f81734aa861aec0f43abfba00a58b135.ppt
- Количество слайдов: 68

Pilot Countries View from the Public Sector Me. TA Ghana Me. TA Philippines Me. TA Jordan Me. TA Uganda Me. TA Kyrgyzstan Me. TA Zambia Me. TA Peru 3/15/2018 Medicines Transparency Alliance 1

Me. TA Ghana Mr. Samuel Boateng Office of Chief Director Ministry of Health (Mo. H) 3/15/2018 Medicines Transparency Alliance 2
Summary Analysis at start of Me. TA What were the needs and issues in your specific sector at the start of Me. TA? l Strong existing enabling and legislative environment BUT q q q q Benefits of improved procurement not translating into affordability and availability for patients (e. g. 2004 WHO/HAI Medicines Survey); Inconsistency with other sectors (private and mission) in terms of how standards (i. e. quality, availability and pricing) are developed and applied; Low consumer awareness due to very little information on quality, availability and prices of medicines in the public domain, Non-adherence to treatment guidelines by prescribers and irrational use of medicines by providers persists despite improved guidance and education; Supply chain performance problems from CMS downstream leading to high stock-out rate; Vulnerability of NHIS to fraud due to inefficient data analysis and dissemination Public health safety concerns arising from counterfeit and/or substandard products 3/15/2018 Medicines Transparency Alliance 3

Major milestones What milestones have been achieved during the Me. TA pilot phase? q Proactive engagement with private sector, development partners & civil society through multi-stakeholder collaboration in information sharing to build consensus around availability, quality, pricing and rational use to increase access to essential medicines for the poor q Periodic monitoring of medicine prices and availability as well as their rational use using WHO standard indicators-2008 q Key Data on medicines from the NHIA made available for systematic analysis and dissemination to improve transparency, accountability, pricing and quality. q Independent monitoring of medicines quality through sentinel testing using GPHF ‘minilabs’ 2009. q Partnership with CSOs to provide more information to consumers, around quality & rational use in medicines advocacy q Data validation of NHIA data carried out; awaiting dissemination 3/15/2018 Medicines Transparency Alliance 4

Successes What were the successes for your sector during the Me. TA pilot phase? q Provided Ghanaian leadership on medicines transparency and accountability through multi-stakeholder engagement as model in West Africa for both effective governance and market efficiency. q Initiated opportunity for pricing and rational use of medicines monitoring mechanism to inform the NHIS q Reduced and/or eliminating the risk of counterfeit and substandard medication in the supply chain. q Provided mechanism for public sector commitment to fight inefficiency and corruption in the drug supply landscape. 3/15/2018 Medicines Transparency Alliance 5

Challenges What challenges has your sector endured during the Me. TA pilot phase? q Mutual suspicions and mistrust between sectors; q Nervousness of all stakeholders about change; q Need to assume a degree of commitment to transparency by all stakeholders and willingness to be mutually accountable. q Sustaining regular open stakeholder dialogue and commitment of all parties in all sectors. q Right to Information Bill yet to be passed into legislation q Dealing with sensitivities of various interest groups in the sector 3/15/2018 Medicines Transparency Alliance 6

Lessons Learned What are the lessons that your sector has learned from Me. TA? q Multi-stakeholder involvement helping to break barriers q Transparency & Accountability in the engagement process is vital for success of Me. TA q The need to accommodate the interests and perceptions of all local stakeholders to reflect the character of the multistakeholder is a key ingredient for its efficiency q The essential elements of a strong, vibrant and sustainable Me. TA in Ghana are: Ø Having the right people connected and committed Ø Willingness of stakeholders to be mutually accountable Ø Ghanaian ownership driven Ø Sustainable political commitment 3/15/2018 Medicines Transparency Alliance 7

Thank you l Name of presenter: Mr. Sam Boateng l Job Title: Office of Chief Director, Mo. H; Member, Ghana GC l Email: samuel. boateng@moh. gov. gh l Mobile number: +233 244 269336 l Website: www. moh. gov. gh 3/15/2018 Medicines Transparency Alliance 8

Me. TA Jordan Presenter Name Job Title 3/15/2018 Medicines Transparency Alliance 9
Overview of Public Health Sector in Jordan l l l Jordan is characterized by a diverse and fragmented public sector. It consists of: MOH, Royal Medical Services, Jordan University Hospital, King Abdullah the Second University Hospital, King Husain Cancer Center and Prince Hamzeh Hospital The public sector covers about 72% of the population Pharmaceutical expenditure as a percent of total health expenditure is 34. 0%, where public accounts for 11. 3% Public pharmaceutical expenditure as a percentage of total pharmaceutical expenditure accounts for 33. 3%. Pharmaceutical expenditure is growing at 17% per annum compared to GDP growth of 3. 3% The public sector is represented at the Jordan Me. TA Council by 9 members out of 18 Source: Jordan National Health Accounts (NHA) 2007 3/15/2018 Medicines Transparency Alliance Me. TA 10
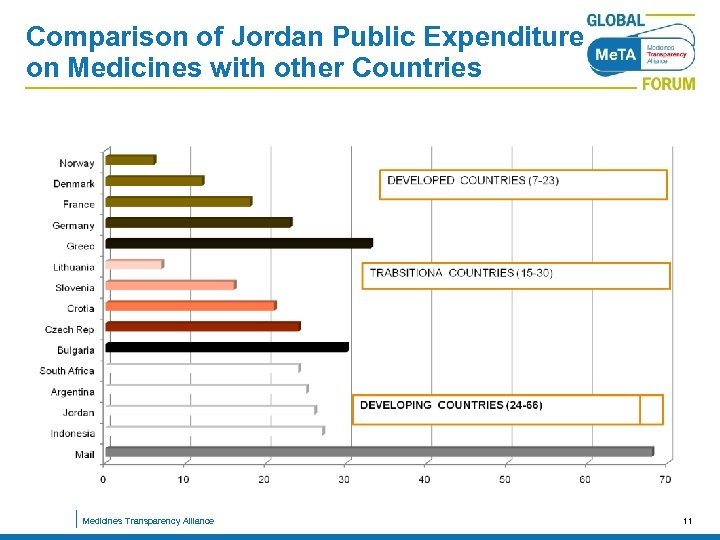
Comparison of Jordan Public Expenditure on Medicines with other Countries Medicines Transparency Alliance 11

Level of Public Sector Engagement l l The Government/ public sector has supported Me. TA since the start of Me. TA The public sector actively participates in all Me. TA activities at the level of the Council, subcommittees and established task forces that work on activities in our national workplan Me. TA Council representatives of the Public sector have acted as facilitators for the Baseline Pharmaceutical Assessment Household and Health Facility survey level II, as well as the Pharmaceutical Sector Scan The Public Sector is fully committed and are the main drivers for the Me. TA process in Jordan 3/15/2018 Medicines Transparency Alliance Me. TA 12

Level of Public Sector Engagement cont. l l l Me. TA is hosted at one of the public health sector institutions/ the High Health Council (HHC) The elected Me. TA Chair is the Secretary General of the HHC Representatives from public sector were nominated to participate in the household and health facility survey level II technical committee. Representative pharmacists from public sector institutions participated in facility survey data collection Most of public sector representative at the Me. TA Council actively participated in the baseline data disclosure survey 3/15/2018 Medicines Transparency Alliance Me. TA 13

Summary Analysis at start of Me. TA The needs and issues in the public sector at the start of Me. TA focused on three main areas: l l l Improving Availability in the public sector through adapting and using transparent evidence based decision making for the Rational Drug List (RDL) Promoting Rational Drug Use through encouraging best practice and developing STGs The need for reliable Data to be used in forecasting etc. especially with the lack of Health IT within the public sector 3/15/2018 Medicines Transparency Alliance 14

Major Milestones l l Developed national Standard Treatment Guidelines (STGs) for essential hypertension for all public primary health care facilities (Expected Output: implementation of pilot STG after discussing implementation strategy with Mo. H) Reviewed TORs and SOPs of various (therapeutic area-related) committees involved in selection of drugs to be included in Rational Drug List (RDL) (Expected Output: adoption of revised criteria for SOPs and Conflict of Interest declarations and increased accountability) Reviewed criteria for adding and deleting drugs to and from RDL (Expected Output: adoption of revised criteria and increased transparency in medicines selection processes) Reviewed classification of drugs (restricted, unrestricted, authorized and unauthorized) in RDL (Expected Output: adoption of revised classifications using transparent evidence based medicine criteria to inform decisions) 3/15/2018 Medicines Transparency Alliance 15

Major Milestones cont. l l l For reliable data on medicines availability and use they advised to have the Pharmaceutical Baseline Assessment Survey-Level II and a WHO/HAI surveys to take place and contributed data to them Supported and contributed to a Supply Chain Mapping Assessment Promoted a Rational Drug Use workshop amongst public sector health workers Advised that a series of training sessions on pharmacoeconomics take place for health professionals -to date two training sessions have already taken place Promote good practice and work on the development of conflict of interest (COI) declaration and management system for all committees to improve accountability 3/15/2018 Medicines Transparency Alliance 16

Successes l l l The Government is highly supportive for the Me. TA initiative in Jordan as mentioned earlier The public sector is committed to Me. TA and they believe in the Me. TA main objective and the five core principles The Government has supported Me. TA and hosted the initiative at one of the governmental institutions -the HHC. This gave the whole process an official identity and led to the fragmented public sector working together towards improving availability through adapting/using evidence based decision making for the Rational Drug List (RDL) and promoting RDU through encouraging best practice 3/15/2018 Medicines Transparency Alliance 17

Challenges At the level of the Pharmaceutical Sector: l l Poor forecasting and estimation of medicines needs which leads to low availability of medicines in public sector High Health expenditure 9. 05% of GDP where 34% out of it is spent on medicines with 2/3 expenditure is out of pocket Direct local purchases by institutions (prices of the private sector) instead of sticking to procuring jointly through JPD Irrational Use of Medicines and absence of national STGs in different disease areas l Weak role and capacity of pharmaco- vigilance system l Lack of private sector transparency 3/15/2018 Medicines Transparency Alliance 18

Challenges cont. General Challenges: l l The two year period for Me. TA pilot is too short and not long enough to measure outcomes and impact on the availability of medicines in the public sector and on Rational Drug Use Changes in Me. TA Council representatives positions/agendas can hinder their participation in effecting medicines policy changes and creates a gap between Council members 3/15/2018 Medicines Transparency Alliance 19

Lessons Learned 1. The main lesson learned is the importance of partnership AND the multi-stakeholder process: l l l That public sector can be more effective in dealing with issues within the medicine supply chain in terms of availability, distribution, RDU when working with the private sector and civil society organizations (CSO), since the private sector secures the country with medicines and the CSO speak on behalf of patients’ and consumers’ and their needs The Me. TA process has opened channels of communication between different stakeholders and between different countries Me. TA has acted as a coordinating and facilitating body for the pharmaceutical sector players and stakeholders 3/15/2018 Medicines Transparency Alliance 20

Lessons Learned cont. 2. We have learned the Importance of Data and Data Disclosure l l l Participated fully in the Pharmaceutical Sector Data Disclosure Survey Contributed fully in the Pharmaceutical Baseline Assessment Survey-Level II: Facility and household and included the Pricing Survey within the baseline assessment facility survey Participated in the Pharmaceutical Sector Scan Survey Agreed to publish all outputs and data on Jordan Me. TA Web site and learned that the media should be more involved Identified gaps and formulated recommendations for the Jordan pharmaceutical sector (based on existing pharmaceutical reform sector studies (WB 2004) and publish on the Jordan Me. TA Web site 3/15/2018 Medicines Transparency Alliance 21

Thank you l Name of presenter: l Job Title: l Email: l Mobile number: +962 l Website: www. meta. jo 3/15/2018 Medicines Transparency Alliance 22

Me. TA Kyrgyzstan Expertise of current Legislation Djusupova Djanyl Deputy director of NDRA of KG 3/15/2018 Medicines Transparency Alliance 23
Summary Analysis at start of Me. TA l l l Since gaining independence of KG when the pharmaceutical sector privatized the legal framework corresponding new function requirements in drug provision has been established NDRA KG has been established However, regulatory tools lacked to ensure transparency and accountability in the pharmaceutical sector if basic legislation was available Survey objective: Analysis of legislation, with emphasis on mechanisms of transparency and accountability, including an assessment of the law in practice and existing contradictions 3/15/2018 Medicines Transparency Alliance 24
Summary Analysis at start of Me. TA The new policy reforms aimed at improving the business environment by removing administrative barriers to business and investment has led to: - A reduction of 30% of licenses and permissions for business activities. Changing the system of control and inspection of business. Reduction to 30% of the bodies monitoring business. A significant impact on regulation of pharmaceutical sector: According to advisory group of BEI project medicines were considered as an ordinary product without regard to their characteristics. The adoption of some recommendations could lead to deregulation in pharmaceutical sector: - Activities of wholesalers and manufacturers of medicines and pharmacies (except pharmacies produced medicines extempore) is no longer licensed - Mo. H standards would be voluntary implemented in pharmaceutical sector - the declaration of compliance with medicines is introducing instead of mandatory certification of medicines - Unilateral recognition of medicine registration Pharmaceutical Inspection has no rights to have unexpected visit , even there are public complaints 3/15/2018 Medicines Transparency Alliance 25

Major milestones l l META initiated a dialogue with the USAID BEI project involving private sector, NGOs, government and WHO to prevent an imbalance in the regulation of the pharmaceutical sector under an authoritarian approach and non-transparency of the policy Expert group on legislation analysis conducted meetings with representatives of different sectors: - l - CSOs sector l l - Public sector: Mo. H, Mo. F, NDRA - Private sector Identified issues and contraventions in legislation were discussed at the Round Table by all parties participated E- group involving all stakeholders was created to discuss legislation issues 3/15/2018 Medicines Transparency Alliance 26

Areas of survey 1. The main normative documents governing the drug provision in KG: 1. 1. Effectiveness of legal documents to ensure transparency of drug provision in KG 1. 2. Legal and regulatory shortcomings and contradictions 2. The patient’s rights on the drug provision and their protection 2. 1. Information public support on medicine issues 2. 2. Services on the drug provision 2. 3. The possibility of appeal by patients of their rights on the drug provision 3. State regulation 3. 1. Regulation of licensing issues concerning the drug provision 3. 2. Bodies authorized to control the drug provision 3. 3. The system of state control efficiency and drug safety 4. Responsibility for the public health Medicines Transparency Alliance
Challenges with legislative features 1. 2. 3. 4. 5. 6. 1. Contradictions in certain normative documents including differences in terminology 2. It is necessary to improve the regulatory framework (conflict of interests of NDRA and exclusion of obsolete rules) 3. Medicines included in the scope of the Law "On the basis of technical regulation in the Kyrgyz Republic” 4. New rules of establishment of regulation status may lead to the fact that regulations of the drug provision will cease to be binding 5. Inadequacy of certain legislation (complication of pharmaceutical inspection, law enforcement pressure on pharmacies) 6. The lack of legislative mechanisms to ensure transparency and accountability in the pharmaceutical sector Medicines Transparency Alliance
Challenges with executive features 1. Procurement: The inaccessibility of information on procurement of medicines, except for procurement announcement Violation of procurement procedures - 2. Licensing system: - No transparency in issue of licenses There is no public information on licensed pharmacies Lack of information management system in the pharmaceutical sector 3. Disciplinary-executive: • - Competence of documentation requirement by certain control agencies (prosecution or other fiscal bodies) Medicines Transparency Alliance

Recommendations of Stakeholders (1) - Increasing the responsibility of officials (administrative, criminal, disciplinary) - Obligatory support information of patients including information on ADR - To introduce standards for the number of pharmacies in a particular area To attract the local government to monitor the activities of pharmacies - Promotion of ethic code of pharmacist - To introduce bar code for medicines - To ensure timely review of list of medicines reimbursed Medicines Transparency Alliance
Recommendations of Stakeholders (2) Tender: - to implement e-procurement (legalize the flow of documents in electronic format). to introduce ethic standards for the members of tender commission to introduce criteria for medicine procurement to procure quality medicines (price should not be sole criterion) Information: Increasing public awareness Information on the DRA website: Licensed pharmacies , Certified products, Registered medicines To introduce provisional regulations on period of medicine certification Medicines Transparency Alliance

Lessons Learned l l Working with different sectors revealed a significant lack of information in the pharmaceutical sector (indicated by all parties) Private sector and NGOs are ready to participate in development and enhancement of legislation through: l l l Establishing working group involving all sectors to address specific laws Establishing groups to lobby for promotion of certain laws The system of regular meetings, newsletters, online forums Involvement of all stakeholders enhances the process of improving the legislation and its implementation Activities META promote confidence-building between the sectors 3/15/2018 Medicines Transparency Alliance 32

Thank you l Djusupova Djanyl l Deputy director of NDRA of KG l Email: ddjanyl@gmail. com l Mobile number: +996 770 221 055 l Skype: l Website: www. pharm. med. kg 3/15/2018 Medicines Transparency Alliance 33

Me. TA Peru Melitón Arce Pedro Yarasca Pedro Tintaya 3/15/2018 Medicines Transparency Alliance 34

Analytical Summary at the beginning of Me. TA What were the needs and problems in this specific sector at the beginning of Me. TA l Problems: – Lax legislation for the Registration and Quality of medicines. – Little transparency in the information of prices. – Promotion and Publicity of medicines l Needs: – Transparency in information of prices of Pharmaceutical Products – Politics of Generic Medicines 3/15/2018 Medicines Transparency Alliance 35
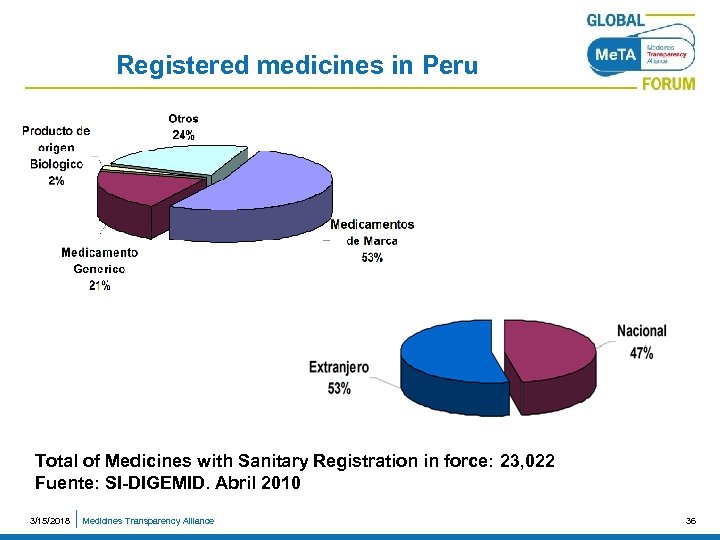
Registered medicines in Peru Total of Medicines with Sanitary Registration in force: 23, 022 Fuente: SI-DIGEMID. Abril 2010 3/15/2018 Medicines Transparency Alliance 36

Main mailstones What milestones have been reached during the Me. TA pilot phase? During the period of the Me. TA pilot phase, the following milestones have been achieved, favored by Me. TA Peru: l Observatoriy of Prices of Pharmaceutical Products: Regulatory framework for the OPPF – Development of the software for prices reports – Technological support : PCs, Server – Informative campaigns for the dissemination of the OPPF: Informative Modules – Aspects that have facilitated to reach this milestones: New legislation of Pharmaceutical Products, medical devices and sanitary products. – Obligatory nature of prescription in International Common Denomination (DCI) – 3/15/2018 Medicines Transparency Alliance 37

Successes What were the successes of the sector during the Me. TA pilot phase? – Elaboration and approval of the normative framework on the Observatory of Prices – Launch of the Observatory of Prices - Currently in Version 2. – Political commitment and support by the Minister of Health – Acceptability of the Observatory of Prices by part of the population 3/15/2018 Medicines Transparency Alliance 38

Examples Informative campaign on Prices of Medicines 3/15/2018 Medicines Transparency Alliance 39

Challenges l What challenges have this sector to confront during the Me. TA Pilot Phase? – To build a better trust among the associates. – The presence of divergent opinions during the process. – Greater capacity in technological and human resources. 3/15/2018 Medicines Transparency Alliance 40

Lessons Learned What lessons has this sector learned of Me. TA? – Recognition of issues that should be revealed to the population (prices, availability, public purchases, information of medicines). – Allowing space for multisectorial dialogue. – Identification of objectives and common strategies among the associates, always respecting their points of view. – It has permitted to identify prominent tissues for the country in matter of medicines. 3/15/2018 Medicines Transparency Alliance 41

Thank you Pedro Yarasca e-mail: pyarasca@digemid. minsa. gob. pe Telf. : 0051 -1 -998704127 www. digemid. minsa. gob. pe 3/15/2018 Medicines Transparency Alliance 42

Me. TA Philippines Usec. Alexander A. Padilla & Robert Louie P. So, MD National Center for Pharmaceutical Access and Management 3/15/2018 Medicines Transparency Alliance 43
Summary Analysis at start of Me. TA l Filipinos suffer from lack of Access l l l l Sustainable Financing Availability Budget Limited entitlements Transparency/ Accountability Pricing Human resource Issues l No outcome indicators for Access to Medicines l Information / Addressing Asymmetry l l Expand understanding of the Philippine Pharmaceutical Situation Engagement with stakeholders for collective strategic efforts to improve sector 3/15/2018 Medicines Transparency Alliance 44

Major milestones DOH in terms of Public- Private Partnerships Advisory Council for Price Regulation l DOH- Valsartan Access Program l Insulin Access Program with Eli Lilly and Sanofi- Aventis l Childhood Cancer Medicine Access Program l Pharmacist Services for Botika ng Barangays l Good Governance for Medicines with WHO l Jump Start Medicines Transparency and Alliance Philippines l 3/15/2018 Medicines Transparency Alliance 45

Successes l Institutional Successes l l l l RA 9502 “Universally Accessible and Cheaper Medicines Act of 2008” Generics promotion Botika ng Barangay P 100 Launching Price Regulation: Maximum drug Retail Price & Government Mediated Access Program (MDRP & G-MAP) Good Governance for Medicines l Electronic- Essential Drug Price Monitoring System (E-EDPMS) l Philippine National Drug Formulary System (PNDFS) DOH support to Me. TA l l 3/15/2018 Financial support in early stages Resource for pharmaceutical scan Office lodged in DOH compound Engagements for memberships at the start of Me. TA Medicines Transparency Alliance 46

Challenges l Revitalizing the National Medicines Policy in the Philippines in line with significant legislation and inputs from stakeholders Strengthening government agencies involved in medicines access: FDA and DOH- NCPAM Improving the general public’s confidence in the use of generic medicines Strengthening community pharmacies through expanding drug list, disseminating best practices and acquiring pharmacist services Assessing impact of drug price reduction initiatives Technical resources are limited Fiscal resources needs to be improved Standard guidelines needs to be further developed l Me. TA Assistance to Public Sector l l l l Engaging local groups to expand Me. TA process. Rolling out public sector initiatives and raising awareness through Me. TA Role of Me. TA as conscience at the grassroots level Me. TA support for consolidating resources in assesing impact/ surveys and technical assistance for capacity building in selling multistakeholder process Collective support for rolling out good governance initiatives Other Challenges l l l 3/15/2018 Me. TA institutionalization and sustainability. Resolving Me. TA personality. Expanding Me. TA acceptance by all sectors Medicines Transparency Alliance 47

Lessons Learned l We were able to share with Me. TA the effective mechanisms to engage the different stakeholders in the Philippine pharmaceutical sector in conceptualizing, creation, implementation and evaluation of State initiatives for improving access to medicines are needed. – – l l Advisory Council for Price regulation Public-Private Partnership Transparency in pharmaceutical data leads to better and more effective policy making and evaluation Me. TA Philippines is effective as a venue for validation (check and Balance): Act as Social Conscience to guide policies and effective strategies 3/15/2018 Medicines Transparency Alliance 48

Thank you l l Alexander A. Padilla Undersecretary in Charge of the National Center for Pharmaceutical Access and Management Robert Louie P. So, MD Project Director of the National Center for Pharmaceutical Access and Management l dohncpam@gmail. com l Mobile number: +63 917 835 2312 3/15/2018 Medicines Transparency Alliance 49
Me. TA Uganda Presenter: Oteba Olowo Martin Job Title: Assistant Commissioner health Services (pharmacy) 3/15/2018 Medicines Transparency Alliance 50

Multi-stakeholder process - milestones l l Establishment of the Me. TA country chapter with a secretariat facilitated by a national coordinator CSO – Government – Private sector multistakeholder national Me. TA council established the National Pharmaceutical Sector Strategic Plan (NPSSP) supported – draft NPSSP produced Facilitating transparency in the medicines registration process – register of medicines regularly updated and accessible on website free of charge 3/15/2018 Medicines Transparency Alliance 51

Multi-stakeholder process milestones l The three year procurement plan evaluated, updated and shared among stakeholders in the health sector 3/15/2018 Medicines Transparency Alliance 52

Major achievements and successes – Me. TA Uganda l l l Joint action in areas hitherto of mutual suspicion achieved Understanding diversity, taking advantage of the opportunities therein for collective and effective policy development and implementation Medicines price monitoring conducted on a quarterly basis Process of computerizing medicines import data started and dialogue for continued support by other partners initiated The NPSSP reviewed through a wider stakeholder process – Document available in draft form (Yet to be costed) 3/15/2018 l Medicines Transparency Alliance 53

Overall challenges during the pilot – Me. Ta Uganda l l l Appreciating the Me. TA core values and value add to the various stakeholders Breaking barriers between stakeholders and opening up opportunities for synergistic actions – time waste Building trust and confidence among the stakeholders Time waste in addressing the individual stakeholder interests and concerns Most project outcomes yet to be evaluated for broader impact 3/15/2018 Medicines Transparency Alliance 54

Lessons Learned – Me. TA Uganda l l Soliciting goodwill and support by stakeholders should not be taken for granted Stakeholder prior understanding of new initiatives provides good grounds for buy in The pilot phase as a consequence of the lag phase has been ultimately shorter than was expected creating pressure in the implementation of agreed on programmes at country Stakeholder engagement provides requisite grounds for mitigating and or eliminating potential conflict and allows for progressive synergy in moving forward agenda in medicines transparency programmes 3/15/2018 Medicines Transparency Alliance 55

Lessons Learnt l Understanding each others interests, mandates and responsibilities and positively sharing the concerns of the other parties. 3/15/2018 Medicines Transparency Alliance 56

Thank you l Name of presenter: Oteba Olowo Martin l Email: l Mobile number: +256 772 512 975 l Skype: l Website: 3/15/2018 orukan 33@hotmail. com Medicines Transparency Alliance 57

Me. TA Zambia Mrs Bernice Mwale Director – Product Registration Pharmaceutical Regulatory Authority 3/15/2018 Medicines Transparency Alliance 58
Summary Analysis at start of Me. TA What were the needs and issues in your specific sector at the start of Me. TA l l l Me. TA was initially to be housed within MOH, later agreed that Transparency International (Z) be host Me. TA very much linked to public sector; MOH key to success of Me. TA, even though not driving Me. TA provides big opportunity to have all different stakeholders on one roundtable to discuss issues that could influence policies/practices l Understanding core principles of Me. TA by members very key l Expected support from CPs l Me. TA members are volunteers, their time is at a premium l The size of Me. TA Council directly relates to Me. TA effectiveness l Targeting issues that directly impacts general public is measure of Me. TA success 3/15/2018 Medicines Transparency Alliance 59

Major milestones l l Government has shown support towards Me. TA as shown by the Minister of Health launching of Me. TA in Zambia and other activities Government has participated in the Me. TA live radio programs after some initial hitches Orientation workshop for Me. TA Council members in PRA mandate/functions Orientation of Me. TA Council members in the national supply chain systems (public and private) l Collaborative meeting held between PRA and its stake holders l Active participation of MOH and its institutions in Forum meeting l Co-chairing of Me. TA Council meetings 3/15/2018 Medicines Transparency Alliance 60

Successes l Me. TA provided another forum for dissemination of information to wider circle of stake holders l Participation in live Me. TA radio programs l MOH representative is Vice Chairperson of Me. To. A Council l l Successful orientation of Me. TA Council members in the functions/mandate of PRA Successful collaboration by PRA with key stakeholders on various issues affecting them Successful orientation of Me. TA Council members by MOH/its institutions on the national supply chain system Active participation of MOH, PRA and MSL IN THE Me. TA Forum meeting 3/15/2018 Medicines Transparency Alliance 61

Challenges (1) l l l Big challenge for government employees to talk about transparency and accountability issues Finding time to attend to Me. TA activities given busy schedules Weakness in the tools that were used to carry-out some surveys; should have been field tested first The surveys were supposed to gather more evidence for advocacy and influencing change Ensuring quality verses spending more money in a short time (to beat financial deadline). It was a challenge for some government officials in the Me. TA sub -committees to follow tendering procedures rather than those followed in government (Comprehensive Tendering Procedures) 3/15/2018 Medicines Transparency Alliance 62
Challenges (2) l l l Balancing Me. TA activities and government duties was a challenge, especially that Me. TA programs do not give sufficient notice The role of MOH in Me. TA is key, however, need to recognise that MOH is not the driver for Me. TA may be expected to address all kinds of issues, even if these are influencing access to affordable essential medicines Most CPs needed to be reminded about Me. TA can be seen as a tool with which to ‘punish’ or ‘expose’ the weaknesses in the public health sector 3/15/2018 Medicines Transparency Alliance 63

Challenges (3) l l l The challenge Me. TA presents is that all stakeholders and concerned citizens want to be counted within Me. TA. This is clearly a difficult situation for Me. TA Council and admin team Me. TA is still in the formative stage and it may feel inadequate to tackle certain issues that impact on access to essential medicines Initial focus was on private sector from the regulatory point of view Misrepresentation of facts by consultant involved in desk review, delayed other Me. TA follow on activities Some of the tools used for data collection appeared to be deficient in certain critical information. 3/15/2018 Medicines Transparency Alliance 64

Lessons Learned (1) l l Me. TA should have an independent stand alone registered secretariat It was an opportunity for government to share information with the general public to discuss health issues, the forum can be used to share information dialogues with other stakeholders to introduce/withdraw new policies, schemes, fees and report illegal practices It was learnt that it is important to identify issues surrounding a survey before it is carried out. Difficult to fit in Me. TA activities 3/15/2018 Medicines Transparency Alliance 65

Lesson Learned (2) l l l There was need to have clear cut issues identified if a change was to be achieved it was a big opportunity for government to have all different stakeholders on one table hence a chance to raise awareness to other sectors. Awareness was also raised through the Me. TA radio programs Me. TA is very much linked to the public health sector. The MOH, even though not driving Me. TA, is key to the success of Me. TA There is a need to advocate for more infrastructure and health personnel to improve quality and accessibility of good quality medicines Focus on one or two issues was very necessary if tangible results were to be achieved The public health sector need not be seen as the only target for Me. TA 3/15/2018 Medicines Transparency Alliance l 66
Lessons Learned (3) l l Knowledge of the purpose and intentions of Me. TA by stakeholders. There is a need for all members and stakeholder to keep in mind the background that resulted in Me. TA The support from Cooperating Partners has been below expectation l Me. TA members are volunteers and their time is at a premium l The size of Me. TA Council directly relates to the effectiveness of Me. TA l l Targeting issues that directly impact the general public is a measure of Me. TA’s success Clearly the time- frame for Me. TA put pressure on members and the Me. TA administrative team. All parties want to succeed. 3/15/2018 Medicines Transparency Alliance 67

Thank you l Name of presenter : Mrs Bernice Mwale l Job Title: Director – Product Registration (PRA) l Email: bcmwale@pra. gov. zm l Mobile number: +260977804353 l Website: www. metazambia. org 3/15/2018 Medicines Transparency Alliance 68
f81734aa861aec0f43abfba00a58b135.ppt