2 Subtype A • HIV-2 subtype B • • CRF 01_AE CRF 02_AG CRF 04_AGHKU (plasma only) CRF 07_BC CRF 11_A 01 GJ CRF 13_A 01 GJU CRF 14_BG
HIV field isolates, reference strains and CRFs currently available • • Subtype G H J K • Group N (to be sourced) • Group O • HIV-2 Subtype A • HIV-2 subtype B • • CRF 01_AE CRF 02_AG CRF 04_AGHKU (plasma only) CRF 07_BC CRF 11_A 01 GJ CRF 13_A 01 GJU CRF 14_BG


