34952b8d865073becbbe0148cfcd04f9.ppt
- Количество слайдов: 26
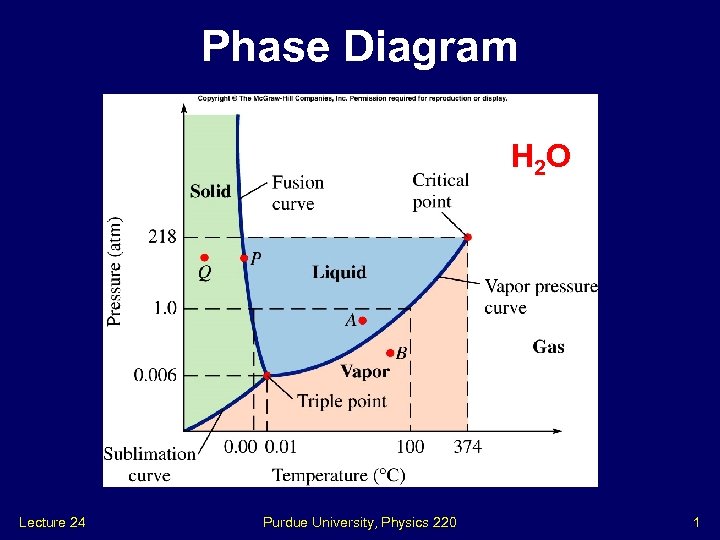 Phase Diagram H 2 O Lecture 24 Purdue University, Physics 220 1
Phase Diagram H 2 O Lecture 24 Purdue University, Physics 220 1
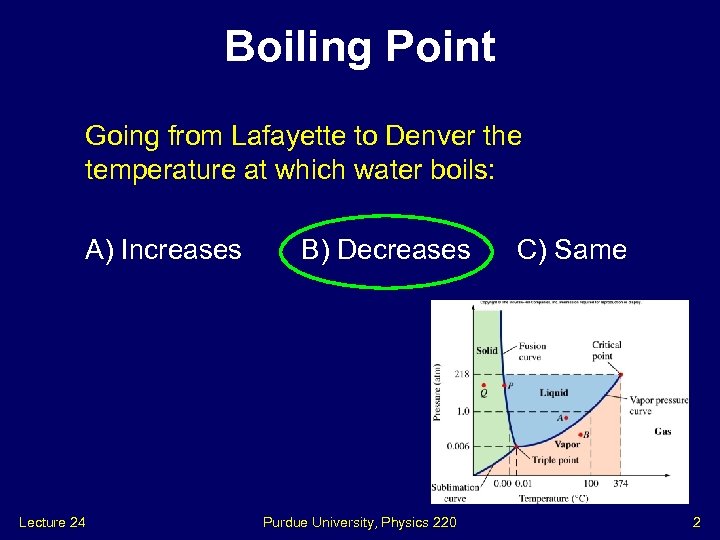 Boiling Point Going from Lafayette to Denver the temperature at which water boils: A) Increases Lecture 24 B) Decreases Purdue University, Physics 220 C) Same 2
Boiling Point Going from Lafayette to Denver the temperature at which water boils: A) Increases Lecture 24 B) Decreases Purdue University, Physics 220 C) Same 2
 Exercise During a tough work out, your body sweats (and evaporates) 1 liter of water to keep cool (37 C). How much cold water would you need to drink (at 2 C) to achieve the same thermal cooling? (recall CV = 4. 2 J/g for water, Lv=2. 2 x 103 J/g) A) 0. 15 liters B) 1. 0 liters C) 15 liters D) 150 liters Qevaporative = L m = 2. 2 x 103 k. J/kg x 1 kg Qc = c m t = 4. 2 k. J/kg. K x 35 K x m m = 2. 2 x 103 / 147 = 15 kg or 15 liters! Lecture 24 Purdue University, Physics 220 3
Exercise During a tough work out, your body sweats (and evaporates) 1 liter of water to keep cool (37 C). How much cold water would you need to drink (at 2 C) to achieve the same thermal cooling? (recall CV = 4. 2 J/g for water, Lv=2. 2 x 103 J/g) A) 0. 15 liters B) 1. 0 liters C) 15 liters D) 150 liters Qevaporative = L m = 2. 2 x 103 k. J/kg x 1 kg Qc = c m t = 4. 2 k. J/kg. K x 35 K x m m = 2. 2 x 103 / 147 = 15 kg or 15 liters! Lecture 24 Purdue University, Physics 220 3
 Exercise How much ice (at 0 C) do you need to add to 0. 5 liters of a water at 25 C, to cool it down to 10 C? (L = 80 cal/g, c = 1 cal/g C) Lecture 24 Purdue University, Physics 220 4
Exercise How much ice (at 0 C) do you need to add to 0. 5 liters of a water at 25 C, to cool it down to 10 C? (L = 80 cal/g, c = 1 cal/g C) Lecture 24 Purdue University, Physics 220 4
 Exercise Ice cube trays are filled with 0. 5 kg of water at 20 C and placed into the freezer. How much energy must be removed from the water to turn it into ice cubes at -5 C? (L = 80 cal/g, cwater = 1 cal/g C, cice = 0. 5 cal/g C) Water going from 20 C to 0 C: Water turning into ice at 0 C: Ice going from 0 C to -5 C: Lecture 24 Purdue University, Physics 220 5
Exercise Ice cube trays are filled with 0. 5 kg of water at 20 C and placed into the freezer. How much energy must be removed from the water to turn it into ice cubes at -5 C? (L = 80 cal/g, cwater = 1 cal/g C, cice = 0. 5 cal/g C) Water going from 20 C to 0 C: Water turning into ice at 0 C: Ice going from 0 C to -5 C: Lecture 24 Purdue University, Physics 220 5
 PHYSICS 220 Lecture 25 Heat Transfer Lecture 25 Purdue University, Physics 220 6
PHYSICS 220 Lecture 25 Heat Transfer Lecture 25 Purdue University, Physics 220 6
 Heat transfer • Conduction • Convection • Electro-magnetic radiation Lecture 25 Purdue University, Physics 220 7
Heat transfer • Conduction • Convection • Electro-magnetic radiation Lecture 25 Purdue University, Physics 220 7
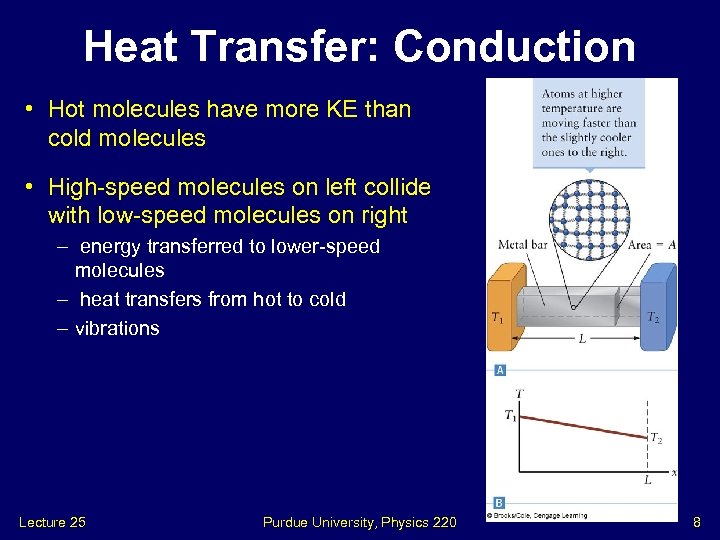 Heat Transfer: Conduction • Hot molecules have more KE than cold molecules • High-speed molecules on left collide with low-speed molecules on right – energy transferred to lower-speed molecules – heat transfers from hot to cold – vibrations Lecture 25 Purdue University, Physics 220 8
Heat Transfer: Conduction • Hot molecules have more KE than cold molecules • High-speed molecules on left collide with low-speed molecules on right – energy transferred to lower-speed molecules – heat transfers from hot to cold – vibrations Lecture 25 Purdue University, Physics 220 8
![Heat Transfer: Conduction • I = rate of heat transfer = Q/t [J/s] I Heat Transfer: Conduction • I = rate of heat transfer = Q/t [J/s] I](https://present5.com/presentation/34952b8d865073becbbe0148cfcd04f9/image-9.jpg) Heat Transfer: Conduction • I = rate of heat transfer = Q/t [J/s] I = k A (TH-TC)/d • Q/t = k A T/ x k = thermal conductivity • Units: J/s*m*C • good conductors…high k e. g. , metal • good insulators … low k e. g. , plastic R = d/(Ak) = thermal resistance TH d = x TC Cold Hot Area A Lecture 25 Purdue University, Physics 220 9
Heat Transfer: Conduction • I = rate of heat transfer = Q/t [J/s] I = k A (TH-TC)/d • Q/t = k A T/ x k = thermal conductivity • Units: J/s*m*C • good conductors…high k e. g. , metal • good insulators … low k e. g. , plastic R = d/(Ak) = thermal resistance TH d = x TC Cold Hot Area A Lecture 25 Purdue University, Physics 220 9
 Conduction Which of the following is an example of conductive heat transfer? A) You stir some hot soup with a silver spoon and notice that the spoon warms up. B) You stand watching a bonfire, but can’t get too close because of the heat. C) Its hard for central air-conditioning in an old house to cool the attic. Lecture 25 Purdue University, Physics 220 10
Conduction Which of the following is an example of conductive heat transfer? A) You stir some hot soup with a silver spoon and notice that the spoon warms up. B) You stand watching a bonfire, but can’t get too close because of the heat. C) Its hard for central air-conditioning in an old house to cool the attic. Lecture 25 Purdue University, Physics 220 10
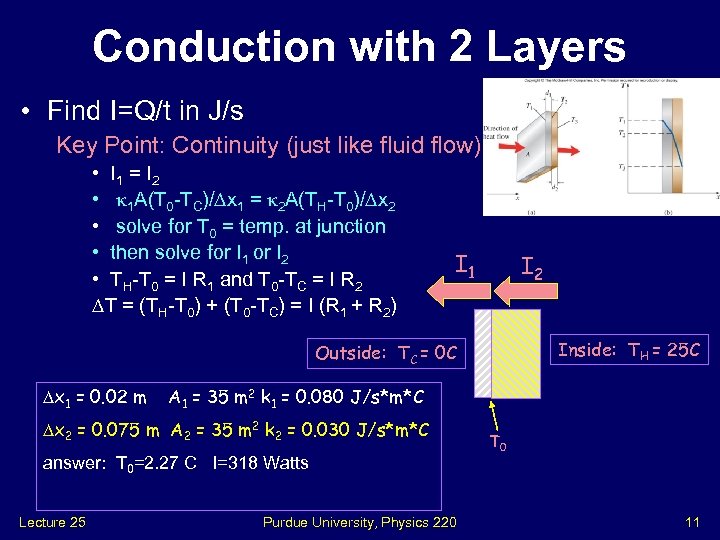 Conduction with 2 Layers • Find I=Q/t in J/s Key Point: Continuity (just like fluid flow) • I 1 = I 2 • k 1 A(T 0 -TC)/ x 1 = k 2 A(TH-T 0)/ x 2 • solve for T 0 = temp. at junction • then solve for I 1 or I 2 • TH-T 0 = I R 1 and T 0 -TC = I R 2 T = (TH-T 0) + (T 0 -TC) = I (R 1 + R 2) I 1 I 2 Inside: TH = 25 C Outside: TC = 0 C x 1 = 0. 02 m A 1 = 35 m 2 k 1 = 0. 080 J/s*m*C x 2 = 0. 075 m A 2 = 35 m 2 k 2 = 0. 030 J/s*m*C answer: T 0=2. 27 C I=318 Watts Lecture 25 Purdue University, Physics 220 T 0 11
Conduction with 2 Layers • Find I=Q/t in J/s Key Point: Continuity (just like fluid flow) • I 1 = I 2 • k 1 A(T 0 -TC)/ x 1 = k 2 A(TH-T 0)/ x 2 • solve for T 0 = temp. at junction • then solve for I 1 or I 2 • TH-T 0 = I R 1 and T 0 -TC = I R 2 T = (TH-T 0) + (T 0 -TC) = I (R 1 + R 2) I 1 I 2 Inside: TH = 25 C Outside: TC = 0 C x 1 = 0. 02 m A 1 = 35 m 2 k 1 = 0. 080 J/s*m*C x 2 = 0. 075 m A 2 = 35 m 2 k 2 = 0. 030 J/s*m*C answer: T 0=2. 27 C I=318 Watts Lecture 25 Purdue University, Physics 220 T 0 11
 i. Clicker Touch the metal base of a chair and the top of a wooden desk in an air-conditioned room, which feels colder? A) Base B) Same C) Desk Both must be the same temperature (room temperature), but metal feels colder because it conducts heat better/faster. Lecture 25 Purdue University, Physics 220 12
i. Clicker Touch the metal base of a chair and the top of a wooden desk in an air-conditioned room, which feels colder? A) Base B) Same C) Desk Both must be the same temperature (room temperature), but metal feels colder because it conducts heat better/faster. Lecture 25 Purdue University, Physics 220 12
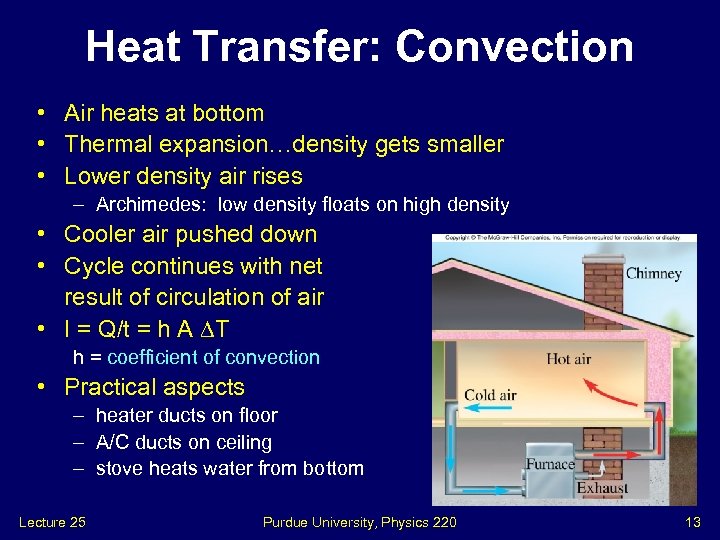 Heat Transfer: Convection • Air heats at bottom • Thermal expansion…density gets smaller • Lower density air rises – Archimedes: low density floats on high density • Cooler air pushed down • Cycle continues with net result of circulation of air • I = Q/t = h A T h = coefficient of convection • Practical aspects – heater ducts on floor – A/C ducts on ceiling – stove heats water from bottom Lecture 25 Purdue University, Physics 220 13
Heat Transfer: Convection • Air heats at bottom • Thermal expansion…density gets smaller • Lower density air rises – Archimedes: low density floats on high density • Cooler air pushed down • Cycle continues with net result of circulation of air • I = Q/t = h A T h = coefficient of convection • Practical aspects – heater ducts on floor – A/C ducts on ceiling – stove heats water from bottom Lecture 25 Purdue University, Physics 220 13
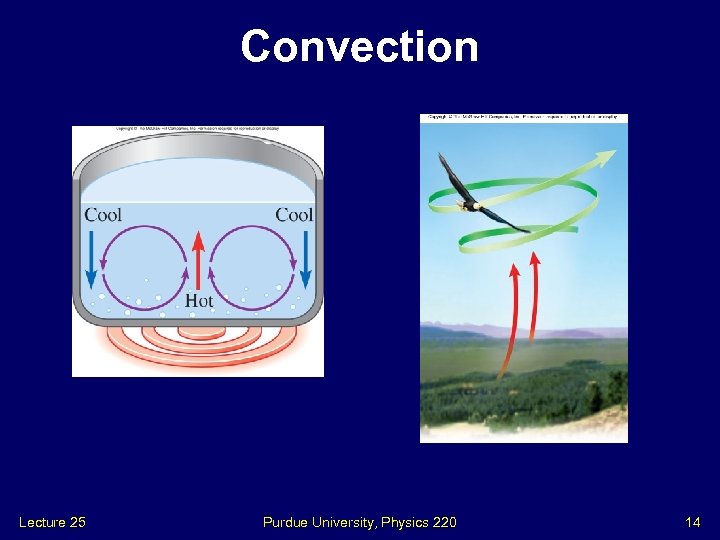 Convection Lecture 25 Purdue University, Physics 220 14
Convection Lecture 25 Purdue University, Physics 220 14
 Convection Which of the following is an example of convective heat transfer? A) You stir some hot soup with a silver spoon and notice that the spoon warms up. B) You stand watching a bonfire, but can’t get too close because of the heat. C) Its hard for central air-conditioning in an old house to cool the attic. Lecture 25 Purdue University, Physics 220 15
Convection Which of the following is an example of convective heat transfer? A) You stir some hot soup with a silver spoon and notice that the spoon warms up. B) You stand watching a bonfire, but can’t get too close because of the heat. C) Its hard for central air-conditioning in an old house to cool the attic. Lecture 25 Purdue University, Physics 220 15
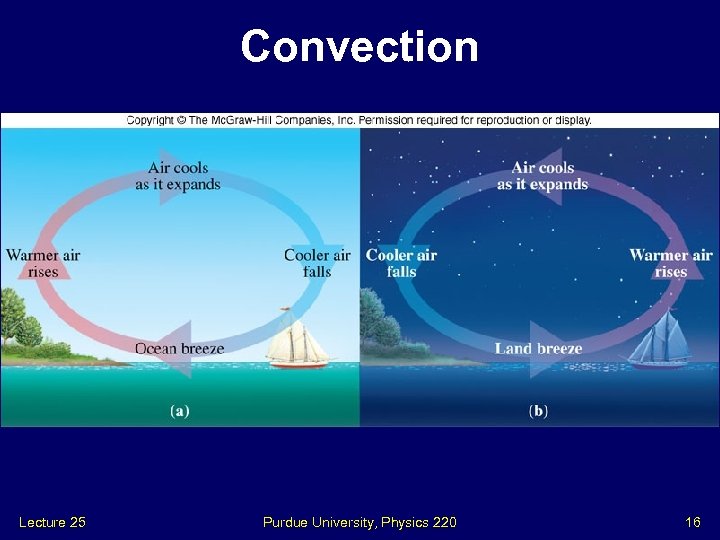 Convection Lecture 25 Purdue University, Physics 220 16
Convection Lecture 25 Purdue University, Physics 220 16
 Heat Transfer: Radiation • All things radiate / absorb electromagnetic energy • Why? - Because vibration or/and acceleration of charged particles (electrons, protons, ions) results in electromagnetic radiation and vice versa. • No “medium” required • How amount of emitted / absorbed radiation depends on body temperature? Lecture 25 Purdue University, Physics 220 17
Heat Transfer: Radiation • All things radiate / absorb electromagnetic energy • Why? - Because vibration or/and acceleration of charged particles (electrons, protons, ions) results in electromagnetic radiation and vice versa. • No “medium” required • How amount of emitted / absorbed radiation depends on body temperature? Lecture 25 Purdue University, Physics 220 17
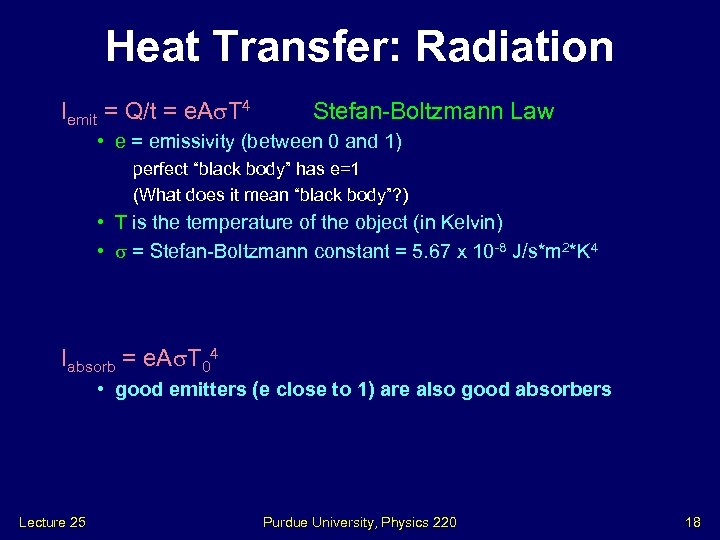 Heat Transfer: Radiation Iemit = Q/t = e. A T 4 Stefan-Boltzmann Law • e = emissivity (between 0 and 1) perfect “black body” has e=1 (What does it mean “black body”? ) • T is the temperature of the object (in Kelvin) • = Stefan-Boltzmann constant = 5. 67 x 10 -8 J/s*m 2*K 4 Iabsorb = e. A T 04 • good emitters (e close to 1) are also good absorbers Lecture 25 Purdue University, Physics 220 18
Heat Transfer: Radiation Iemit = Q/t = e. A T 4 Stefan-Boltzmann Law • e = emissivity (between 0 and 1) perfect “black body” has e=1 (What does it mean “black body”? ) • T is the temperature of the object (in Kelvin) • = Stefan-Boltzmann constant = 5. 67 x 10 -8 J/s*m 2*K 4 Iabsorb = e. A T 04 • good emitters (e close to 1) are also good absorbers Lecture 25 Purdue University, Physics 220 18
 Question One day during the winter, the sun has been shining all day. Toward sunset a light snow begins to fall. It collects without melting on a cement playground, but it melts immediately upon contact on a black asphalt road adjacent to the playground. How do you explain this. The black asphalt absorbs more heat from the sun. The black asphalt has an emissivity of 1 and absorbs energy from the surrounding (from the sun) compared to a smaller number for the cement. As a result, it is at a higher temperature than the cement and melts the snow. Lecture 25 Purdue University, Physics 220 19
Question One day during the winter, the sun has been shining all day. Toward sunset a light snow begins to fall. It collects without melting on a cement playground, but it melts immediately upon contact on a black asphalt road adjacent to the playground. How do you explain this. The black asphalt absorbs more heat from the sun. The black asphalt has an emissivity of 1 and absorbs energy from the surrounding (from the sun) compared to a smaller number for the cement. As a result, it is at a higher temperature than the cement and melts the snow. Lecture 25 Purdue University, Physics 220 19
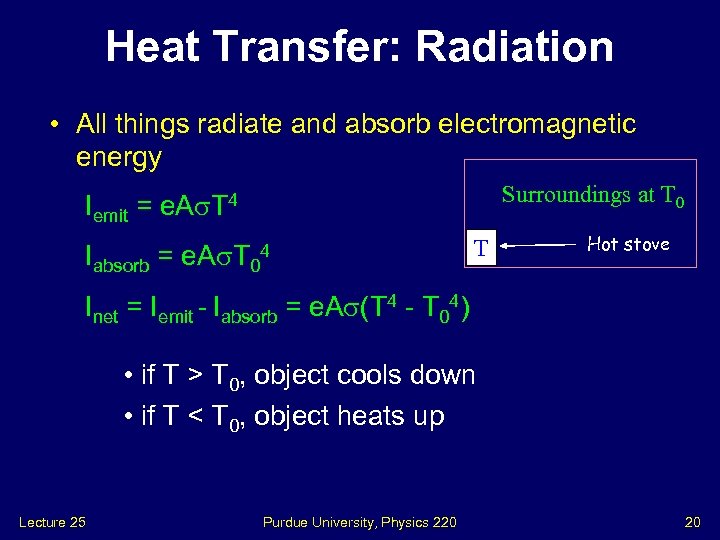 Heat Transfer: Radiation • All things radiate and absorb electromagnetic energy Surroundings at T 0 Iemit = e. A T 4 Iabsorb = e. A T 0 4 T Hot stove Inet = Iemit - Iabsorb = e. A (T 4 - T 04) • if T > T 0, object cools down • if T < T 0, object heats up Lecture 25 Purdue University, Physics 220 20
Heat Transfer: Radiation • All things radiate and absorb electromagnetic energy Surroundings at T 0 Iemit = e. A T 4 Iabsorb = e. A T 0 4 T Hot stove Inet = Iemit - Iabsorb = e. A (T 4 - T 04) • if T > T 0, object cools down • if T < T 0, object heats up Lecture 25 Purdue University, Physics 220 20
 Exercise The Earth has a surface temperature around 270 K and an emissivity of 0. 8, while space has a temperature of around 2 K. What is the net power radiated by the earth into free space? (Radii of the Earth and the Sun are RE = 6. 38× 106 m, RS = 7× 108 m. ) Inet = Iemit - Iabsorb = e. A (T 4 - T 04) Lecture 25 Purdue University, Physics 220 21
Exercise The Earth has a surface temperature around 270 K and an emissivity of 0. 8, while space has a temperature of around 2 K. What is the net power radiated by the earth into free space? (Radii of the Earth and the Sun are RE = 6. 38× 106 m, RS = 7× 108 m. ) Inet = Iemit - Iabsorb = e. A (T 4 - T 04) Lecture 25 Purdue University, Physics 220 21
 Radiation Which of the following is an example of radiative heat transfer? A) You stir some hot soup with a silver spoon and notice that the spoon warms up. B) You stand watching a bonfire, but cant get too close because of the heat. C) Its hard for central air-conditioning in an old house to cool the attic. Lecture 25 Purdue University, Physics 220 22
Radiation Which of the following is an example of radiative heat transfer? A) You stir some hot soup with a silver spoon and notice that the spoon warms up. B) You stand watching a bonfire, but cant get too close because of the heat. C) Its hard for central air-conditioning in an old house to cool the attic. Lecture 25 Purdue University, Physics 220 22
 i. Clicker Amount of radiation emitted by the Earth is A) less B) equal C) more (than) the amount of radiation absorbed by the Earth from the Sun. Lecture 25 Purdue University, Physics 220 23
i. Clicker Amount of radiation emitted by the Earth is A) less B) equal C) more (than) the amount of radiation absorbed by the Earth from the Sun. Lecture 25 Purdue University, Physics 220 23
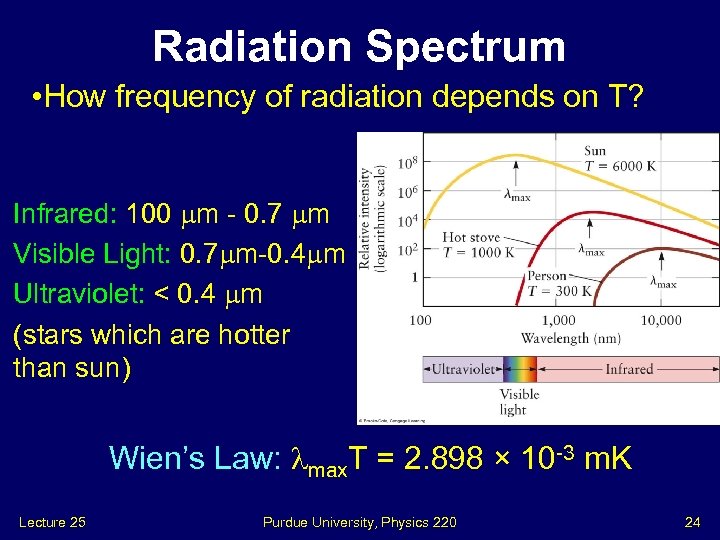 Radiation Spectrum • How frequency of radiation depends on T? Infrared: 100 m - 0. 7 m Visible Light: 0. 7 m-0. 4 m Ultraviolet: < 0. 4 m (stars which are hotter than sun) Wien’s Law: max. T = 2. 898 × 10 -3 m. K Lecture 25 Purdue University, Physics 220 24
Radiation Spectrum • How frequency of radiation depends on T? Infrared: 100 m - 0. 7 m Visible Light: 0. 7 m-0. 4 m Ultraviolet: < 0. 4 m (stars which are hotter than sun) Wien’s Law: max. T = 2. 898 × 10 -3 m. K Lecture 25 Purdue University, Physics 220 24
 Night vision goggles Lecture 25 Purdue University, Physics 220 25
Night vision goggles Lecture 25 Purdue University, Physics 220 25
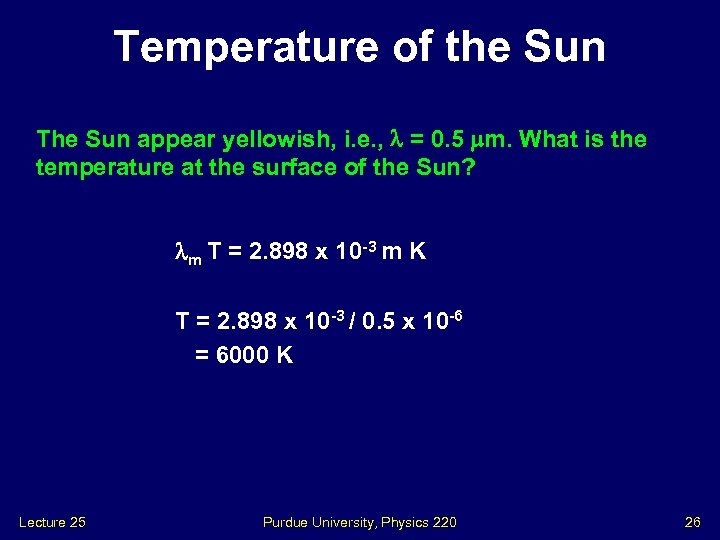 Temperature of the Sun The Sun appear yellowish, i. e. , = 0. 5 m. What is the temperature at the surface of the Sun? m T = 2. 898 x 10 -3 m K T = 2. 898 x 10 -3 / 0. 5 x 10 -6 = 6000 K Lecture 25 Purdue University, Physics 220 26
Temperature of the Sun The Sun appear yellowish, i. e. , = 0. 5 m. What is the temperature at the surface of the Sun? m T = 2. 898 x 10 -3 m K T = 2. 898 x 10 -3 / 0. 5 x 10 -6 = 6000 K Lecture 25 Purdue University, Physics 220 26


