450c9b2aa61c4b2c551ef1d7308e2b82.ppt
- Количество слайдов: 20
 Phase Changes Physics 102 Professor Lee Carkner Lecture 4 Session: 104884
Phase Changes Physics 102 Professor Lee Carkner Lecture 4 Session: 104884
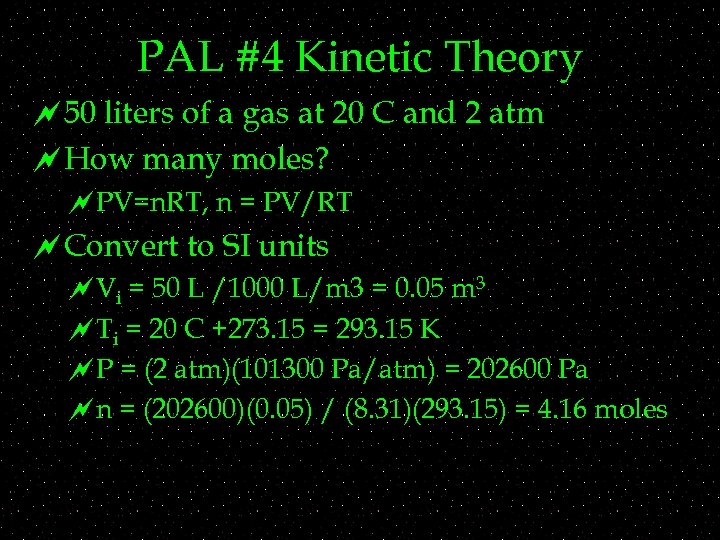 PAL #4 Kinetic Theory ~50 liters of a gas at 20 C and 2 atm ~How many moles? ~PV=n. RT, n = PV/RT ~Convert to SI units ~Vi = 50 L /1000 L/m 3 = 0. 05 m 3 ~Ti = 20 C +273. 15 = 293. 15 K ~P = (2 atm)(101300 Pa/atm) = 202600 Pa ~n = (202600)(0. 05) / (8. 31)(293. 15) = 4. 16 moles
PAL #4 Kinetic Theory ~50 liters of a gas at 20 C and 2 atm ~How many moles? ~PV=n. RT, n = PV/RT ~Convert to SI units ~Vi = 50 L /1000 L/m 3 = 0. 05 m 3 ~Ti = 20 C +273. 15 = 293. 15 K ~P = (2 atm)(101300 Pa/atm) = 202600 Pa ~n = (202600)(0. 05) / (8. 31)(293. 15) = 4. 16 moles
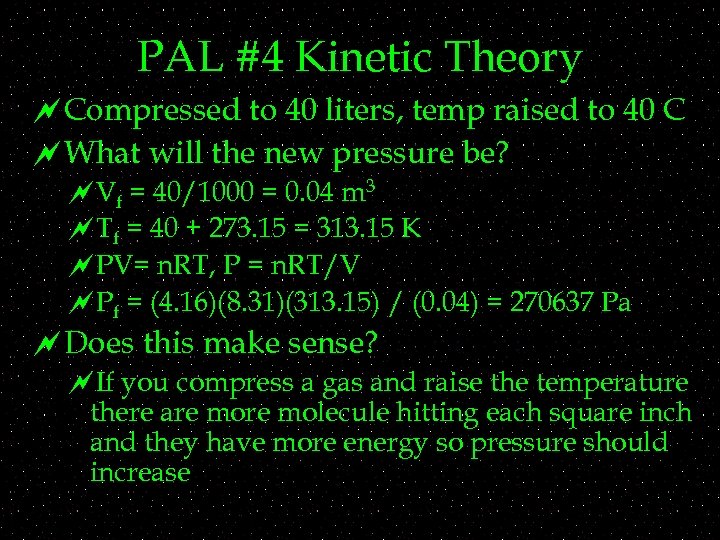 PAL #4 Kinetic Theory ~Compressed to 40 liters, temp raised to 40 C ~What will the new pressure be? ~Vf = 40/1000 = 0. 04 m 3 ~Tf = 40 + 273. 15 = 313. 15 K ~PV= n. RT, P = n. RT/V ~Pf = (4. 16)(8. 31)(313. 15) / (0. 04) = 270637 Pa ~Does this make sense? ~If you compress a gas and raise the temperature there are molecule hitting each square inch and they have more energy so pressure should increase
PAL #4 Kinetic Theory ~Compressed to 40 liters, temp raised to 40 C ~What will the new pressure be? ~Vf = 40/1000 = 0. 04 m 3 ~Tf = 40 + 273. 15 = 313. 15 K ~PV= n. RT, P = n. RT/V ~Pf = (4. 16)(8. 31)(313. 15) / (0. 04) = 270637 Pa ~Does this make sense? ~If you compress a gas and raise the temperature there are molecule hitting each square inch and they have more energy so pressure should increase
 Phase Change ~ ~Once ice starts to melt its temperature does not change. ~ ~solid to liquid -- melting ~ ~How much energy does this take? ~Each substance has a latent heat ~Represented by L
Phase Change ~ ~Once ice starts to melt its temperature does not change. ~ ~solid to liquid -- melting ~ ~How much energy does this take? ~Each substance has a latent heat ~Represented by L
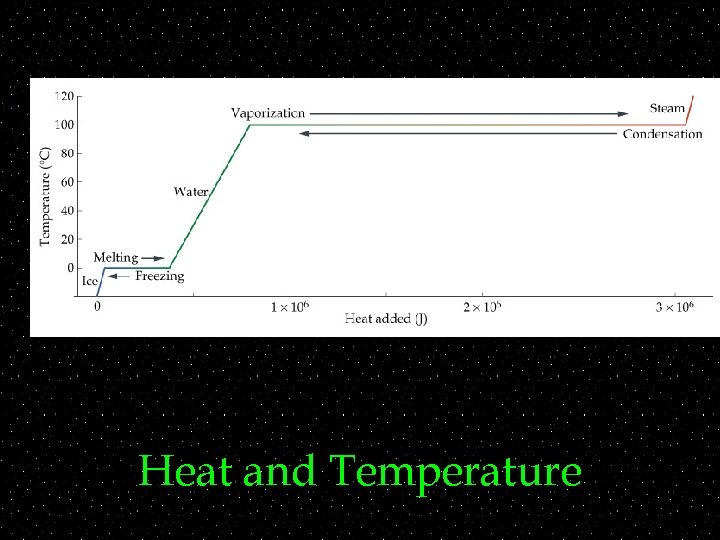 Heat and Temperature
Heat and Temperature
 Phase Change and Heat ~ ~ heat of fusion ~ ~ heat of vaporization ~ Amount of heat: Q = m. L ~ ~ Q = cm. DT ~ ~ Q = m. L ~ Must add all heats together § Note that you must make m. L gained positive and m. L lost negative !
Phase Change and Heat ~ ~ heat of fusion ~ ~ heat of vaporization ~ Amount of heat: Q = m. L ~ ~ Q = cm. DT ~ ~ Q = m. L ~ Must add all heats together § Note that you must make m. L gained positive and m. L lost negative !
 The Final State ~ ~ Its hard to know what terms to put in your heat balance since you are not sure if you will get a phase change ~ ~ e. g. liquid water at 120 C or ice at 5 C ~ Can also do some quick initial computations to try and find the final state ~ ~ n. b. , when a phase change happens, the specific heat changes ~ e. g. , once ice melts it becomes water
The Final State ~ ~ Its hard to know what terms to put in your heat balance since you are not sure if you will get a phase change ~ ~ e. g. liquid water at 120 C or ice at 5 C ~ Can also do some quick initial computations to try and find the final state ~ ~ n. b. , when a phase change happens, the specific heat changes ~ e. g. , once ice melts it becomes water
 Phase Change Calorimetry 1. 2. Write down a “Q” for each heat necessary to reach the final state 3. ~ Make sure you: ~ Write DT = Tf –Ti ~ ~ Assign a sign to each m. L term ~ ~ Check units
Phase Change Calorimetry 1. 2. Write down a “Q” for each heat necessary to reach the final state 3. ~ Make sure you: ~ Write DT = Tf –Ti ~ ~ Assign a sign to each m. L term ~ ~ Check units
 Melting Ice Cube ~Consider a 10 g ice cube at -10 C in 100 g of water at 10 C, what is final T? Assume ice does not melt ~ ~(0. 01)(2090)(Tf-(-10) + (0. 1)(4186)(Tf-10) =0 ~ ~Wrong! Ice can’t be at greater than 0 C ~ ~(0. 01)(2090)(0 -(-10)) +(0. 01)(33. 5 X 104)+ (0. 01)(4186)(Tf 0)+(0. 1)(4186)(Tf-10) = 0 ~ ~This T is OK since all that is left is water ~ ~If we got a T less that 0 it would mean that only a fraction of the ice melted (set Tf = 0 and solve for mmelt)
Melting Ice Cube ~Consider a 10 g ice cube at -10 C in 100 g of water at 10 C, what is final T? Assume ice does not melt ~ ~(0. 01)(2090)(Tf-(-10) + (0. 1)(4186)(Tf-10) =0 ~ ~Wrong! Ice can’t be at greater than 0 C ~ ~(0. 01)(2090)(0 -(-10)) +(0. 01)(33. 5 X 104)+ (0. 01)(4186)(Tf 0)+(0. 1)(4186)(Tf-10) = 0 ~ ~This T is OK since all that is left is water ~ ~If we got a T less that 0 it would mean that only a fraction of the ice melted (set Tf = 0 and solve for mmelt)
 Phase Change and Pressure ~ ~Boiling point depends on both temperature and pressure ~Examples: ~ ~pressure cooker ~ ~Easier for molecules to escape to vapor phase
Phase Change and Pressure ~ ~Boiling point depends on both temperature and pressure ~Examples: ~ ~pressure cooker ~ ~Easier for molecules to escape to vapor phase
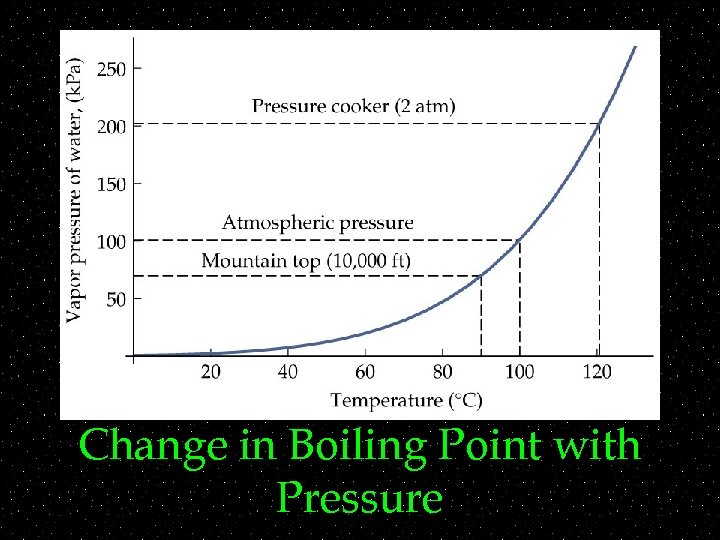 Change in Boiling Point with Pressure
Change in Boiling Point with Pressure
 Phase Diagram ~ ~Usually high P, low T produces a solid and low P, high T produces gas ~ ~Water is an exception, increasing pressure on ice produces water ~ ~This causes ice skates to melt ice and freezing water to expand produce frost heaves
Phase Diagram ~ ~Usually high P, low T produces a solid and low P, high T produces gas ~ ~Water is an exception, increasing pressure on ice produces water ~ ~This causes ice skates to melt ice and freezing water to expand produce frost heaves
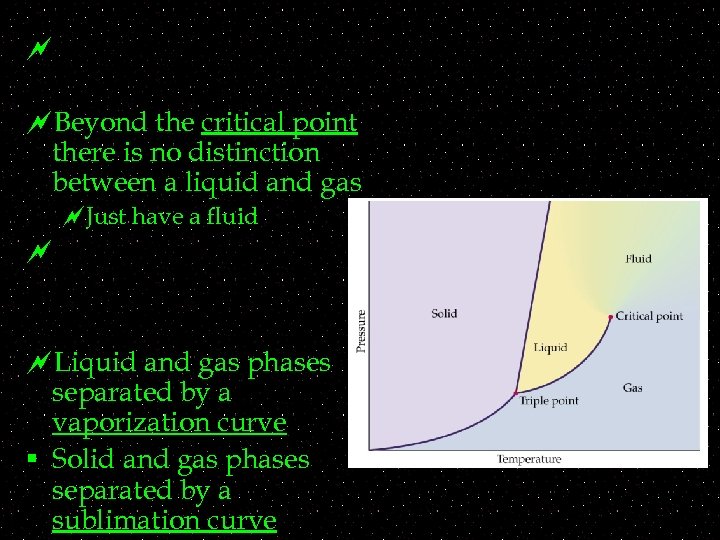 ~ ~Beyond the critical point there is no distinction between a liquid and gas ~Just have a fluid ~ ~Liquid and gas phases separated by a vaporization curve § Solid and gas phases separated by a sublimation curve
~ ~Beyond the critical point there is no distinction between a liquid and gas ~Just have a fluid ~ ~Liquid and gas phases separated by a vaporization curve § Solid and gas phases separated by a sublimation curve
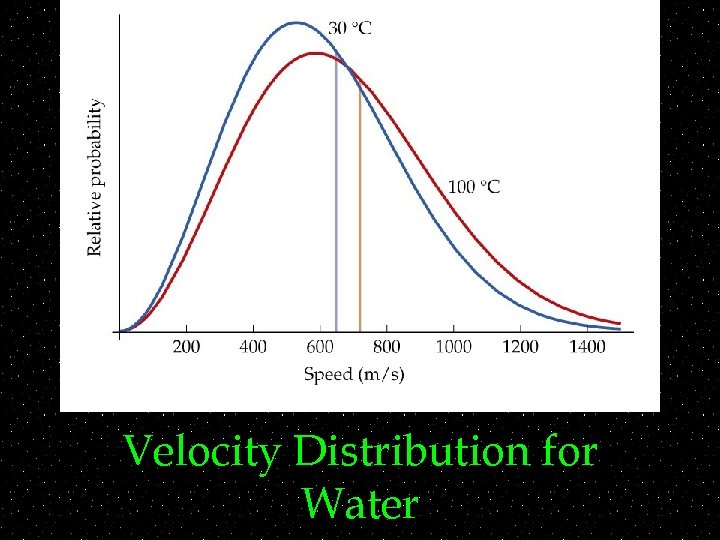 Velocity Distribution for Water
Velocity Distribution for Water
 Evaporation ~How can water evaporate (become gas) if it is not at the boiling point? ~ ~Evaporation only works if the air above is not saturated with vapor ~ ~Pressure is too high ~This is why it is hard to cool off on humid days and easier on windy days §
Evaporation ~How can water evaporate (become gas) if it is not at the boiling point? ~ ~Evaporation only works if the air above is not saturated with vapor ~ ~Pressure is too high ~This is why it is hard to cool off on humid days and easier on windy days §
 Next Time ~Read: 15. 1 -15. 3 ~Homework: Ch 14: P 25, 28, Ch 15: P 1, 2
Next Time ~Read: 15. 1 -15. 3 ~Homework: Ch 14: P 25, 28, Ch 15: P 1, 2
 If a fixed amount of gas at constant temperature undergoes a increase in volume, what happens to the pressure? a) b) c) d) e) It goes up It goes down It stays the same It depends on the value of R It depends on the number of moles
If a fixed amount of gas at constant temperature undergoes a increase in volume, what happens to the pressure? a) b) c) d) e) It goes up It goes down It stays the same It depends on the value of R It depends on the number of moles
 If a fixed amount of gas at constant pressure undergoes an increase in volume, what happens to the temperature? a) b) c) d) e) It goes up It goes down It stays the same It depends on the value of R It depends on the number of moles
If a fixed amount of gas at constant pressure undergoes an increase in volume, what happens to the temperature? a) b) c) d) e) It goes up It goes down It stays the same It depends on the value of R It depends on the number of moles
 Consider two rooms of a house, room A and room B. If the (otherwise identical) molecules in room B have twice as much average kinetic energy than the ones in A, how does the temperature of room A compare to the temperature of room B? A) B) C) D) E) TA = T B TA = 2 T B TA = ½ T B TA = √ 2 TB TA = (3/2) TB
Consider two rooms of a house, room A and room B. If the (otherwise identical) molecules in room B have twice as much average kinetic energy than the ones in A, how does the temperature of room A compare to the temperature of room B? A) B) C) D) E) TA = T B TA = 2 T B TA = ½ T B TA = √ 2 TB TA = (3/2) TB
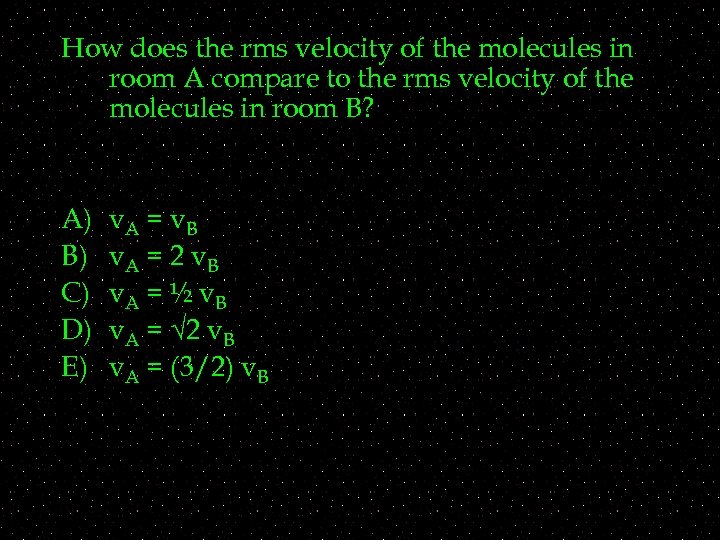 How does the rms velocity of the molecules in room A compare to the rms velocity of the molecules in room B? A) B) C) D) E) v. A = v. B v. A = 2 v. B v. A = ½ v. B v. A = √ 2 v. B v. A = (3/2) v. B
How does the rms velocity of the molecules in room A compare to the rms velocity of the molecules in room B? A) B) C) D) E) v. A = v. B v. A = 2 v. B v. A = ½ v. B v. A = √ 2 v. B v. A = (3/2) v. B


