d2feb2040f4487d5471bf17c257dc939.ppt
- Количество слайдов: 21
 Ph. USE 2008 Manchester 2008 -10 -12 / 2008 -10 -15 Michael Knoessl Implementing CDISC at Boehringer Ingelheim Michael Knoessl 1 17 March 2018
Ph. USE 2008 Manchester 2008 -10 -12 / 2008 -10 -15 Michael Knoessl Implementing CDISC at Boehringer Ingelheim Michael Knoessl 1 17 March 2018
 CDISC – Impact 2004 – 07 – 21 B. C. Before CDISC Michael Knoessl According to CDISC 2 17 March 2018
CDISC – Impact 2004 – 07 – 21 B. C. Before CDISC Michael Knoessl According to CDISC 2 17 March 2018
 Agenda Motivation and Target Approach: Design Example Results: Enhancing SDTM Michael Knoessl 3 17 March 2018
Agenda Motivation and Target Approach: Design Example Results: Enhancing SDTM Michael Knoessl 3 17 March 2018
 Motivation • Issue: analyses on pooled databases (PDB) are increasing • Need: all clinical data of a project/substance available in one standardized structure for all clinical data • Task: investigate the CDISC data standards for usability for analysis & reporting Michael Knoessl 4 17 March 2018
Motivation • Issue: analyses on pooled databases (PDB) are increasing • Need: all clinical data of a project/substance available in one standardized structure for all clinical data • Task: investigate the CDISC data standards for usability for analysis & reporting Michael Knoessl 4 17 March 2018
 Motivation 1) need for efficient pooled databases (PDB) 2) need to submit data in CDISC standard Implementing CDISC at BI ICBI Michael Knoessl 5 17 March 2018
Motivation 1) need for efficient pooled databases (PDB) 2) need to submit data in CDISC standard Implementing CDISC at BI ICBI Michael Knoessl 5 17 March 2018
 Target design and implementation of a CLINICAL DATA MODEL based on CDISC principles Michael Knoessl 6 17 March 2018
Target design and implementation of a CLINICAL DATA MODEL based on CDISC principles Michael Knoessl 6 17 March 2018
 Target CLINICAL DATA MODEL • corporate wide across trials, projects, substances • integral part of the in-house clinical data business process • operational for single trials and pooled databases (PDB) - data quality check - Analysis Data Set (ADa. M / ADS) creation - ad hoc analysis • CDISC principles of SDTM, ADa. M, ODM, CT Michael Knoessl 7 17 March 2018
Target CLINICAL DATA MODEL • corporate wide across trials, projects, substances • integral part of the in-house clinical data business process • operational for single trials and pooled databases (PDB) - data quality check - Analysis Data Set (ADa. M / ADS) creation - ad hoc analysis • CDISC principles of SDTM, ADa. M, ODM, CT Michael Knoessl 7 17 March 2018
 Agenda Motivation and Target Approach: Design Example Results: Enhancing SDTM Michael Knoessl 8 17 March 2018
Agenda Motivation and Target Approach: Design Example Results: Enhancing SDTM Michael Knoessl 8 17 March 2018
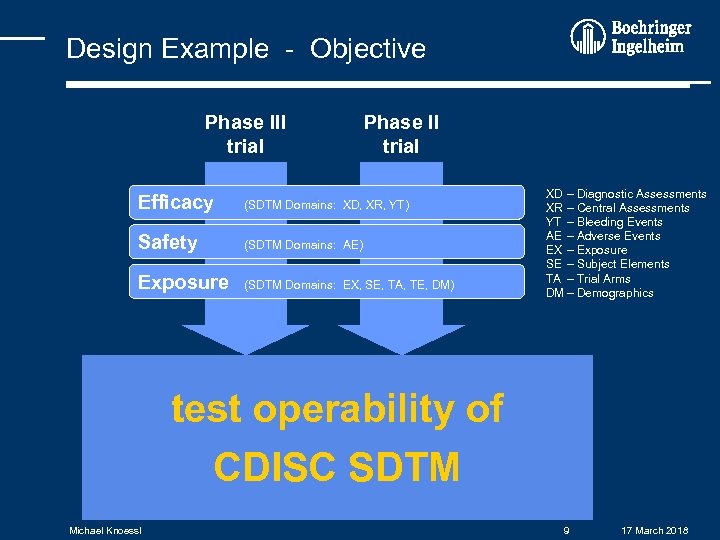 Design Example - Objective Phase III trial Phase II trial Efficacy (SDTM Domains: XD, XR, YT) Safety (SDTM Domains: AE) Exposure (SDTM Domains: EX, SE, TA, TE, DM) XD – Diagnostic Assessments XR – Central Assessments YT – Bleeding Events AE – Adverse Events EX – Exposure SE – Subject Elements TA – Trial Arms DM – Demographics test operability of CDISC SDTM Michael Knoessl 9 17 March 2018
Design Example - Objective Phase III trial Phase II trial Efficacy (SDTM Domains: XD, XR, YT) Safety (SDTM Domains: AE) Exposure (SDTM Domains: EX, SE, TA, TE, DM) XD – Diagnostic Assessments XR – Central Assessments YT – Bleeding Events AE – Adverse Events EX – Exposure SE – Subject Elements TA – Trial Arms DM – Demographics test operability of CDISC SDTM Michael Knoessl 9 17 March 2018
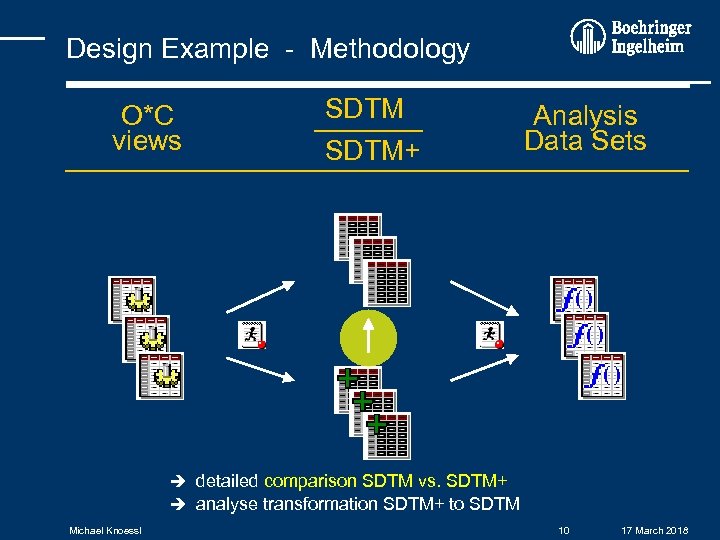 Design Example - Methodology O*C views SDTM+ Analysis Data Sets detailed comparison SDTM vs. SDTM+ analyse transformation SDTM+ to SDTM Michael Knoessl 10 17 March 2018
Design Example - Methodology O*C views SDTM+ Analysis Data Sets detailed comparison SDTM vs. SDTM+ analyse transformation SDTM+ to SDTM Michael Knoessl 10 17 March 2018
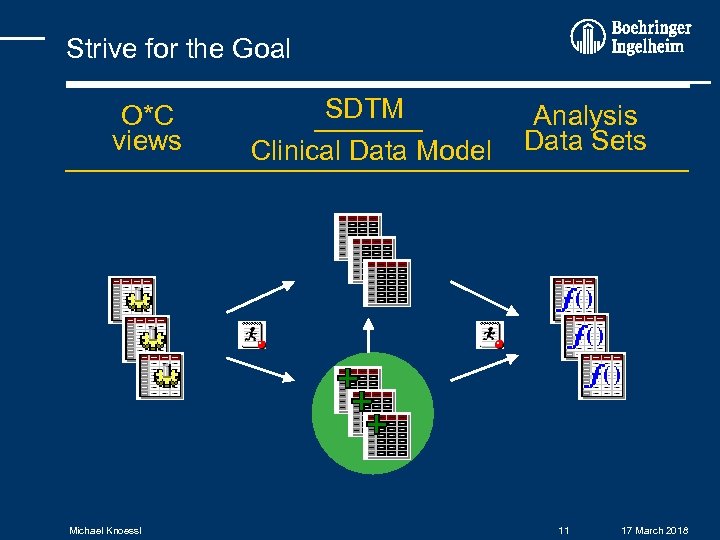 Strive for the Goal O*C views Michael Knoessl SDTM Clinical Data Model SDTM+ Analysis Data Sets 11 17 March 2018
Strive for the Goal O*C views Michael Knoessl SDTM Clinical Data Model SDTM+ Analysis Data Sets 11 17 March 2018
 Agenda Motivation and Target Approach: Design Example Results: Enhancing SDTM Michael Knoessl 12 17 March 2018
Agenda Motivation and Target Approach: Design Example Results: Enhancing SDTM Michael Knoessl 12 17 March 2018
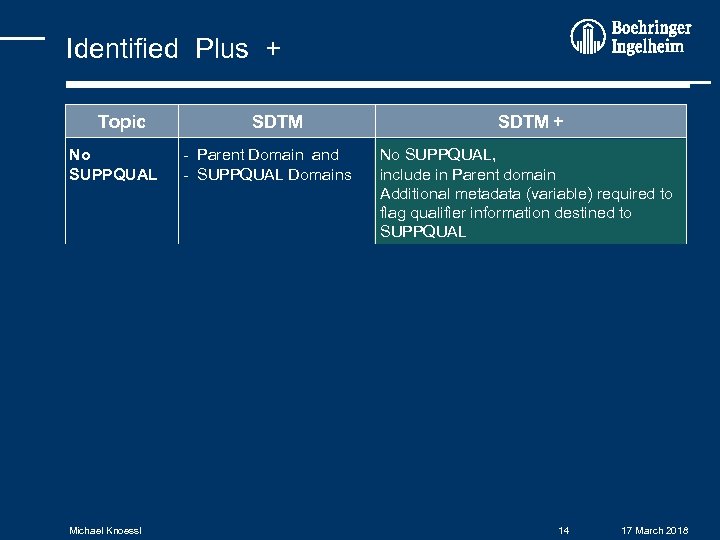
 Identified Plus + Topic SDTM + NUM – CHAR variables in general have: - CHAR - NUM variables Example: --ORRES + "--ORRESN" Code – Decode only Decode (CHAR) have: - Code (NUM) - Decode (CHAR) - associated SAS format dates / times all dates are CHAR (ISO 8601) have: - ISO 8601 dates (CHAR) and - SAS dates (NUM) date / time imputation reported date / time (ISO 8601) have: - reported date / time - imputation rule Missing SDTM definitions no definition for some BI-variables t. b. d. - keep as plus variables, or - modify reporting tools Michael Knoessl Example: TSORT - treatment sort order 13 17 March 2018
Identified Plus + Topic SDTM + NUM – CHAR variables in general have: - CHAR - NUM variables Example: --ORRES + "--ORRESN" Code – Decode only Decode (CHAR) have: - Code (NUM) - Decode (CHAR) - associated SAS format dates / times all dates are CHAR (ISO 8601) have: - ISO 8601 dates (CHAR) and - SAS dates (NUM) date / time imputation reported date / time (ISO 8601) have: - reported date / time - imputation rule Missing SDTM definitions no definition for some BI-variables t. b. d. - keep as plus variables, or - modify reporting tools Michael Knoessl Example: TSORT - treatment sort order 13 17 March 2018
 Identified Plus + Topic No SUPPQUAL SDTM - Parent Domain and - SUPPQUAL Domains SDTM + No SUPPQUAL, include in Parent domain Additional metadata (variable) required to flag qualifier information destined to SUPPQUAL Tracking of no place for: same patient - Previous Trial No. in multiple - Previous Patient No. trials - Main + Follow-Up Trial No. - Main + Follow-Up Patient No. Key concept Keys to be defined based on clinical content to provide relationality across domains STUDYID USUBJID DOMAIN --SEQ --REFID --GRPID --SPID needed for - reporting and - to facilitate pooling only relate to SUPPQUAL, CO, RELREC Michael Knoessl 14 17 March 2018
Identified Plus + Topic No SUPPQUAL SDTM - Parent Domain and - SUPPQUAL Domains SDTM + No SUPPQUAL, include in Parent domain Additional metadata (variable) required to flag qualifier information destined to SUPPQUAL Tracking of no place for: same patient - Previous Trial No. in multiple - Previous Patient No. trials - Main + Follow-Up Trial No. - Main + Follow-Up Patient No. Key concept Keys to be defined based on clinical content to provide relationality across domains STUDYID USUBJID DOMAIN --SEQ --REFID --GRPID --SPID needed for - reporting and - to facilitate pooling only relate to SUPPQUAL, CO, RELREC Michael Knoessl 14 17 March 2018
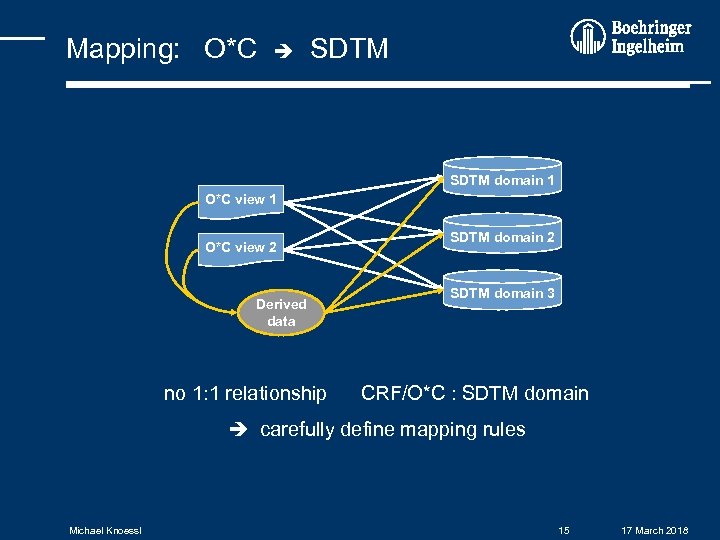 Mapping: O*C SDTM domain 1 O*C view 2 Derived data no 1: 1 relationship SDTM domain 2 SDTM domain 3 CRF/O*C : SDTM domain carefully define mapping rules Michael Knoessl 15 17 March 2018
Mapping: O*C SDTM domain 1 O*C view 2 Derived data no 1: 1 relationship SDTM domain 2 SDTM domain 3 CRF/O*C : SDTM domain carefully define mapping rules Michael Knoessl 15 17 March 2018
 Additional Results • Variable renaming is exception - rule is complex transformations • CDISC SDTM does not cover all analysis & reporting relevant data additional records e. g. IE additional domains e. g. efficacy CT - Controlled Terminology • consistency between variable and content • pooling requirement • CDISC CT does not provide sufficient cover yet define BI CT to comply with CDISC CT consolidation of existing GLIB formats Michael Knoessl 16 17 March 2018
Additional Results • Variable renaming is exception - rule is complex transformations • CDISC SDTM does not cover all analysis & reporting relevant data additional records e. g. IE additional domains e. g. efficacy CT - Controlled Terminology • consistency between variable and content • pooling requirement • CDISC CT does not provide sufficient cover yet define BI CT to comply with CDISC CT consolidation of existing GLIB formats Michael Knoessl 16 17 March 2018
 Impact on … Overall Risks and Benefits - Risks - Benefits … Processes, Systems - Clinical Data Flow - Systems (O*C, LSH) - External Data - Legacy Data … Compliance - Functions / Roles - SOPs and Guidelines - Training - Validation Michael Knoessl … Resources, Commitment - other BI Initiatives - Effort and Cost - Organisation … Current Standards - Global Library - Macros 17 17 March 2018
Impact on … Overall Risks and Benefits - Risks - Benefits … Processes, Systems - Clinical Data Flow - Systems (O*C, LSH) - External Data - Legacy Data … Compliance - Functions / Roles - SOPs and Guidelines - Training - Validation Michael Knoessl … Resources, Commitment - other BI Initiatives - Effort and Cost - Organisation … Current Standards - Global Library - Macros 17 17 March 2018
 Design Example - Objective Phase III trial Phase II trial Efficacy (SDTM Domains: XD, XR, YT) Safety (SDTM Domains: AE) Exposure (SDTM Domains: EX, SE, TA, TE, DM) XD – Diagnostic Assessments XR – Central Assessments YT – Bleeding Events AE – Adverse Events EX – Exposure SE – Subject Elements TA – Trial Arms DM – Demographics test operability of CDISC SDTM Michael Knoessl 18 17 March 2018
Design Example - Objective Phase III trial Phase II trial Efficacy (SDTM Domains: XD, XR, YT) Safety (SDTM Domains: AE) Exposure (SDTM Domains: EX, SE, TA, TE, DM) XD – Diagnostic Assessments XR – Central Assessments YT – Bleeding Events AE – Adverse Events EX – Exposure SE – Subject Elements TA – Trial Arms DM – Demographics test operability of CDISC SDTM Michael Knoessl 18 17 March 2018
 Operability of CDISC SDTM ? Michael Knoessl 19 17 March 2018
Operability of CDISC SDTM ? Michael Knoessl 19 17 March 2018
 Benefit of Enhanced SDTM Clinical Data Model - creation of pooled databases (PDB) - facilitate analysis & reporting Michael Knoessl 20 17 March 2018
Benefit of Enhanced SDTM Clinical Data Model - creation of pooled databases (PDB) - facilitate analysis & reporting Michael Knoessl 20 17 March 2018
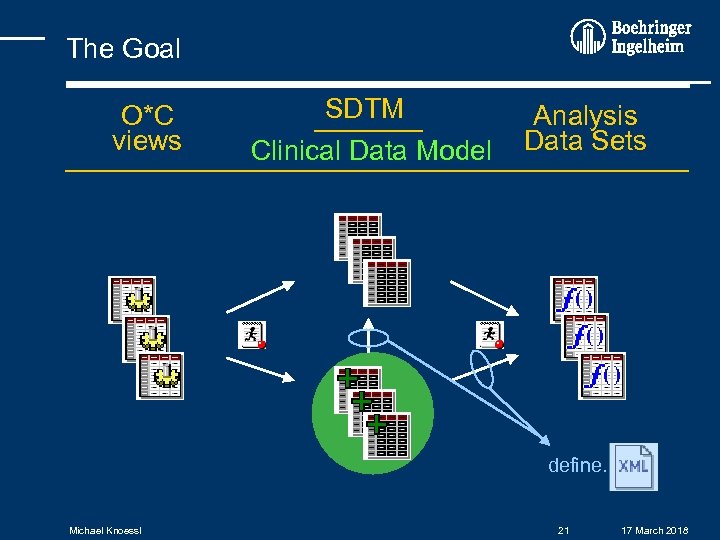 The Goal O*C views SDTM Clinical Data Model SDTM+ Analysis Data Sets define. Michael Knoessl 21 17 March 2018
The Goal O*C views SDTM Clinical Data Model SDTM+ Analysis Data Sets define. Michael Knoessl 21 17 March 2018


