8ea3565035a13d95c0f8e2887ad808c3.ppt
- Количество слайдов: 22

Phase III trial of capecitabine + oxaliplatin (XELOX) vs. 5 -fluorouracil (5 -FU), folinic acid (FA), and oxaliplatin (FOLFOX-4) as 2 nd-line treatment for patients with metastatic colorectal cancer (MCRC) Rothenberg ML, 1 Navarro M, 2 Butts C, 3 Bang Y, 4 Cox JV, 5 Goel R, 6 Gollins S, 7 Siu LL, 8 Cunningham D 9 on behalf of the NO 16967 Study Group 1 Vanderbilt Ingram Cancer Center, Nashville, USA; 2 Hospital Duran I Reynals, Barcelona, Spain; 3 Cross Cancer Institute, Edmonton, Canada; 4 Seoul National University Hospital, Seoul, Republic of Korea; 5 Texas Oncology, PA & US Oncology Research, Dallas, USA; 6 The Ottawa Hospital, Ottawa, Canada; 7 North Wales Cancer Treatment Centre Glan Clwyd Hos, Rhyl, UK; 8 Princess Margaret Hospital, Toronto, Canada; 9 The Royal Marsden Hospital, Sutton, UK
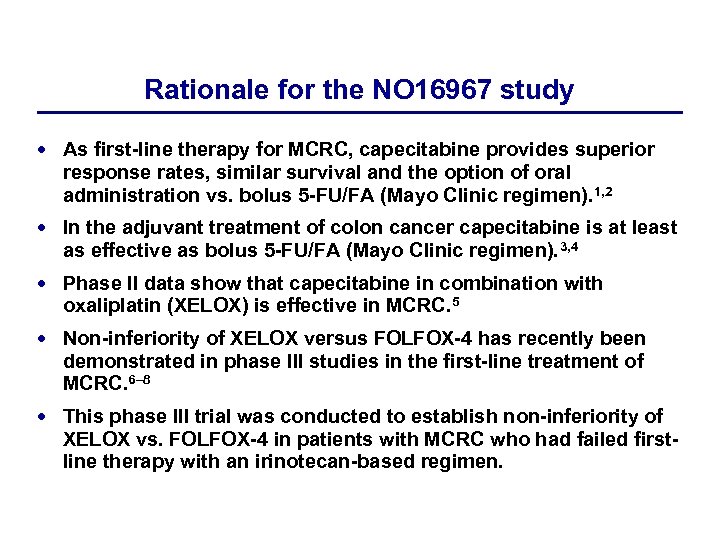
Rationale for the NO 16967 study As first-line therapy for MCRC, capecitabine provides superior response rates, similar survival and the option of oral administration vs. bolus 5 -FU/FA (Mayo Clinic regimen). 1, 2 In the adjuvant treatment of colon cancer capecitabine is at least as effective as bolus 5 -FU/FA (Mayo Clinic regimen). 3, 4 Phase II data show that capecitabine in combination with oxaliplatin (XELOX) is effective in MCRC. 5 Non-inferiority of XELOX versus FOLFOX-4 has recently been demonstrated in phase III studies in the first-line treatment of MCRC. 6– 8 This phase III trial was conducted to establish non-inferiority of XELOX vs. FOLFOX-4 in patients with MCRC who had failed firstline therapy with an irinotecan-based regimen.

XELOX vs. FOLFOX-4 in the second-line treatment of MCRC: study design The primary endpoint of this study was progression-free survival (PFS) defined as time to progression or death. Randomization was stratified by geographic region, ECOG performance status (0 vs. 1 or 2), number of metastatic sites (organs) at baseline (1 vs. >1), alkaline phosphatase level (normal vs. above normal), and reason for termination of prior irinotecan-based therapy (clinical failure vs. toxicity).
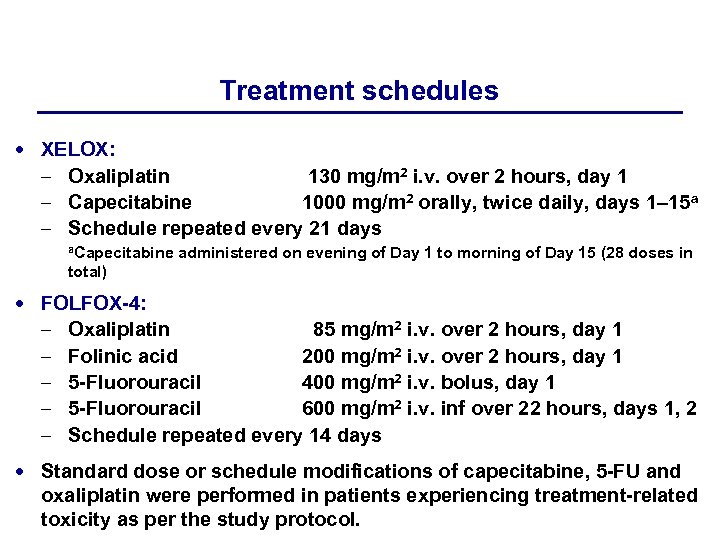
Treatment schedules XELOX: – Oxaliplatin 130 mg/m 2 i. v. over 2 hours, day 1 – Capecitabine 1000 mg/m 2 orally, twice daily, days 1– 15 a – Schedule repeated every 21 days a. Capecitabine administered on evening of Day 1 to morning of Day 15 (28 doses in total) FOLFOX-4: – – – Oxaliplatin 85 mg/m 2 i. v. over 2 hours, day 1 Folinic acid 200 mg/m 2 i. v. over 2 hours, day 1 5 -Fluorouracil 400 mg/m 2 i. v. bolus, day 1 5 -Fluorouracil 600 mg/m 2 i. v. inf over 22 hours, days 1, 2 Schedule repeated every 14 days Standard dose or schedule modifications of capecitabine, 5 -FU and oxaliplatin were performed in patients experiencing treatment-related toxicity as per the study protocol.
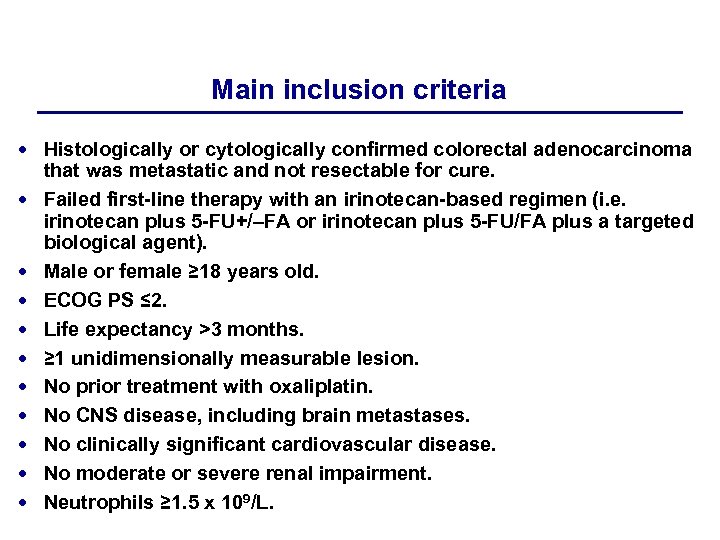
Main inclusion criteria Histologically or cytologically confirmed colorectal adenocarcinoma that was metastatic and not resectable for cure. Failed first-line therapy with an irinotecan-based regimen (i. e. irinotecan plus 5 -FU+/–FA or irinotecan plus 5 -FU/FA plus a targeted biological agent). Male or female ≥ 18 years old. ECOG PS ≤ 2. Life expectancy >3 months. ≥ 1 unidimensionally measurable lesion. No prior treatment with oxaliplatin. No CNS disease, including brain metastases. No clinically significant cardiovascular disease. No moderate or severe renal impairment. Neutrophils ≥ 1. 5 x 109/L.
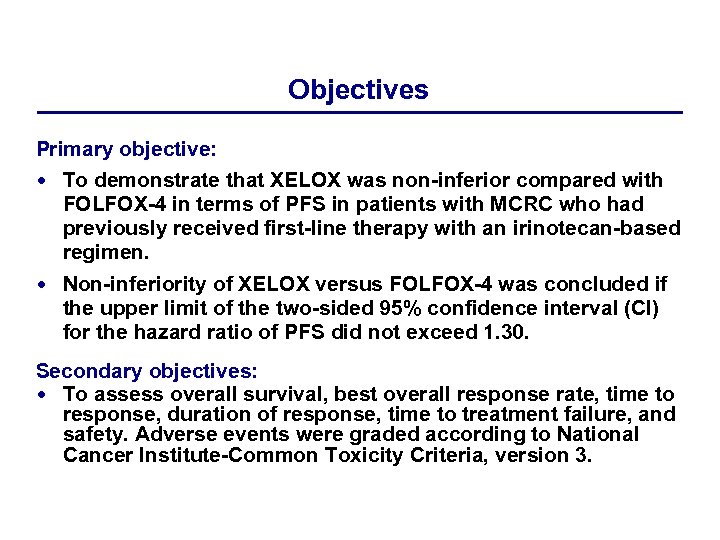
Objectives Primary objective: To demonstrate that XELOX was non-inferior compared with FOLFOX-4 in terms of PFS in patients with MCRC who had previously received first-line therapy with an irinotecan-based regimen. Non-inferiority of XELOX versus FOLFOX-4 was concluded if the upper limit of the two-sided 95% confidence interval (CI) for the hazard ratio of PFS did not exceed 1. 30. Secondary objectives: To assess overall survival, best overall response rate, time to response, duration of response, time to treatment failure, and safety. Adverse events were graded according to National Cancer Institute-Common Toxicity Criteria, version 3.
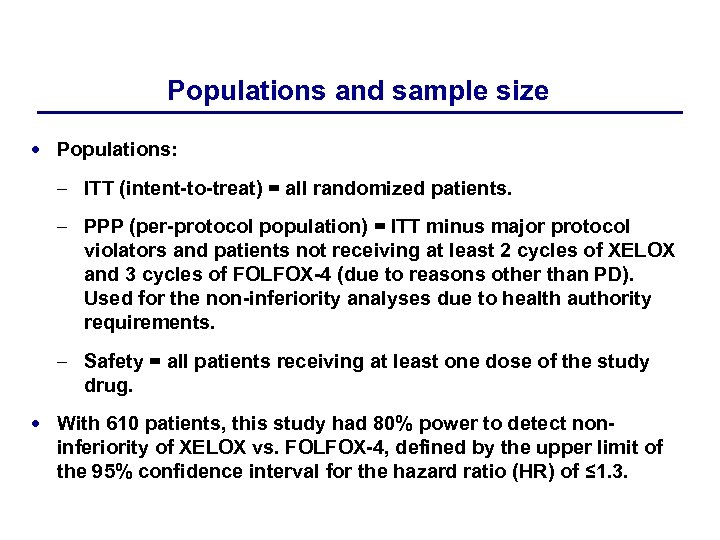
Populations and sample size Populations: – ITT (intent-to-treat) = all randomized patients. – PPP (per-protocol population) = ITT minus major protocol violators and patients not receiving at least 2 cycles of XELOX and 3 cycles of FOLFOX-4 (due to reasons other than PD). Used for the non-inferiority analyses due to health authority requirements. – Safety = all patients receiving at least one dose of the study drug. With 610 patients, this study had 80% power to detect non- inferiority of XELOX vs. FOLFOX-4, defined by the upper limit of the 95% confidence interval for the hazard ratio (HR) of ≤ 1. 3.
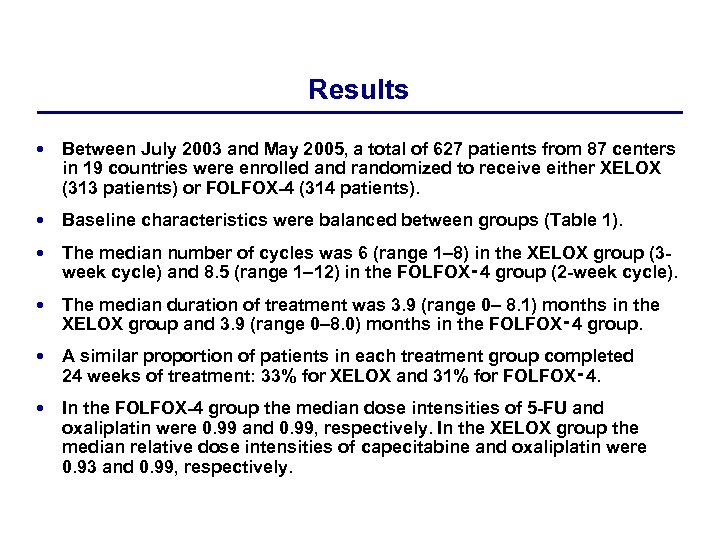
Results Between July 2003 and May 2005, a total of 627 patients from 87 centers in 19 countries were enrolled and randomized to receive either XELOX (313 patients) or FOLFOX-4 (314 patients). Baseline characteristics were balanced between groups (Table 1). The median number of cycles was 6 (range 1– 8) in the XELOX group (3 week cycle) and 8. 5 (range 1– 12) in the FOLFOX‑ 4 group (2 -week cycle). The median duration of treatment was 3. 9 (range 0– 8. 1) months in the XELOX group and 3. 9 (range 0– 8. 0) months in the FOLFOX‑ 4 group. A similar proportion of patients in each treatment group completed 24 weeks of treatment: 33% for XELOX and 31% for FOLFOX‑ 4. In the FOLFOX-4 group the median dose intensities of 5 -FU and oxaliplatin were 0. 99 and 0. 99, respectively. In the XELOX group the median relative dose intensities of capecitabine and oxaliplatin were 0. 93 and 0. 99, respectively.
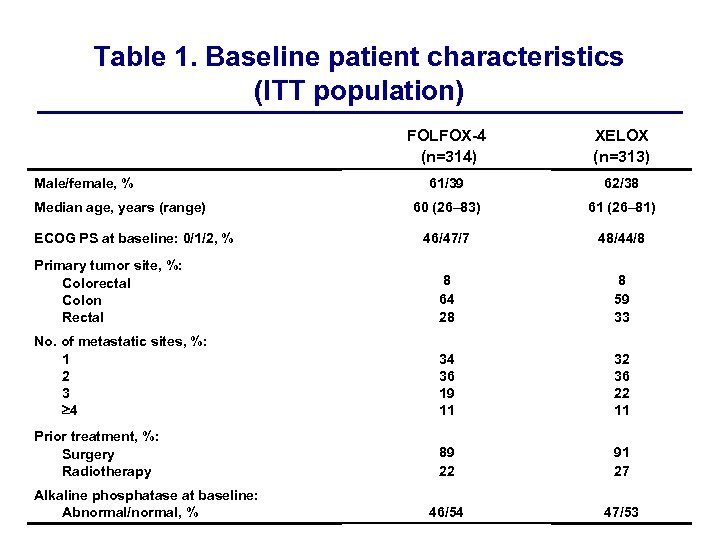
Table 1. Baseline patient characteristics (ITT population) FOLFOX-4 (n=314) XELOX (n=313) 61/39 62/38 60 (26– 83) 61 (26– 81) 46/47/7 48/44/8 8 64 28 8 59 33 No. of metastatic sites, %: 1 2 3 4 34 36 19 11 32 36 22 11 Prior treatment, %: Surgery Radiotherapy 89 22 91 27 46/54 47/53 Male/female, % Median age, years (range) ECOG PS at baseline: 0/1/2, % Primary tumor site, %: Colorectal Colon Rectal Alkaline phosphatase at baseline: Abnormal/normal, %
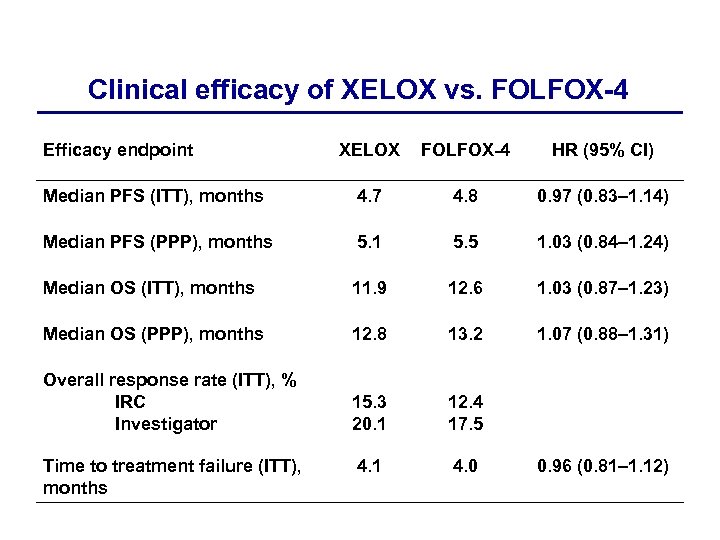
Clinical efficacy of XELOX vs. FOLFOX-4 Efficacy endpoint XELOX FOLFOX-4 HR (95% CI) Median PFS (ITT), months 4. 7 4. 8 0. 97 (0. 83– 1. 14) Median PFS (PPP), months 5. 1 5. 5 1. 03 (0. 84– 1. 24) Median OS (ITT), months 11. 9 12. 6 1. 03 (0. 87– 1. 23) Median OS (PPP), months 12. 8 13. 2 1. 07 (0. 88– 1. 31) Overall response rate (ITT), % IRC Investigator 15. 3 20. 1 12. 4 17. 5 4. 1 4. 0 Time to treatment failure (ITT), months 0. 96 (0. 81– 1. 12)
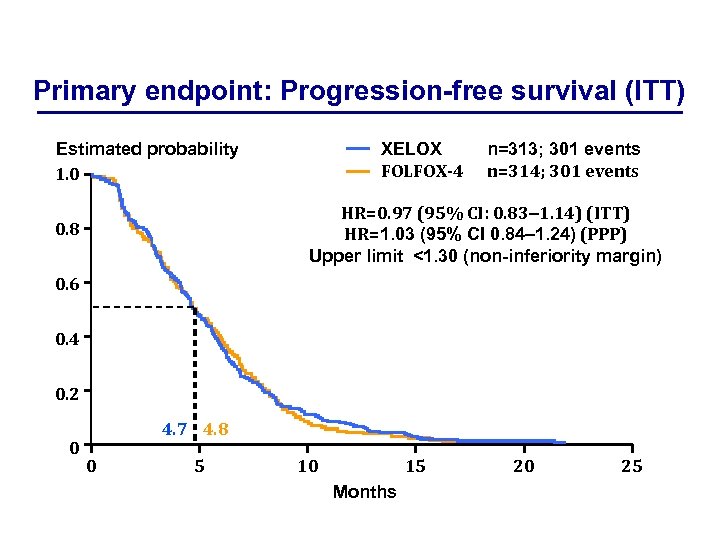
Primary endpoint: Progression-free survival (ITT) XELOX FOLFOX-4 Estimated probability 1. 0 n=313; 301 events n=314; 301 events HR=0. 97 (95% CI: 0. 83– 1. 14) (ITT) HR=1. 03 (95% CI 0. 84– 1. 24) (PPP) Upper limit <1. 30 (non-inferiority margin) 0. 8 0. 6 0. 4 0. 2 0 4. 7 4. 8 0 5 10 15 Months 20 25
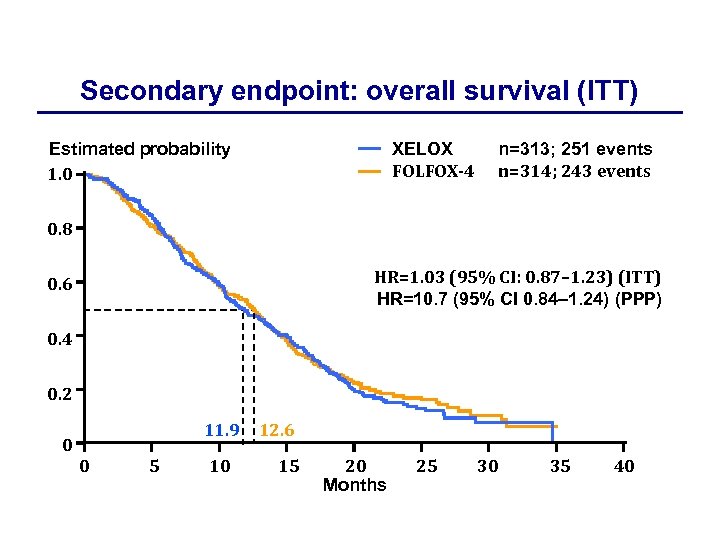
Secondary endpoint: overall survival (ITT) XELOX FOLFOX-4 Estimated probability 1. 0 n=313; 251 events n=314; 243 events 0. 8 HR=1. 03 (95% CI: 0. 87– 1. 23) (ITT) HR=10. 7 (95% CI 0. 84– 1. 24) (PPP) 0. 6 0. 4 0. 2 0 11. 9 0 5 10 12. 6 15 20 Months 25 30 35 40
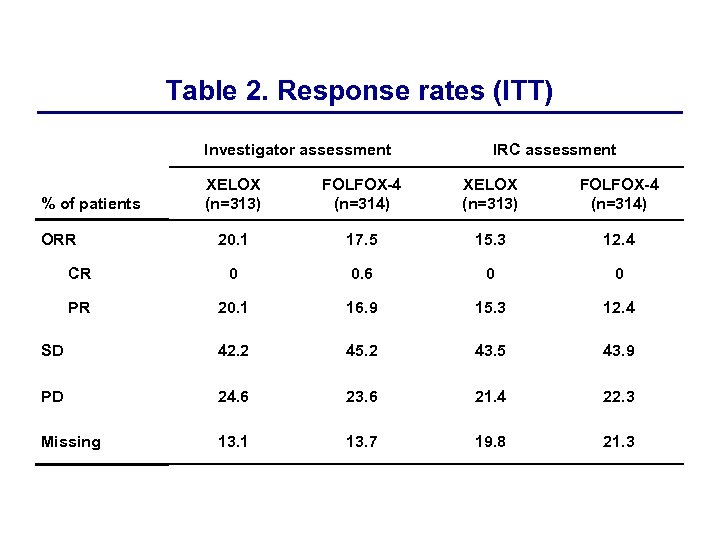
Table 2. Response rates (ITT) Investigator assessment % of patients XELOX (n=313) FOLFOX-4 (n=314) 20. 1 17. 5 15. 3 12. 4 CR 0 0. 6 0 0 PR 20. 1 16. 9 15. 3 12. 4 SD 42. 2 45. 2 43. 5 43. 9 PD 24. 6 23. 6 21. 4 22. 3 Missing 13. 1 13. 7 19. 8 21. 3 ORR Intent-to-treat IRC assessment

Adverse events The overall profiles of adverse events with XELOX and FOLFOX -4 were similar, although there were differences in the rates at which individual events were reported (Table 4).
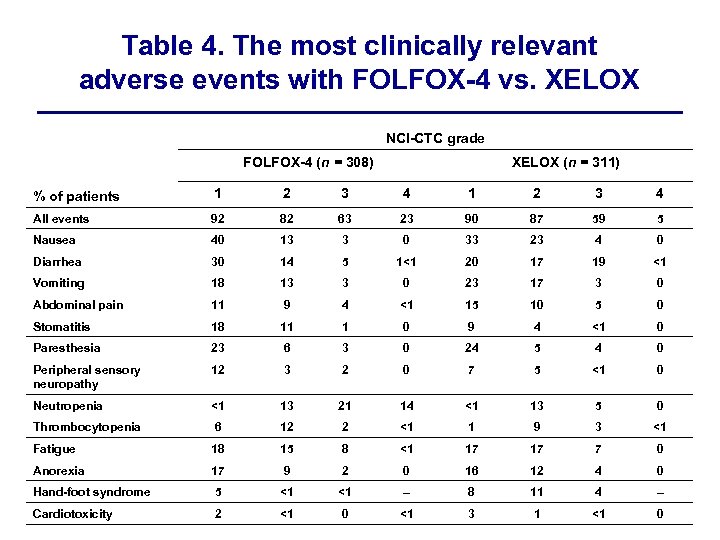
Table 4. The most clinically relevant adverse events with FOLFOX-4 vs. XELOX NCI-CTC grade FOLFOX-4 (n = 308) XELOX (n = 311) % of patients 1 2 3 4 All events 92 82 63 23 90 87 59 5 Nausea 40 13 3 0 33 23 4 0 Diarrhea 30 14 5 1<1 20 17 19 <1 Vomiting 18 13 3 0 23 17 3 0 Abdominal pain 11 9 4 <1 15 10 5 0 Stomatitis 18 11 1 0 9 4 <1 0 Paresthesia 23 6 3 0 24 5 4 0 Peripheral sensory neuropathy 12 3 2 0 7 5 <1 0 Neutropenia <1 13 21 14 <1 13 5 0 Thrombocytopenia 6 12 2 <1 1 9 3 <1 Fatigue 18 15 8 <1 17 17 7 0 Anorexia 17 9 2 0 16 12 4 0 Hand-foot syndrome 5 <1 <1 – 8 11 4 – Cardiotoxicity 2 <1 0 <1 3 1 <1 0

Hyperbilirubinemia (laboratory abnormality) was reported in 31% (all grade) and 2% (grade 3/4) of patients in the FOLFOX-4 group and in 34% (all grade) and 3% (grade 3/4) of those in the XELOX group.
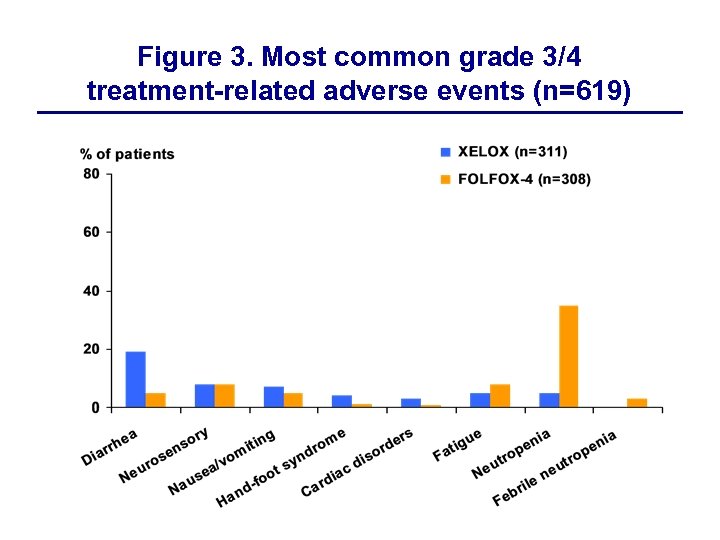
Figure 3. Most common grade 3/4 treatment-related adverse events (n=619)
Discontinuations and mortality During study treatment, more FOLFOX-4 -treated patients (n=143; 46%) than those on XELOX (n=117; 38%) withdrew because of insufficient therapeutic response. More patients in the XELOX group withdrew because of adverse events (n=63, 20% vs. n=41, 13% with FOLFOX-4). The 60 -day all-cause mortality rate was 3. 9% in XELOX- and 4. 2% in FOLFOX-4 -treated patients.
Conclusions Progression-free survival with XELOX is non-inferior to FOLFOX-4 as second-line treatment for MCRC. Results for overall survival and response rates were also similar between the XELOX and FOLFOX-4 groups. The safety profile was similar to previous studies, with no unexpected toxicities. These findings support those reported from a similar study in the first-line setting. 8 XELOX is an effective and well-tolerated alternative to FOLFOX-4 as second-line therapy in MCRC.

Collaborators Belgium: Peeters M, Vergauwe P, Kerger J, Van Laethem J-C, Hendlisz A; Canada: Butts C, Wilson J, Colwell B, Goel R, Couture F, Dalfen R, Siu L, Lesperance B, Dube P, Siddiqui J, Vincent M, Wierzbicki R, Aucoin N, Salim M, Dueck D-A, Findlay B; Croatia: Mrsic-Krmpotic Z, Vrdoljak E; Finland: Pyrhoenen S, Hietanen T; France: Mineur L, Dorval E, Brunet R, Michel P, Debrigode C, Smith D, Adhoute X, Tubiana-Mathieu N; Germany: Gregor M; Greece: Geirgoulias V, Katsos I; Israel: Shani A, Gabizon A, Man S, Aderka D, Klein B, Figer A, Brenner B; Italy: Aprile G, La. Bianca R, Pasquini E, Ravaioli A; Poland: Szczylik C, Pawlega J, Nowacki M, Wojtukiewicz M; Republic of Korea: Bang Y-J, Kim TW, Lee YY, Chung HC, Hong DS, Kang J; Serbia: Radulovic S; Slovakia: Salek T; Slovenia: Ocvirk J; South Africa: Du Toit JA, De Bruyne R, Cullis S, Alberts A, Malan J; Spain: Navarro Garcia M, Gravalos C, Manzano H, Cubedo R, Garcia Carbonero R; Taiwan: Su W-C, Hsieh R-K, Yang T-S; USA: Hwang J, Cobb P, Rader M, Piroso E, Woodson M, Patel R, Chakrabarti A, Cox U, Baez L; UK: Wilson G, Gollins S, Smith D, Susnerwala D.

Acknowledgements Sincere thanks to: The patients and their families The co-investigators The research nurses and data managers The study management team at Roche
References 1. Van Cutsem E, et al. Br J Cancer 2004; 90: 1190− 7. 2. Cassidy J, et al. Ann Oncol 2002; 13: 566− 75. 3. Twelves C, et al. NEJM 2005; 352: 2696− 704. Cassidy J, et al. Br J Cancer 2006; 94: 1122− 9. 5. Cassidy J, et al. J Clin Oncol 2004; 22: 2084– 91. 6. Martoni AA, et al. Eur J Cancer 2006; 42: 3161− 8. 7. Bennouna J, et al. Proc ASCO GI Cancers Symposium 2007; 221: (Abst 272). 8. Cassidy J, et al. Proc ASCO 2007 (Abst 4030). Presented at the ASCO Annual Meeting, 1− 5 June, 2007
8ea3565035a13d95c0f8e2887ad808c3.ppt