Lecture No.2.ppt
- Количество слайдов: 26
 PHYSICAL CHEMISTRY OF NANOSTRUCTURED SYSTEMS 1 Dr. TERESA FERNANDEZ ALDAMA ¨SAMARA UNIVERSITY¨
PHYSICAL CHEMISTRY OF NANOSTRUCTURED SYSTEMS 1 Dr. TERESA FERNANDEZ ALDAMA ¨SAMARA UNIVERSITY¨
 2 LECTURE No. 2 CARBON BASED MATERIALS
2 LECTURE No. 2 CARBON BASED MATERIALS
 3 OBJECTIVES q To describe the structure and the most important characteristics of fullerenes, their formation and properties. q To give the most important applications.
3 OBJECTIVES q To describe the structure and the most important characteristics of fullerenes, their formation and properties. q To give the most important applications.
 4 OUTLINE q Fullerenes. The structure and its characteristics. q Types of fullerenes. q Mechanism of formation. q Chemical properties. q Applications.
4 OUTLINE q Fullerenes. The structure and its characteristics. q Types of fullerenes. q Mechanism of formation. q Chemical properties. q Applications.
 5 Importance of the carbon atoms q The most studied chemical element q Forms organic compounds with: H, O and N q Applications in Medicine, Biology, energy production and conservation of environment q Two types of materials: graphite, which we use in the pencil mines, and diamond, crystalline cubic structure.
5 Importance of the carbon atoms q The most studied chemical element q Forms organic compounds with: H, O and N q Applications in Medicine, Biology, energy production and conservation of environment q Two types of materials: graphite, which we use in the pencil mines, and diamond, crystalline cubic structure.
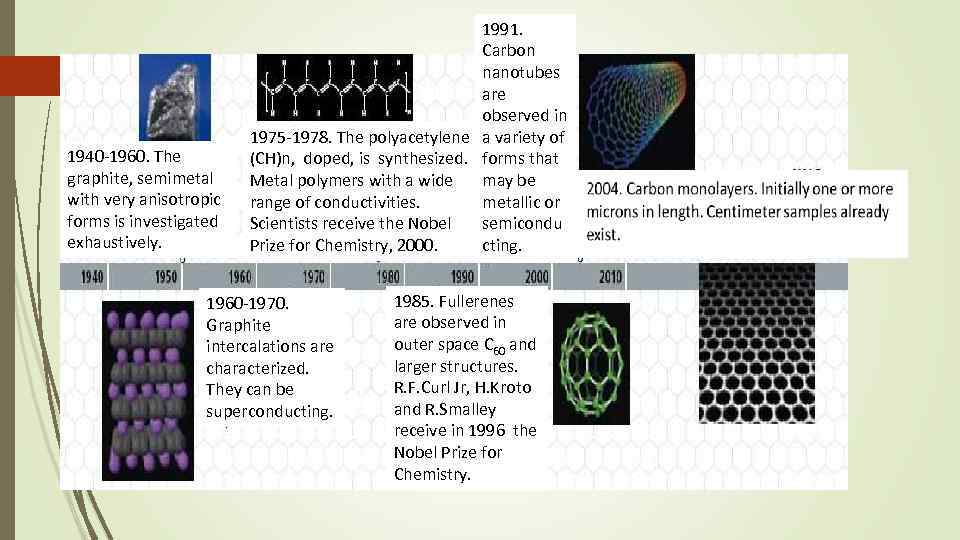 6 1940 -1960. The graphite, semimetal with very anisotropic forms is investigated exhaustively. 1991. Carbon nanotubes are observed in 1975 -1978. The polyacetylene a variety of (CH)n, doped, is synthesized. forms that Metal polymers with a wide may be range of conductivities. metallic or Scientists receive the Nobel semicondu Prize for Chemistry, 2000. cting. 1960 -1970. Graphite intercalations are characterized. They can be superconducting. 1985. Fullerenes are observed in outer space C 60 and larger structures. R. F. Curl Jr, H. Kroto and R. Smalley receive in 1996 the Nobel Prize for Chemistry.
6 1940 -1960. The graphite, semimetal with very anisotropic forms is investigated exhaustively. 1991. Carbon nanotubes are observed in 1975 -1978. The polyacetylene a variety of (CH)n, doped, is synthesized. forms that Metal polymers with a wide may be range of conductivities. metallic or Scientists receive the Nobel semicondu Prize for Chemistry, 2000. cting. 1960 -1970. Graphite intercalations are characterized. They can be superconducting. 1985. Fullerenes are observed in outer space C 60 and larger structures. R. F. Curl Jr, H. Kroto and R. Smalley receive in 1996 the Nobel Prize for Chemistry.
 7 Carbon based materials Fullerenes Nanotubes Graphene Graphane
7 Carbon based materials Fullerenes Nanotubes Graphene Graphane
 8 Fullerenes q They were discovered in 1985 by Harold Kroto, James R. Heath, Sean O'Brien, Robert Curl, and Richard Smalley at Rice University, USA (Nobel Prize in 1991). q The unique electronic structure of fullerenes defines their unique properties including: üchemical resistance, ühigh strength, üthermal and electrical conductivity (Applications)
8 Fullerenes q They were discovered in 1985 by Harold Kroto, James R. Heath, Sean O'Brien, Robert Curl, and Richard Smalley at Rice University, USA (Nobel Prize in 1991). q The unique electronic structure of fullerenes defines their unique properties including: üchemical resistance, ühigh strength, üthermal and electrical conductivity (Applications)
 9 Characteristics of Fullerenes q Structural beauty and versatility to form new compounds. q Forms like spheres, ellipsoids or cylinders: ü Sphericals Bucky spheres ü Cylindrical Buckytubes or nanotubes
9 Characteristics of Fullerenes q Structural beauty and versatility to form new compounds. q Forms like spheres, ellipsoids or cylinders: ü Sphericals Bucky spheres ü Cylindrical Buckytubes or nanotubes
 10 Characteristics of Fullerenes Geodesic dome (Buckminster Fuller)
10 Characteristics of Fullerenes Geodesic dome (Buckminster Fuller)
 11 Characteristics of Fullerenes Geodesic dome (Buckminster Fuller) (Buckminsterfullerenes)
11 Characteristics of Fullerenes Geodesic dome (Buckminster Fuller) (Buckminsterfullerenes)
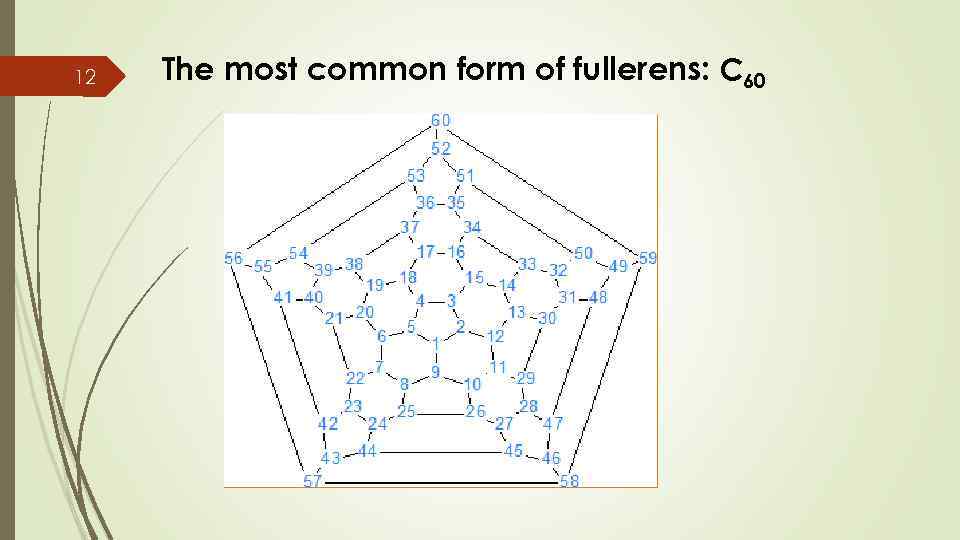 12 The most common form of fullerens: C 60
12 The most common form of fullerens: C 60
 13 Characteristics of C 60 q There are 60 carbon atoms bonding together like hexagons and pentagons in a succer ball. q It consists in 20 hexagons and 12 pentagons.
13 Characteristics of C 60 q There are 60 carbon atoms bonding together like hexagons and pentagons in a succer ball. q It consists in 20 hexagons and 12 pentagons.
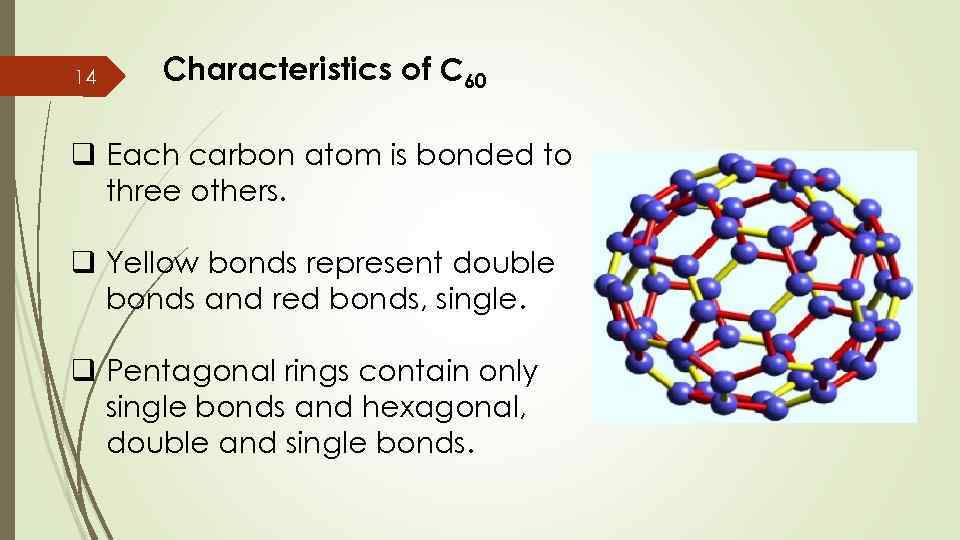 14 Characteristics of C 60 q Each carbon atom is bonded to three others. q Yellow bonds represent double bonds and red bonds, single. q Pentagonal rings contain only single bonds and hexagonal, double and single bonds.
14 Characteristics of C 60 q Each carbon atom is bonded to three others. q Yellow bonds represent double bonds and red bonds, single. q Pentagonal rings contain only single bonds and hexagonal, double and single bonds.
 15 Characteristics of C 60 q Double bonds have shorter bond lenght: Ø Instability in the pentagonal rings Ø Poor delocalization of electrons Molecule reactivity q Strong and resistant carbon macromolecule. It resists extraordinary pressures. q There are different structures: C 20, C 26, C 36, C 50, C 60, C 72, C 76, C 80, C 82, C 84, up to C 540.
15 Characteristics of C 60 q Double bonds have shorter bond lenght: Ø Instability in the pentagonal rings Ø Poor delocalization of electrons Molecule reactivity q Strong and resistant carbon macromolecule. It resists extraordinary pressures. q There are different structures: C 20, C 26, C 36, C 50, C 60, C 72, C 76, C 80, C 82, C 84, up to C 540.
 16 Physical properties § Density: 1, 72 g/cm 3 § Poorly soluble in most solvents (toluene and carbon disulfide. § Solutions of pure buckminsterfullerene have an intense purple color. § Thermal conductivity (300 K): 0. 4 W m 1 K 1 § Electrical conductivity: 1. 7 10 7 Cm § Boiling temperature: 1180 С § Great tensile strength
16 Physical properties § Density: 1, 72 g/cm 3 § Poorly soluble in most solvents (toluene and carbon disulfide. § Solutions of pure buckminsterfullerene have an intense purple color. § Thermal conductivity (300 K): 0. 4 W m 1 K 1 § Electrical conductivity: 1. 7 10 7 Cm § Boiling temperature: 1180 С § Great tensile strength
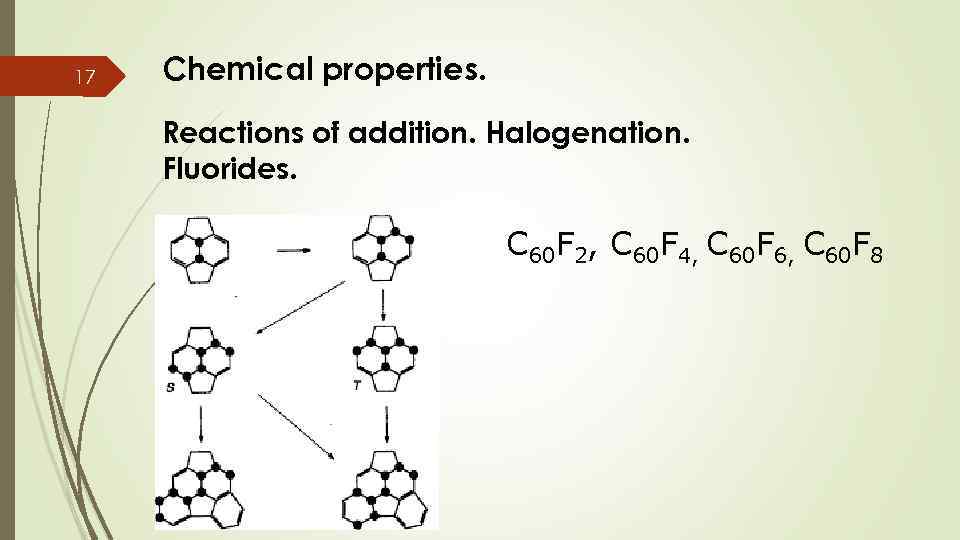 17 Chemical properties. Reactions of addition. Halogenation. Fluorides. C 60 F 2, C 60 F 4, C 60 F 6, C 60 F 8
17 Chemical properties. Reactions of addition. Halogenation. Fluorides. C 60 F 2, C 60 F 4, C 60 F 6, C 60 F 8
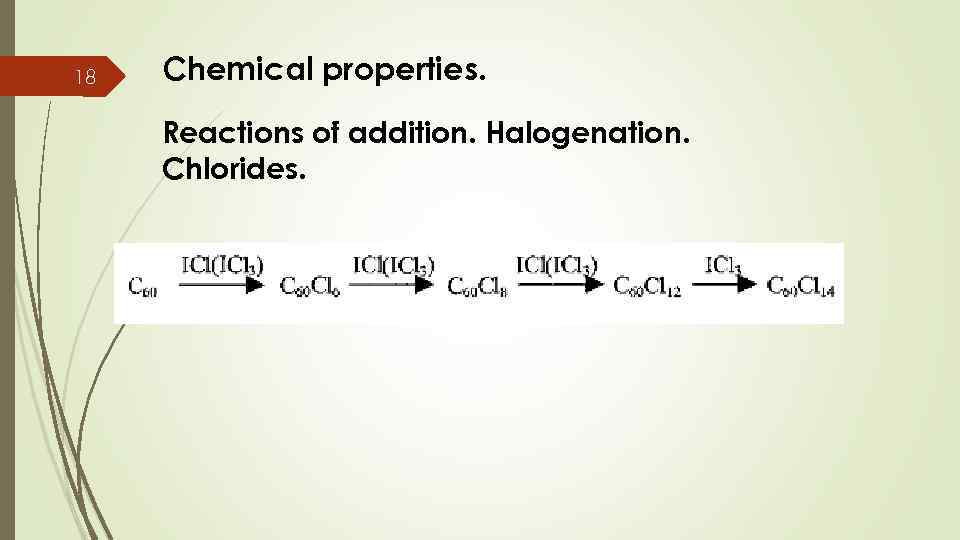 18 Chemical properties. Reactions of addition. Halogenation. Chlorides.
18 Chemical properties. Reactions of addition. Halogenation. Chlorides.
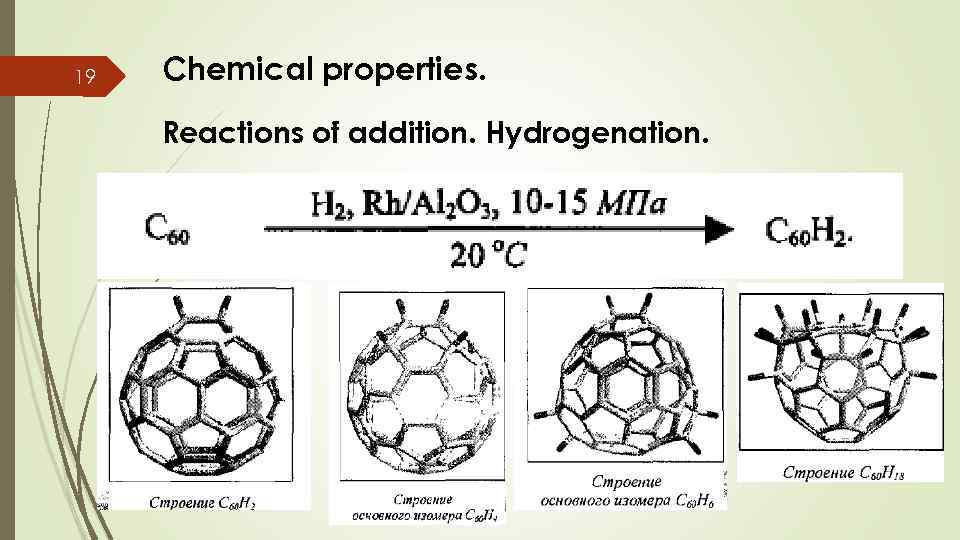 19 Chemical properties. Reactions of addition. Hydrogenation.
19 Chemical properties. Reactions of addition. Hydrogenation.
 20 Chemical properties. Endohedral fullerenes They are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. Molecular conteiners
20 Chemical properties. Endohedral fullerenes They are fullerenes that have additional atoms, ions, or clusters enclosed within their inner spheres. Molecular conteiners
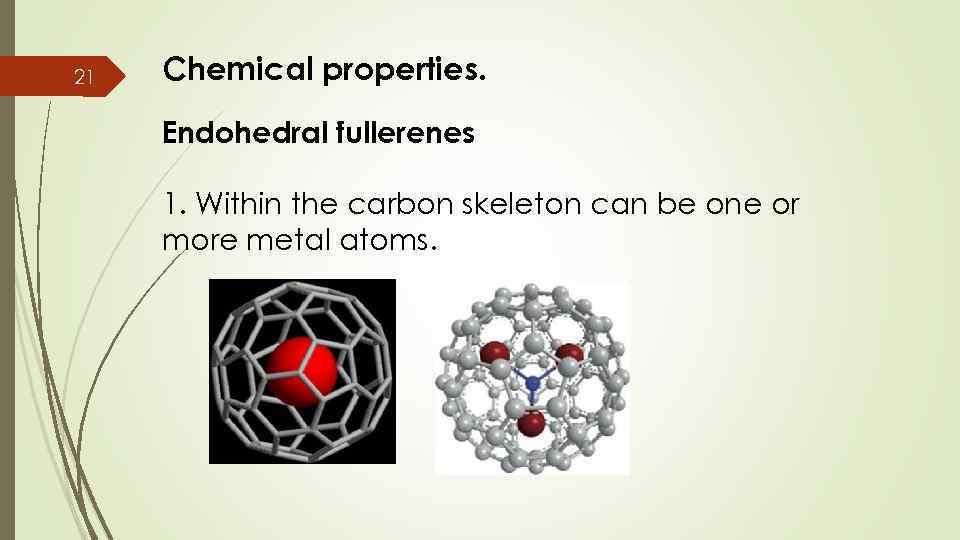 21 Chemical properties. Endohedral fullerenes 1. Within the carbon skeleton can be one or more metal atoms.
21 Chemical properties. Endohedral fullerenes 1. Within the carbon skeleton can be one or more metal atoms.
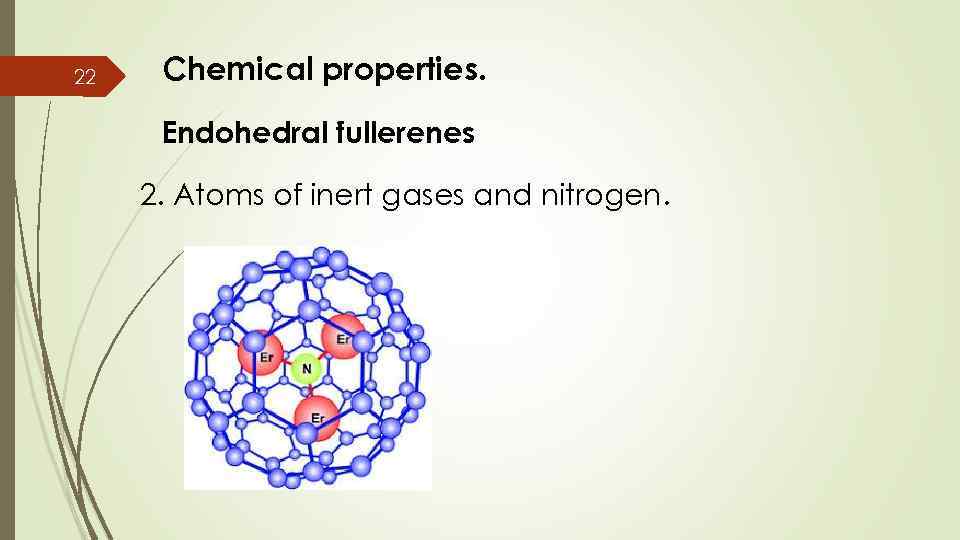 22 Chemical properties. Endohedral fullerenes 2. Atoms of inert gases and nitrogen.
22 Chemical properties. Endohedral fullerenes 2. Atoms of inert gases and nitrogen.
 23 Chemical properties. Endohedral fullerenes The first C 60 complex was synthesized in 1985 and called lanthanum C 60 La@C 60 K 2(K@C 59 B)
23 Chemical properties. Endohedral fullerenes The first C 60 complex was synthesized in 1985 and called lanthanum C 60 La@C 60 K 2(K@C 59 B)
 24 Applications q Electronics, chemistry, medicine, optics q As the basis to produce batteries q Optical gates q As additives for rocket fuel, lubricant.
24 Applications q Electronics, chemistry, medicine, optics q As the basis to produce batteries q Optical gates q As additives for rocket fuel, lubricant.
 25 Control questions 1. Describe in briefly what is fullerenes? 2. Mention the main characteristics of fullerenes. 3. Explain the structure of C 60 4. Mention some physical properties of fullerenes. 5. Mention some chemical properties of fullerenes and explain one of them.
25 Control questions 1. Describe in briefly what is fullerenes? 2. Mention the main characteristics of fullerenes. 3. Explain the structure of C 60 4. Mention some physical properties of fullerenes. 5. Mention some chemical properties of fullerenes and explain one of them.
 26 THANK YOU FOR YOUR ATTENTION!
26 THANK YOU FOR YOUR ATTENTION!


