aa8fe79a0c0d0ca1dc5aaa46ed0c4e12.ppt
- Количество слайдов: 9
 Physics 101: Lecture 28 The Transfer of Heat l Today’s lecture will cover Textbook Chapter 13 èThe Transfer of Heat » Convection » Conduction » Radiation Physics 101: Lecture 28, Pg 1
Physics 101: Lecture 28 The Transfer of Heat l Today’s lecture will cover Textbook Chapter 13 èThe Transfer of Heat » Convection » Conduction » Radiation Physics 101: Lecture 28, Pg 1
 Concept Question Which of the following is an example of convective, conductive and radiative heat transfer? 1. You stir some hot soup with a silver spoon and notice that the spoon warms up. 2. You stand watching a bonfire, but can’t get too close because of the heat. 3. Its hard for central air-conditioning in an old house to cool the attic. Physics 101: Lecture 28, Pg 2
Concept Question Which of the following is an example of convective, conductive and radiative heat transfer? 1. You stir some hot soup with a silver spoon and notice that the spoon warms up. 2. You stand watching a bonfire, but can’t get too close because of the heat. 3. Its hard for central air-conditioning in an old house to cool the attic. Physics 101: Lecture 28, Pg 2
 Heat Transfer: Convection l Convection: heat is transferred by the bulk movement of a gas or liquid. Example: Fluid is sitting on a burner is heated from below. The fluid right above the flame is getting hot and thus expands: V increases => density decrease Thus, the hotter fluid experiences a net force upward (buoyant force) l FB = rcold V g > rhot V g Archimedes Principle: low density floats on high density => warmer fluid moves upward and is replaced by cooler fluid -> fluid is warmed -> moves upward -> and so on l Cycle continues with net result of circulation of fluid that carries heat. l Practical aspects: èheater ducts on floor èA/C ducts on ceiling Physics 101: Lecture 28, Pg 3 èstove heats water from bottom
Heat Transfer: Convection l Convection: heat is transferred by the bulk movement of a gas or liquid. Example: Fluid is sitting on a burner is heated from below. The fluid right above the flame is getting hot and thus expands: V increases => density decrease Thus, the hotter fluid experiences a net force upward (buoyant force) l FB = rcold V g > rhot V g Archimedes Principle: low density floats on high density => warmer fluid moves upward and is replaced by cooler fluid -> fluid is warmed -> moves upward -> and so on l Cycle continues with net result of circulation of fluid that carries heat. l Practical aspects: èheater ducts on floor èA/C ducts on ceiling Physics 101: Lecture 28, Pg 3 èstove heats water from bottom
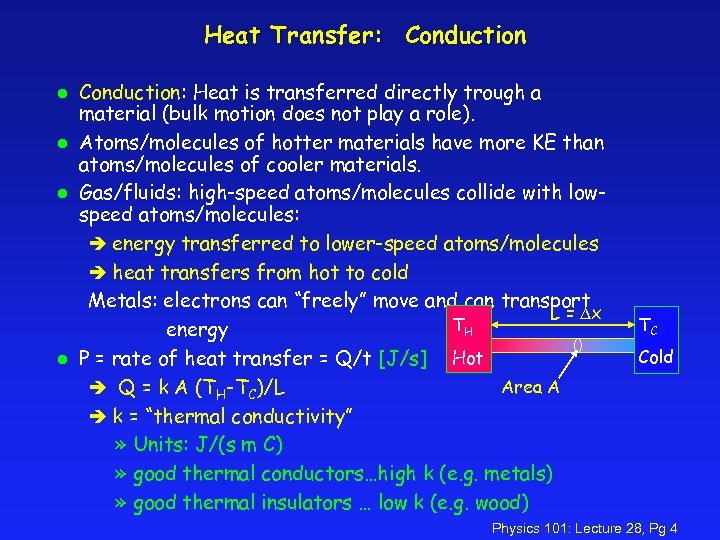 Heat Transfer: Conduction l l Conduction: Heat is transferred directly trough a material (bulk motion does not play a role). Atoms/molecules of hotter materials have more KE than atoms/molecules of cooler materials. Gas/fluids: high-speed atoms/molecules collide with lowspeed atoms/molecules: è energy transferred to lower-speed atoms/molecules è heat transfers from hot to cold Metals: electrons can “freely” move and can transport L = Dx TH energy P = rate of heat transfer = Q/t [J/s] Hot Area A è Q = k A (TH-TC)/L è k = “thermal conductivity” » Units: J/(s m C) » good thermal conductors…high k (e. g. metals) » good thermal insulators … low k (e. g. wood) TC Cold Physics 101: Lecture 28, Pg 4
Heat Transfer: Conduction l l Conduction: Heat is transferred directly trough a material (bulk motion does not play a role). Atoms/molecules of hotter materials have more KE than atoms/molecules of cooler materials. Gas/fluids: high-speed atoms/molecules collide with lowspeed atoms/molecules: è energy transferred to lower-speed atoms/molecules è heat transfers from hot to cold Metals: electrons can “freely” move and can transport L = Dx TH energy P = rate of heat transfer = Q/t [J/s] Hot Area A è Q = k A (TH-TC)/L è k = “thermal conductivity” » Units: J/(s m C) » good thermal conductors…high k (e. g. metals) » good thermal insulators … low k (e. g. wood) TC Cold Physics 101: Lecture 28, Pg 4
 l Example with 2 layers: find P=Q/t in J/s èKey Point: Continuity (just like fluid flow) » P 1 = P 2 » k 1 A(T 0 -TC)/Dx 1 = k 2 A(TH-T 0)/Dx 2 » solve for T 0 = temp. at junction » then solve for P 1 or P 2 n answers: T 0=5. 8 C P=265 Watts P 1 P 2 Inside: TH = 25 C Outside: TC = 4 C T 0 Dx 1 = 0. 019 m A 1 = 35 m 2 k 1 = 0. 080 J/s-m-C Dx 2 = 0. 076 m A 2 = 35 m 2 k 2 = 0. 030 J/s-m-C Physics 101: Lecture 28, Pg 5
l Example with 2 layers: find P=Q/t in J/s èKey Point: Continuity (just like fluid flow) » P 1 = P 2 » k 1 A(T 0 -TC)/Dx 1 = k 2 A(TH-T 0)/Dx 2 » solve for T 0 = temp. at junction » then solve for P 1 or P 2 n answers: T 0=5. 8 C P=265 Watts P 1 P 2 Inside: TH = 25 C Outside: TC = 4 C T 0 Dx 1 = 0. 019 m A 1 = 35 m 2 k 1 = 0. 080 J/s-m-C Dx 2 = 0. 076 m A 2 = 35 m 2 k 2 = 0. 030 J/s-m-C Physics 101: Lecture 28, Pg 5
 Heat Transfer: Radiation l Radiation: All things radiate electromagnetic energy. è emit = Q/t = e. A T 4 P Surroundings at T 0 » e = emissivity (between 0 and 1) n perfect “black body” has e=1 Hot stove T » T is Kelvin temperature » = Stefan-Boltzmann constant = 5. 67 x 10 -8 J/(s m 2 K 4) èNo “medium” required l All things absorb electromagnetic energy from surroundings. 4 è Pabsorb = e. A T 0 » good emitters (e close to 1) are also good absorbers Physics 101: Lecture 28, Pg 6
Heat Transfer: Radiation l Radiation: All things radiate electromagnetic energy. è emit = Q/t = e. A T 4 P Surroundings at T 0 » e = emissivity (between 0 and 1) n perfect “black body” has e=1 Hot stove T » T is Kelvin temperature » = Stefan-Boltzmann constant = 5. 67 x 10 -8 J/(s m 2 K 4) èNo “medium” required l All things absorb electromagnetic energy from surroundings. 4 è Pabsorb = e. A T 0 » good emitters (e close to 1) are also good absorbers Physics 101: Lecture 28, Pg 6
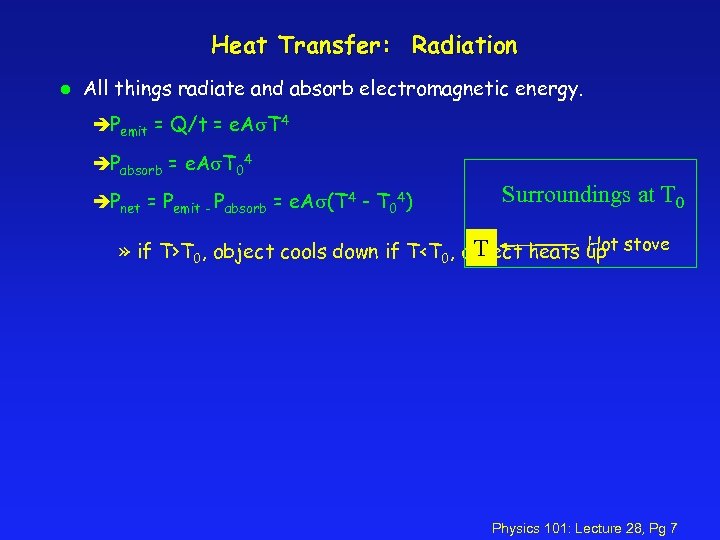 Heat Transfer: Radiation l All things radiate and absorb electromagnetic energy. èPemit = Q/t = e. A T 4 èPabsorb = e. A T 04 èPnet = Pemit - Pabsorb = e. A (T 4 - T 04) Surroundings at T 0 Hot T » if T>T 0, object cools down if T
Heat Transfer: Radiation l All things radiate and absorb electromagnetic energy. èPemit = Q/t = e. A T 4 èPabsorb = e. A T 04 èPnet = Pemit - Pabsorb = e. A (T 4 - T 04) Surroundings at T 0 Hot T » if T>T 0, object cools down if T
 NASA’s Thermal Imaging System l http: //mars. jpl. nasa. gov/ During day time the sun heats the surface of Earth or Mars. At night the materials on the surface emit this thermal energy again in form of radiation in the infrared. Since different materials emit differently, a whole spectrum of infrared radiation is measured which is used to identify the material. Physics 101: Lecture 28, Pg 8
NASA’s Thermal Imaging System l http: //mars. jpl. nasa. gov/ During day time the sun heats the surface of Earth or Mars. At night the materials on the surface emit this thermal energy again in form of radiation in the infrared. Since different materials emit differently, a whole spectrum of infrared radiation is measured which is used to identify the material. Physics 101: Lecture 28, Pg 8
 Concept Question One day during the winter, the sun has been shining all day. Toward sunset a light snow begins to fall. It collects without melting on a cement playground, but it melts immediately upon contact on a black asphalt road adjacent to the playground. How do you explain this. Black (asphalt) absorbs electromagnetic waves (radiation) more readily than white (cement) does (emissivity is larger). Hence, the black has more radiation to emit because it has absorbed more. As a result, it releases more radiation into the snow, causing the snow to heat up, and melt. Physics 101: Lecture 28, Pg 9
Concept Question One day during the winter, the sun has been shining all day. Toward sunset a light snow begins to fall. It collects without melting on a cement playground, but it melts immediately upon contact on a black asphalt road adjacent to the playground. How do you explain this. Black (asphalt) absorbs electromagnetic waves (radiation) more readily than white (cement) does (emissivity is larger). Hence, the black has more radiation to emit because it has absorbed more. As a result, it releases more radiation into the snow, causing the snow to heat up, and melt. Physics 101: Lecture 28, Pg 9


