2686c7587df4002b4dbd2912d8eb812d.ppt
- Количество слайдов: 71

Pharmacotherapy for Overactive Bladder Rationale for Treatment Choice David A. Ginsberg, M. D. Assistant Professor of Urology USC Keck School of Medicine


Overactive Bladder Terminology OAB = bladder contracting w/o pt’s permission OAB definition per ICS based on symptoms • Urgency, with or without urge incontinence, usually with frequency and nocturia • In the absence of pathologic or metabolic conditions that might explain these symptoms ICS = International Continence Society (www. icsoffice. org)
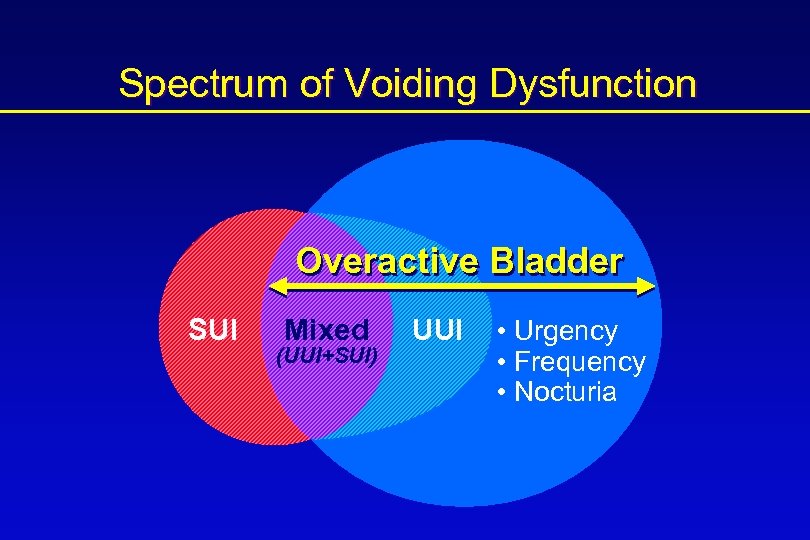
Spectrum of Voiding Dysfunction Overactive Bladder SUI z Mixed (UUI+SUI) UUI • Urgency • Frequency • Nocturia
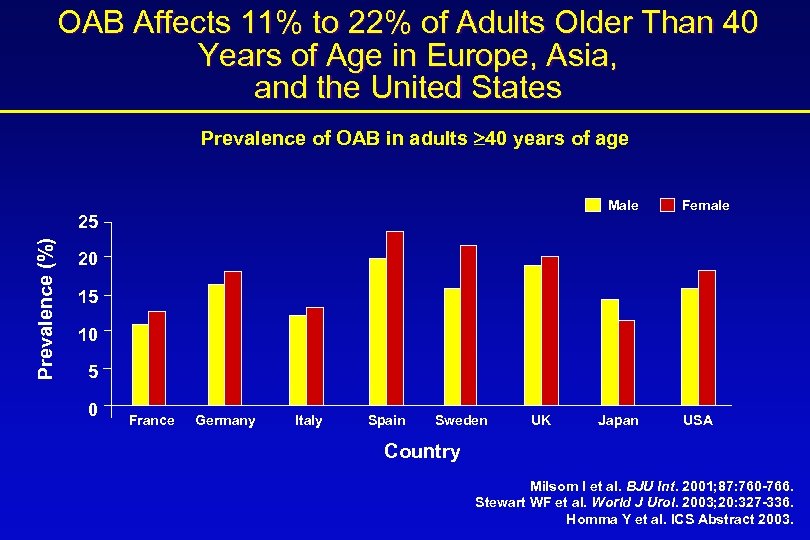
OAB Affects 11% to 22% of Adults Older Than 40 Years of Age in Europe, Asia, and the United States Prevalence of OAB in adults 40 years of age Male Prevalence (%) 25 Female 20 15 10 5 0 France Germany Italy Spain Sweden UK Japan USA Country Milsom I et al. BJU Int. 2001; 87: 760 -766. Stewart WF et al. World J Urol. 2003; 20: 327 -336. Homma Y et al. ICS Abstract 2003.
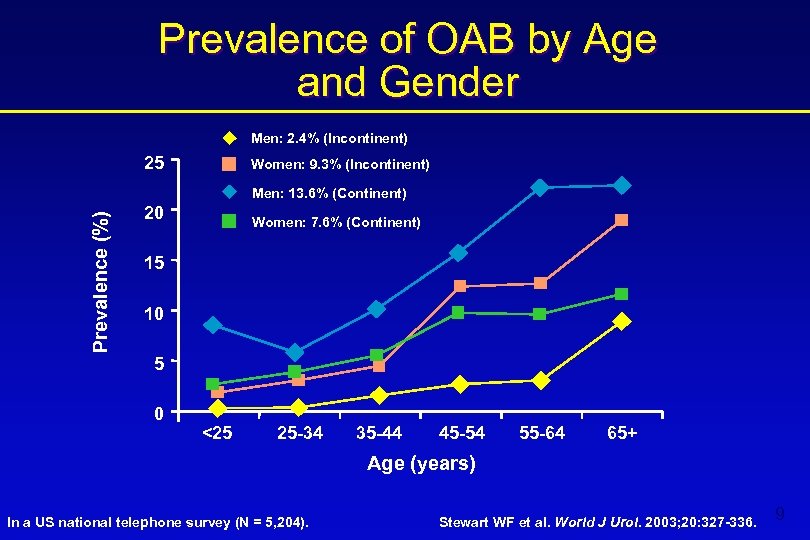
Prevalence of OAB by Age and Gender Men: 2. 4% (Incontinent) 25 Women: 9. 3% (Incontinent) Prevalence (%) Men: 13. 6% (Continent) 20 Women: 7. 6% (Continent) 15 10 5 0 <25 25 -34 35 -44 45 -54 55 -64 65+ Age (years) In a US national telephone survey (N = 5, 204). Stewart WF et al. World J Urol. 2003; 20: 327 -336. 9

Incidence Underreported • “Tip of the iceberg” • Increasing incidence in an aging population
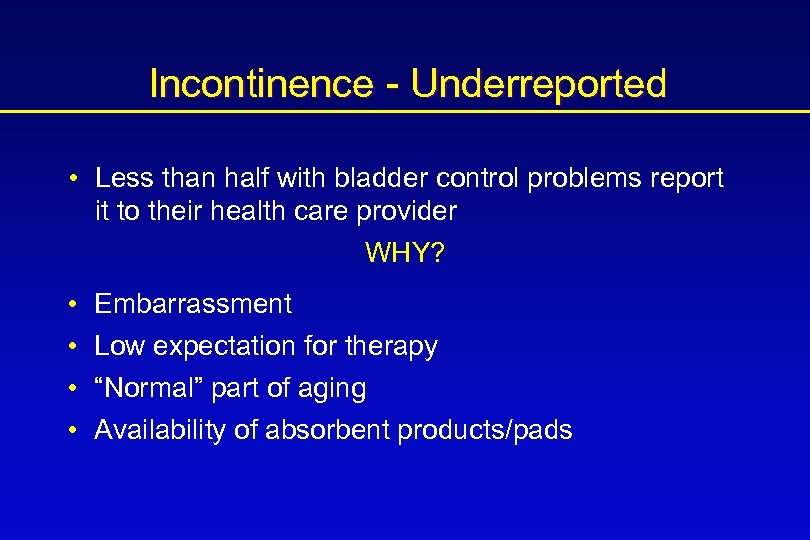
Incontinence - Underreported • Less than half with bladder control problems report it to their health care provider WHY? • • Embarrassment Low expectation for therapy “Normal” part of aging Availability of absorbent products/pads
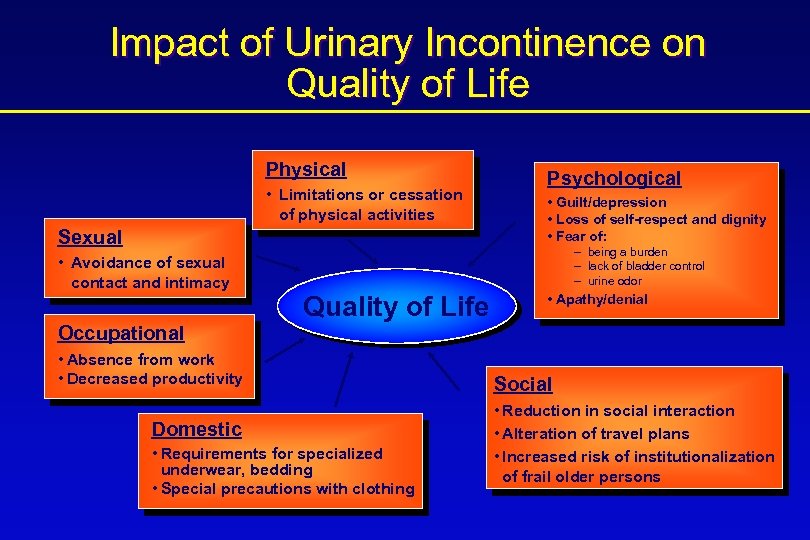
Impact of Urinary Incontinence on Quality of Life Physical • Limitations or cessation of physical activities Sexual • Avoidance of sexual contact and intimacy Occupational Psychological • Guilt/depression • Loss of self-respect and dignity • Fear of: - being a burden - lack of bladder control - urine odor Quality of Life • Absence from work • Decreased productivity Domestic • Requirements for specialized underwear, bedding • Special precautions with clothing • Apathy/denial Social • Reduction in social interaction • Alteration of travel plans • Increased risk of institutionalization of frail older persons
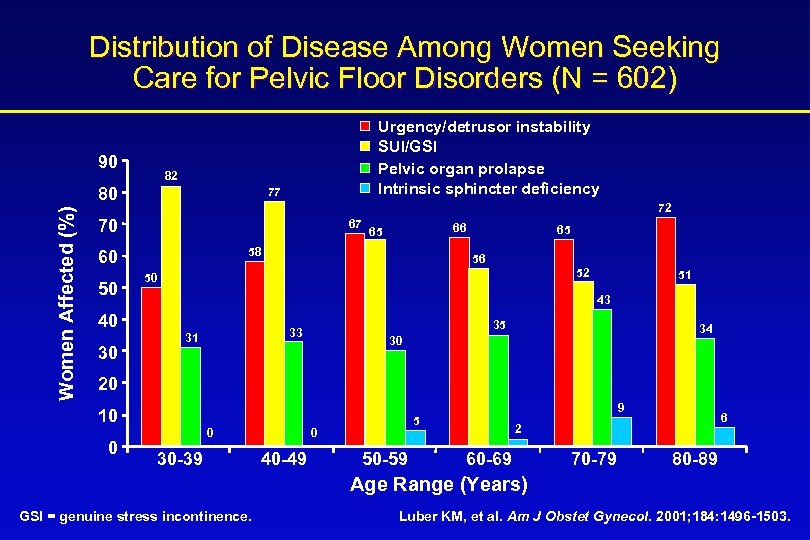
Distribution of Disease Among Women Seeking Care for Pelvic Floor Disorders (N = 602) 90 82 80 Women Affected (%) Urgency/detrusor instability SUI/GSI Pelvic organ prolapse Intrinsic sphincter deficiency 77 72 70 67 58 60 50 65 56 52 50 51 43 40 30 66 65 35 33 31 34 30 20 10 0 0 30 -39 0 40 -49 9 5 50 -59 6 2 60 -69 70 -79 80 -89 Age Range (Years) GSI = genuine stress incontinence. Luber KM, et al. Am J Obstet Gynecol. 2001; 184: 1496 -1503.

Diagnosis of Overactive Bladder • Most cases of overactive bladder can be diagnosed based on: – patient history, symptom assessment – physical examination – urinalysis • Initiation of noninvasive treatment does not require an extensive further workup

History • How long? How old when started? • How much (volume)? Degree of bother? • Characteristics of leakage? – Activity related? – Day and night, wet pads at night = instability – Urgency? • Suppressible = probably SUI • not suppressible (urge incontinence) = instability • Other: fluid intake, UTI’s, pain, hematuria, LE swelling, medications
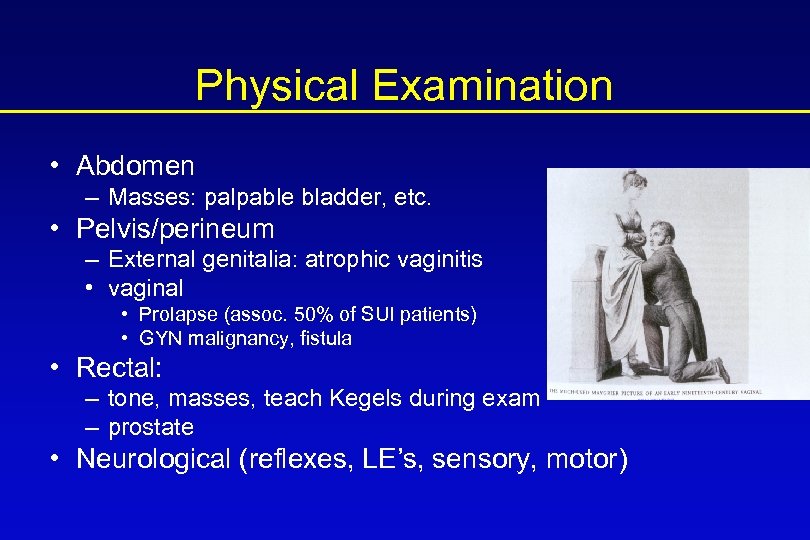
Physical Examination • Abdomen – Masses: palpable bladder, etc. • Pelvis/perineum – External genitalia: atrophic vaginitis • vaginal • Prolapse (assoc. 50% of SUI patients) • GYN malignancy, fistula • Rectal: – tone, masses, teach Kegels during exam – prostate • Neurological (reflexes, LE’s, sensory, motor)
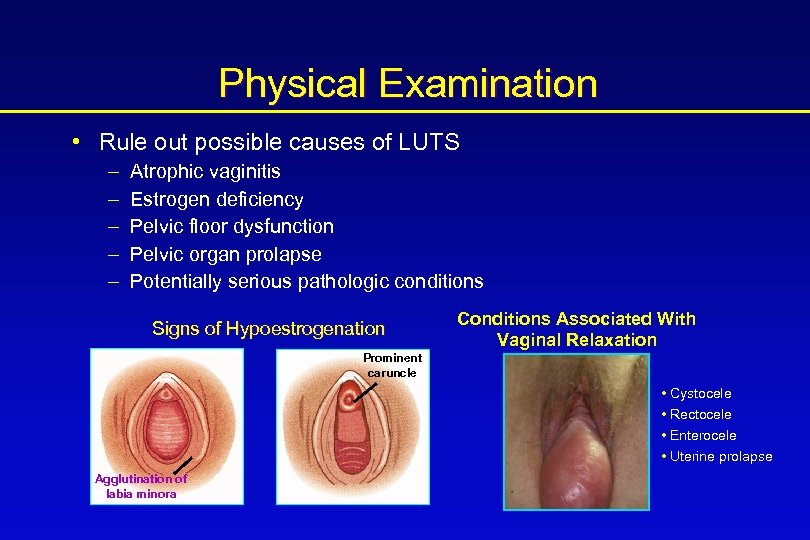
Physical Examination • Rule out possible causes of LUTS – – – Atrophic vaginitis Estrogen deficiency Pelvic floor dysfunction Pelvic organ prolapse Potentially serious pathologic conditions Signs of Hypoestrogenation Conditions Associated With Vaginal Relaxation Prominent caruncle • Cystocele • Rectocele • Enterocele • Uterine prolapse Agglutination of labia minora

Laboratory Tests • Urinalysis – – Dipstick Culture Microscopic examination Look for hematuria, pyuria, bacteriuria, glucosuria, proteinuria • Appropriate blood work – Glucose – Electrolytes – Prostate specific antigen (PSA) in men Fantl JA, et al. Agency for Healthcare Policy and Research; 1996. AHCPR publication 96 -0686.
Laboratory Tests • Rule out possible causes of LUTS – Urinary tract infection (UTI) or sexually transmitted disease (STD) – Diabetes mellitus – LUT tumor or kidney stones – Potentially serious pathologic conditions Refer to appropriate specialist.

Stress Incontinence, Overactive Bladder, and Mixed Symptoms
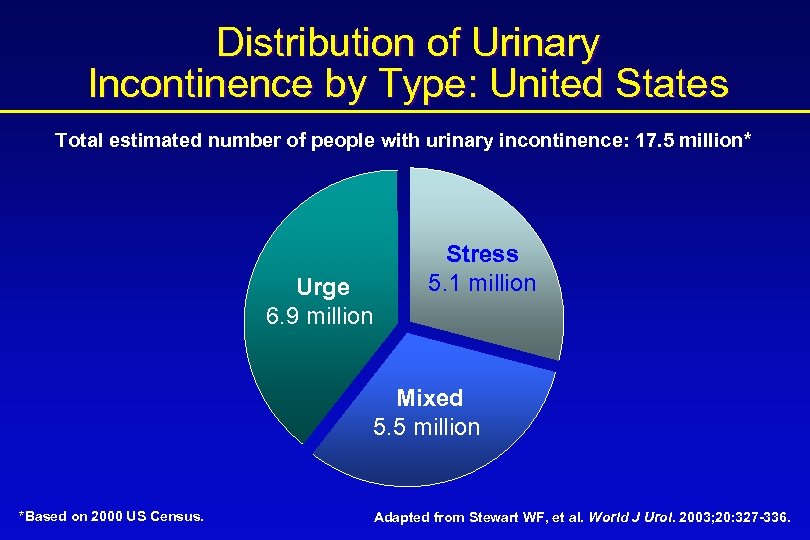
Distribution of Urinary Incontinence by Type: United States Total estimated number of people with urinary incontinence: 17. 5 million* Urge 6. 9 million Stress 5. 1 million Mixed 5. 5 million *Based on 2000 US Census. Adapted from Stewart WF, et al. World J Urol. 2003; 20: 327 -336.
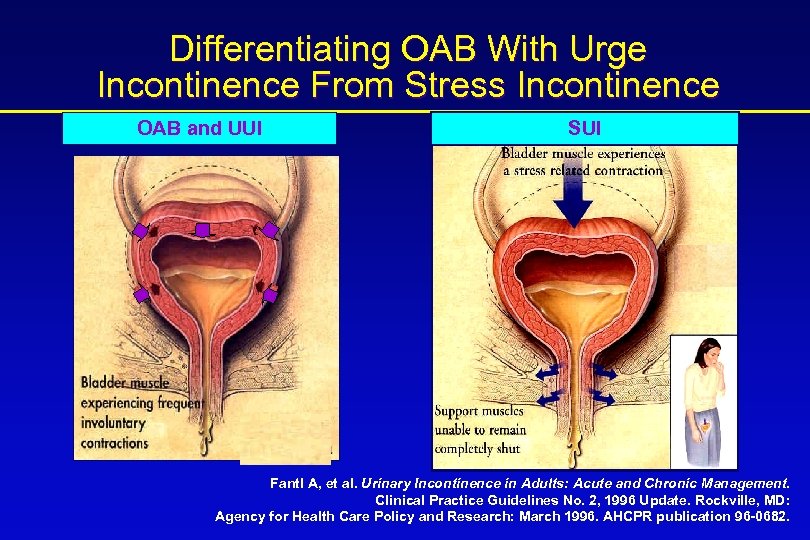
Differentiating OAB With Urge Incontinence From Stress Incontinence OAB and UUI SUI Fantl A, et al. Urinary Incontinence in Adults: Acute and Chronic Management. Clinical Practice Guidelines No. 2, 1996 Update. Rockville, MD: Agency for Health Care Policy and Research: March 1996. AHCPR publication 96 -0682.
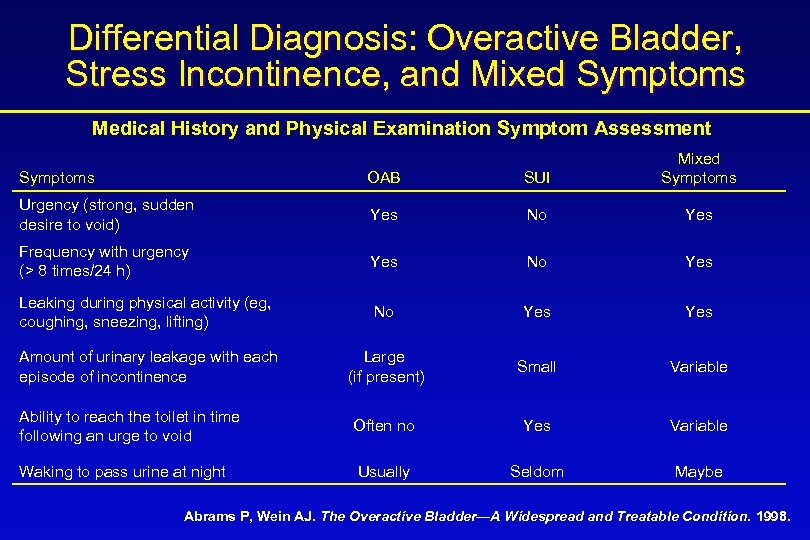
Differential Diagnosis: Overactive Bladder, Stress Incontinence, and Mixed Symptoms Medical History and Physical Examination Symptom Assessment Symptoms OAB SUI Mixed Symptoms Urgency (strong, sudden desire to void) Yes No Yes Frequency with urgency (> 8 times/24 h) Yes No Yes Leaking during physical activity (eg, coughing, sneezing, lifting) No Yes Amount of urinary leakage with each episode of incontinence Large (if present) Small Variable Ability to reach the toilet in time following an urge to void Often no Yes Variable Waking to pass urine at night Usually Seldom Maybe Abrams P, Wein AJ. The Overactive Bladder—A Widespread and Treatable Condition. 1998.
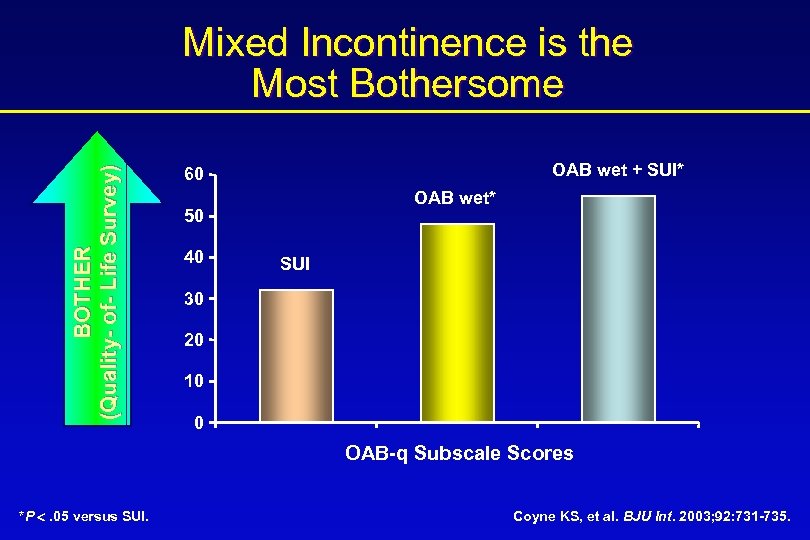
BOTHER (Quality- of- Life Survey) Mixed Incontinence is the Most Bothersome OAB wet + SUI* 60 OAB wet* 50 40 SUI 30 20 10 0 OAB-q Subscale Scores *P . 05 versus SUI. Coyne KS, et al. BJU Int. 2003; 92: 731 -735.

Treatment for Overactive Bladder • Pads • Behavioral therapy • Medications • Neuromodulation • Surgery
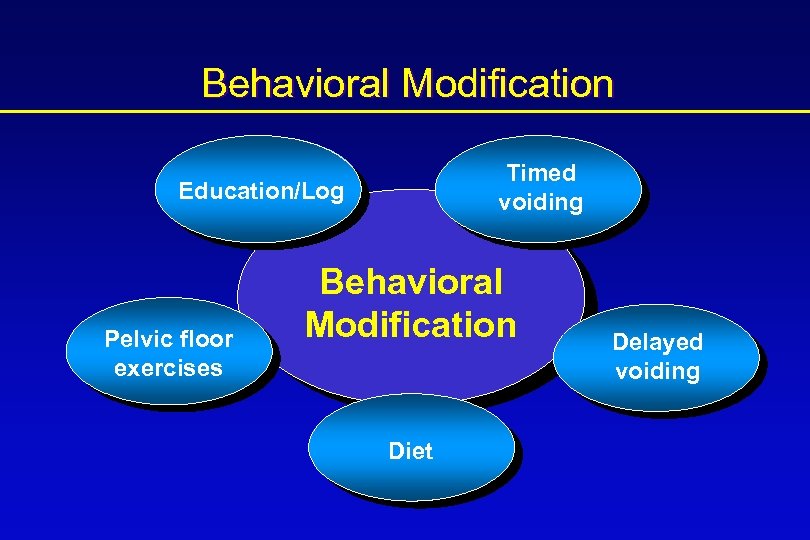
Behavioral Modification Timed voiding Education/Log Pelvic floor exercises Behavioral Modification Diet Delayed voiding

Diet Modification • Avoid food/beverages irritating to the bladder (coffee, caffeine, etc. ) • Manage fluid intake • Stop evening fluids • Avoid constipation

Bladder Training • Modify bladder function • Methods – bladder diary – gradually increase void interval – teach coping strategies • Strengthen pelvic floor muscles and improving bladder stability

Management of Overactive Bladder • Behavioral therapies 1 • Pharmacologic therapy • Combined pharmacologic and behavioral therapy provides improved outcomes 2, 3 1. Mattiasson A. Urology. 2000; 55(suppl 5 a): 12 -13. 2. Mattiasson A. Neuro Urodyn. 2001; 20: 403 -404. 3. Burgio et al. JAGS. 2000; 48: 370 -374.

Behavioral Modification Burgio, et al • 197 women with urge incontinence • Modified crossover design • Initially on monotherapy of either – Behavioral therapy – Drug therapy (oxy 2. 5 -15 mg/d) • Combined therapy offered after 8 weeks if not content with monotherapy alone Burgio et al. JAGS. 2000; 48: 370 -374

Behavioral Modification • Behavioral therapy – 57. 5% reduction in incontinence – 8 pts crossed over – 88. 5% reduction in incontinence when meds added (p=0. 034) • Medical therapy – 72. 7% reduction in incontinence – 27 pts crossed over – 84. 3% reduction in incontinence when meds added (p=0. 001) • Conclusion: combining drug & behavioral therapy in a stepped program can produce added benefit for patients with UUI Burgio et al. JAGS. 2000; 48: 370 -374

Pharmacologic Therapy for the Treatment of OAB • Antimuscarinic agents are the mainstay for treating OAB • OAB symptoms relieved by – inhibition of involuntary bladder contractions – increased bladder capacity • Treatment can be limited by side effects such as dry mouth, GI effects (eg, constipation), and CNS effects
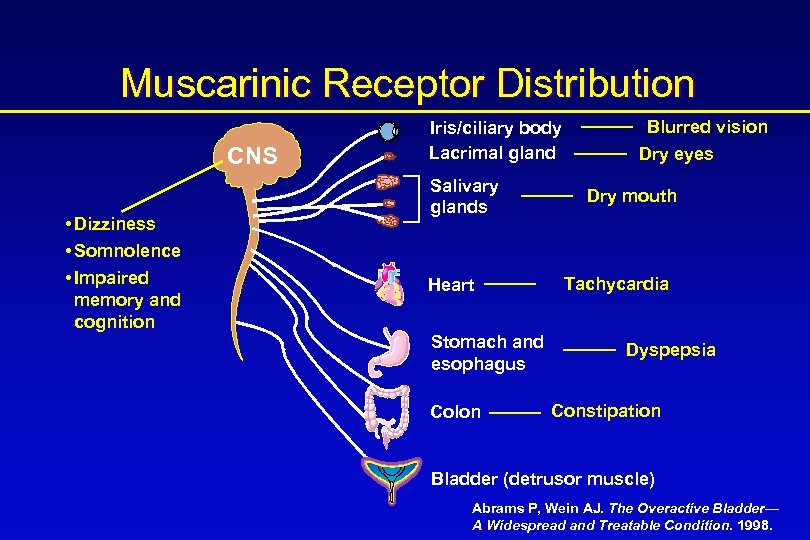
Muscarinic Receptor Distribution CNS • Dizziness • Somnolence • Impaired memory and cognition Iris/ciliary body Lacrimal gland Salivary glands Heart Stomach and esophagus Colon Blurred vision Dry eyes Dry mouth Tachycardia Dyspepsia Constipation Bladder (detrusor muscle) Abrams P, Wein AJ. The Overactive Bladder— A Widespread and Treatable Condition. 1998.

Anticholinergics A Delicate Balance Efficacy • Less frequency • Less UUI • Increased voided volume Adverse effects • Dry mouth • Constipation • CNS
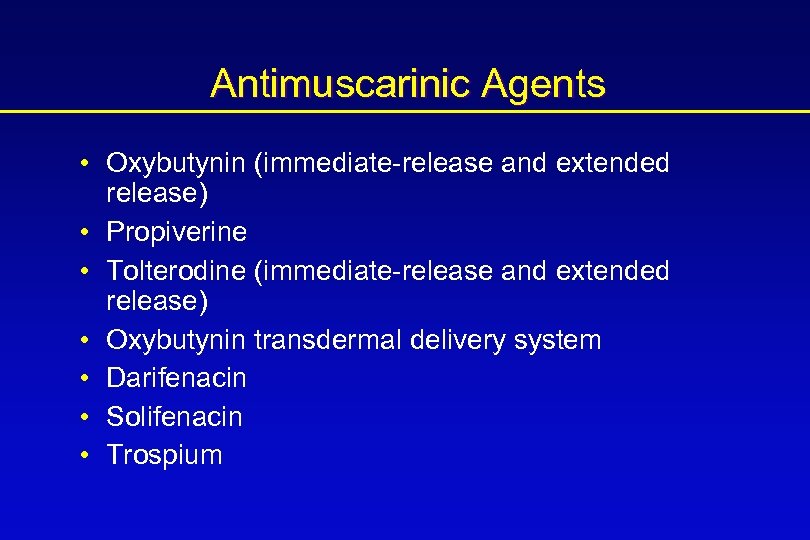
Antimuscarinic Agents • Oxybutynin (immediate-release and extended release) • Propiverine • Tolterodine (immediate-release and extended release) • Oxybutynin transdermal delivery system • Darifenacin • Solifenacin • Trospium

Tolterodine SR Registration Trial Van Kerrebroeck P et al. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001; 57: 414 -421.
Detrol® LA (tolterodine tartrate extended release capsules) Tolterodine SR in the Treatment of OAB: Study Design and Inclusion Criteria Registration Trial: Van Kerrebroeck et al • Multicenter (167 sites across North America, Europe, Australia, and New Zealand), randomized, double-blind, active- and placebo-controlled trial in adult patients with symptoms of OAB • Adult patients aged 18 or older with symptoms of OAB for >6 months • Symptoms of urinary frequency (average of >8 micturitions/24 h) and urge incontinence (>5 incontinence episodes/wk) Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
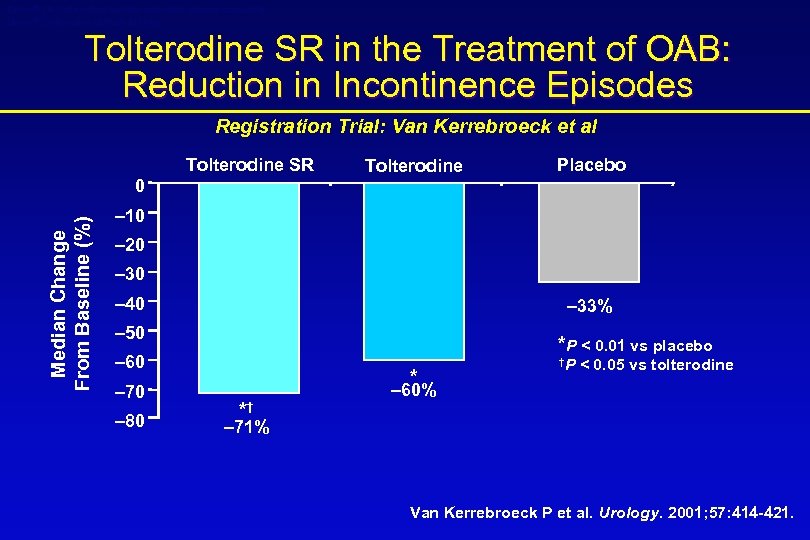
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Reduction in Incontinence Episodes Registration Trial: Van Kerrebroeck et al Tolterodine SR Tolterodine Placebo Median Change From Baseline (%) 0 – 10 – 20 – 30 – 40 – 33% – 50 *P < 0. 01 vs placebo – 60 – 70 – 80 * *† †P < 0. 05 vs tolterodine – 60% – 71% Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
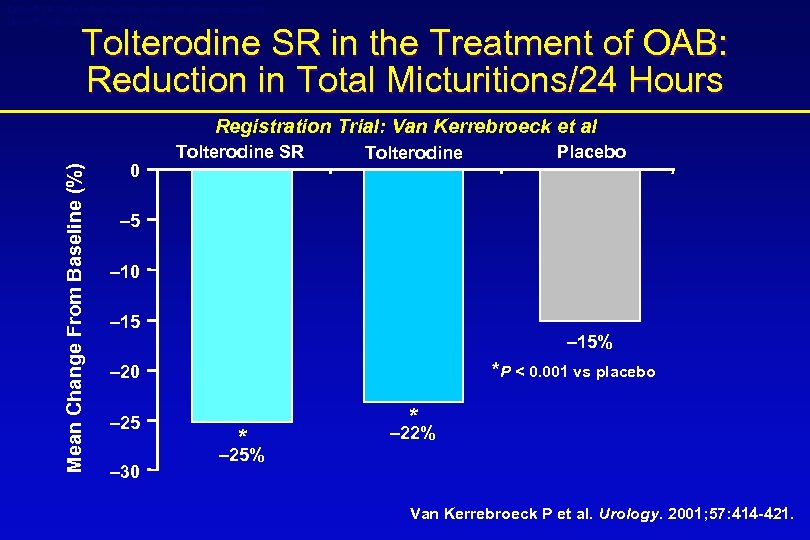
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Reduction in Total Micturitions/24 Hours Mean Change From Baseline (%) Registration Trial: Van Kerrebroeck et al 0 Tolterodine SR Tolterodine Placebo – 5 – 10 – 15% *P < 0. 001 vs placebo – 20 – 25 – 30 * * – 22% – 25% Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
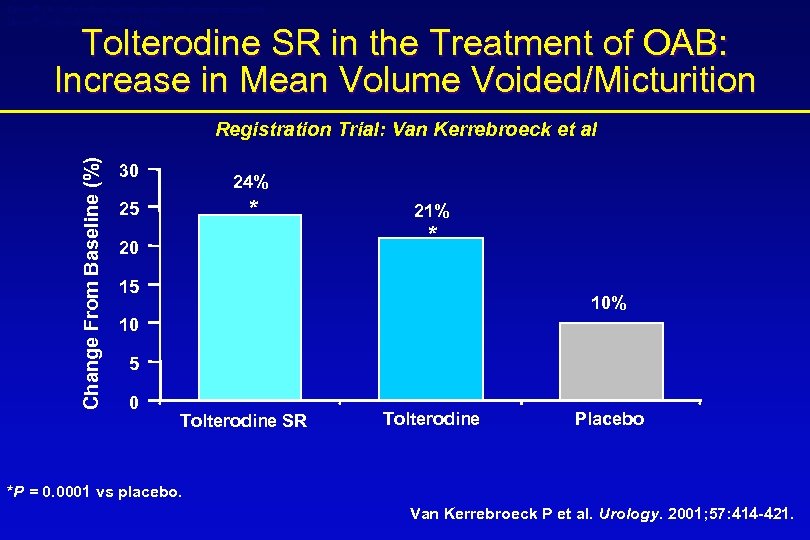
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Increase in Mean Volume Voided/Micturition Change From Baseline (%) Registration Trial: Van Kerrebroeck et al 30 24% * 25 20 21% * 15 10% 10 5 0 Tolterodine SR Tolterodine Placebo *P = 0. 0001 vs placebo. Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
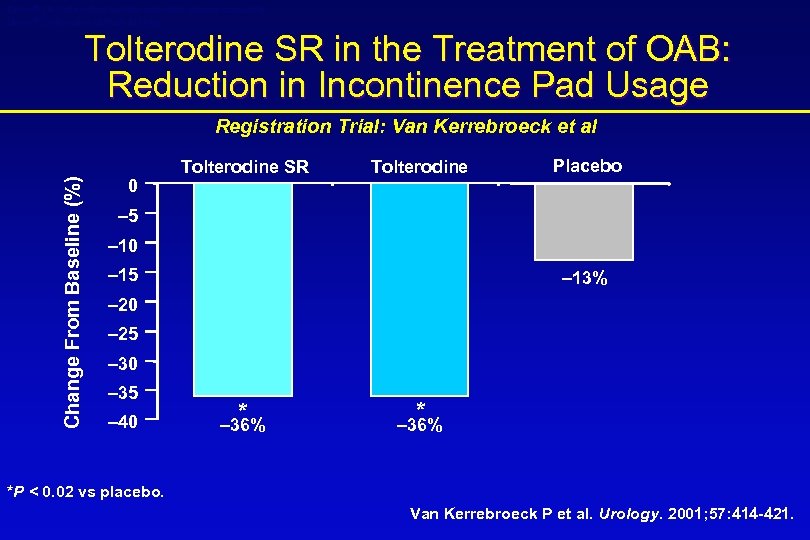
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Reduction in Incontinence Pad Usage Change From Baseline (%) Registration Trial: Van Kerrebroeck et al 0 Tolterodine SR Tolterodine Placebo – 5 – 10 – 15 – 13% – 20 – 25 – 30 – 35 – 40 * – 36% *P < 0. 02 vs placebo. Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
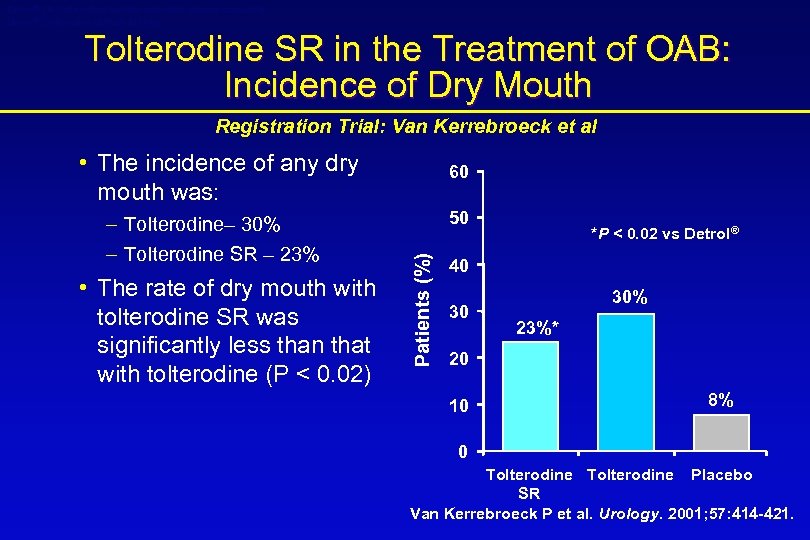
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Incidence of Dry Mouth Registration Trial: Van Kerrebroeck et al • The incidence of any dry mouth was: • The rate of dry mouth with tolterodine SR was significantly less than that with tolterodine (P < 0. 02) 50 Patients (%) – Tolterodine– 30% – Tolterodine SR – 23% 60 *P < 0. 02 vs Detrol® 40 30 30% 23%* 20 10 8% 0 Tolterodine Placebo SR Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
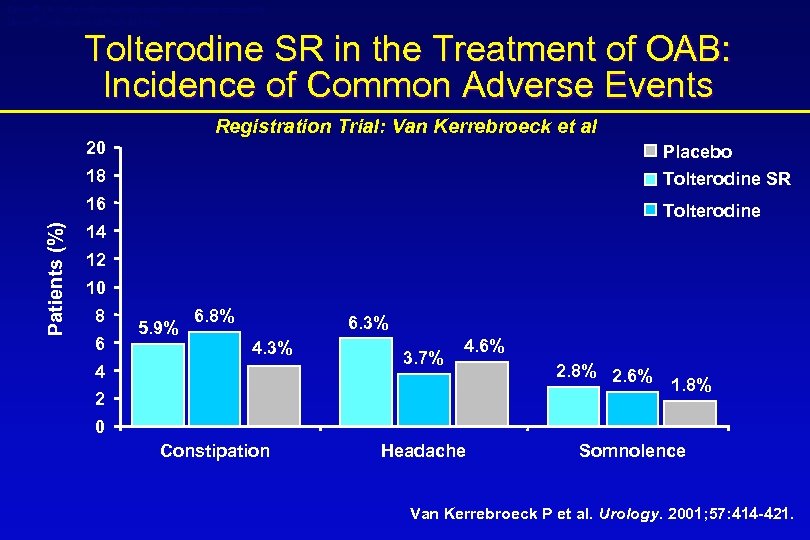
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Incidence of Common Adverse Events Registration Trial: Van Kerrebroeck et al Placebo 18 Tolterodine SR 16 Patients (%) 20 Tolterodine 14 12 10 8 6 5. 9% 6. 8% 6. 3% 4 3. 7% 4. 6% 2 2. 8% 2. 6% 1. 8% 0 Constipation Headache Somnolence Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.
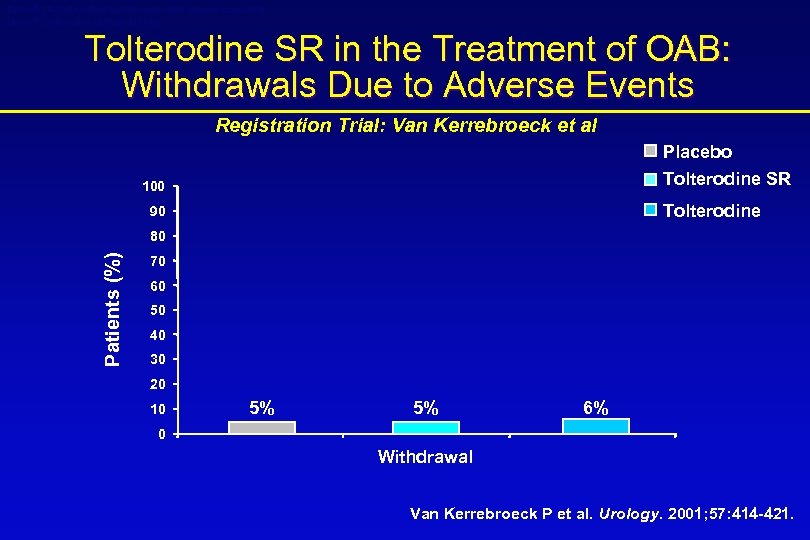
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Withdrawals Due to Adverse Events Registration Trial: Van Kerrebroeck et al Placebo Tolterodine SR 100 Tolterodine 90 Patients (%) 80 70 60 50 40 30 20 10 5% 5% 6% 0 Withdrawal Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.

Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR in the Treatment of OAB: Study Conclusions Registration Trial: Van Kerrebroeck et al • A 12 -week multinational study comparing tolterodine SR 4 mg once daily, and tolterodine 2 mg twice daily with placebo in 1, 529 patients • Compared with placebo, tolterodine SR and tolterodine were significantly more effective in improving: – – Incontinence episodes/week Total micturitions/24 hours Mean volume voided/micturition Incontinence pad usage/24 hours Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.

007 Registration Trial: 12 -Month Extension Kreder K et al. Long-term safety, tolerability and efficacy of extended-release tolterodine in the treatment of overactive bladder. Eur Urol. 2002; 41: 588 -595.
Detrol® LA (tolterodine tartrate extended release capsules) Study Design 007 Registration Trial 12 -Month Extension: Kreder et al • 12 -Month open-label, uncontrolled, nonrandomized extension (N = 1, 337) – 759 patients completed 12 -month extension • Eligibility – Those who completed the 12 -week double-blind phase • Primary end point – Safety and tolerability of tolterodine SR 4 mg qd • Secondary end points – Efficacy – Persistency Kreder K et al. Eur Urol. 2002; 41: 588 -595.
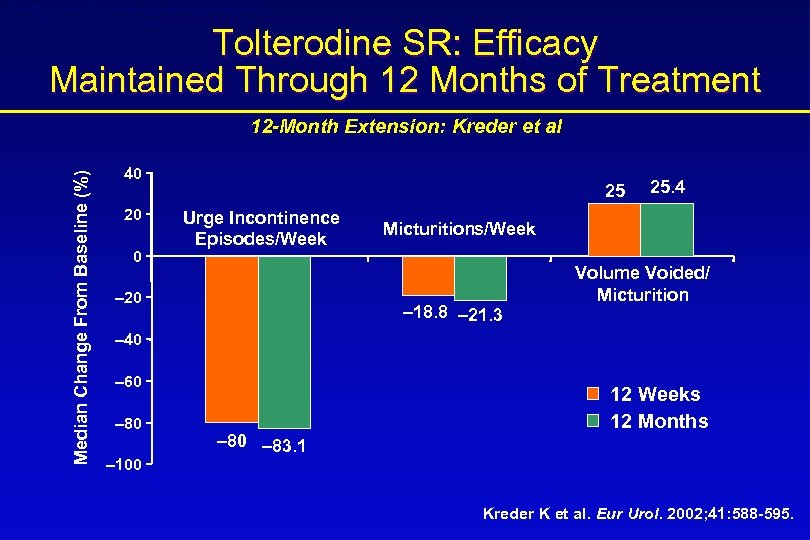
Detrol® LA (tolterodine tartrate extended release capsules) Detrol® (tolterodine tartrate tablets) Tolterodine SR: Efficacy Maintained Through 12 Months of Treatment Median Change From Baseline (%) 12 -Month Extension: Kreder et al 40 20 0 25 Urge Incontinence Episodes/Week – 20 25. 4 Micturitions/Week – 18. 8 – 21. 3 Volume Voided/ Micturition – 40 – 60 – 80 – 100 – 83. 1 12 Weeks 12 Months Kreder K et al. Eur Urol. 2002; 41: 588 -595.
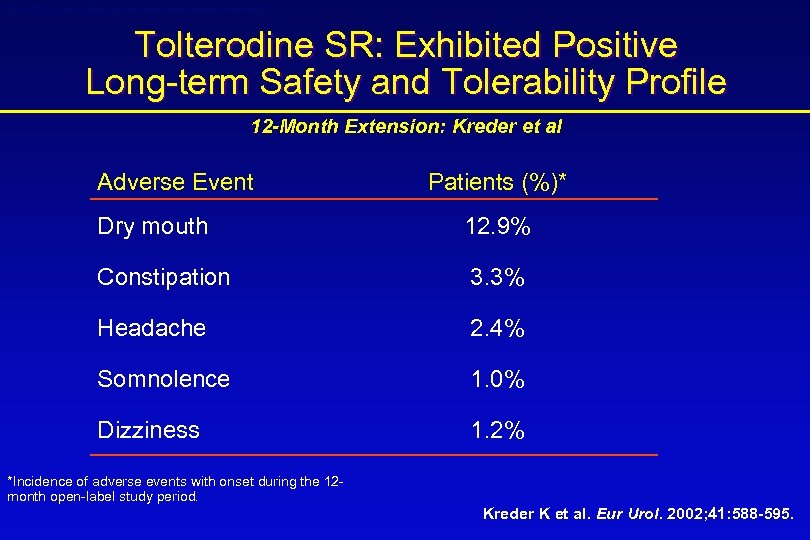
Detrol® LA (tolterodine tartrate extended release capsules) Tolterodine SR: Exhibited Positive Long-term Safety and Tolerability Profile 12 -Month Extension: Kreder et al Adverse Event Patients (%)* Dry mouth 12. 9% Constipation 3. 3% Headache 2. 4% Somnolence 1. 0% Dizziness 1. 2% *Incidence of adverse events with onset during the 12 month open-label study period. Kreder K et al. Eur Urol. 2002; 41: 588 -595.

Detrol® LA (tolterodine tartrate extended release capsules) Conclusions 12 -Month Extension: Kreder et al • Efficacy of tolterodine SR was maintained for at least 1 year with continued treatment • Improvements from baseline in all micturition variables comparable to those observed in the 12 -week double-blind phase • Long-term treatment with tolterodine SR was well tolerated • 71% of patients completed 12 months of therapy Kreder K et al. Eur Urol. 2002; 41: 588 -595.

STAT Speed of Onset Therapeutic Assessment Trial

Questions • How rapidly does medication work? • Does effectiveness increase over time? • Is there a difference between patients naïve and nonnaïve to prior anticholinergic therapy?

Patients • 1138 patients – 302 men, 836 women – 735 naïve, 403 non-naïve • Minimum age of 18 years (range 19 -91) • Diagnosis of OAB – Frequency (>8 voids/24 hours) – Urgency – With or without urge incontinence • 911 patients (79. 4%) completed the study

Methods • Prospective • Multicenter • 12 -weeks duration • Efficacy assessed at 1, 4 & 12 weeks using – Micturition diary – Patient perception of improvement – Physician perception of improvement

Medication • Treated with tolterodine extended-release (ER) • 4 mg daily for 12 weeks • If previously on anticholinergics then had 7 -day washout
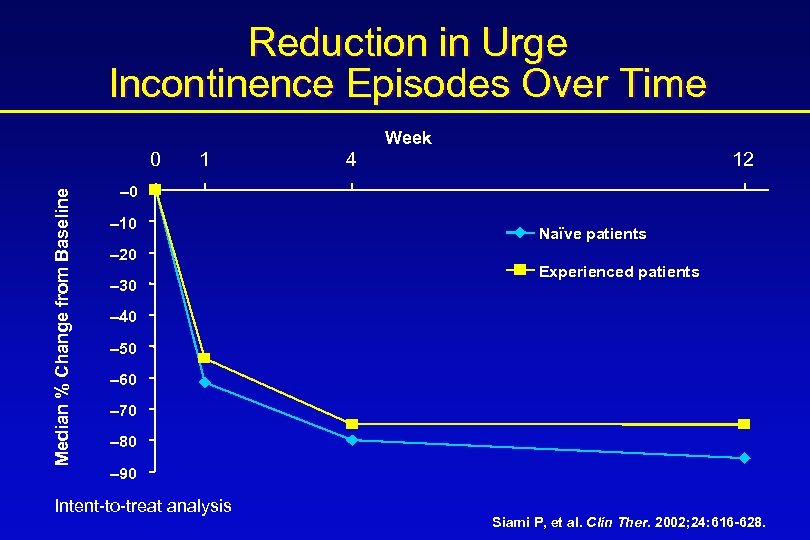
Reduction in Urge Incontinence Episodes Over Time Week Median % Change from Baseline 0 1 4 12 – 0 – 10 – 20 – 30 Naïve patients Experienced patients – 40 – 50 – 60 – 70 – 80 – 90 Intent-to-treat analysis Siami P, et al. Clin Ther. 2002; 24: 616 -628.
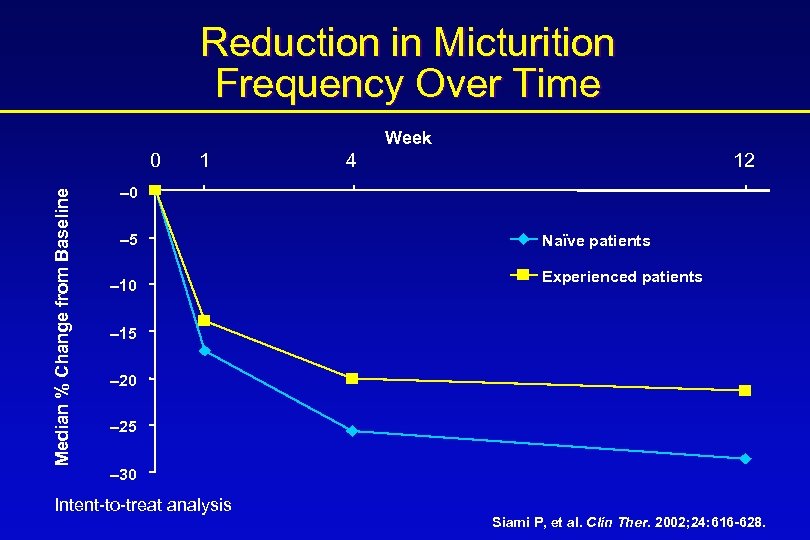
Reduction in Micturition Frequency Over Time Week Median % Change from Baseline 0 1 4 12 – 0 – 5 – 10 Naïve patients Experienced patients – 15 – 20 – 25 – 30 Intent-to-treat analysis Siami P, et al. Clin Ther. 2002; 24: 616 -628.
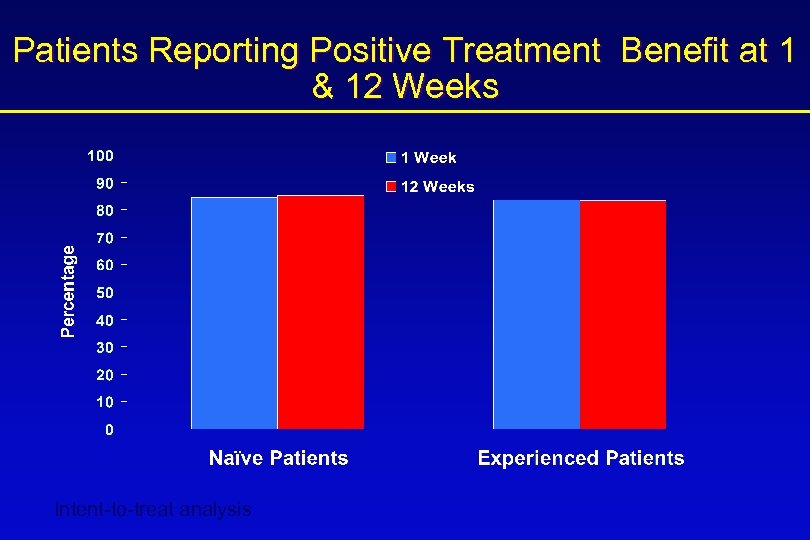
Patients Reporting Positive Treatment Benefit at 1 & 12 Weeks Intent-to-treat analysis
STAT Summary • Improvements in efficacy based on patients’ bladder diaries were seen at 1 week, with further improvements noted at 12 weeks • Perception of treatment benefit by both patients and physicians was maintained at 12 weeks • After only 1 week of treatment, ~85% of patients and physicians reported benefit from treatment – half of those patients who reported no treatment benefit at 1 week reported benefit at 12 weeks

Efficacy and Tolerability of Tolterodine SR Versus Oxybutynin IR in Japanese and Korean Patients
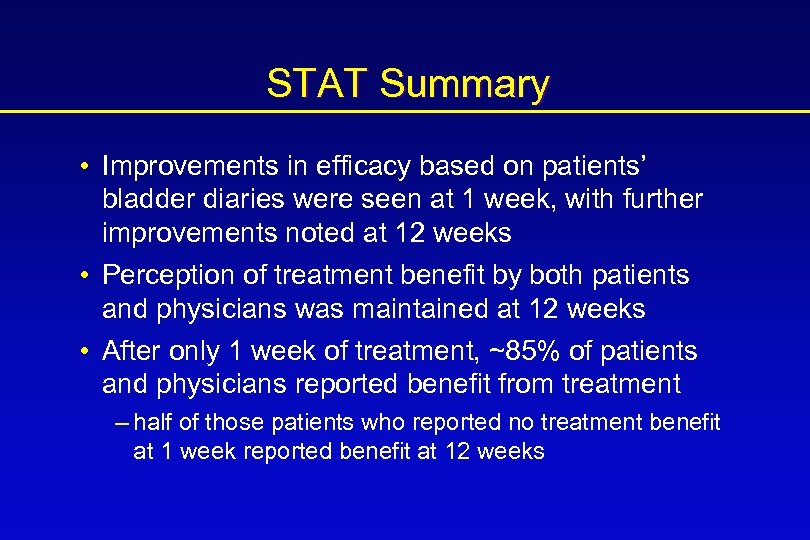
Efficacy Change in Mean Voiding Diary End Points Incontinence episode/wk No. voids/24 h Tolterodine SR (n = 239) Tolterodine IR (n = 244) Placebo (n = 122) – 78. 6% – 76. 5% – 46. 4% – 2. 0 – 2. 1 – 1. 1 • Significant reduction between treatment and placebo group • No significant difference between Detrol® LA and oxybutynin IR Homma Y et al. BJU Int. 2003; 92: 741 -747.
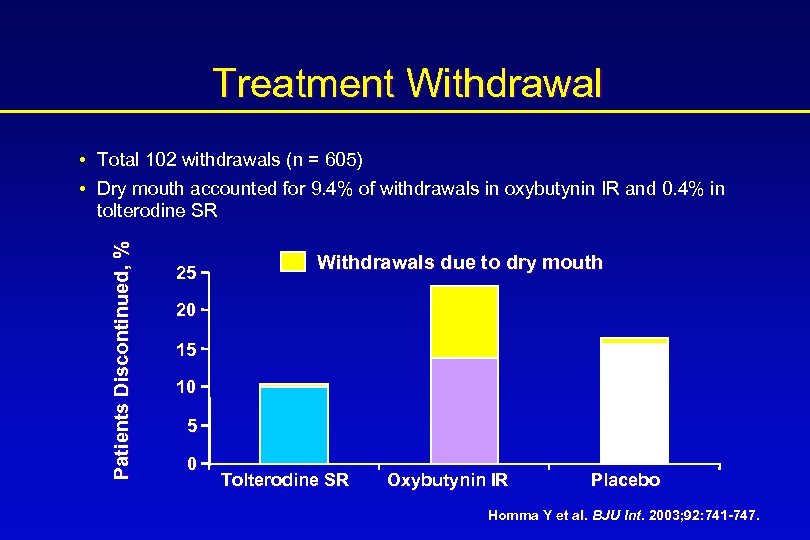
Treatment Withdrawal Patients Discontinued, % • Total 102 withdrawals (n = 605) • Dry mouth accounted for 9. 4% of withdrawals in oxybutynin IR and 0. 4% in tolterodine SR 25 Withdrawals due to dry mouth 20 15 10 5 0 Tolterodine SR Oxybutynin IR Placebo Homma Y et al. BJU Int. 2003; 92: 741 -747.

Japanese and Korean Study: Conclusions • Tolterodine SR has similar efficacy but is better tolerated than oxybutynin IR in Japanese and Korean patients with OAB • Improved tolerability of tolterodine SR resulted in fewer discontinuations of therapy compared with oxybutynin IR Homma Y et al. BJU Int. 2003; 92: 741 -747.

MERIT Mixed Incontinence Effectiveness Research Investigating Tolterodine
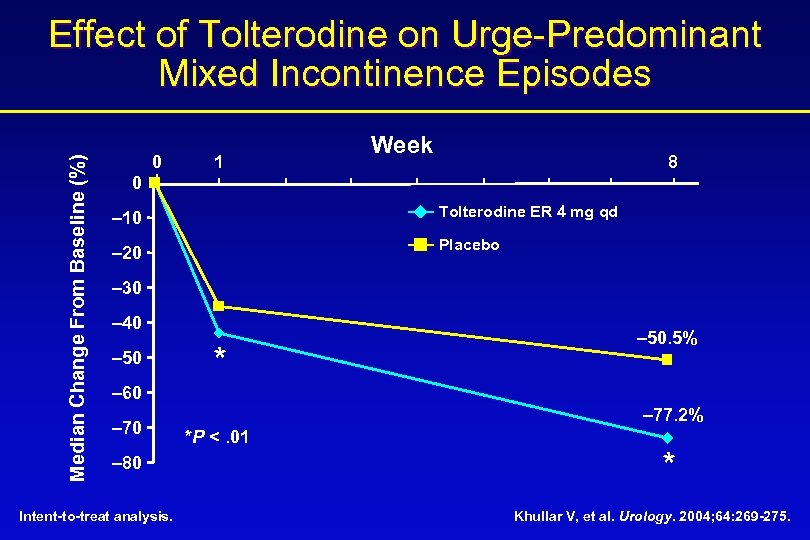
Median Change From Baseline (%) Effect of Tolterodine on Urge-Predominant Mixed Incontinence Episodes 0 1 Week 8 0 – 10 Tolterodine ER 4 mg qd – 20 Placebo – 30 – 40 – 50 * – 50. 5% – 60 – 70 – 80 Intent-to-treat analysis. – 77. 2% *P <. 01 * Khullar V, et al. Urology. 2004; 64: 269 -275.
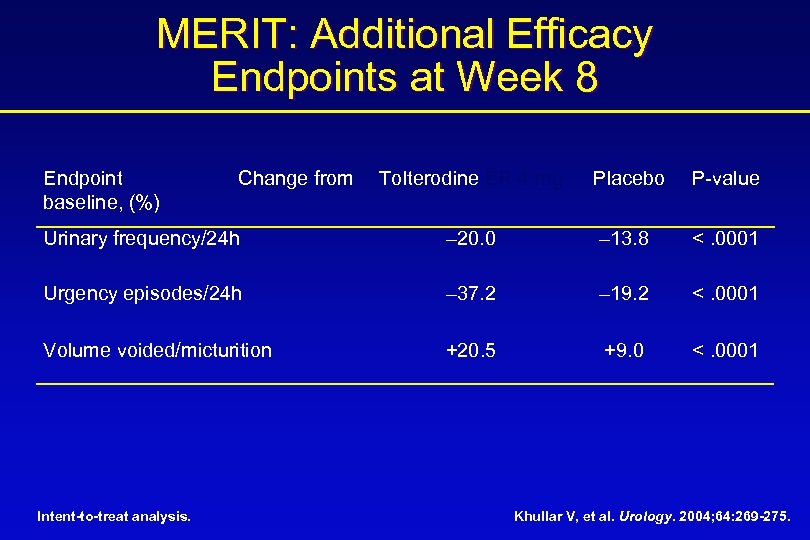
MERIT: Additional Efficacy Endpoints at Week 8 Endpoint baseline, (%) Tolterodine ER 4 mg Placebo P-value Urinary frequency/24 h – 20. 0 – 13. 8 <. 0001 Urgency episodes/24 h – 37. 2 – 19. 2 <. 0001 Volume voided/micturition +20. 5 +9. 0 <. 0001 Intent-to-treat analysis. Change from Khullar V, et al. Urology. 2004; 64: 269 -275.
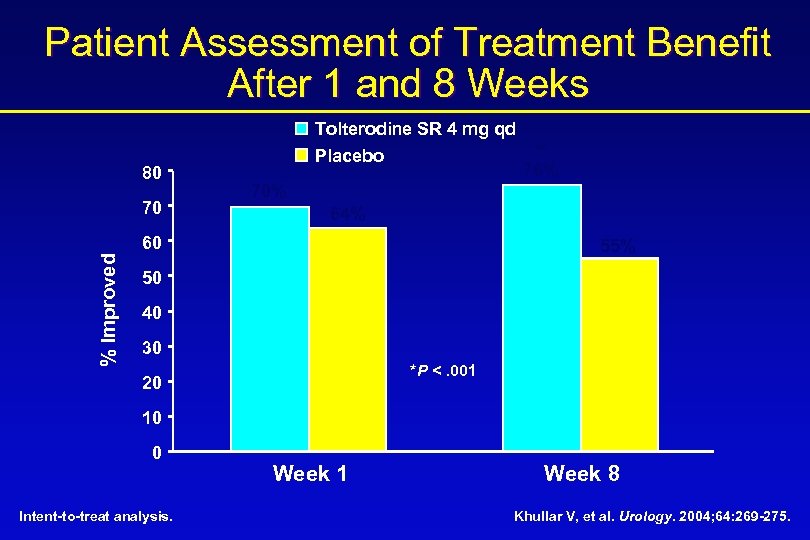
Patient Assessment of Treatment Benefit After 1 and 8 Weeks 80 70 Tolterodine SR 4 mg qd Placebo 70% 64% 60 % Improved * 76% 55% 50 40 30 *P <. 001 20 10 0 Intent-to-treat analysis. Week 1 Week 8 Khullar V, et al. Urology. 2004; 64: 269 -275.
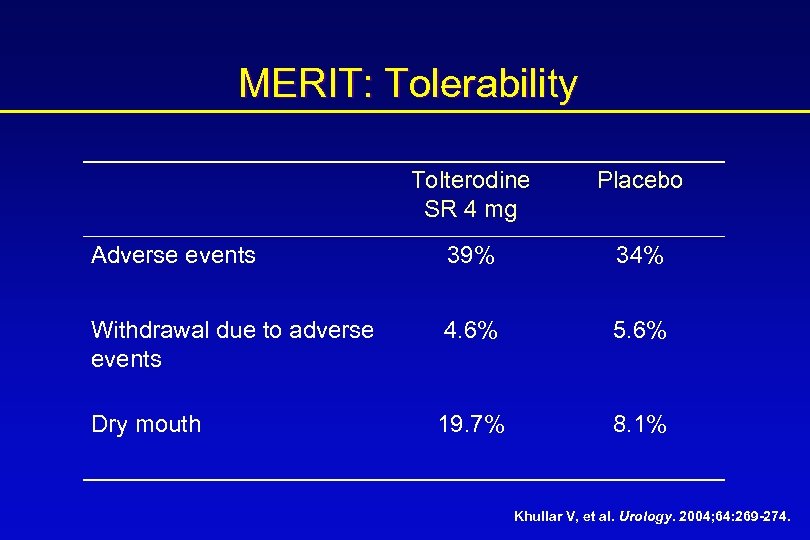
MERIT: Tolerability Tolterodine SR 4 mg Placebo Adverse events 39% 34% Withdrawal due to adverse events 4. 6% 5. 6% Dry mouth 19. 7% 8. 1% Khullar V, et al. Urology. 2004; 64: 269 -274.
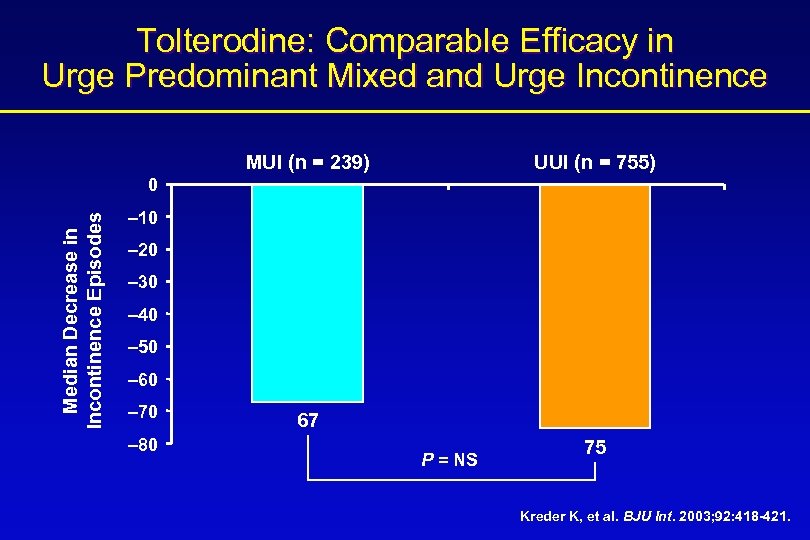
Tolterodine: Comparable Efficacy in Urge Predominant Mixed and Urge Incontinence MUI (n = 239) UUI (n = 755) Median Decrease in Incontinence Episodes 0 – 10 – 20 – 30 – 40 – 50 – 60 – 70 – 80 67 P = NS 75 Kreder K, et al. BJU Int. 2003; 92: 418 -421.
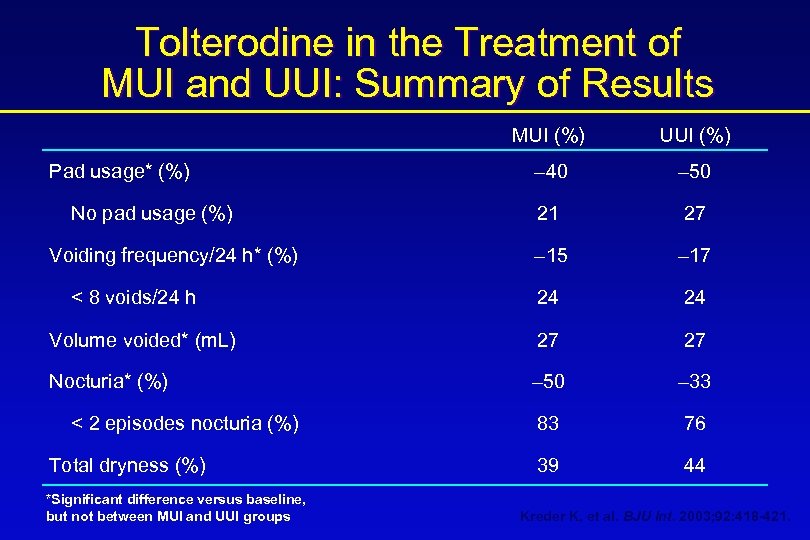
Tolterodine in the Treatment of MUI and UUI: Summary of Results MUI (%) UUI (%) – 40 – 50 21 27 – 15 – 17 24 24 Volume voided* (m. L) 27 27 Nocturia* (%) – 50 – 33 83 76 39 44 Pad usage* (%) No pad usage (%) Voiding frequency/24 h* (%) < 8 voids/24 h < 2 episodes nocturia (%) Total dryness (%) *Significant difference versus baseline, but not between MUI and UUI groups Kreder K, et al. BJU Int. 2003; 92: 418 -421.
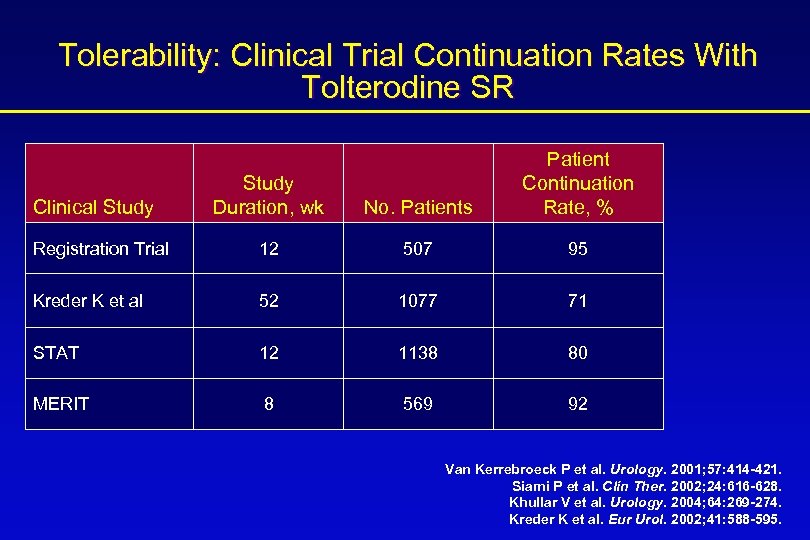
Tolerability: Clinical Trial Continuation Rates With Tolterodine SR Study Duration, wk No. Patients Patient Continuation Rate, % Registration Trial 12 507 95 Kreder K et al 52 1077 71 STAT 12 1138 80 MERIT 8 569 92 Clinical Study Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421. Siami P et al. Clin Ther. 2002; 24: 616 -628. Khullar V et al. Urology. 2004; 64: 269 -274. Kreder K et al. Eur Urol. 2002; 41: 588 -595.
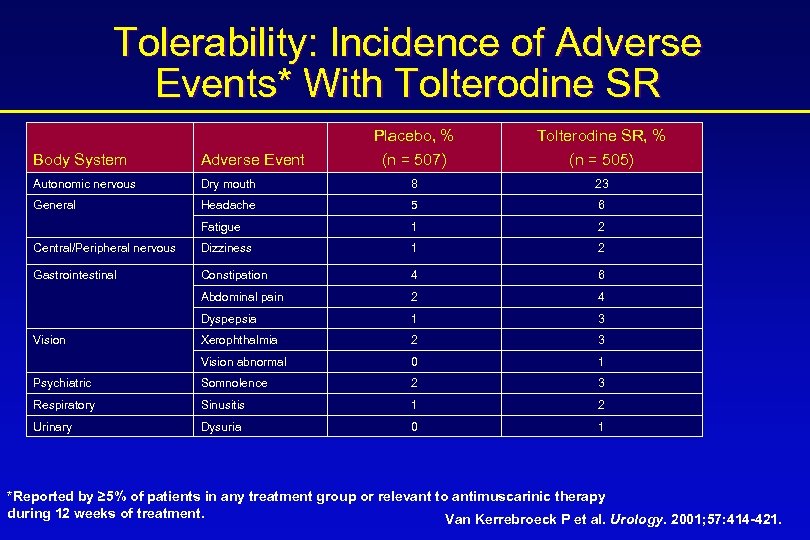
Tolerability: Incidence of Adverse Events* With Tolterodine SR Placebo, % (n = 507) Tolterodine SR, % (n = 505) Body System Adverse Event Autonomic nervous Dry mouth 8 23 General Headache 5 6 Fatigue 1 2 Central/Peripheral nervous Dizziness 1 2 Gastrointestinal Constipation 4 6 Abdominal pain 2 4 Dyspepsia 1 3 Xerophthalmia 2 3 Vision abnormal 0 1 Psychiatric Somnolence 2 3 Respiratory Sinusitis 1 2 Urinary Dysuria 0 1 Vision *Reported by ≥ 5% of patients in any treatment group or relevant to antimuscarinic therapy during 12 weeks of treatment. Van Kerrebroeck P et al. Urology. 2001; 57: 414 -421.

Summary • • OAB is a highly prevalent condition As our population ages, rates will increase OAB has a large impact on our patient’s quality of life Tolterodine SR is an effective therapy shown to significantly reduce incontinence episodes, reduce urgency and frequency, increase void volume, and reduce pad usage • The efficacy of tolterodine SR can be seen as early as 1 week and the effect is maintained with long-term therapy • Patients perceive benefit with therapy and improvements in quality of life have been demonstrated

2686c7587df4002b4dbd2912d8eb812d.ppt