a0b7a2ba10d357f4cf2b193389a71fdf.ppt
- Количество слайдов: 53
 PHYSICAL PROPERTIES: GLASS AND SOIL CHAPTER 4
PHYSICAL PROPERTIES: GLASS AND SOIL CHAPTER 4
 Distinctive Properties Forensic Scientist determines properties that identify and individualize substance Physical Properties Chemical Properties Standard system of measurement
Distinctive Properties Forensic Scientist determines properties that identify and individualize substance Physical Properties Chemical Properties Standard system of measurement
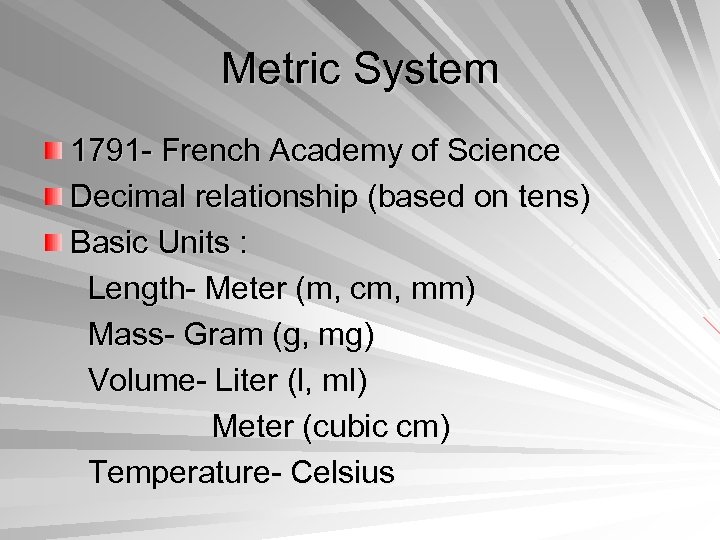 Metric System 1791 - French Academy of Science Decimal relationship (based on tens) Basic Units : Length- Meter (m, cm, mm) Mass- Gram (g, mg) Volume- Liter (l, ml) Meter (cubic cm) Temperature- Celsius
Metric System 1791 - French Academy of Science Decimal relationship (based on tens) Basic Units : Length- Meter (m, cm, mm) Mass- Gram (g, mg) Volume- Liter (l, ml) Meter (cubic cm) Temperature- Celsius
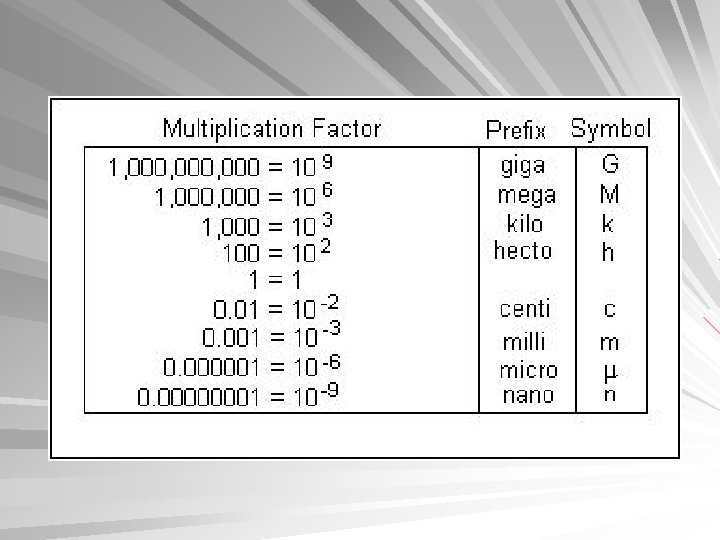
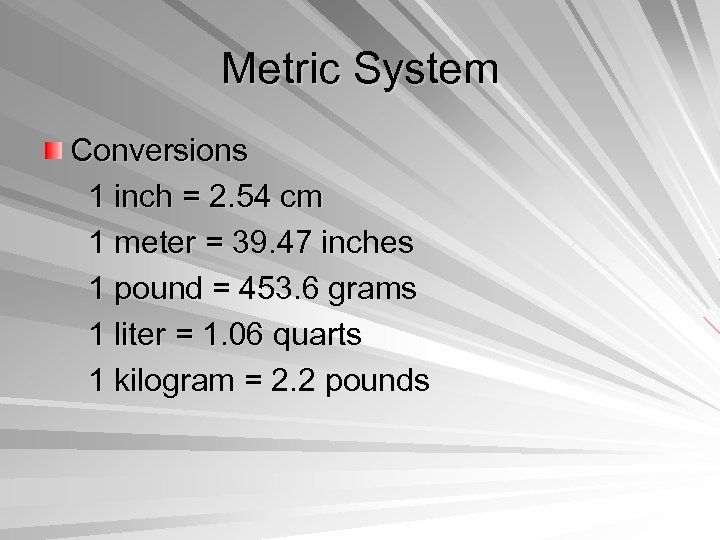 Metric System Conversions 1 inch = 2. 54 cm 1 meter = 39. 47 inches 1 pound = 453. 6 grams 1 liter = 1. 06 quarts 1 kilogram = 2. 2 pounds
Metric System Conversions 1 inch = 2. 54 cm 1 meter = 39. 47 inches 1 pound = 453. 6 grams 1 liter = 1. 06 quarts 1 kilogram = 2. 2 pounds
 Chemical Properties Behavior of a substance when it reacts with another substance Ex- Wood burning reacting with oxygen Ex- Heroin reacting with Marquis reagent
Chemical Properties Behavior of a substance when it reacts with another substance Ex- Wood burning reacting with oxygen Ex- Heroin reacting with Marquis reagent
 Physical Properties Describe behavior of substance without altering its composition, without reference to another substance Boiling point, Melting point, Weight, Mass, Density, Volume, Color, Refractive index
Physical Properties Describe behavior of substance without altering its composition, without reference to another substance Boiling point, Melting point, Weight, Mass, Density, Volume, Color, Refractive index
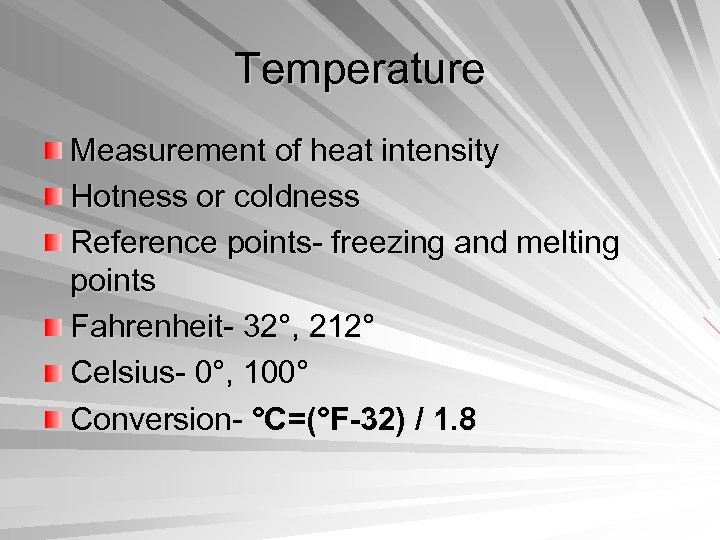 Temperature Measurement of heat intensity Hotness or coldness Reference points- freezing and melting points Fahrenheit- 32°, 212° Celsius- 0°, 100° Conversion- °C=(°F-32) / 1. 8
Temperature Measurement of heat intensity Hotness or coldness Reference points- freezing and melting points Fahrenheit- 32°, 212° Celsius- 0°, 100° Conversion- °C=(°F-32) / 1. 8
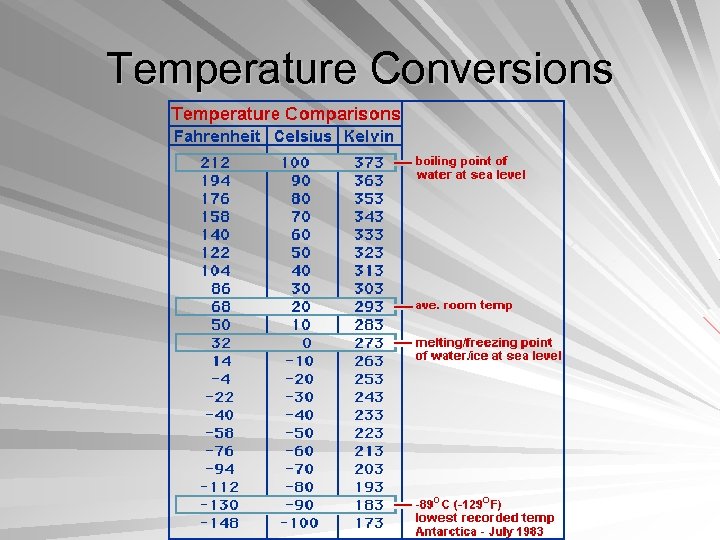 Temperature Conversions
Temperature Conversions
 Weight Depends on mass and force of gravity on that mass Ex: Weight=180 lbs – earth’s gravity pulling with force of 180 lbs. but on moon weight= 30 lbs. Weight = mass x gravity Weigh on scale
Weight Depends on mass and force of gravity on that mass Ex: Weight=180 lbs – earth’s gravity pulling with force of 180 lbs. but on moon weight= 30 lbs. Weight = mass x gravity Weigh on scale
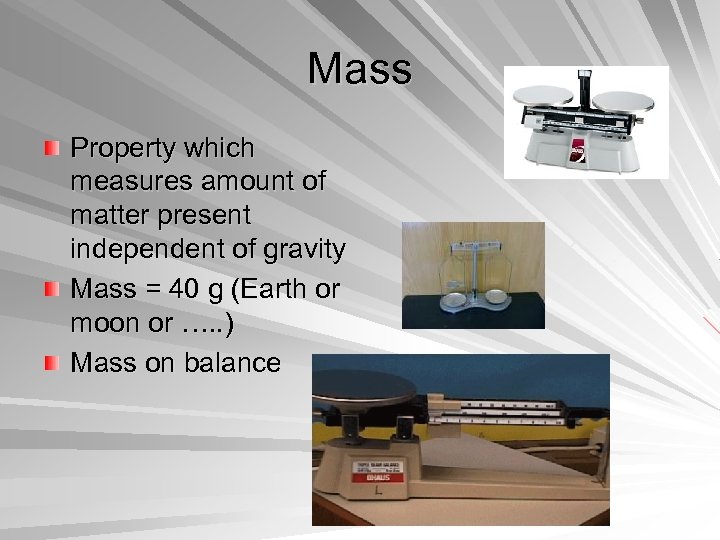 Mass Property which measures amount of matter present independent of gravity Mass = 40 g (Earth or moon or …. . ) Mass on balance
Mass Property which measures amount of matter present independent of gravity Mass = 40 g (Earth or moon or …. . ) Mass on balance
 Density Mass per unit volume Density = mass/volume Intensive Property-property not dependent on size of material Characteristic property used for identification Solids > Liquids > Gases
Density Mass per unit volume Density = mass/volume Intensive Property-property not dependent on size of material Characteristic property used for identification Solids > Liquids > Gases
 Refractive Index Refraction- Bending of a light wave as it passes from one medium to another due to change in velocity Light waves travel through air 300 million meters per second Slowed when penetrate another medium i. e. water or glass
Refractive Index Refraction- Bending of a light wave as it passes from one medium to another due to change in velocity Light waves travel through air 300 million meters per second Slowed when penetrate another medium i. e. water or glass
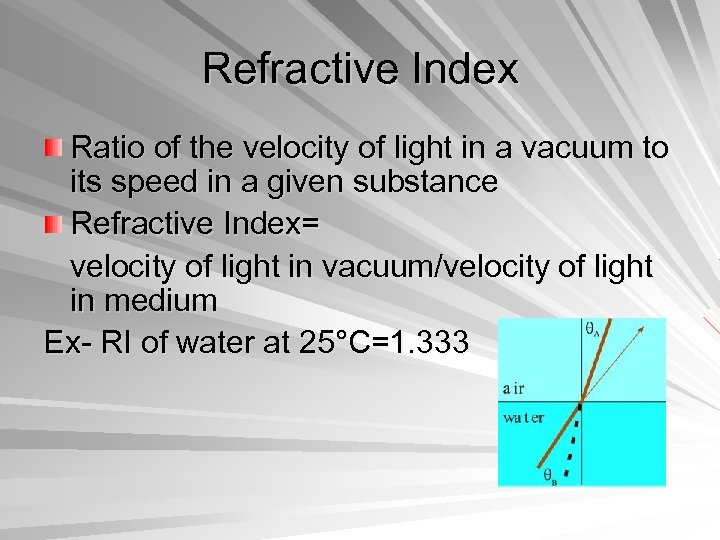 Refractive Index Ratio of the velocity of light in a vacuum to its speed in a given substance Refractive Index= velocity of light in vacuum/velocity of light in medium Ex- RI of water at 25°C=1. 333
Refractive Index Ratio of the velocity of light in a vacuum to its speed in a given substance Refractive Index= velocity of light in vacuum/velocity of light in medium Ex- RI of water at 25°C=1. 333
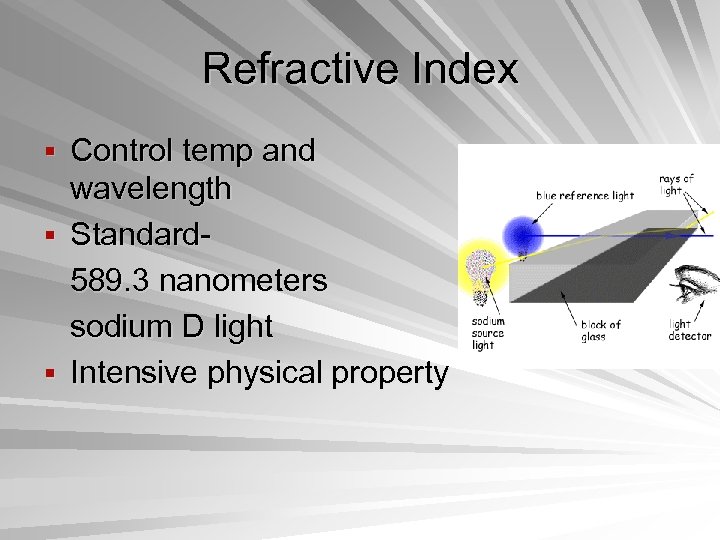 Refractive Index § Control temp and wavelength § Standard 589. 3 nanometers sodium D light § Intensive physical property
Refractive Index § Control temp and wavelength § Standard 589. 3 nanometers sodium D light § Intensive physical property
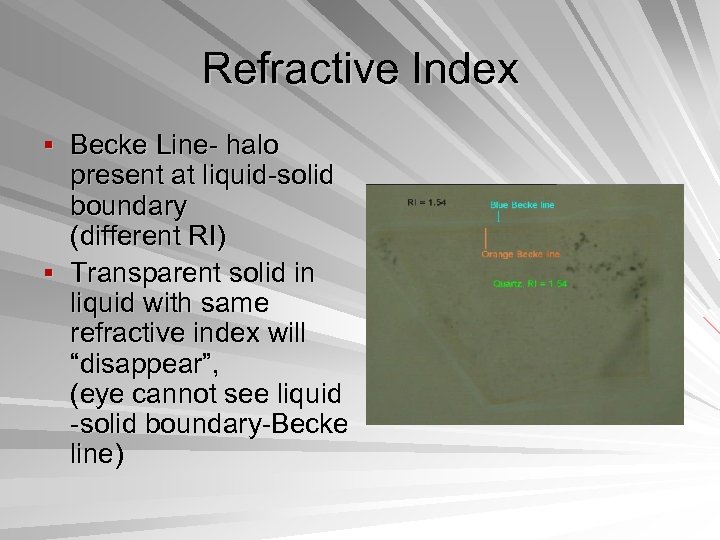 Refractive Index § Becke Line- halo present at liquid-solid boundary (different RI) § Transparent solid in liquid with same refractive index will “disappear”, (eye cannot see liquid -solid boundary-Becke line)
Refractive Index § Becke Line- halo present at liquid-solid boundary (different RI) § Transparent solid in liquid with same refractive index will “disappear”, (eye cannot see liquid -solid boundary-Becke line)
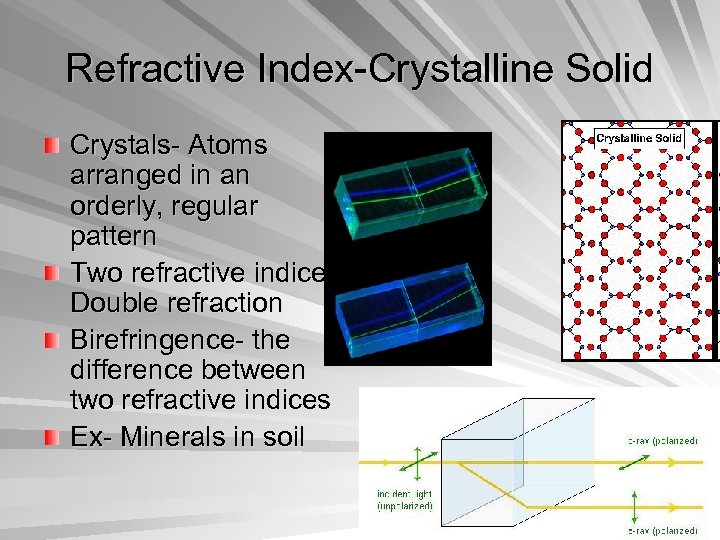 Refractive Index-Crystalline Solid Crystals- Atoms arranged in an orderly, regular pattern Two refractive indices, Double refraction Birefringence- the difference between two refractive indices Ex- Minerals in soil
Refractive Index-Crystalline Solid Crystals- Atoms arranged in an orderly, regular pattern Two refractive indices, Double refraction Birefringence- the difference between two refractive indices Ex- Minerals in soil
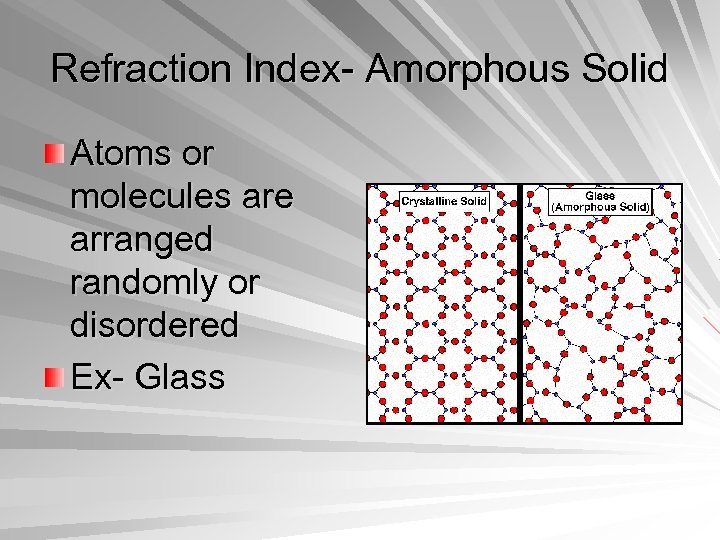 Refraction Index- Amorphous Solid Atoms or molecules are arranged randomly or disordered Ex- Glass
Refraction Index- Amorphous Solid Atoms or molecules are arranged randomly or disordered Ex- Glass
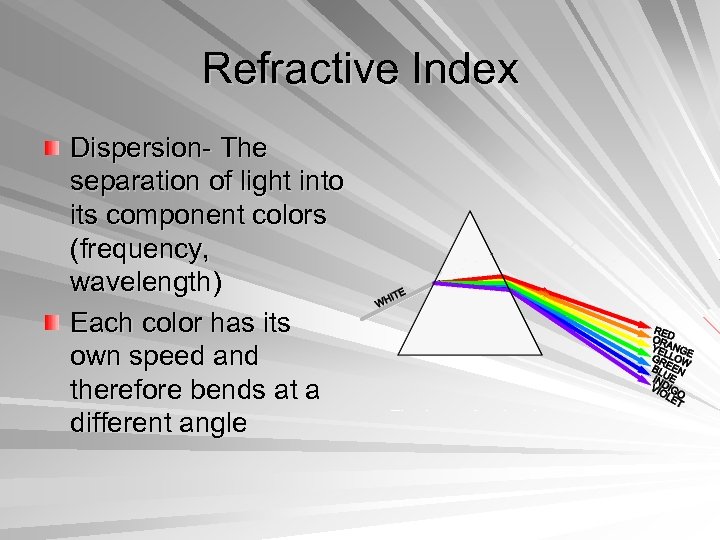 Refractive Index Dispersion- The separation of light into its component colors (frequency, wavelength) Each color has its own speed and therefore bends at a different angle
Refractive Index Dispersion- The separation of light into its component colors (frequency, wavelength) Each color has its own speed and therefore bends at a different angle
 Glass As Evidence Type of Glass Source of Glass Class or Individual How broken? Direction, sequence, speed, size of projectile Link a suspect to a crime scene - Glass from CS on suspect, Hit and Run, Fingerprints, Blood
Glass As Evidence Type of Glass Source of Glass Class or Individual How broken? Direction, sequence, speed, size of projectile Link a suspect to a crime scene - Glass from CS on suspect, Hit and Run, Fingerprints, Blood
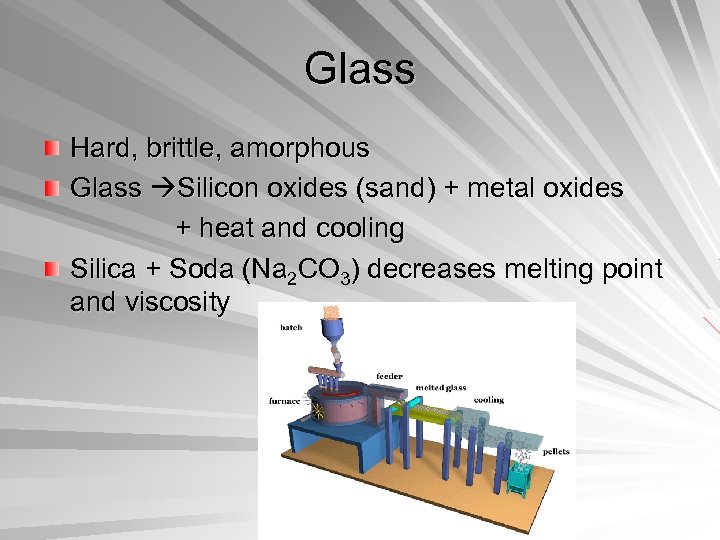 Glass Hard, brittle, amorphous Glass Silicon oxides (sand) + metal oxides + heat and cooling Silica + Soda (Na 2 CO 3) decreases melting point and viscosity
Glass Hard, brittle, amorphous Glass Silicon oxides (sand) + metal oxides + heat and cooling Silica + Soda (Na 2 CO 3) decreases melting point and viscosity
 Glass Silicon oxides + metal oxides (sodium, calcium, magnesium, aluminum) + soda + lime (Ca. O) “Sodalime” Glass Decreased solubilty Ex- windows, bottle glass, lighting
Glass Silicon oxides + metal oxides (sodium, calcium, magnesium, aluminum) + soda + lime (Ca. O) “Sodalime” Glass Decreased solubilty Ex- windows, bottle glass, lighting
 Glass Different oxide Different types of glass Ex- Borosilicates (boron oxide) heat resistant glass- headlights, Pyrex
Glass Different oxide Different types of glass Ex- Borosilicates (boron oxide) heat resistant glass- headlights, Pyrex
 Glass Tempered- Rapid heating and cooling strengthens glass “dices” instead of shattering when broken Side and rear windows of U. S. made cars and some foreign windshields
Glass Tempered- Rapid heating and cooling strengthens glass “dices” instead of shattering when broken Side and rear windows of U. S. made cars and some foreign windshields
 Glass Laminated Glass- Plastic sandwiched between ordinary glass U. S. windshields
Glass Laminated Glass- Plastic sandwiched between ordinary glass U. S. windshields
 Comparing Glass Fragments Individualized Characteristics: DIFFICULT! Matching irregular shapes, striations, ream patterns Class Characteristics: Chemical composition * Density * Refractive Index
Comparing Glass Fragments Individualized Characteristics: DIFFICULT! Matching irregular shapes, striations, ream patterns Class Characteristics: Chemical composition * Density * Refractive Index
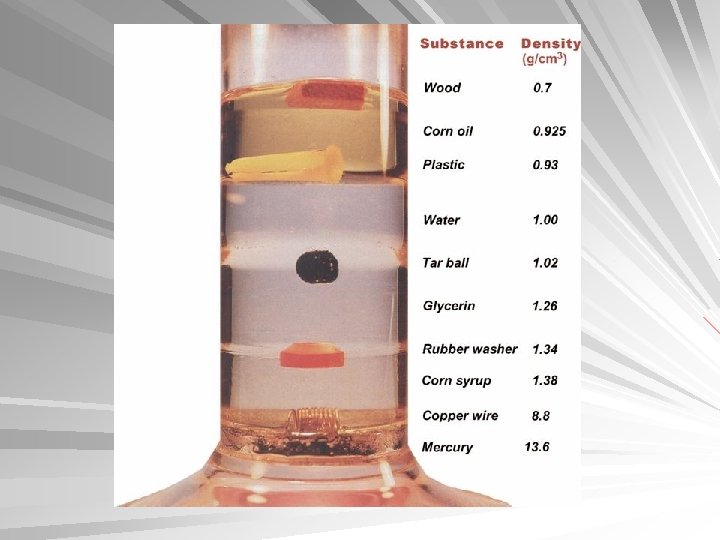
 Density Glass particles in a liquid will sink, float or be suspended depending whether they are more or less dense than the liquid Flotation- Standard/reference suspended in bromoform/bromobenzene mixture compared to unknown Sensitivity of 0. 001 g/ml Window glass is not homogeneous (0. 0003 g/ml)
Density Glass particles in a liquid will sink, float or be suspended depending whether they are more or less dense than the liquid Flotation- Standard/reference suspended in bromoform/bromobenzene mixture compared to unknown Sensitivity of 0. 001 g/ml Window glass is not homogeneous (0. 0003 g/ml)
 Refractive Index Immersion Method- Glass in immersion liquid with same RI causes Becke line to disappear (match point) Hot- stage microscope Glass immersed in silicone oil, heated 0. 2°C/minute until match point GRIM 2 (Glass Refractive Index Measurement)automated computer/video system FBI Database of comparison densities and RI’s CSI determines probability of common source
Refractive Index Immersion Method- Glass in immersion liquid with same RI causes Becke line to disappear (match point) Hot- stage microscope Glass immersed in silicone oil, heated 0. 2°C/minute until match point GRIM 2 (Glass Refractive Index Measurement)automated computer/video system FBI Database of comparison densities and RI’s CSI determines probability of common source
 Annealing Slowly heating and cooling of glass which changes RI Tempered glassgreat change Nontempered glasssmall change
Annealing Slowly heating and cooling of glass which changes RI Tempered glassgreat change Nontempered glasssmall change
 Glass Fractures Force causes glass to bend and then fracture CS reconstruction- type of force, direction and order of impact(s)
Glass Fractures Force causes glass to bend and then fracture CS reconstruction- type of force, direction and order of impact(s)
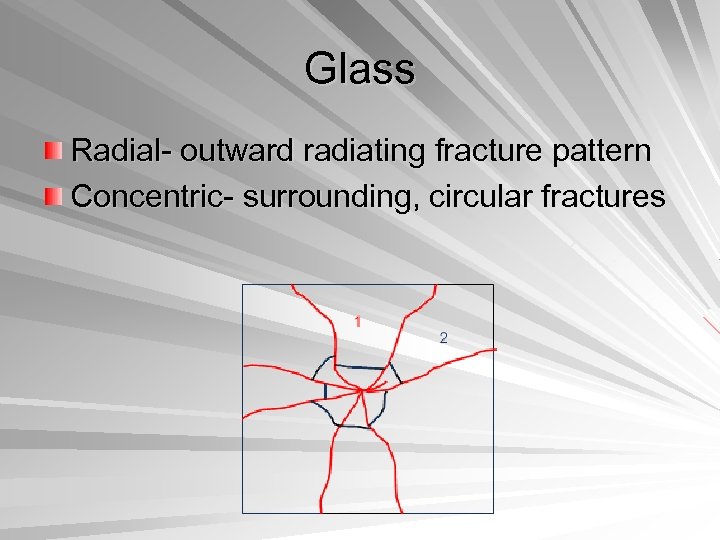 Glass Radial- outward radiating fracture pattern Concentric- surrounding, circular fractures
Glass Radial- outward radiating fracture pattern Concentric- surrounding, circular fractures
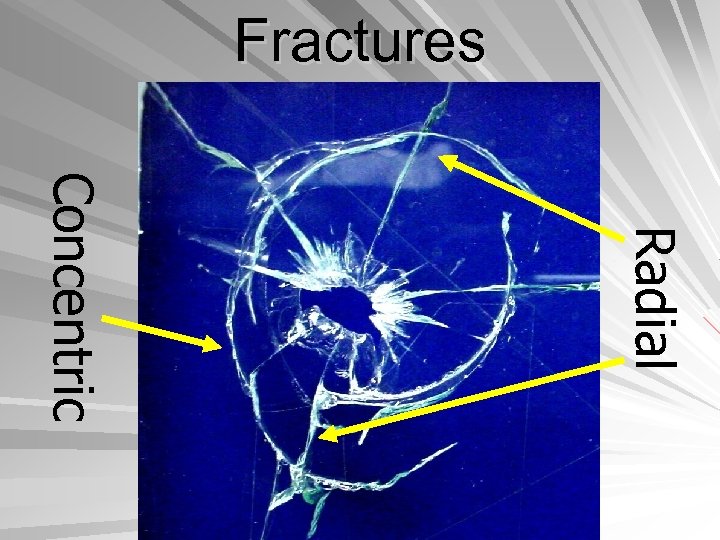 Fractures Radial Concentric
Fractures Radial Concentric
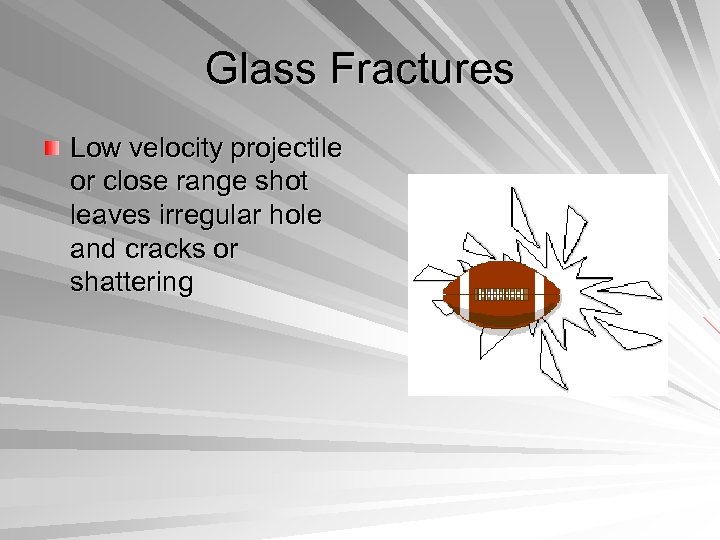 Glass Fractures Low velocity projectile or close range shot leaves irregular hole and cracks or shattering
Glass Fractures Low velocity projectile or close range shot leaves irregular hole and cracks or shattering
 Glass Fractures High velocity projectile (bullet) usually leaves round, cratershaped hole surrounded by near symmetrical radial and concentric cracks n A high velocity projectile hole is inevitably wider at the exit side (high velocity)
Glass Fractures High velocity projectile (bullet) usually leaves round, cratershaped hole surrounded by near symmetrical radial and concentric cracks n A high velocity projectile hole is inevitably wider at the exit side (high velocity)

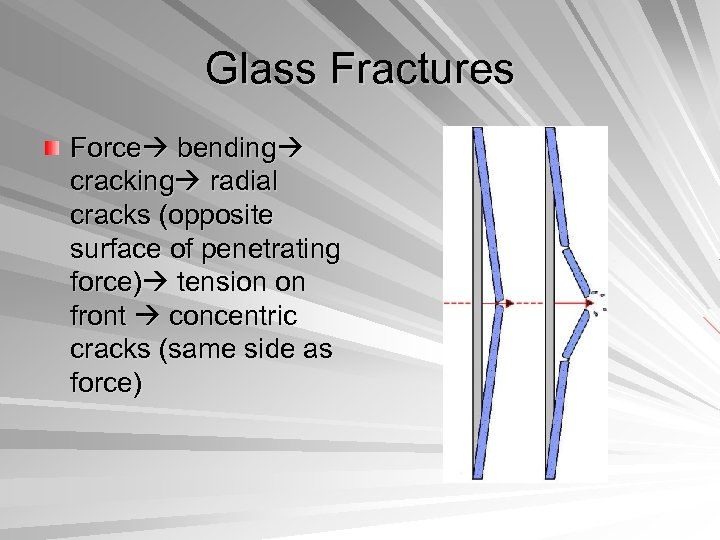 Glass Fractures Force bending cracking radial cracks (opposite surface of penetrating force) tension on front concentric cracks (same side as force)
Glass Fractures Force bending cracking radial cracks (opposite surface of penetrating force) tension on front concentric cracks (same side as force)

 Stress Marks- Conchoidal Lines n. The perpendicular edge of stress marks always face the surface on which the crack originated n 3 R rule- Radial cracks form a right angle on the Reverse (opposite) side of the force n. Concentric cracks form a right angle on the side of the force
Stress Marks- Conchoidal Lines n. The perpendicular edge of stress marks always face the surface on which the crack originated n 3 R rule- Radial cracks form a right angle on the Reverse (opposite) side of the force n. Concentric cracks form a right angle on the side of the force
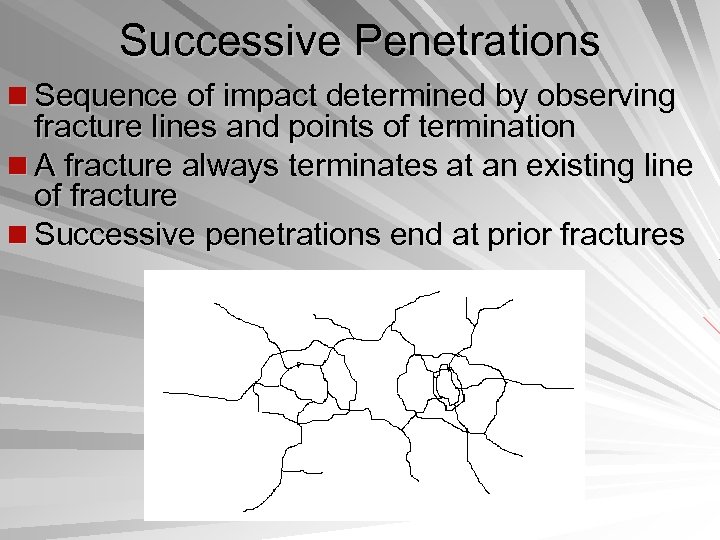 Successive Penetrations n Sequence of impact determined by observing fracture lines and points of termination n A fracture always terminates at an existing line of fracture n Successive penetrations end at prior fractures
Successive Penetrations n Sequence of impact determined by observing fracture lines and points of termination n A fracture always terminates at an existing line of fracture n Successive penetrations end at prior fractures


 Collection and Preservation of Glass Evidence All fragments at CS &/or possessed by suspect should be collected- Individualization by piecing fragments together Broken glass w/interior/exterior noted (dirt, paint, grease, putty)- direction of impact Standard/Reference from CS (1 sq. inch) Hit & Run- headlight, reflectors Package in solid container Clothing/shoes wrapped in paper
Collection and Preservation of Glass Evidence All fragments at CS &/or possessed by suspect should be collected- Individualization by piecing fragments together Broken glass w/interior/exterior noted (dirt, paint, grease, putty)- direction of impact Standard/Reference from CS (1 sq. inch) Hit & Run- headlight, reflectors Package in solid container Clothing/shoes wrapped in paper
 Forensic Characteristics of Soil Disintegrated surface material that lies on or near the earth’s surface (natural, artificial) Rocks, minerals, vegetation, animal matter, glass, paint chips, asphalt, brick, cinders Forensic Geologist Associated w/crime = Physical Evidence
Forensic Characteristics of Soil Disintegrated surface material that lies on or near the earth’s surface (natural, artificial) Rocks, minerals, vegetation, animal matter, glass, paint chips, asphalt, brick, cinders Forensic Geologist Associated w/crime = Physical Evidence
 Soil Associative Evidencelinks suspect to crime scene Comparativecollected evidence to crime scene Crime scene clothes- shoesvehicle- suspect- tools -? Class or Individual?
Soil Associative Evidencelinks suspect to crime scene Comparativecollected evidence to crime scene Crime scene clothes- shoesvehicle- suspect- tools -? Class or Individual?
 Soil First compare color (1100), texture Soils from different locations, regions Observe dried samples Minerals (2200/40) - color, geometric shape, density, RI, birefringence Particle size Rocks- mineral content, grain size Geologist checks comparative points, frequency
Soil First compare color (1100), texture Soils from different locations, regions Observe dried samples Minerals (2200/40) - color, geometric shape, density, RI, birefringence Particle size Rocks- mineral content, grain size Geologist checks comparative points, frequency
 Soil Examination Gross Examination
Soil Examination Gross Examination
 Soil Examination Microscopic
Soil Examination Microscopic
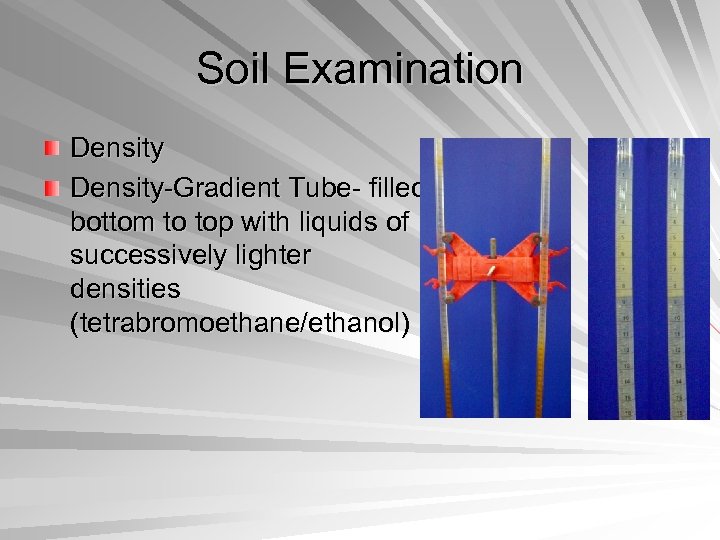 Soil Examination Density-Gradient Tube- filled bottom to top with liquids of successively lighter densities (tetrabromoethane/ethanol)
Soil Examination Density-Gradient Tube- filled bottom to top with liquids of successively lighter densities (tetrabromoethane/ethanol)
 Density Gradient Assemble two identical density columns with varying levels of liquids in them Drop equal amounts of soil samples (a control and unknown) in the columns Allow the sample to disperse throughout the columns for comparison If the two samples are of the same origin, then they will produce nearly identical gradient tubes Nondefinitive, Use with other tests
Density Gradient Assemble two identical density columns with varying levels of liquids in them Drop equal amounts of soil samples (a control and unknown) in the columns Allow the sample to disperse throughout the columns for comparison If the two samples are of the same origin, then they will produce nearly identical gradient tubes Nondefinitive, Use with other tests
 Density Gradient
Density Gradient
 Collection and Preservation of Soil Evidence Standard/reference samples (100 yd. ) Top-surface layer 1 -2 Tablespoons Plastic vials Clothes/shoes wrapped in paper Cars collect soil over long periods Intact, layers
Collection and Preservation of Soil Evidence Standard/reference samples (100 yd. ) Top-surface layer 1 -2 Tablespoons Plastic vials Clothes/shoes wrapped in paper Cars collect soil over long periods Intact, layers
 http: //investigation. discovery. com/videos/s olved-trace-evidence. html
http: //investigation. discovery. com/videos/s olved-trace-evidence. html


