83d1add129ba7a9b56878d259b352201.ppt
- Количество слайдов: 28
 Photochemical transformation reactions Direct photolysis = transformation of a compound due to its absorption of UV light Indirect photolysis = transformation of a compound due to its interaction with a reactant generated by the influence of UV light (photosensitizer or reactive oxygen species)
Photochemical transformation reactions Direct photolysis = transformation of a compound due to its absorption of UV light Indirect photolysis = transformation of a compound due to its interaction with a reactant generated by the influence of UV light (photosensitizer or reactive oxygen species)
 Direct photolysis and light absorption Types of orbitals: bonding: (single) or (double) non-bonding: n (often lone pairs on hetero atoms such as N, O) anti-bonding: * (single) or * (double) Absorption of light causes electronic transitions: important transitions are usually n to * or to *
Direct photolysis and light absorption Types of orbitals: bonding: (single) or (double) non-bonding: n (often lone pairs on hetero atoms such as N, O) anti-bonding: * (single) or * (double) Absorption of light causes electronic transitions: important transitions are usually n to * or to *
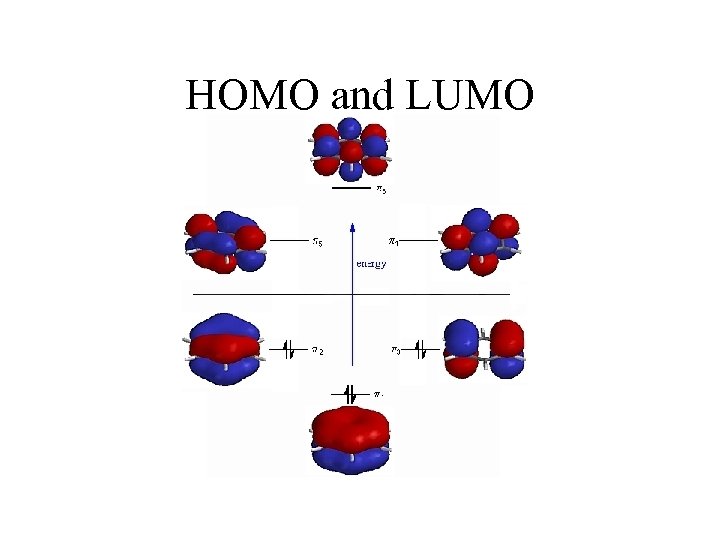 HOMO and LUMO
HOMO and LUMO
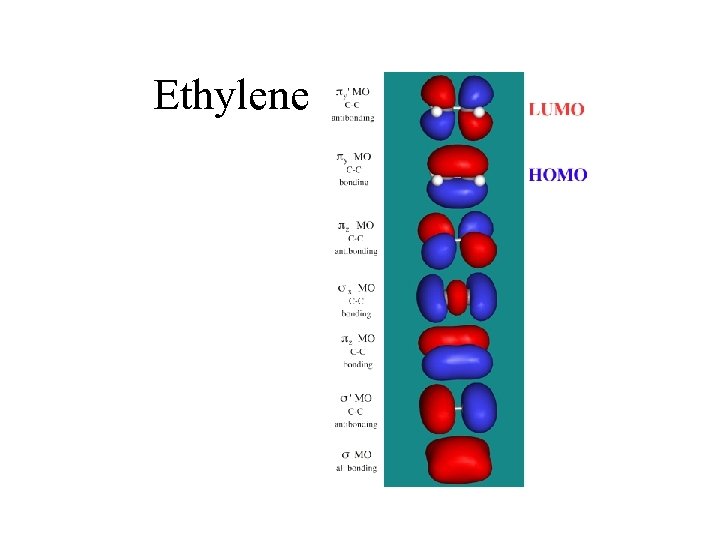 Ethylene
Ethylene
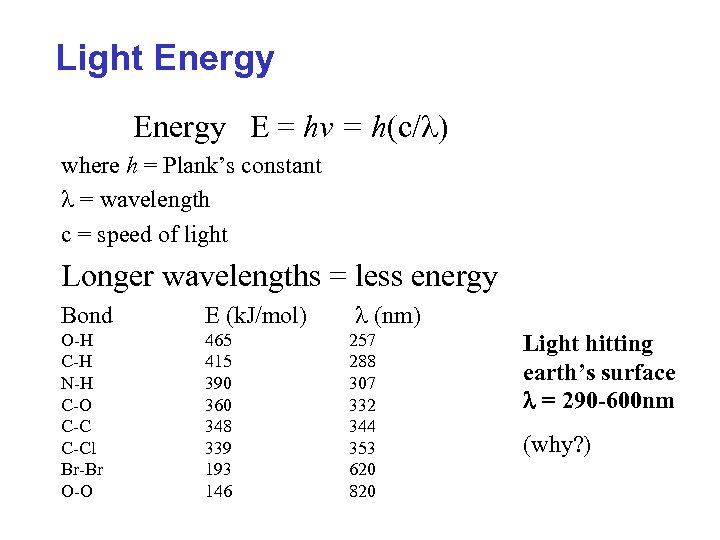 Light Energy E = hv = h(c/l) where h = Plank’s constant l = wavelength c = speed of light Longer wavelengths = less energy Bond E (k. J/mol) O-H C-H N-H C-O C-Cl Br-Br O-O 465 415 390 360 348 339 193 146 l (nm) 257 288 307 332 344 353 620 820 Light hitting earth’s surface l = 290 -600 nm (why? )
Light Energy E = hv = h(c/l) where h = Plank’s constant l = wavelength c = speed of light Longer wavelengths = less energy Bond E (k. J/mol) O-H C-H N-H C-O C-Cl Br-Br O-O 465 415 390 360 348 339 193 146 l (nm) 257 288 307 332 344 353 620 820 Light hitting earth’s surface l = 290 -600 nm (why? )
 Light absorption Beer-Lambert Law where: A = absorbance I = light intensity (emerging vs. incident) l = wavelength a = absorption coefficient of the medium e = absorption coefficient of the compound l = path length
Light absorption Beer-Lambert Law where: A = absorbance I = light intensity (emerging vs. incident) l = wavelength a = absorption coefficient of the medium e = absorption coefficient of the compound l = path length
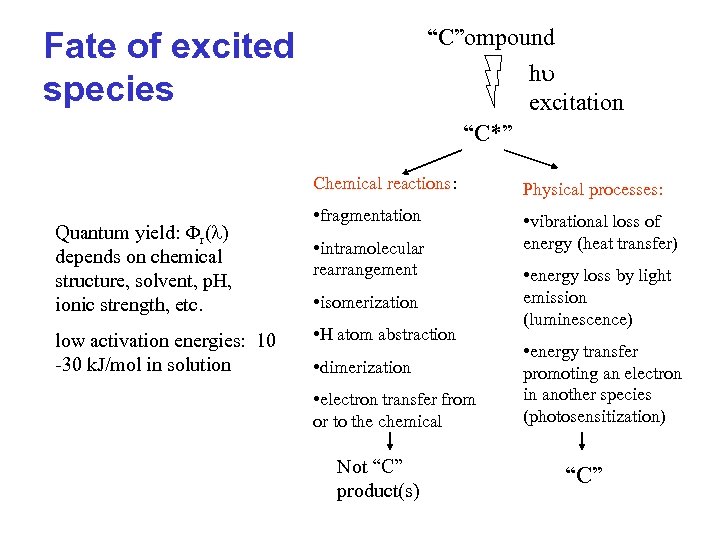 “C”ompound h excitation “C*” Fate of excited species Chemical reactions: Quantum yield: r(l) depends on chemical structure, solvent, p. H, ionic strength, etc. low activation energies: 10 -30 k. J/mol in solution Physical processes: • fragmentation • vibrational loss of energy (heat transfer) • intramolecular rearrangement • isomerization • H atom abstraction • dimerization • electron transfer from or to the chemical Not “C” product(s) • energy loss by light emission (luminescence) • energy transfer promoting an electron in another species (photosensitization) “C”
“C”ompound h excitation “C*” Fate of excited species Chemical reactions: Quantum yield: r(l) depends on chemical structure, solvent, p. H, ionic strength, etc. low activation energies: 10 -30 k. J/mol in solution Physical processes: • fragmentation • vibrational loss of energy (heat transfer) • intramolecular rearrangement • isomerization • H atom abstraction • dimerization • electron transfer from or to the chemical Not “C” product(s) • energy loss by light emission (luminescence) • energy transfer promoting an electron in another species (photosensitization) “C”
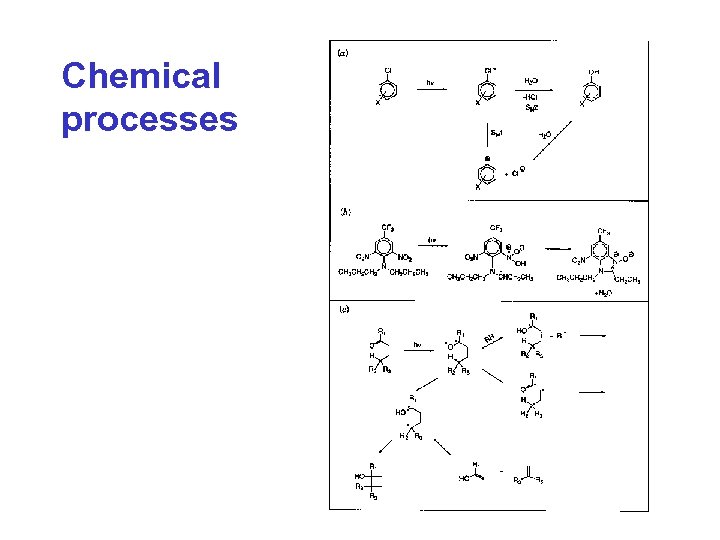 Chemical processes
Chemical processes
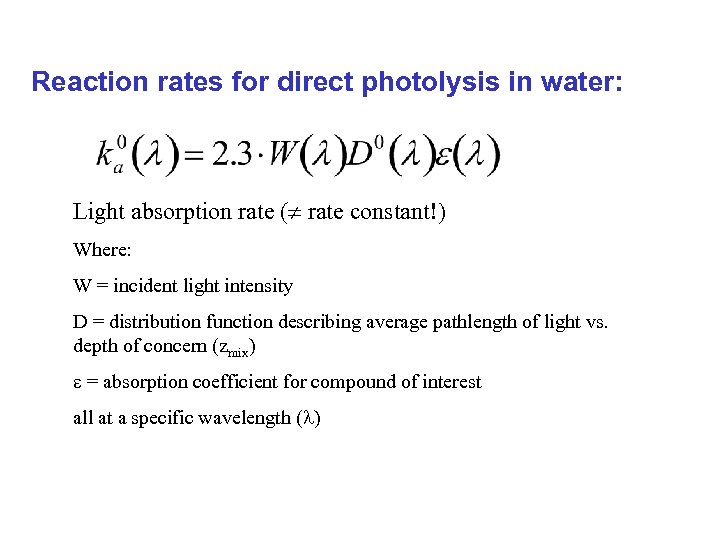 Reaction rates for direct photolysis in water: Light absorption rate ( rate constant!) Where: W = incident light intensity D = distribution function describing average pathlength of light vs. depth of concern (zmix) e = absorption coefficient for compound of interest all at a specific wavelength (l)
Reaction rates for direct photolysis in water: Light absorption rate ( rate constant!) Where: W = incident light intensity D = distribution function describing average pathlength of light vs. depth of concern (zmix) e = absorption coefficient for compound of interest all at a specific wavelength (l)
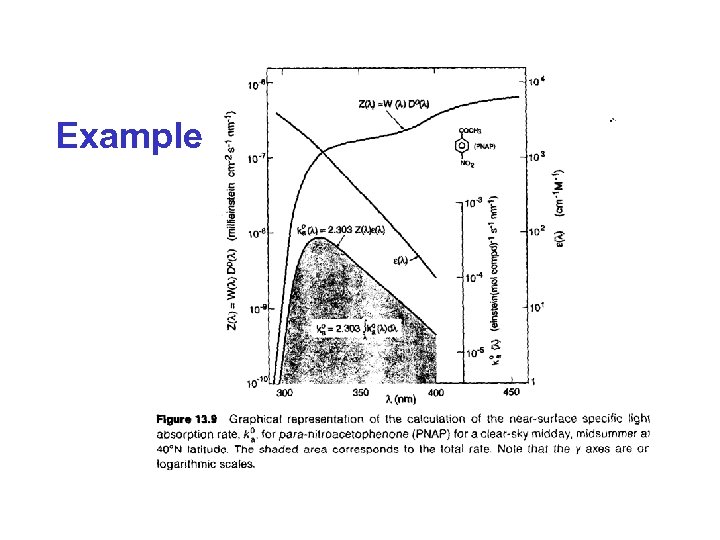 Example
Example
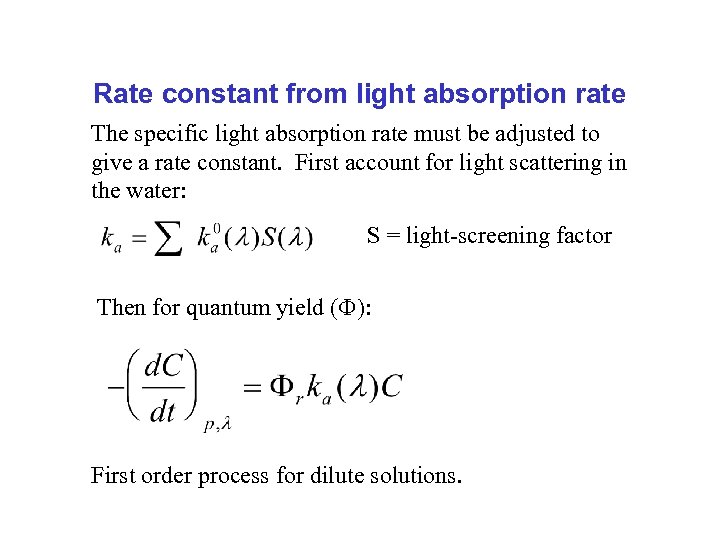 Rate constant from light absorption rate The specific light absorption rate must be adjusted to give a rate constant. First account for light scattering in the water: S = light-screening factor Then for quantum yield ( ): First order process for dilute solutions.
Rate constant from light absorption rate The specific light absorption rate must be adjusted to give a rate constant. First account for light scattering in the water: S = light-screening factor Then for quantum yield ( ): First order process for dilute solutions.
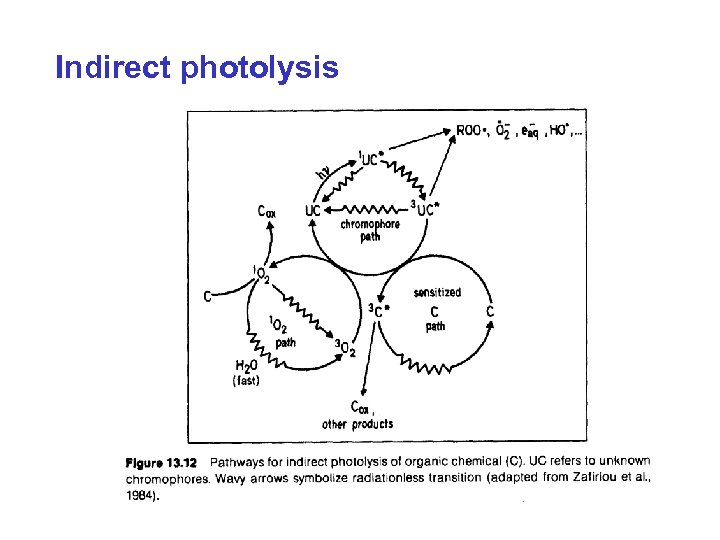 Indirect photolysis
Indirect photolysis
![Important reactants (electrophiles) Singlet oxygen (1 O 2) [1 O 2 ]ss = 7 Important reactants (electrophiles) Singlet oxygen (1 O 2) [1 O 2 ]ss = 7](https://present5.com/presentation/83d1add129ba7a9b56878d259b352201/image-13.jpg) Important reactants (electrophiles) Singlet oxygen (1 O 2) [1 O 2 ]ss = 7 to 11 10 -14 M summer day, mid-latitude electrophile, important for Diels-Alder rxns (electron-rich double bonds) oxidation of reduced sulfur groups, anilines, and phenols. Peroxy Radicals (ROO • ) formed by addition of 3 O 2 to excited chromophores or radicals usually react via abstraction of H with alkyl phenols, aromatic amines, thiophenols, imines reactivity depends on R both are electrophiles, thus electron donating groups increase reactivity
Important reactants (electrophiles) Singlet oxygen (1 O 2) [1 O 2 ]ss = 7 to 11 10 -14 M summer day, mid-latitude electrophile, important for Diels-Alder rxns (electron-rich double bonds) oxidation of reduced sulfur groups, anilines, and phenols. Peroxy Radicals (ROO • ) formed by addition of 3 O 2 to excited chromophores or radicals usually react via abstraction of H with alkyl phenols, aromatic amines, thiophenols, imines reactivity depends on R both are electrophiles, thus electron donating groups increase reactivity
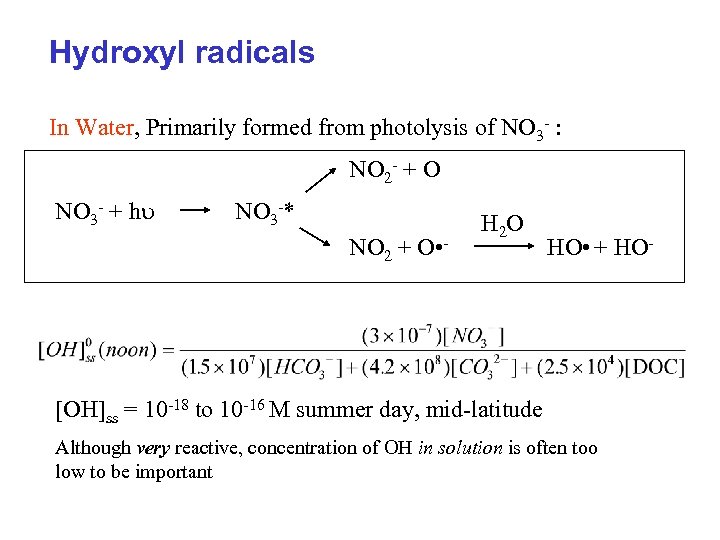 Hydroxyl radicals In Water, Primarily formed from photolysis of NO 3 - : NO 2 - + O NO 3 - + h NO 3 -* NO 2 + O • - H 2 O HO • + HO- [OH]ss = 10 -18 to 10 -16 M summer day, mid-latitude Although very reactive, concentration of OH in solution is often too low to be important
Hydroxyl radicals In Water, Primarily formed from photolysis of NO 3 - : NO 2 - + O NO 3 - + h NO 3 -* NO 2 + O • - H 2 O HO • + HO- [OH]ss = 10 -18 to 10 -16 M summer day, mid-latitude Although very reactive, concentration of OH in solution is often too low to be important
 Reactions of OH in solution H abstraction: aliphatic hydrogen, leaving stable radical. RH + OH • R • + H 2 O Methyl < primary < secondary < tertiary benzylic or allylic H Addition to multiple bonds: electron donating groups will increase reaction rate.
Reactions of OH in solution H abstraction: aliphatic hydrogen, leaving stable radical. RH + OH • R • + H 2 O Methyl < primary < secondary < tertiary benzylic or allylic H Addition to multiple bonds: electron donating groups will increase reaction rate.
 Tropospheric photochemistry: Ozone in the upper atmosphere or stratosphere acts as a protective layer screening harmful ultraviolet light (UV). Ozone found in the lower atmosphere or troposphere does not act as an essential screen but as a pollutant. About 8 % of the total column ozone is in the troposphere. Ozone is a green house gas and possibly contributes to the global warming. Ozone is harmful for human being and crops in the troposphere. Ozone oxidizes many chemical substances in the troposphere. Ozone is continually monitored at many urban locations.
Tropospheric photochemistry: Ozone in the upper atmosphere or stratosphere acts as a protective layer screening harmful ultraviolet light (UV). Ozone found in the lower atmosphere or troposphere does not act as an essential screen but as a pollutant. About 8 % of the total column ozone is in the troposphere. Ozone is a green house gas and possibly contributes to the global warming. Ozone is harmful for human being and crops in the troposphere. Ozone oxidizes many chemical substances in the troposphere. Ozone is continually monitored at many urban locations.
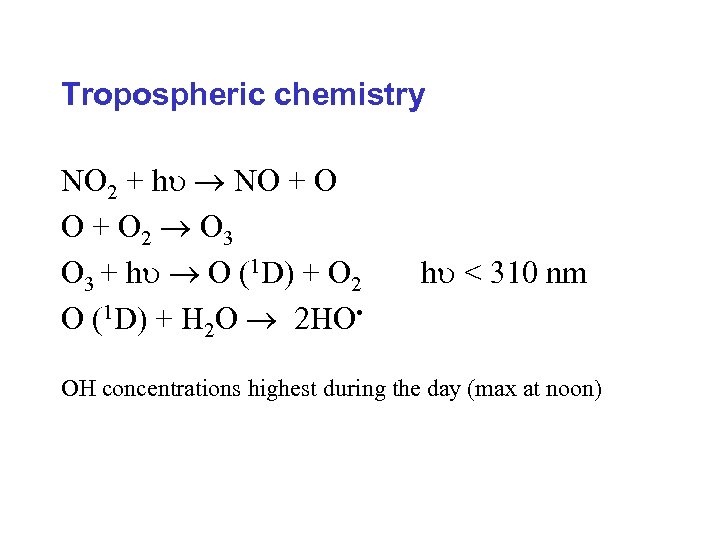 Tropospheric chemistry NO 2 + h NO + O 2 O 3 + h O (1 D) + O 2 O (1 D) + H 2 O 2 HO • h < 310 nm OH concentrations highest during the day (max at noon)
Tropospheric chemistry NO 2 + h NO + O 2 O 3 + h O (1 D) + O 2 O (1 D) + H 2 O 2 HO • h < 310 nm OH concentrations highest during the day (max at noon)
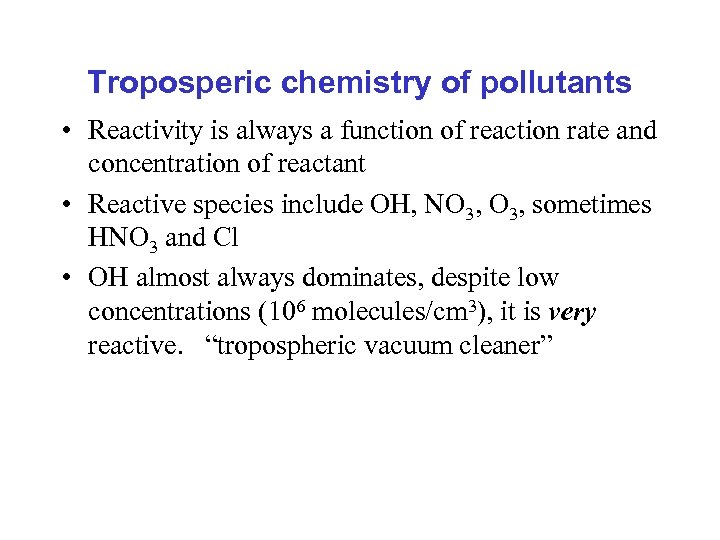 Troposperic chemistry of pollutants • Reactivity is always a function of reaction rate and concentration of reactant • Reactive species include OH, NO 3, sometimes HNO 3 and Cl • OH almost always dominates, despite low concentrations (106 molecules/cm 3), it is very reactive. “tropospheric vacuum cleaner”
Troposperic chemistry of pollutants • Reactivity is always a function of reaction rate and concentration of reactant • Reactive species include OH, NO 3, sometimes HNO 3 and Cl • OH almost always dominates, despite low concentrations (106 molecules/cm 3), it is very reactive. “tropospheric vacuum cleaner”
 Other Tropospheric Reactants • Reactions with NO 3 important for compounds containing (non-aromatic) double bonds, fused rings (PAHs), and S atoms. NO 3 concentrations peak at night. • O 3 reacts with (non-aromatic) double bonds. O 3 concentrations are higher during the day but can still be substantial at night. • Cl atoms can be generated in marine environments at conc’s up to 104 molecules/cm 3. May be important reactants in some situations.
Other Tropospheric Reactants • Reactions with NO 3 important for compounds containing (non-aromatic) double bonds, fused rings (PAHs), and S atoms. NO 3 concentrations peak at night. • O 3 reacts with (non-aromatic) double bonds. O 3 concentrations are higher during the day but can still be substantial at night. • Cl atoms can be generated in marine environments at conc’s up to 104 molecules/cm 3. May be important reactants in some situations.
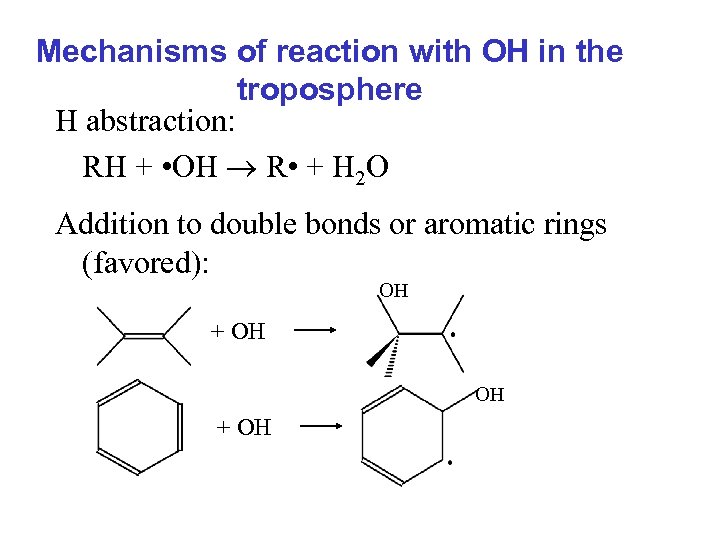 Mechanisms of reaction with OH in the troposphere H abstraction: RH + • OH R • + H 2 O Addition to double bonds or aromatic rings (favored): OH + OH
Mechanisms of reaction with OH in the troposphere H abstraction: RH + • OH R • + H 2 O Addition to double bonds or aromatic rings (favored): OH + OH
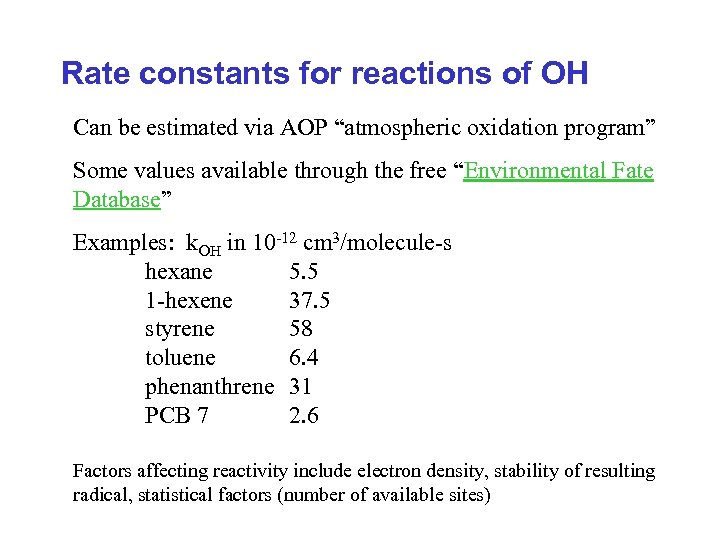 Rate constants for reactions of OH Can be estimated via AOP “atmospheric oxidation program” Some values available through the free “Environmental Fate Database” Examples: k. OH in 10 -12 cm 3/molecule-s hexane 5. 5 1 -hexene 37. 5 styrene 58 toluene 6. 4 phenanthrene 31 PCB 7 2. 6 Factors affecting reactivity include electron density, stability of resulting radical, statistical factors (number of available sites)
Rate constants for reactions of OH Can be estimated via AOP “atmospheric oxidation program” Some values available through the free “Environmental Fate Database” Examples: k. OH in 10 -12 cm 3/molecule-s hexane 5. 5 1 -hexene 37. 5 styrene 58 toluene 6. 4 phenanthrene 31 PCB 7 2. 6 Factors affecting reactivity include electron density, stability of resulting radical, statistical factors (number of available sites)
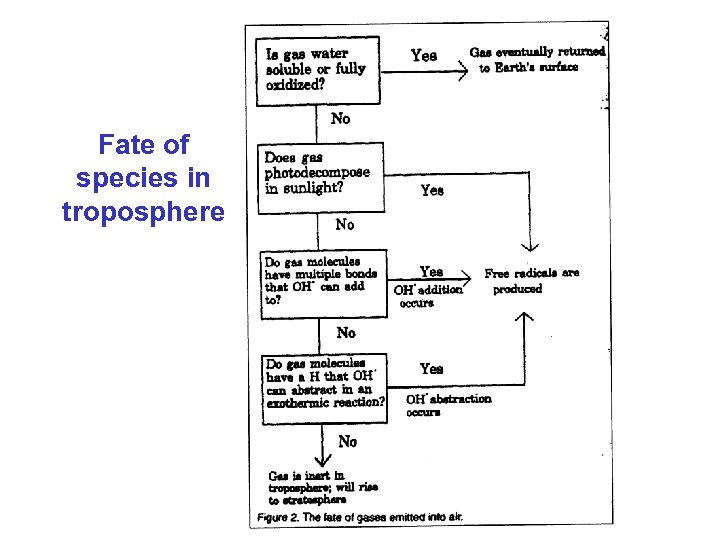 Fate of species in troposphere
Fate of species in troposphere
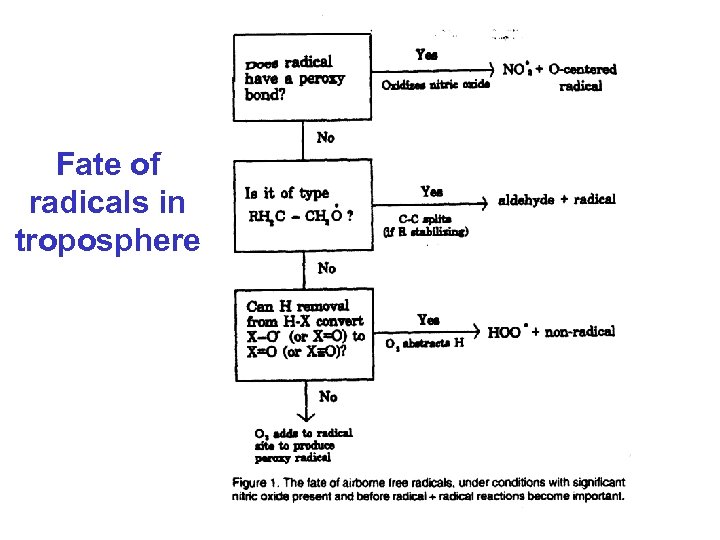 Fate of radicals in troposphere
Fate of radicals in troposphere
 Reactions of PCBs with OH during atmospheric transport • Laboratory-measured rate constants between 5. 0 - 0. 4 1012 cm 3 s-1 (Anderson and Hites, 1996) Þ Half-lives for gas-phase PCBs of 0. 5 to 7 days at relevant OH concentrations • Single most important sink for PCBs on global scale (? ) • Reactivity decreases as number of chlorines increases.
Reactions of PCBs with OH during atmospheric transport • Laboratory-measured rate constants between 5. 0 - 0. 4 1012 cm 3 s-1 (Anderson and Hites, 1996) Þ Half-lives for gas-phase PCBs of 0. 5 to 7 days at relevant OH concentrations • Single most important sink for PCBs on global scale (? ) • Reactivity decreases as number of chlorines increases.
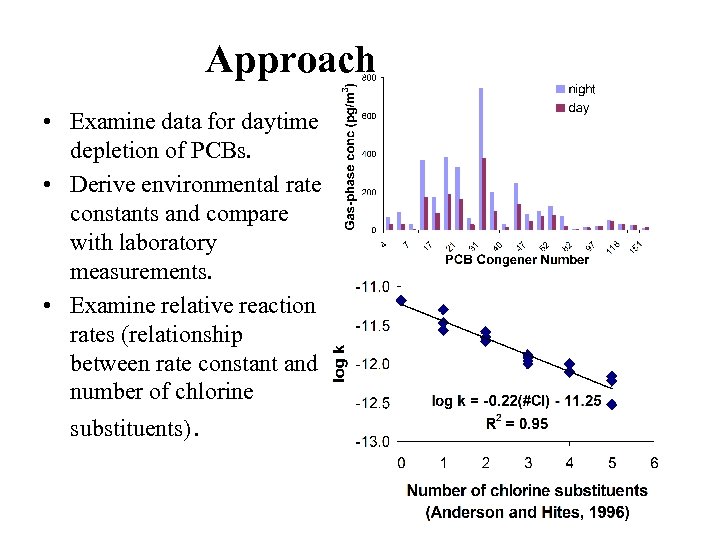 Approach • Examine data for daytime depletion of PCBs. • Derive environmental rate constants and compare with laboratory measurements. • Examine relative reaction rates (relationship between rate constant and number of chlorine substituents).
Approach • Examine data for daytime depletion of PCBs. • Derive environmental rate constants and compare with laboratory measurements. • Examine relative reaction rates (relationship between rate constant and number of chlorine substituents).
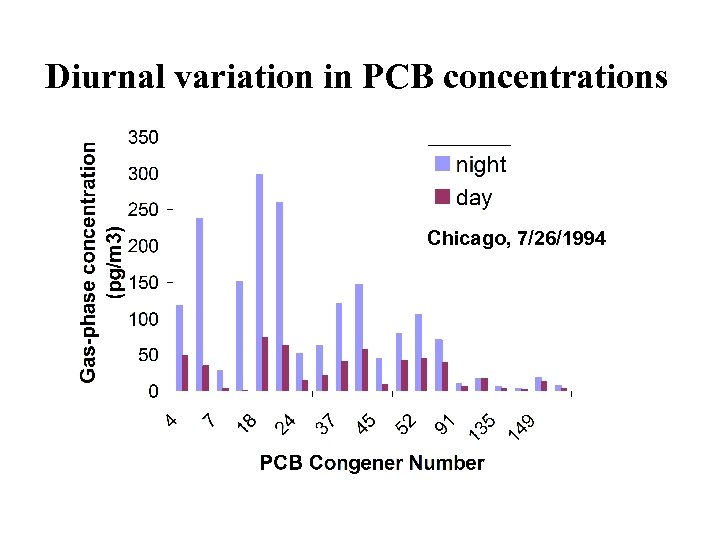 Diurnal variation in PCB concentrations Chicago, 7/26/1994
Diurnal variation in PCB concentrations Chicago, 7/26/1994
![Environmental rate constants: kobs = ke[OH] Assume OH = 3 106 molecules/cm 3 Environmental rate constants: kobs = ke[OH] Assume OH = 3 106 molecules/cm 3](https://present5.com/presentation/83d1add129ba7a9b56878d259b352201/image-27.jpg) Environmental rate constants: kobs = ke[OH] Assume OH = 3 106 molecules/cm 3
Environmental rate constants: kobs = ke[OH] Assume OH = 3 106 molecules/cm 3
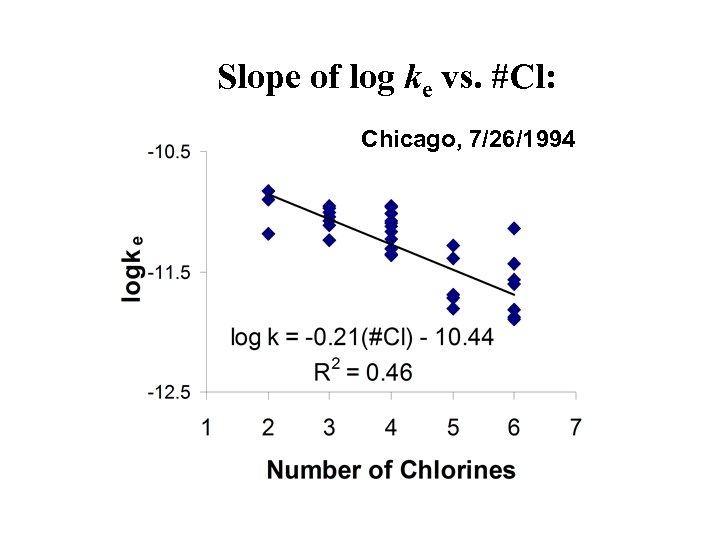 Slope of log ke vs. #Cl: Chicago, 7/26/1994
Slope of log ke vs. #Cl: Chicago, 7/26/1994


