62ebca33af4433696311f963775d867f.ppt
- Количество слайдов: 98

Pharmacology By Prof. Dr. Amany Ibrahim El Brairy Professor of Pharmacology H 107

Why we study Pharmacology? n Drug information : knowledge, skills, and attitude n Rationale and safe prescribing in dental practice throughout the dental graduate career n Self and continuous learner
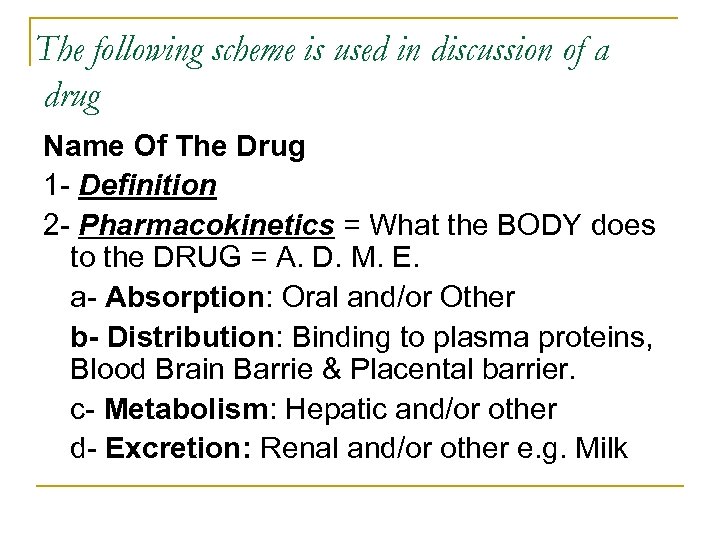
The following scheme is used in discussion of a drug Name Of The Drug 1 Definition 2 Pharmacokinetics = What the BODY does to the DRUG = A. D. M. E. a Absorption: Oral and/or Other b- Distribution: Binding to plasma proteins, Blood Brain Barrie & Placental barrier. c Metabolism: Hepatic and/or other d Excretion: Renal and/or other e. g. Milk

3 Pharmacodynamics = What the DRUG does to the BODY a Mechanism of action b Pharmacological actions: Desirable = Therapeutic effects = Uses Undesirable = Adverse effects = Side effects and toxicity 4 Pharmacotherapeutics: a Therapeutic uses = Indications b Dosage 5 Side effects and toxicity: a Manifestations b Management 6 Contraindications 7 Drug interactions.

Types of drug names Chemical: e. g. acetyl salicylic acid. Generic (scientific): e. g. aspirin. Commercial (Trade): e. g. Rivo, Aspocid, Rhonal. . etc.

PHARMACOKINETIC What the Body dose to the Drug? Absorption, Distribution, Metabolism , Excretion ADME
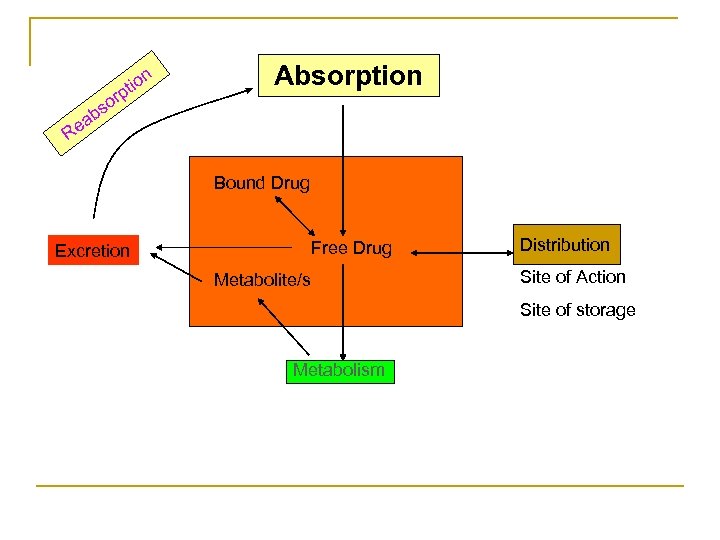
n tio p or s Absorption b ea R Bound Drug Excretion Free Drug Metabolite/s Distribution Site of Action Site of storage Metabolism

The study of pharmacokinetics is important to: 1 Design a proper dosage schedule (dose, route, frequency of administration) 2 Determine the drug’s bioavailability.
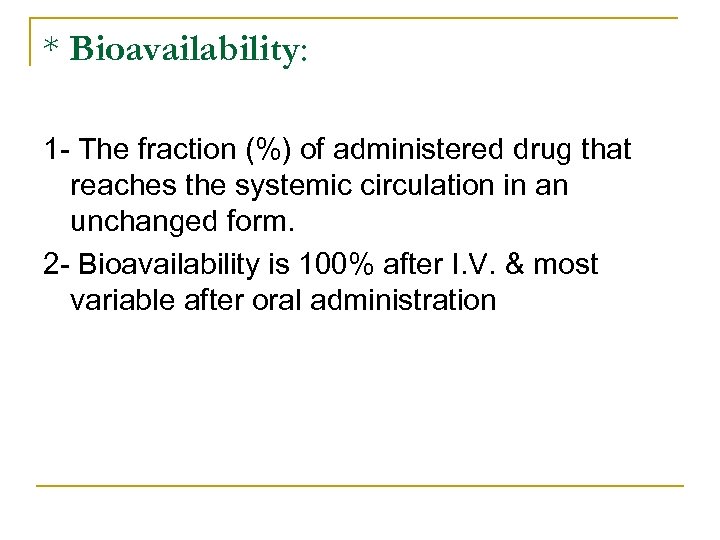
* Bioavailability: 1 The fraction (%) of administered drug that reaches the systemic circulation in an unchanged form. 2 Bioavailability is 100% after I. V. & most variable after oral administration
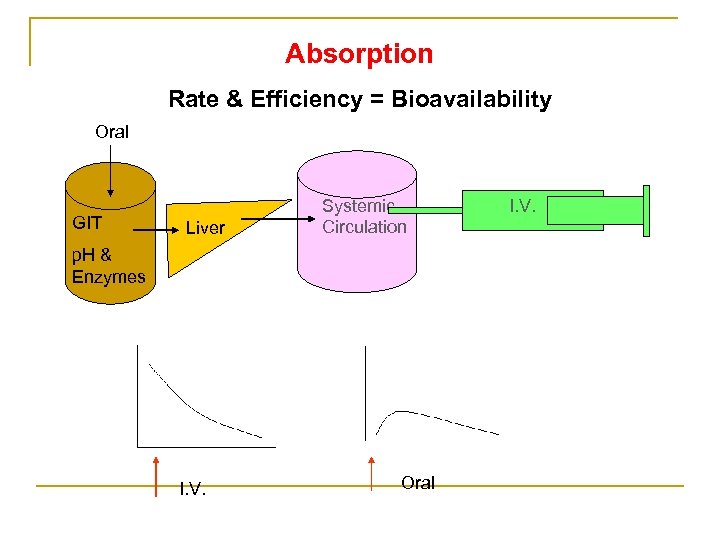
Absorption Rate & Efficiency = Bioavailability Oral GIT Liver Systemic Circulation p. H & Enzymes I. V. Oral I. V.
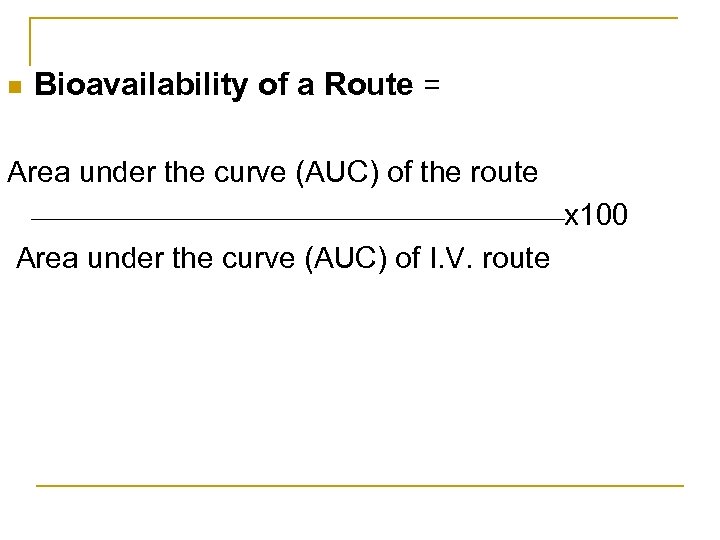
n Bioavailability of a Route = Area under the curve (AUC) of the route x 100 Area under the curve (AUC) of I. V. route
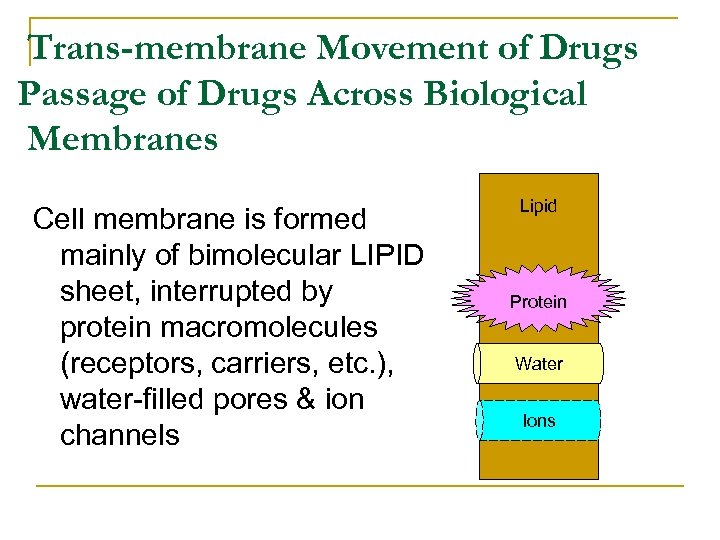
Trans-membrane Movement of Drugs Passage of Drugs Across Biological Membranes Cell membrane is formed mainly of bimolecular LIPID sheet, interrupted by protein macromolecules (receptors, carriers, etc. ), water filled pores & ion channels Lipid Protein Water Ions
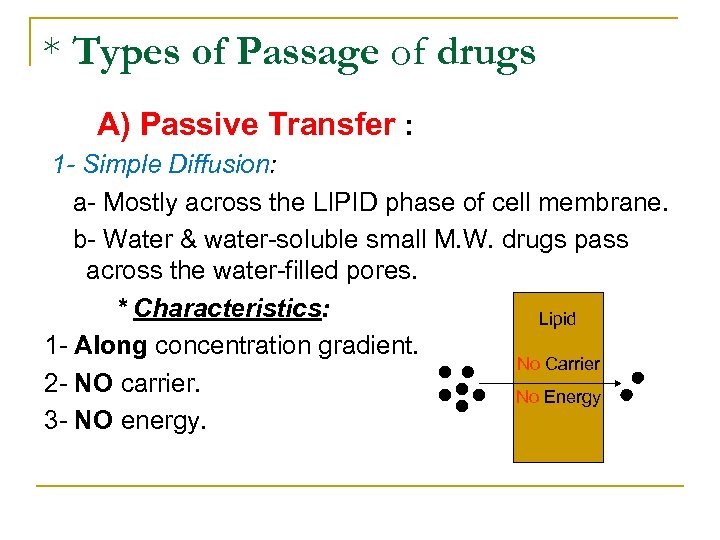
* Types of Passage of drugs A) Passive Transfer : 1 - Simple Diffusion: a Mostly across the LIPID phase of cell membrane. b Water & water soluble small M. W. drugs pass across the water filled pores. * Characteristics: Lipid 1 Along concentration gradient. No Carrier 2 NO carrier. No Energy 3 NO energy.

Factors & Forces: 1 -Gradient (Concentration of drugs ) Higher gradient = Higher rate of passage across the membrane. 2 -Molecular weight & size: The smaller is the faster.
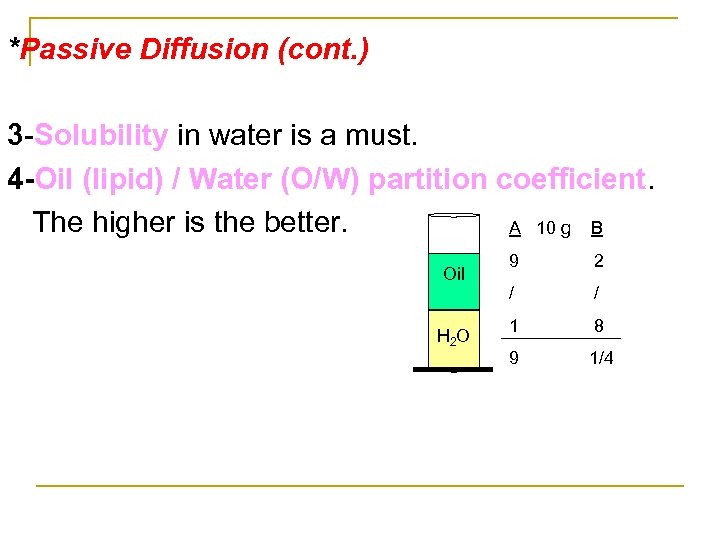
*Passive Diffusion (cont. ) 3 Solubility in water is a must. 4 -Oil (lipid) / Water (O/W) partition coefficient. The higher is the better. A 10 g B Oil H 2 O 9 2 / / 1 8 9 1/4

*Passive Diffusion (cont. ) 5 -Ionization: n It depends upon p. H of the medium & p. Ka of the drug (p. H at which 50% of drug is ionized) n Low Ionization = High lipid solubility = Better passage. n Drugs are non ionized when they are present in a similar medium (Acidic drugs in acid medium and basic drugs in alkaline medium).
*Effect of p. H on Oral Absorption & Renal Excretion of Drugs: For weak base and acid drugs: a The unionized (non polar) form is lipid soluble and easily absorbed. b Ionized (polar) form of drugs is lipid insoluble and not easily absorbed but easily excreted. n Weak acid drugs are more unionized in acid & more ionized in alkaline media. n Weak base drugs are more unionized in alkaline & more ionized in acid media.

n n n When drugs are present in a reverse medium → Ionized → not lipid soluble → not absorbed → Excreted. Aspirin is better absorbed in acid medium e. g. Stomach. Alkalinization of urine by sodium acetate, or citrate its urinary excretion. Ephedrine is better absorbed in alkaline medium e. g. Intestine. Acidification of urine by ammonium chloride its urinary excretion.
2 - Filtration: Passage of drugs through Capillary endothelium & Glomeruli * Characteristics: n Along hydrostatic and osmotic gradients Circulation n. No carrier n. No energy
2 - Filtration (cont. ) * Factors & Forces: 1 Molecular weight 2 Not bound to plasma proteins 3 Hydrostatic and osmotic gradients 4 Blood flow
B) Special Transfer: 1 Facilitated Diffusion: Along concentration gradient Needs Carrier Site for Saturation No energy Example: Glucose uptake Carrier No Energy
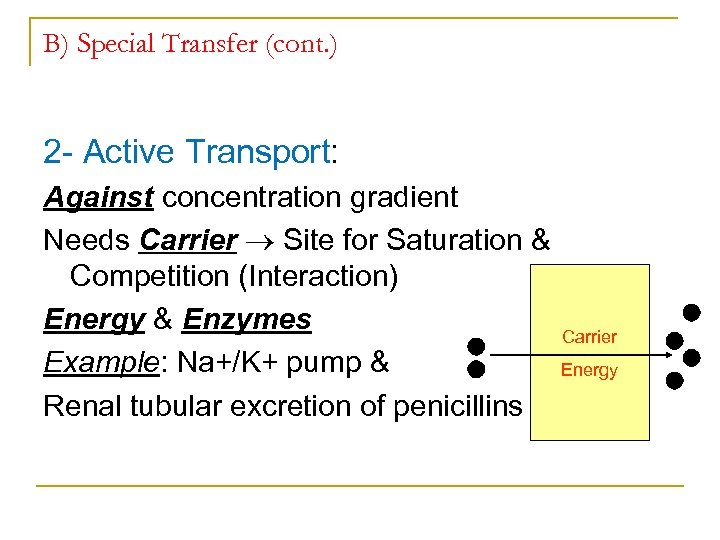
B) Special Transfer (cont. ) 2 Active Transport: Against concentration gradient Needs Carrier Site for Saturation & Competition (Interaction) Energy & Enzymes Example: Na+/K+ pump & Renal tubular excretion of penicillins Carrier Energy
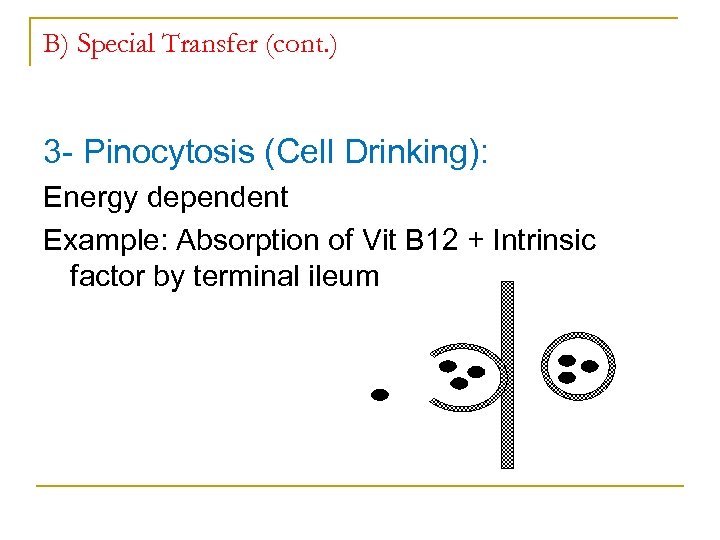
B) Special Transfer (cont. ) 3 Pinocytosis (Cell Drinking): Energy dependent Example: Absorption of Vit B 12 + Intrinsic factor by terminal ileum
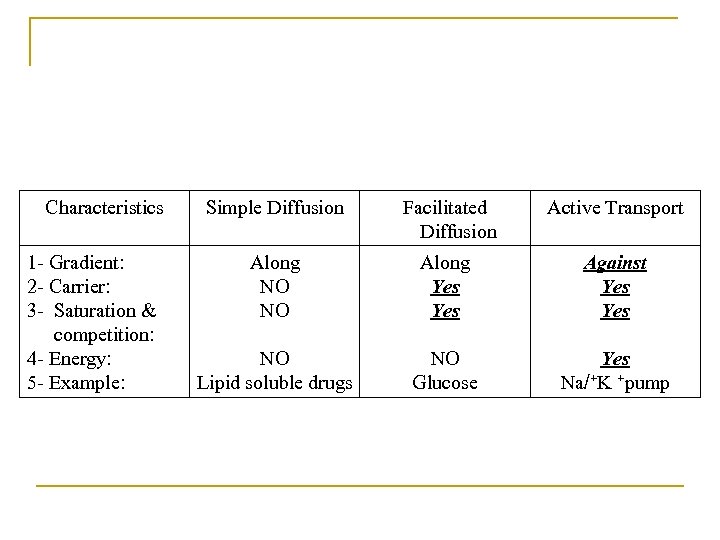
Characteristics 1 - Gradient: 2 - Carrier: 3 - Saturation & competition: 4 - Energy: 5 - Example: Simple Diffusion Facilitated Diffusion Active Transport Along NO NO Along Yes Against Yes NO Lipid soluble drugs NO Glucose Yes Na/+K +pump

I- Absorption n Transfer of drugs from their site of administration to the systemic circulation
*Factors Affecting Absorption: A) Factors Related to the Patient 1 Route of Administration: I. V. > I. M. > S. C. > Oral > Skin 2 Absorbing Surface: a Vascularity: Alveoli > Skeletal muscle > Subcutaneous b Surface area: Alveoli > Intestine > Stomach c State of health: Diarrhea & mal absorption inhibit Oral absorption

3 Systemic circulation: Shock & Heart failure Absorption 4 Specific factors: Intrinsic factor for Vit B 12 5 -Presence of other drugs & food: n a Adrenaline S. C. V. C. Absorption of Local anesthetics Long duration of action n b Milk (Calcium) Oral absorption of Tetracyclines (Antibiotic)

B (Factors Related to the Drug: 1 Water and lipid solubility: Drugs MUST be Water soluble as well as Lipid soluble. Drugs must be completely dissolved in water to be absorbed. Drugs insoluble in water e. g. Barium chloride (Ba. Cl 2) are NOT absorbed. More lipid solubility High Lipid/Water partition coefficient Better absorption

2 -Ionization : Non ionized drugs are more lipid soluble Better absorption Depends on p. Ka of the drug & p. H of the medium. Tertiary amines Non ionized Better absorption. Streptomycin has high p. Ka Always ionized Not absorbed Sulfaguanidine Not ionized yet not lipid soluble Poor absorption

3 Valency: Ferrous iron (Fe 2+) is better absorbed than Ferric Iron (Fe 3+). 4 Nature: Inorganic (small molecules) > Organic (Big molecules) 5 Pharmaceutical Preparation: a Dosage form: Solution > Suspension >Tablet b Shape & size of particles and rates of disintegration & dissolution of tables: Rapid with paracetamol & propranolol Slow with digoxin c Excepient (Filler)e. g. Ca. CO 3 & Ca Phosphate Absorption of Tetracyclines.
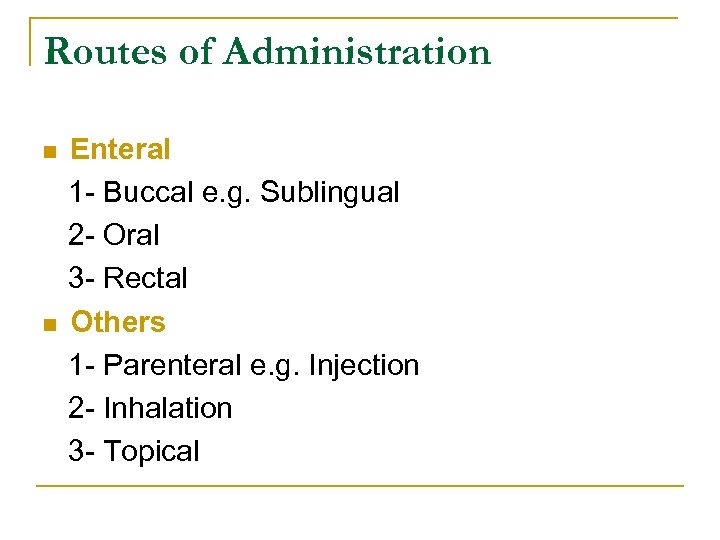
Routes of Administration n n Enteral 1 Buccal e. g. Sublingual 2 Oral 3 Rectal Others 1 Parenteral e. g. Injection 2 Inhalation 3 Topical

Effect Of Administered Drug: 1 Systemic (General): If drug is absorbed and distributed 2 Local (Topical): If drug is not absorbed nor distributed
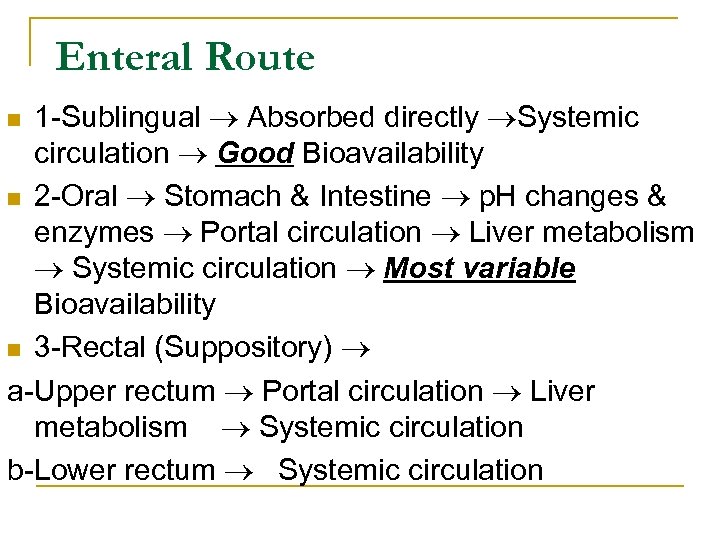
Enteral Route 1 Sublingual Absorbed directly Systemic circulation Good Bioavailability n 2 Oral Stomach & Intestine p. H changes & enzymes Portal circulation Liver metabolism Systemic circulation Most variable Bioavailability n 3 Rectal (Suppository) a Upper rectum Portal circulation Liver metabolism Systemic circulation b Lower rectum Systemic circulation n

1 - Oral Route Characteristics: 1 Suitable: a Small amount or volume b Palatable: If bad taste Dilute with milk or fruit juice Use sugar coated or effervescent form b Non irritant: If Mild irritant Take after meal Use enteric coated form: Covered with acid resistant coat

Advantages: Convenient (Safe, easy & economic) Disadvantages: 1 NOT in emergency Delayed onset 2 NOT in uncooperative patients e. g. coma, insane or very young 3 NOT in vomiting or severe diarrhea 4 NOT in very irritant drugs e. g. Emetine HCl 5 NOT in unabsorbed drugs when systemic effect is wanted 6 NOT for drugs with extensive First Pass Effect (Metabolism): a p. H changes: Benzyl penicillin is destroyed by gastric acidity b Digestive enzymes: Insulin c Hepatic enzymes: Nitroglycerin
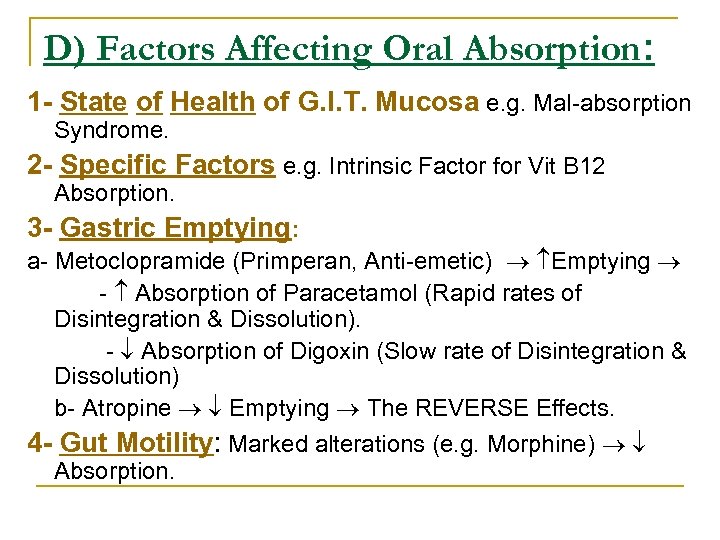
D) Factors Affecting Oral Absorption: 1 - State of Health of G. I. T. Mucosa e. g. Mal absorption Syndrome. 2 - Specific Factors e. g. Intrinsic Factor for Vit B 12 Absorption. 3 - Gastric Emptying: a Metoclopramide (Primperan, Anti emetic) Emptying Absorption of Paracetamol (Rapid rates of Disintegration & Dissolution). Absorption of Digoxin (Slow rate of Disintegration & Dissolution) b Atropine Emptying The REVERSE Effects. 4 - Gut Motility: Marked alterations (e. g. Morphine) Absorption.

5 - p. H a Gastric Acidity Absorption of Salicylates & Barbiturates b Intestinal Alkalinity Absorption of Ephedrine & Amphetamine. 6 - Presence of FOOD & Other DRUGS: a Bad Food dilutes Drugs & may compete with them for absorption e. g. amino acids compete for the same carrier of L DOPA b Good with IRRITANT drugs e. g. aspirin & iron. c Milk (Ca 2+) & Anti acids Interfere with Tetracycline absorption. d Tea (Tannic Acid) & Tetracycline Iron absorption. e Cholestyramine & Activated Charcoal Absorption of Most Drugs.

7 - First Pass Effect (Pre-Systemic Metabolism): Bioavailability a Gut First Pass Effect: Gastric Acidity: Benzyl Penicillin. Digestive Enzymes: Insulin & Pituitary hormones Mucosal enzymes: Tyramine, L DOPA, Methyldopa &Chlorpromazine Flora: Histamine c Hepatic First Pass Effect: Extensive: Nitroglycerine, Lidocaine & Natural sex hormones. Partial: Propranolol & Morphine. Minimal: Atenolol, Nadolol & Barbitone. d How to OVERCOME Hepatic First Pass Metabolism? Increase the oral dose of the drug e. g. Morphine & Propranolol Use other routes (NOT ORAL) e. g. Sublingual “Nitroglycerine”. 8 - Factors related to the DRUG e. g. Lipid Solubility.

2 -Sublingual (Pellet or Linguat) *Example: Isoprenaline & Nitroglycerine. *Advantages: 1 Easy. 2 Escape gut and hepatic first pass effect Good bioavailability. 3 Rapid onset. 4 Proper control of dose by either spitting or swallowing excess of the drug.
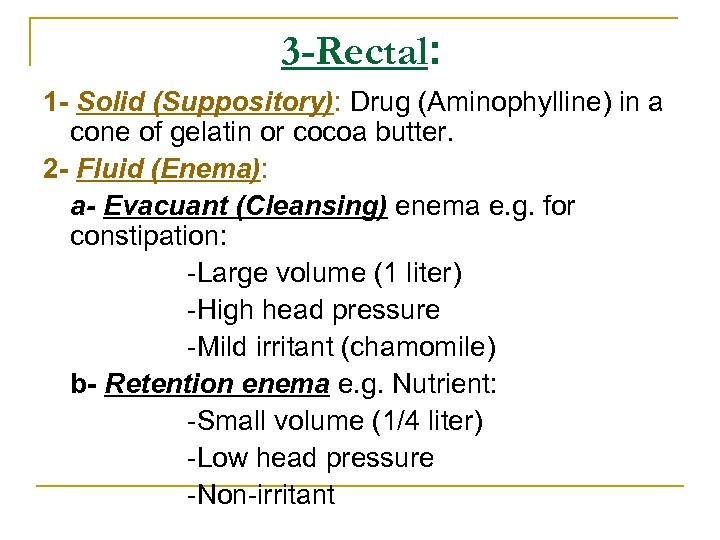
3 -Rectal: 1 - Solid (Suppository): Drug (Aminophylline) in a cone of gelatin or cocoa butter. 2 - Fluid (Enema): a- Evacuant (Cleansing) enema e. g. for constipation: Large volume (1 liter) High head pressure Mild irritant (chamomile) b- Retention enema e. g. Nutrient: Small volume (1/4 liter) Low head pressure Non irritant

B) Advantages: a Escape gut & hepatic first pass effects b Useful in patients with vomiting c Useful in uncooperative patients e. g. coma & young children d Useful in mild irritant drugs e. g. aspirin and aminophylline e Useful in large volume drugs
Parenteral Routes All drugs must be STERILE and PYROGEN-FREE A)Subcutaneous Pellet Implantation: Sterile pellet under the skin Fibrosis Slow absorption Long duration e. g. some hormones (Contraceptives). B)Intradermal Injection (I. D. ): e. g. Sensitivity tests & Vaccinations.

C)Subcutaneous Injection (S. C. ): 1 -Drugs should be: a Non irritant (If irritant or oily Inflammation) b Aqueous Solution or fine suspension. 2 -Absorption can by Enhanced by: a Use a solution b Massage of injection area c Application of heat d Add hyaluronidase enzyme 3 -Absorption can be Slowed by: a Use a suspension b Application of cold c Add adrenaline (V. C. ) to local anesthetics d Add gelatin to heparin
D) Intramuscular (I. M. ): 1 Drugs can be: Solution, suspension, oily, non irritant or mild irritant. 2 Better absorption than S. C. 3 Some drugs (Diazepam & Phenytoin) Bound to muscle proteins Irregular absorption.
E) Intravenous (I. V. ): Either SLOW bolus injection or Infusion (Drip) method. n Water solution ONLY. n Advantages: Useful in Emergencies a 100% bioavailability b Immediate onset c High plasma concentration d Useful for Irritant & Large volume drugs n
Disadvantages: MOST DANGEROUS ROUTE a If Allergy Anaphylactic shock b If Very Irritant Thrombophlebitis c If Extravasation of irritant drug Severe pain and inflammation d If Rapid I. V. Velocity reaction Cardiac problems (Aminophylline) e Pyrogenic reaction by phospho lipo protein of microorganisms f Transmission of diseases e. g. Viral Hepatitis C & AIDS. n
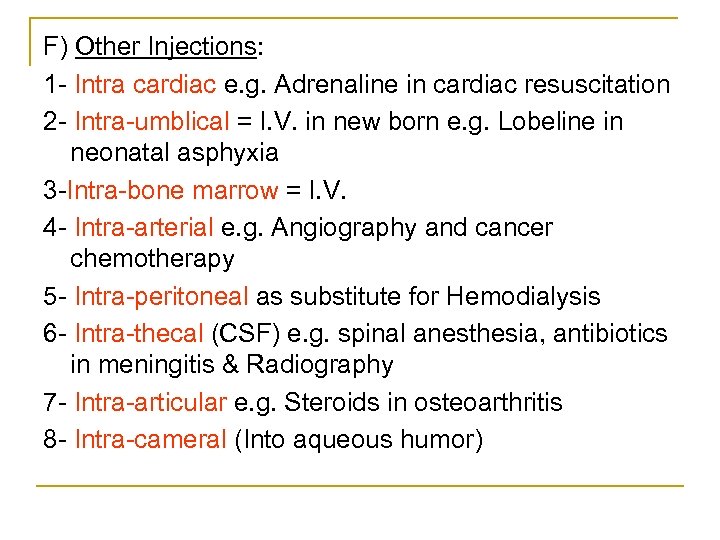
F) Other Injections: 1 Intra cardiac e. g. Adrenaline in cardiac resuscitation 2 Intra umblical = I. V. in new born e. g. Lobeline in neonatal asphyxia 3 Intra bone marrow = I. V. 4 Intra arterial e. g. Angiography and cancer chemotherapy 5 Intra peritoneal as substitute for Hemodialysis 6 Intra thecal (CSF) e. g. spinal anesthesia, antibiotics in meningitis & Radiography 7 Intra articular e. g. Steroids in osteoarthritis 8 Intra cameral (Into aqueous humor)
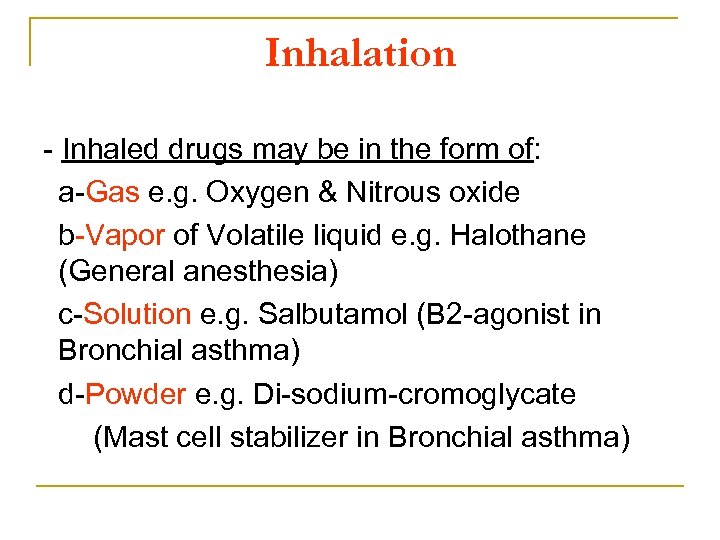
Inhalation Inhaled drugs may be in the form of: a Gas e. g. Oxygen & Nitrous oxide b Vapor of Volatile liquid e. g. Halothane (General anesthesia) c Solution e. g. Salbutamol (B 2 agonist in Bronchial asthma) d Powder e. g. Di sodium cromoglycate (Mast cell stabilizer in Bronchial asthma)

Excellent absorption because of: a Wide surface area b High vascularity c Thin porous membrane of the alveoli
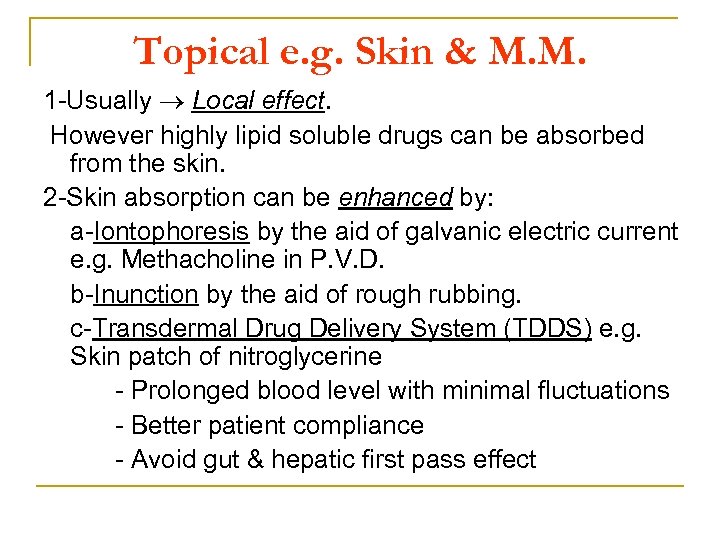
Topical e. g. Skin & M. M. 1 Usually Local effect. However highly lipid soluble drugs can be absorbed from the skin. 2 Skin absorption can be enhanced by: a Iontophoresis by the aid of galvanic electric current e. g. Methacholine in P. V. D. b Inunction by the aid of rough rubbing. c Transdermal Drug Delivery System (TDDS) e. g. Skin patch of nitroglycerine Prolonged blood level with minimal fluctuations Better patient compliance Avoid gut & hepatic first pass effect

3 Usually skin absorption is not wanted and harmful: a Estrogen hormone in females Cancer breast. b Cortisone in infants Moon face. c Insecticides Toxicity.
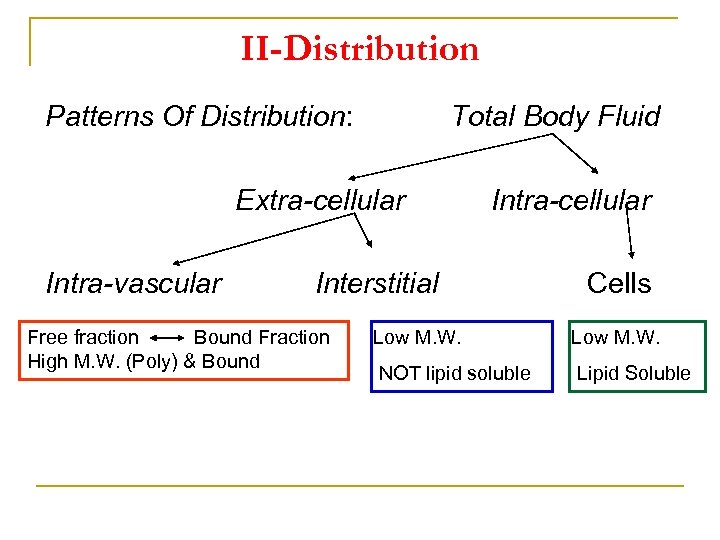
II-Distribution Patterns Of Distribution: Total Body Fluid Extra-cellular Intra-vascular Intra-cellular Interstitial Free fraction Bound Fraction High M. W. (Poly) & Bound Cells Low M. W. NOT lipid soluble Lipid Soluble
A) Binding To Plasma Proteins: A fraction of Most drugs binds Reversibly to plasma proteins Mainly albumin. The Bound fraction of the drug n NOT Active n NOT Filtered n NOT Metabolized n NOT Excreted Depot Form. n More binding = More Depot = Longer duration. n

The Free fraction of the drug n Active, n Metabolized n Excreted. n There equilibrium between the bound & the free fractions of the drug.
Drugs extensively bound to plasma proteins e. g. Thiopentone (I. V. Anesthesia) have to be injected rather Rapidly I. V. n Drugs have specific binding sites on plasma proteins = Non functioning receptors Site for competition & drug interactions. n
Site for Drug Interactions: n Aspirin (NSAID) & Sulfa drugs displace: 1 Oral Anti coagulants e. g. Warfarin Hemorrhage. 2 Oral Hypoglycemics e. g. Tolbutamide Hypoglycemia. 3 Bilirubin in neonates Jaundice & Kernictrus. n
B) Patterns Of Distribution: 1 Intra vascular (Single Compartment): Drug is retained in the blood compartment. Drugs that can NOT filtrate through capillary endothelium. Examples High MW > 500 e. g. Polypeptides (Plasma proteins & Drugs bound to plasma proteins) & Polysaccharides (Heparin & Dextrans).
2 Extra cellular (Two compartments = Intra vascular + Interstitial): Drugs that can filtrate (Small MW) but can NOT pass cell membrane (Not lipid soluble). Ionized form of drugs (Neostigmine), Mannitol, Na+, Cl & SO 4.
3 All over the body (Multi compartment = Intra + Extra cellular): Drugs that can filtrate (Small MW) & Can pass cell membrane (Lipid soluble). Non ionized form of drugd (Physostigmine), Alcohol, Aspirin & Barbiturates.

4 Tissue Reservoirs: a Hair: Arsenic b Thyroid: Iodine c Heart: Digitalis d Liver: Vit B 12 & Chloroquine e Fat: Thiopentone f Bone: Ca 2+
5 Blood Brain Barriers: Lipid cellular barrier composed of Brain Capillary Endothelium (Which lacks the water channels) and the adjacent Glial tissue. Only lipid soluble Non ionized drugs can pass B. B. B. along their concentration gradient. Inflammation (Meningitis) increases permeability of B. B. B. Penicillins can pass inflamed meninges but NOT normal ones.
6 Placental Barrier: Lipid cellular barrier composed of Epithelium of Fetal Villi & Capillary endothelium. Rich in enzymatic activity e. g. M. A. O. Drugs that pass placental barrier may cause: n During pregnancy Teratogenicity e. g. Thalidomide & Tetracyclines n During Labor Neonatal asphyxia e. g. Morphine & Barbiturates.
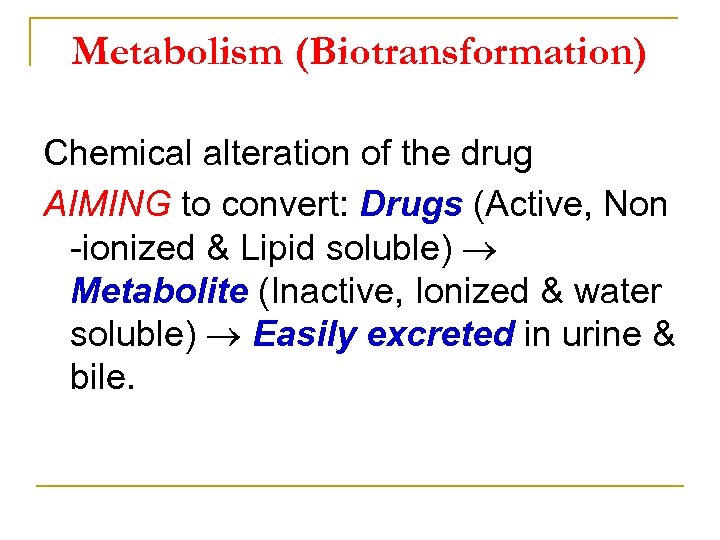
Metabolism (Biotransformation) Chemical alteration of the drug AIMING to convert: Drugs (Active, Non ionized & Lipid soluble) Metabolite (Inactive, Ionized & water soluble) Easily excreted in urine & bile.
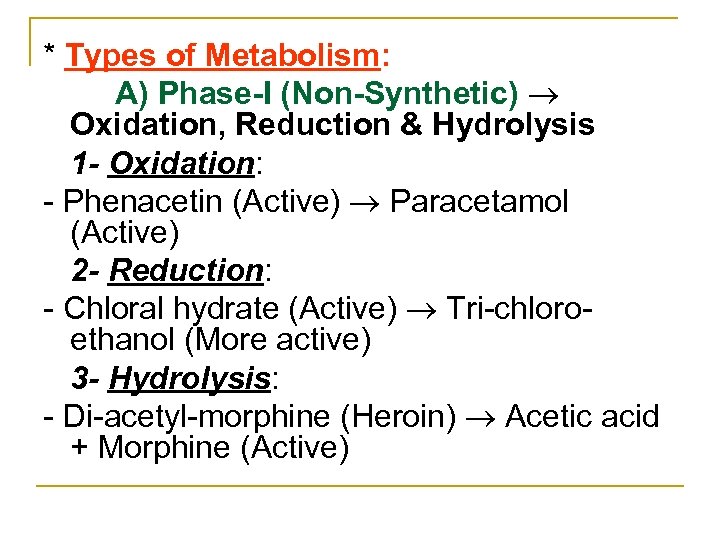
* Types of Metabolism: A) Phase-I (Non-Synthetic) Oxidation, Reduction & Hydrolysis 1 - Oxidation: Phenacetin (Active) Paracetamol (Active) 2 - Reduction: Chloral hydrate (Active) Tri chloro ethanol (More active) 3 - Hydrolysis: Di acetyl morphine (Heroin) Acetic acid + Morphine (Active)
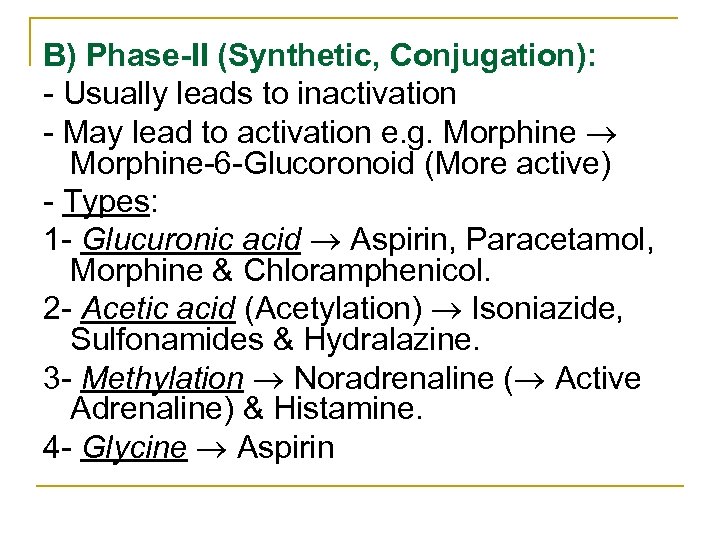
B) Phase-II (Synthetic, Conjugation): Usually leads to inactivation May lead to activation e. g. Morphine 6 Glucoronoid (More active) Types: 1 Glucuronic acid Aspirin, Paracetamol, Morphine & Chloramphenicol. 2 Acetic acid (Acetylation) Isoniazide, Sulfonamides & Hydralazine. 3 Methylation Noradrenaline ( Active Adrenaline) & Histamine. 4 Glycine Aspirin
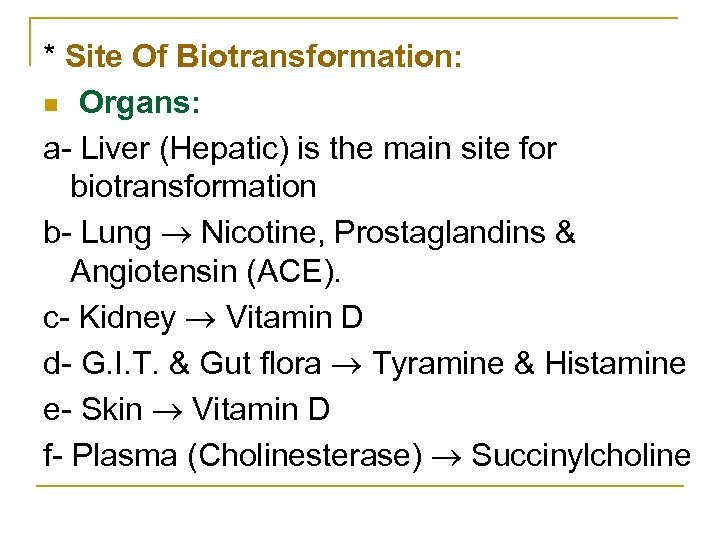
* Site Of Biotransformation: n Organs: a Liver (Hepatic) is the main site for biotransformation b Lung Nicotine, Prostaglandins & Angiotensin (ACE). c Kidney Vitamin D d G. I. T. & Gut flora Tyramine & Histamine e Skin Vitamin D f Plasma (Cholinesterase) Succinylcholine

* Factors Affecting Hepatic Microsomal Enzymes A) Hepatic Microsomal Enzyme Inducers (Activators): Examples: Phenytoin, Carbamazepine, Rifampicin, Testosterone, Cortisol & Tobacco smoking. They Metabolism of other drugs e. g. Oral anti coagulants, Oral hypoglycemics & Oral contraceptives Their duration of action. They Their own metabolism (Auto induction) Tolerance.
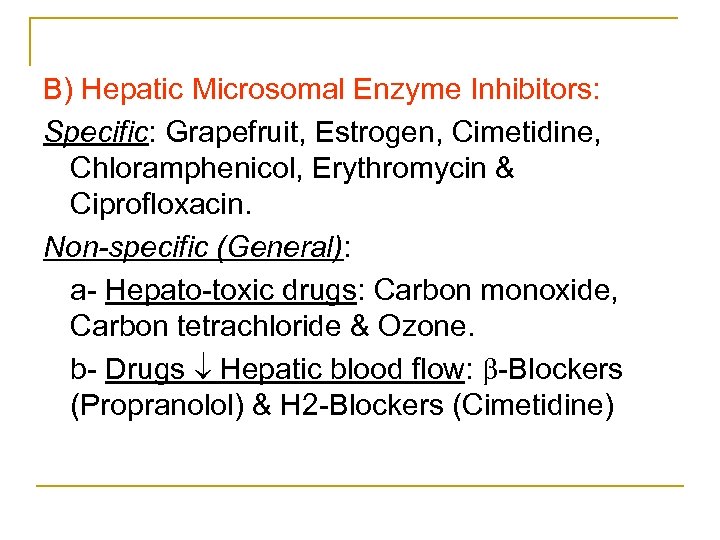
B) Hepatic Microsomal Enzyme Inhibitors: Specific: Grapefruit, Estrogen, Cimetidine, Chloramphenicol, Erythromycin & Ciprofloxacin. Non-specific (General): a Hepato toxic drugs: Carbon monoxide, Carbon tetrachloride & Ozone. b Drugs Hepatic blood flow: Blockers (Propranolol) & H 2 Blockers (Cimetidine)

C) Age: H. M. E. Activity is inhibited in extremities of age. Premature neonate can NOT conjugate chloramphenicol Fatal Grey Baby Syndrome. D) Liver disease, Starvation & Cancer H. M. E. Activity E) Genetic Abnormality (Idiosyncrasy): Favism & Abnormal Pseudo Ch. E.
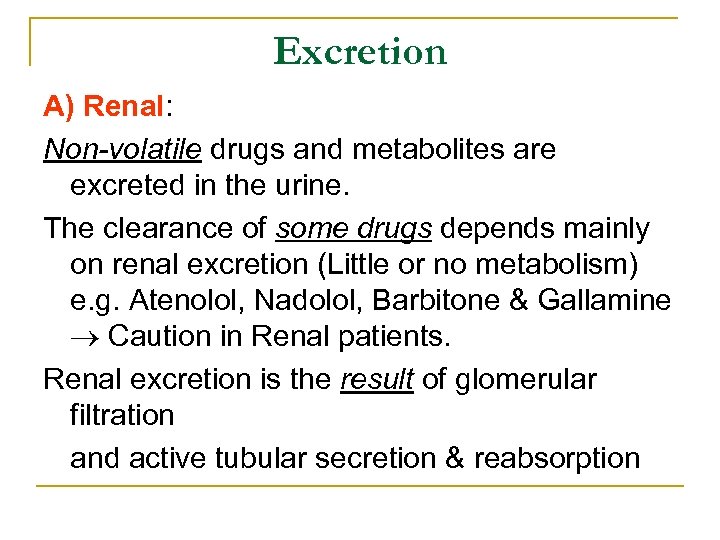
Excretion A) Renal: Non-volatile drugs and metabolites are excreted in the urine. The clearance of some drugs depends mainly on renal excretion (Little or no metabolism) e. g. Atenolol, Nadolol, Barbitone & Gallamine Caution in Renal patients. Renal excretion is the result of glomerular filtration and active tubular secretion & reabsorption

Passive Glomerular filtration for water soluble Non bound drugs with M. W. < 500 e. g. Mannitol. Ø Active Tubular Excretion (Saturable & Site for competition & Drug Interaction): Weak acid drugs e. g. Penicillin, Frusemide, Uric acid & Probenecid. Weak base drugs e. g. Digoxin & Quinidine Ø
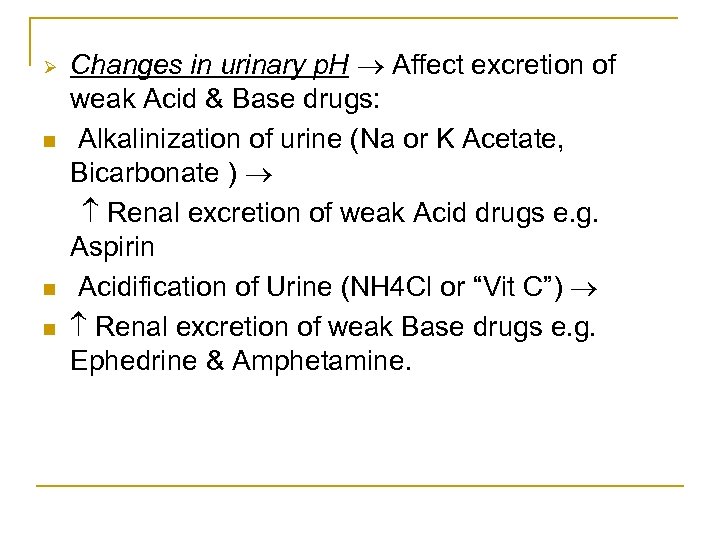
Ø n n n Changes in urinary p. H Affect excretion of weak Acid & Base drugs: Alkalinization of urine (Na or K Acetate, Bicarbonate ) Renal excretion of weak Acid drugs e. g. Aspirin Acidification of Urine (NH 4 Cl or “Vit C”) Renal excretion of weak Base drugs e. g. Ephedrine & Amphetamine.

B) Lung Gases (CO 2) & Volatile Liquids (Halothane) n
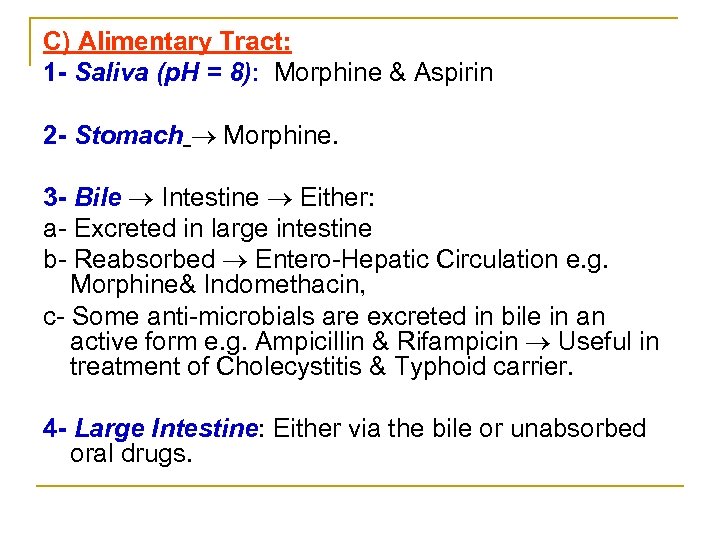
C) Alimentary Tract: 1 - Saliva (p. H = 8): Morphine & Aspirin 2 - Stomach Morphine. 3 - Bile Intestine Either: a Excreted in large intestine b Reabsorbed Entero Hepatic Circulation e. g. Morphine& Indomethacin, c Some anti microbials are excreted in bile in an active form e. g. Ampicillin & Rifampicin Useful in treatment of Cholecystitis & Typhoid carrier. 4 - Large Intestine: Either via the bile or unabsorbed oral drugs.

D) Skin Glands: 1 - Sweat Vit B 1, Hg, As & Rifampicin Red discoloration of sweat. 2 - Milk May affect suckling baby e. g. Morphine, nicotine, Purgatives, Tetracyclines & Chloramphenicol.
PHARMACODYNAMICS (What the DRUG does to the BODY ) n n This science deals with Mechanism & pharmacological Actions of drugs. Drugs are chemical substances that modify increase or decease already present cell function but do not create a new one. However, genetic engineering and gene therapy may change this concept.

* Types of Drug Action: 1 - Local or Topical Action: NO Absorption from site of administration NO Distribution NO Systemic actions. The drug acts at site of application. Examples: Most of eye & ear drops, intra articular injections & skin ointment.

2 - Systemic or General Action: The drug is absorbed and distributed from site of administration. Examples: Oral aspirin, Subcutaneous (SC) adrenaline & Sublingual (SL) isoprenaline. 3 - Reflex or Remote Action: The drug acts at a site to provoke an effect away from its site of action. Examples : SC Camphor Irritation Reflex Respiratory center = Reflex Analeptic.

* Mechanism of Drug Action: 1 - Physical: a Adsorption: Kaolin & Activated charcoal in diarrhea. b Osmotic: Mg. SO 4 as a purgative. c Demulcent: Liquorice as an anti tussive. d Astringent: Tannic acid mouth wash in gingivitis 2 - Chemical: a Neutralization: Na. HCO 3 (Antacid) + HCl in treatment of hyperacidity. b Chelation: Organic compound + Heavy metal Non toxic easy excreted complex. Dimercaprol (British Anti Lewisite) for Mercury (Hg)

3 - Interference with Cell Division: Anti cancer drugs e. g. Nitrogen mustard. 4 - Interference with Metabolic Pathway: Sulfonamides compete with PABA in bacteria Synthesis of folic acid. 5 - Inhibition of Enzymes: Physostigmine ( Cholinesterase) & Aspirin ( Cyclooxygenase, COX).

6 - Action on Ion Channel: Local anesthetics block Sodium (Na+) channels. Calcium channel blockers (CCB) e. g. Verapamil block L type of voltage gated calcium channels of heart & blood vessels. Some pharmacologists consider ion channels as an especial type of receptors.
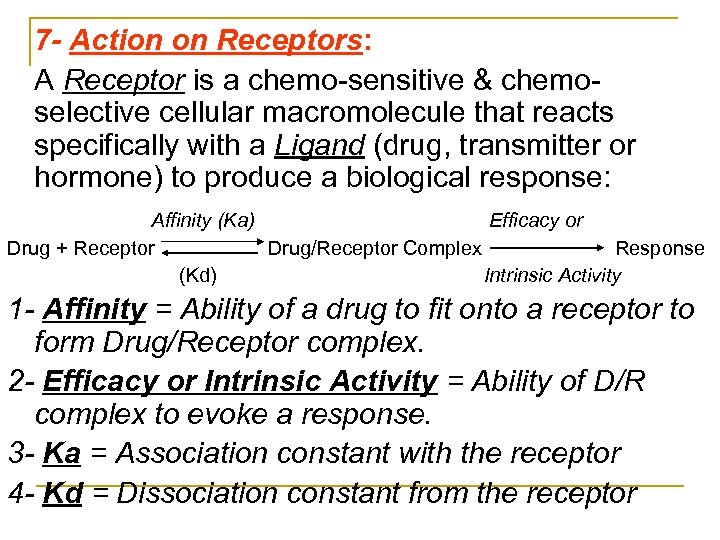
7 - Action on Receptors: A Receptor is a chemo sensitive & chemo selective cellular macromolecule that reacts specifically with a Ligand (drug, transmitter or hormone) to produce a biological response: Affinity (Ka) Efficacy or Drug + Receptor Drug/Receptor Complex Response (Kd) Intrinsic Activity 1 - Affinity = Ability of a drug to fit onto a receptor to form Drug/Receptor complex. 2 - Efficacy or Intrinsic Activity = Ability of D/R complex to evoke a response. 3 - Ka = Association constant with the receptor 4 - Kd = Dissociation constant from the receptor
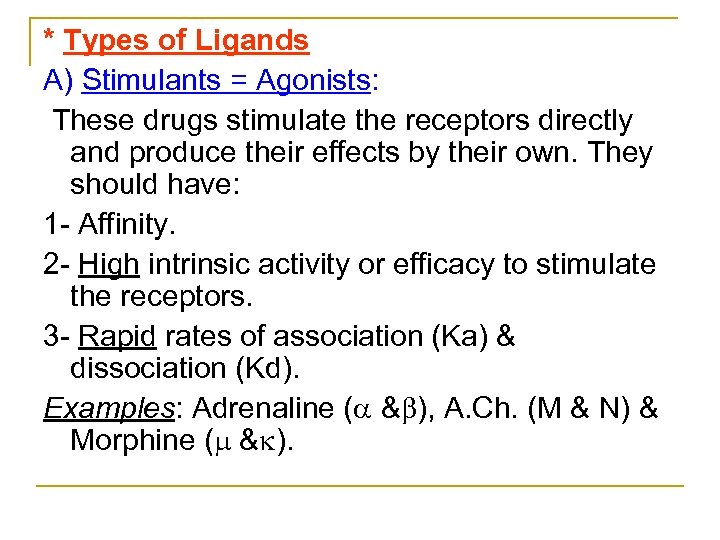
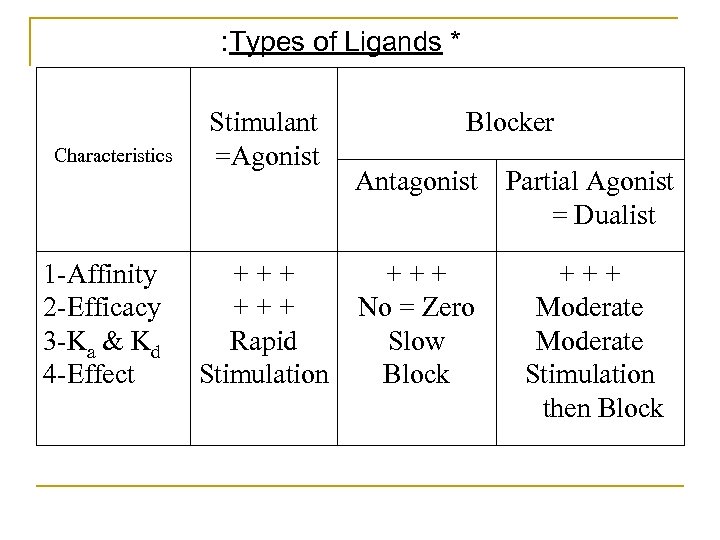
* Types of Ligands A) Stimulants = Agonists: These drugs stimulate the receptors directly and produce their effects by their own. They should have: 1 Affinity. 2 High intrinsic activity or efficacy to stimulate the receptors. 3 Rapid rates of association (Ka) & dissociation (Kd). Examples: Adrenaline ( & ), A. Ch. (M & N) & Morphine ( & ).

B) Blockers: These drugs produce their effects indirectly by blocking the receptors and blocking the actions of internal chemical transmitters and/or hormones. They are: 1 Antagonists: They should have: a Affinity. b No = Zero efficacy No dose/response curve c Slow dissociation from receptors. They block the action of agonists. Examples: Prazosin, propranolol, atropine & naloxone

2 Partial Agonists = Dualists: They should have: a Affinity. b Low intrinsic activity = Weak efficacy Less maximum response (Emax) than agonists. c Moderates of association & dissociation. They produce initial stimulation then block of the receptor If used alone Weak stimulation of the receptor Weak response If used in presence of an agonist Block the action of the agonist. Examples: Ergotamine ( + 5 HT), Oxprenolol ( ), Nicotine (NN) & Succinylcholine (N M ).
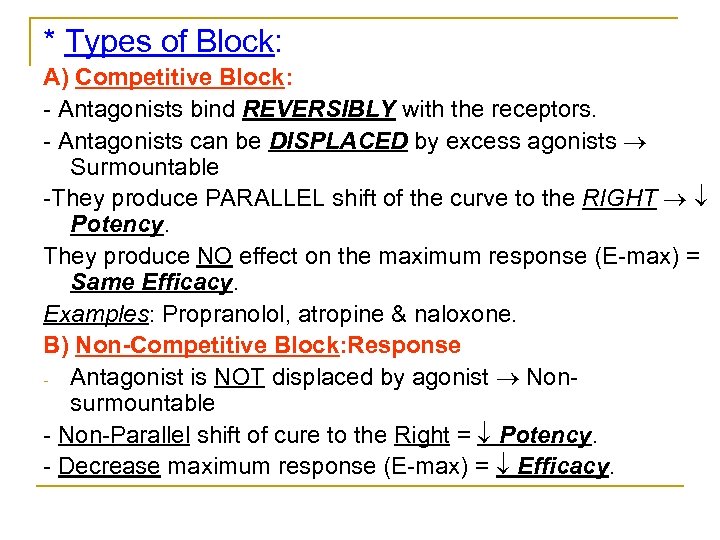
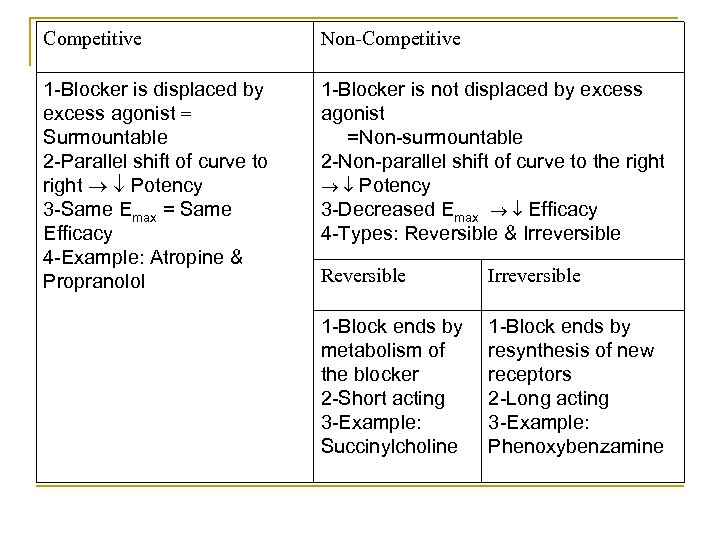
* Types of Block: A) Competitive Block: Antagonists bind REVERSIBLY with the receptors. Antagonists can be DISPLACED by excess agonists Surmountable They produce PARALLEL shift of the curve to the RIGHT Potency. They produce NO effect on the maximum response (E max) = Same Efficacy. Examples: Propranolol, atropine & naloxone. B) Non-Competitive Block: Response Antagonist is NOT displaced by agonist Non surmountable Non Parallel shift of cure to the Right = Potency. Decrease maximum response (E max) = Efficacy.

- Types of Non-Competitive Block : a REVERSIBLE : The antagonist binds REVERSIBLY to the receptor. The block ends by the Metabolism of the blocker. Usually of Short duration of action. Examples : succinylcholine. b IRREVERSIBLE : The antagonist binds COVALENTLY to the receptor. The block ends by Resynthesis of new receptors. Usually of Long duration of action. Examples: organophosphorus compounds.
: Types of Ligands * Characteristics 1 -Affinity 2 -Efficacy 3 -Ka & Kd 4 -Effect Stimulant =Agonist +++ Rapid Stimulation Blocker Antagonist Partial Agonist = Dualist +++ No = Zero Slow Block +++ Moderate Stimulation then Block
Competitive Non-Competitive 1 Blocker is displaced by excess agonist = Surmountable 2 Parallel shift of curve to right Potency 3 Same Emax = Same Efficacy 4 Example: Atropine & Propranolol 1 Blocker is not displaced by excess agonist =Non surmountable 2 Non parallel shift of curve to the right Potency 3 Decreased Emax Efficacy 4 Types: Reversible & Irreversible Reversible Irreversible 1 Block ends by metabolism of the blocker 2 Short acting 3 Example: Succinylcholine 1 Block ends by resynthesis of new receptors 2 Long acting 3 Example: Phenoxybenzamine

NB (Chronic Use of Drugs Affects the No. & Sensitivity of Receptors: Long use of Agonists No. & Sensitivity of Receptors Down Regulation. Long use of Antagonists or drugs that transmission No. & Sensitivity of Receptors Up Regulation.

Doses of Drugs (Posology) 1 Therapeutic Dose: Average dose calculated for an Adult, Male, 20 60 year old & 70 Kg body weight. 2 Initial Dose; Initial large dose aiming to reach therapeutic plasma concentration 3 Maintenance Dose: Small daily dose required to replace eliminated drug from the body to maintain the achieved therapeutic plasma concentration. 4 Maximal Tolerated Dose; Highest dose without toxic effects. 5 Lethal or Fatal Dose: Dose that kill the patient or an experimental animal

6 - Therapeutic Index: Ratio = LD 50 / ED 50 LD 50 = Lethal dose in 50% of animals ED 50 = Effective dose in 50% of animals A good guide to determine & compare SAFETY of drugs The Higher therapeutic index The Safer the drug
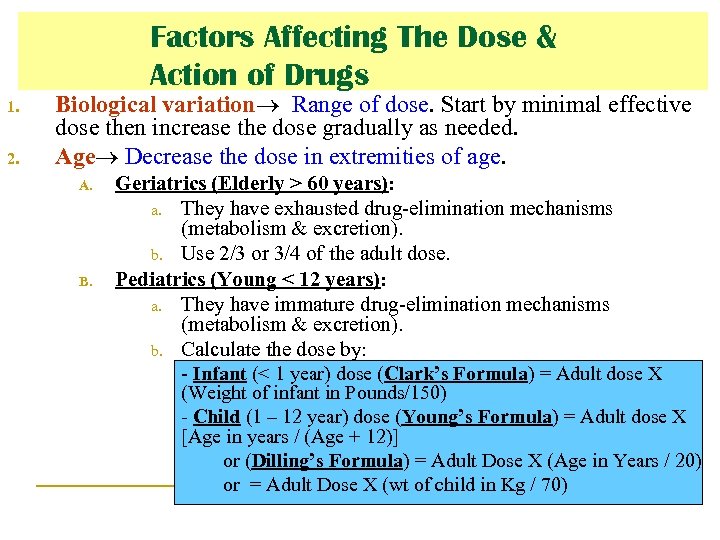
Factors Affecting The Dose & Action of Drugs 1. 2. Biological variation Range of dose. Start by minimal effective dose then increase the dose gradually as needed. Age Decrease the dose in extremities of age. A. B. Geriatrics (Elderly > 60 years): a. They have exhausted drug-elimination mechanisms (metabolism & excretion). b. Use 2/3 or 3/4 of the adult dose. Pediatrics (Young < 12 years): a. They have immature drug-elimination mechanisms (metabolism & excretion). b. Calculate the dose by: Infant (< 1 year) dose (Clark’s Formula) = Adult dose X (Weight of infant in Pounds/150) - Child (1 – 12 year) dose (Young’s Formula) = Adult dose X [Age in years / (Age + 12)] or (Dilling’s Formula) = Adult Dose X (Age in Years / 20) or = Adult Dose X (wt of child in Kg / 70)

Factors Affecting The Dose & Action of Drugs (cont. ) 3. Body Weight & Surface Area: a. b. c. 4. Skeletal muscle weight is more important than fat or edema. Surface area = Height in cm X weight in Kg / 3600 Surface area is more accurate in calculating doses for children & infants. Sex: a. b. Males need higher doses than females: § Males have bulky muscle tissue & Androgens (HME Inducers) § Females have bulky fat tissue & Estrogen (HME Inhibitor) Some drugs are contraindicated in Females during physiological periods: § Menstruation: Aspirin & Cathartics Bleeding § Pregnancy: Sex hormones, Oxytocics (Ergotamine) & Teratogens (Phenytoin) § Labor: Barbiturates & Morphine Neonatal asphyxia § Lactation: Drugs excreted in milk eg Purgatives, Tetracyclines & Chloramphenicol
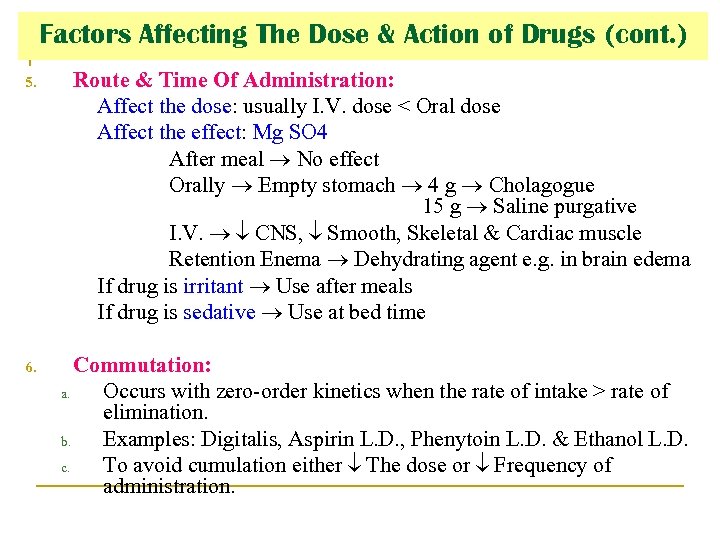
Factors Affecting The Dose & Action of Drugs (cont. ) 5. Route & Time Of Administration: Affect the dose: usually I. V. dose < Oral dose Affect the effect: Mg SO 4 After meal No effect Orally Empty stomach 4 g Cholagogue 15 g Saline purgative I. V. CNS, Smooth, Skeletal & Cardiac muscle Retention Enema Dehydrating agent e. g. in brain edema If drug is irritant Use after meals If drug is sedative Use at bed time 6. Commutation: a. Occurs with zero-order kinetics when the rate of intake > rate of elimination. b. Examples: Digitalis, Aspirin L. D. , Phenytoin L. D. & Ethanol L. D. c. To avoid cumulation either The dose or Frequency of administration.

Factors Affecting The Dose & Action of Drugs (cont. ) 7. Psychological Effect: Some patients improve by Psychological (Suggestion) rather than Pharmacological effect of the drug (Placebo effect). Placebo (Dummy medication) is an inert substance (Lactose, starch, etc. ) used in a dosage form (Tablet, capsule, etc). Useful in: § § Treatment of patients by psychological suggestion As a comparison when testing new drugs

Factors Affecting The Dose & Action of Drugs (cont. ) 8. Pathological Condition: a. Some drugs act ONLY in presence of disease: § § b. Pathology may cause supersensitivity: § § c. Aspirin acts as an antipyretic ONLY in fever Digitalis acts as a diuretic ONLY in heart failure a- Adrenaline in thyrotoxicosis b- -Blockers in bronchial asthma Pathology may affect drug kinetics: Achlorhydria Intrinsic factor Absorption of Vit B-12 Pernicious anemia. Liver and/or kidney disease may affect the dose of some drugs.
Factors Affecting The Dose & Action of Drugs (cont. ) Idiosyncrasy (Pharmacogenetics) Abnormal response 9. Supersensitivity (Intolerance) Dose of the drug 10. Tolerance Dose of the drug 11. Drug interactions Summation, Synergism, Antagonism & Reversal. 8.
62ebca33af4433696311f963775d867f.ppt