a788667a9246888dcc8352cf5600da01.ppt
- Количество слайдов: 76
 Ph. RMA White Paper on ADME Pharmacogenomics: Survey Results Lisa A. Shipley, Ph. D. Advisory Committee for Pharmaceutical Science and Clinical Pharmacology March 18, 2008
Ph. RMA White Paper on ADME Pharmacogenomics: Survey Results Lisa A. Shipley, Ph. D. Advisory Committee for Pharmaceutical Science and Clinical Pharmacology March 18, 2008
 Goals of the White Paper n n Present a pharmaceutical industry perspective of the recent and future utility of pharmacogenomics (PGx) related to the ADME properties of drugs for drug development and utilization Offer perspectives on the current state of practices, strategies, knowledge and key information gaps that need to be addressed in order to fulfill the promise of PGx in targeted medicine Does not intend to provide best practice for ADME PGx in drug development or utilization, nor to address ethical ramifications of ADME PGx Second half of paper discusses current understanding of clinically significant polymorphisms of drug metabolism enzymes and transporters
Goals of the White Paper n n Present a pharmaceutical industry perspective of the recent and future utility of pharmacogenomics (PGx) related to the ADME properties of drugs for drug development and utilization Offer perspectives on the current state of practices, strategies, knowledge and key information gaps that need to be addressed in order to fulfill the promise of PGx in targeted medicine Does not intend to provide best practice for ADME PGx in drug development or utilization, nor to address ethical ramifications of ADME PGx Second half of paper discusses current understanding of clinically significant polymorphisms of drug metabolism enzymes and transporters
 Approach n To establish a cross-industry perspective on the current utility of ADME PGx, Ph. RMA conducted a survey of recent (2003 -2005) major pharmaceutical company practices
Approach n To establish a cross-industry perspective on the current utility of ADME PGx, Ph. RMA conducted a survey of recent (2003 -2005) major pharmaceutical company practices
 The Survey n n n Assembled a series of questions to elicit broad information about current pharmaceutical company ADME PGx practices Survey respondents were instructed to base all answers on clinical trials that initiated during the period 2003 -2005 Companies were also asked to provide citations for peerreviewed original PGx ADME research published by industry scientists (up to 3/company), and examples of how PGx ADME information has been used for internal decision-making and regulatory interactions Ph. RMA staff solicited responses from Ph. RMA member companies and aggregated data, to preserve companies’ anonymity (except for citations of published papers) Not every company answered every question Some questions have been reordered for ease of presentation
The Survey n n n Assembled a series of questions to elicit broad information about current pharmaceutical company ADME PGx practices Survey respondents were instructed to base all answers on clinical trials that initiated during the period 2003 -2005 Companies were also asked to provide citations for peerreviewed original PGx ADME research published by industry scientists (up to 3/company), and examples of how PGx ADME information has been used for internal decision-making and regulatory interactions Ph. RMA staff solicited responses from Ph. RMA member companies and aggregated data, to preserve companies’ anonymity (except for citations of published papers) Not every company answered every question Some questions have been reordered for ease of presentation
 Participating Companies n n n n Abbott Laboratories Amgen Astra-Zeneca Bristol Myers Squibb Glaxo. Smith. Kline Johnson & Johnson Lilly n n n n Merck Millennium Novartis Pfizer Sanofi-Aventis Schering-Plough Wyeth
Participating Companies n n n n Abbott Laboratories Amgen Astra-Zeneca Bristol Myers Squibb Glaxo. Smith. Kline Johnson & Johnson Lilly n n n n Merck Millennium Novartis Pfizer Sanofi-Aventis Schering-Plough Wyeth
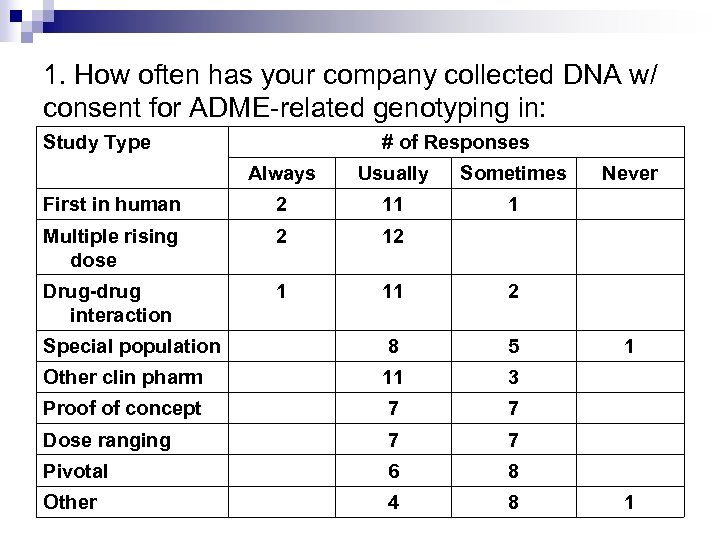 1. How often has your company collected DNA w/ consent for ADME-related genotyping in: Study Type # of Responses Always Usually Sometimes First in human 2 11 1 Multiple rising dose 2 12 Drug-drug interaction 1 11 2 Special population 8 5 Other clin pharm 11 3 Proof of concept 7 7 Dose ranging 7 7 Pivotal 6 8 Other 4 8 Never 1 1
1. How often has your company collected DNA w/ consent for ADME-related genotyping in: Study Type # of Responses Always Usually Sometimes First in human 2 11 1 Multiple rising dose 2 12 Drug-drug interaction 1 11 2 Special population 8 5 Other clin pharm 11 3 Proof of concept 7 7 Dose ranging 7 7 Pivotal 6 8 Other 4 8 Never 1 1
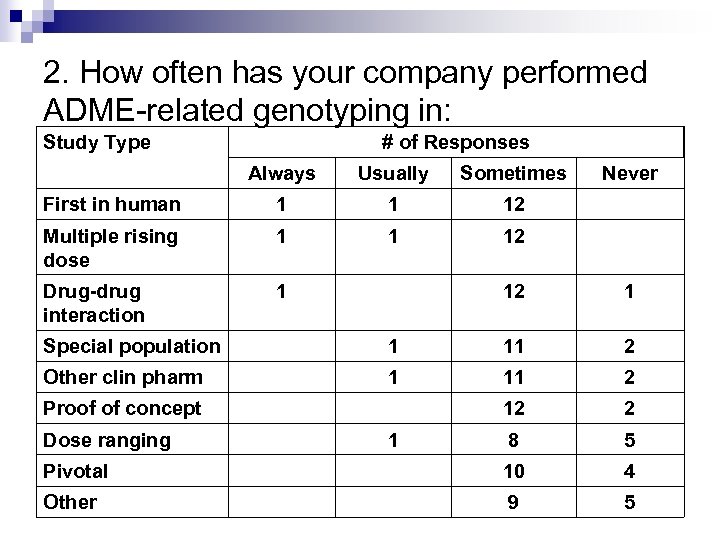 2. How often has your company performed ADME-related genotyping in: Study Type # of Responses Always Usually Sometimes First in human 1 1 12 Multiple rising dose 1 1 12 Drug-drug interaction 1 Never 12 1 Special population 1 11 2 Other clin pharm 1 11 2 12 2 8 5 Pivotal 10 4 Other 9 5 Proof of concept Dose ranging 1
2. How often has your company performed ADME-related genotyping in: Study Type # of Responses Always Usually Sometimes First in human 1 1 12 Multiple rising dose 1 1 12 Drug-drug interaction 1 Never 12 1 Special population 1 11 2 Other clin pharm 1 11 2 12 2 8 5 Pivotal 10 4 Other 9 5 Proof of concept Dose ranging 1
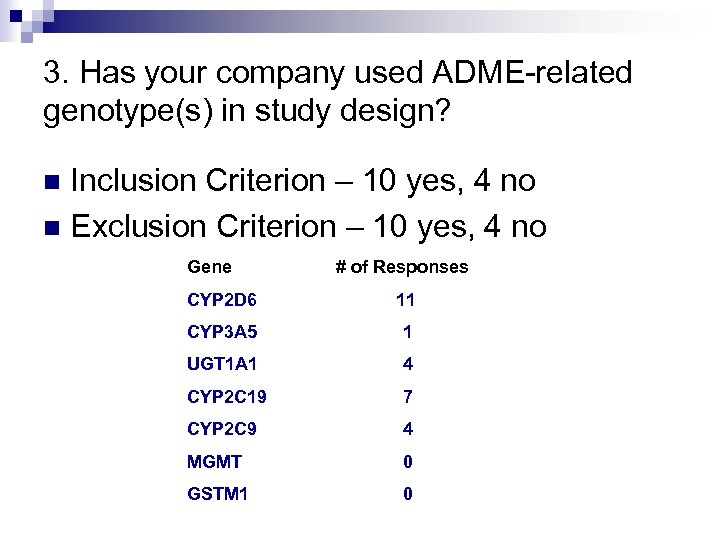 3. Has your company used ADME-related genotype(s) in study design? Inclusion Criterion – 10 yes, 4 no n Exclusion Criterion – 10 yes, 4 no n Gene # of Responses CYP 2 D 6 11 CYP 3 A 5 1 UGT 1 A 1 4 CYP 2 C 19 7 CYP 2 C 9 4 MGMT 0 GSTM 1 0
3. Has your company used ADME-related genotype(s) in study design? Inclusion Criterion – 10 yes, 4 no n Exclusion Criterion – 10 yes, 4 no n Gene # of Responses CYP 2 D 6 11 CYP 3 A 5 1 UGT 1 A 1 4 CYP 2 C 19 7 CYP 2 C 9 4 MGMT 0 GSTM 1 0
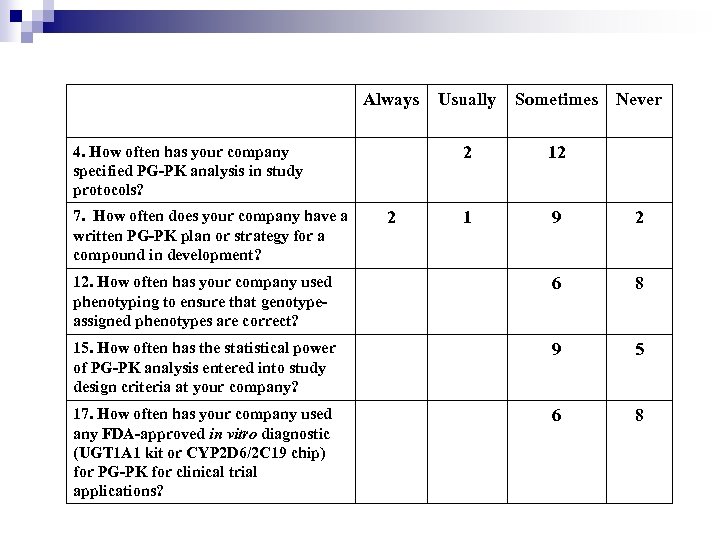 Always Usually Sometimes 2 12 1 9 2 12. How often has your company used phenotyping to ensure that genotypeassigned phenotypes are correct? 6 8 15. How often has the statistical power of PG-PK analysis entered into study design criteria at your company? 9 5 17. How often has your company used any FDA-approved in vitro diagnostic (UGT 1 A 1 kit or CYP 2 D 6/2 C 19 chip) for PG-PK for clinical trial applications? 6 8 4. How often has your company specified PG-PK analysis in study protocols? 7. How often does your company have a written PG-PK plan or strategy for a compound in development? 2 Never
Always Usually Sometimes 2 12 1 9 2 12. How often has your company used phenotyping to ensure that genotypeassigned phenotypes are correct? 6 8 15. How often has the statistical power of PG-PK analysis entered into study design criteria at your company? 9 5 17. How often has your company used any FDA-approved in vitro diagnostic (UGT 1 A 1 kit or CYP 2 D 6/2 C 19 chip) for PG-PK for clinical trial applications? 6 8 4. How often has your company specified PG-PK analysis in study protocols? 7. How often does your company have a written PG-PK plan or strategy for a compound in development? 2 Never
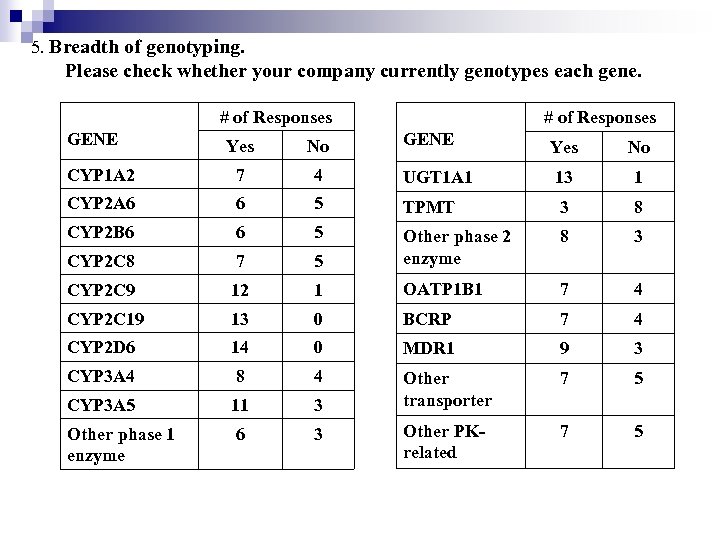 5. Breadth of genotyping. Please check whether your company currently genotypes each gene. # of Responses GENE Yes No 4 UGT 1 A 1 13 1 6 5 TPMT 3 8 CYP 2 B 6 6 5 8 3 CYP 2 C 8 7 5 Other phase 2 enzyme CYP 2 C 9 12 1 OATP 1 B 1 7 4 CYP 2 C 19 13 0 BCRP 7 4 CYP 2 D 6 14 0 MDR 1 9 3 CYP 3 A 4 8 4 7 5 CYP 3 A 5 11 3 Other transporter Other phase 1 enzyme 6 3 Other PKrelated 7 5 Yes No CYP 1 A 2 7 CYP 2 A 6
5. Breadth of genotyping. Please check whether your company currently genotypes each gene. # of Responses GENE Yes No 4 UGT 1 A 1 13 1 6 5 TPMT 3 8 CYP 2 B 6 6 5 8 3 CYP 2 C 8 7 5 Other phase 2 enzyme CYP 2 C 9 12 1 OATP 1 B 1 7 4 CYP 2 C 19 13 0 BCRP 7 4 CYP 2 D 6 14 0 MDR 1 9 3 CYP 3 A 4 8 4 7 5 CYP 3 A 5 11 3 Other transporter Other phase 1 enzyme 6 3 Other PKrelated 7 5 Yes No CYP 1 A 2 7 CYP 2 A 6
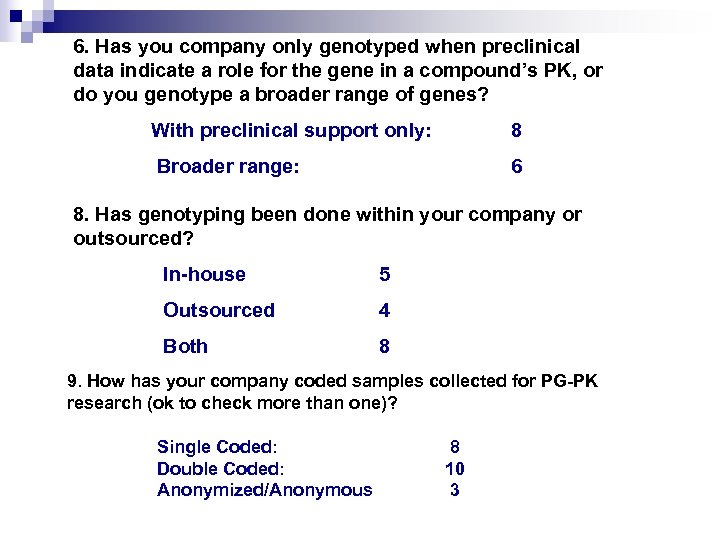 6. Has you company only genotyped when preclinical data indicate a role for the gene in a compound’s PK, or do you genotype a broader range of genes? With preclinical support only: 8 Broader range: 6 8. Has genotyping been done within your company or outsourced? In-house 5 Outsourced 4 Both 8 9. How has your company coded samples collected for PG-PK research (ok to check more than one)? Single Coded: Double Coded: Anonymized/Anonymous 8 10 3
6. Has you company only genotyped when preclinical data indicate a role for the gene in a compound’s PK, or do you genotype a broader range of genes? With preclinical support only: 8 Broader range: 6 8. Has genotyping been done within your company or outsourced? In-house 5 Outsourced 4 Both 8 9. How has your company coded samples collected for PG-PK research (ok to check more than one)? Single Coded: Double Coded: Anonymized/Anonymous 8 10 3
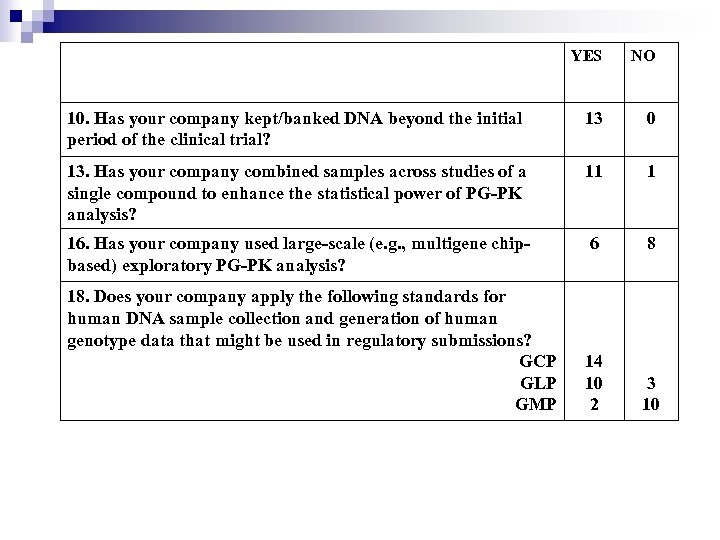 YES NO 10. Has your company kept/banked DNA beyond the initial period of the clinical trial? 13 0 13. Has your company combined samples across studies of a single compound to enhance the statistical power of PG-PK analysis? 11 1 16. Has your company used large-scale (e. g. , multigene chipbased) exploratory PG-PK analysis? 6 8 14 10 2 3 10 18. Does your company apply the following standards for human DNA sample collection and generation of human genotype data that might be used in regulatory submissions? GCP GLP GMP
YES NO 10. Has your company kept/banked DNA beyond the initial period of the clinical trial? 13 0 13. Has your company combined samples across studies of a single compound to enhance the statistical power of PG-PK analysis? 11 1 16. Has your company used large-scale (e. g. , multigene chipbased) exploratory PG-PK analysis? 6 8 14 10 2 3 10 18. Does your company apply the following standards for human DNA sample collection and generation of human genotype data that might be used in regulatory submissions? GCP GLP GMP
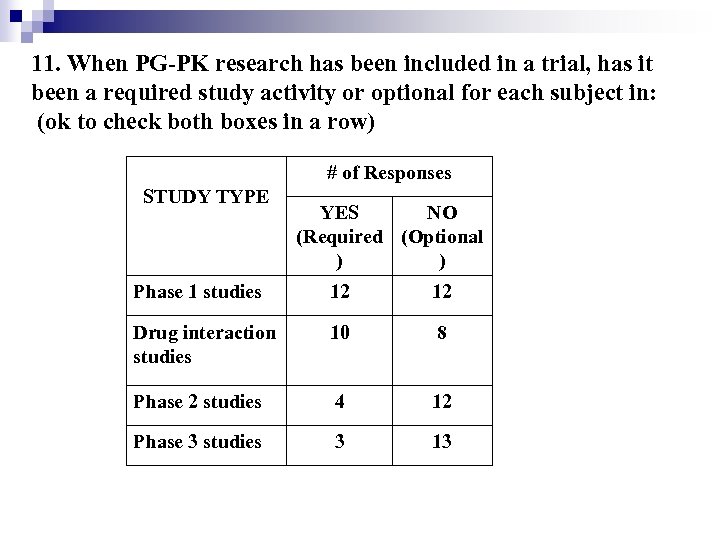 11. When PG-PK research has been included in a trial, has it been a required study activity or optional for each subject in: (ok to check both boxes in a row) # of Responses STUDY TYPE YES NO (Required (Optional ) ) Phase 1 studies 12 12 Drug interaction studies 10 8 Phase 2 studies 4 12 Phase 3 studies 3 13
11. When PG-PK research has been included in a trial, has it been a required study activity or optional for each subject in: (ok to check both boxes in a row) # of Responses STUDY TYPE YES NO (Required (Optional ) ) Phase 1 studies 12 12 Drug interaction studies 10 8 Phase 2 studies 4 12 Phase 3 studies 3 13
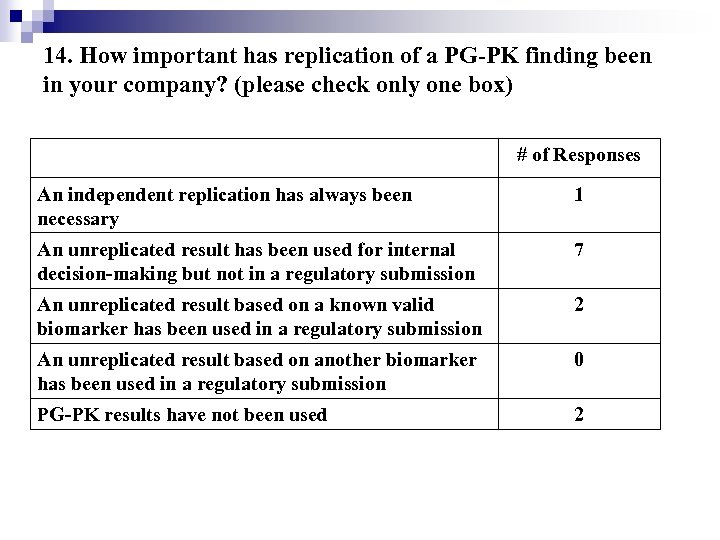 14. How important has replication of a PG-PK finding been in your company? (please check only one box) # of Responses An independent replication has always been necessary 1 An unreplicated result has been used for internal decision-making but not in a regulatory submission 7 An unreplicated result based on a known valid biomarker has been used in a regulatory submission 2 An unreplicated result based on another biomarker has been used in a regulatory submission 0 PG-PK results have not been used 2
14. How important has replication of a PG-PK finding been in your company? (please check only one box) # of Responses An independent replication has always been necessary 1 An unreplicated result has been used for internal decision-making but not in a regulatory submission 7 An unreplicated result based on a known valid biomarker has been used in a regulatory submission 2 An unreplicated result based on another biomarker has been used in a regulatory submission 0 PG-PK results have not been used 2
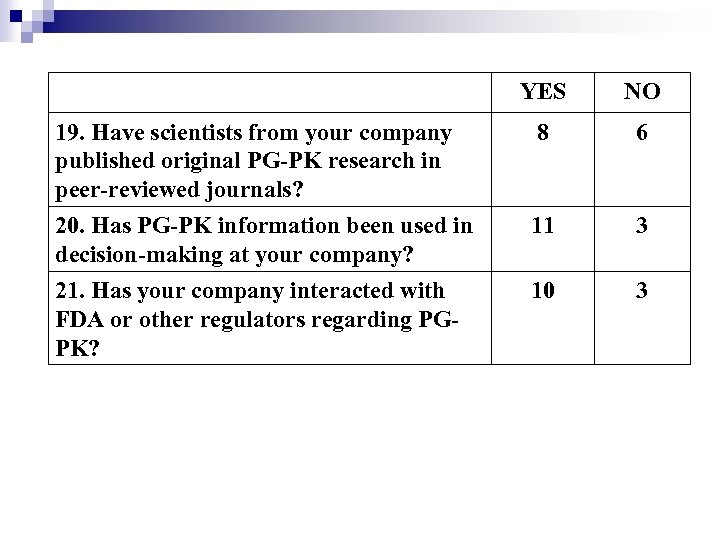 YES NO 19. Have scientists from your company published original PG-PK research in peer-reviewed journals? 8 6 20. Has PG-PK information been used in decision-making at your company? 11 3 21. Has your company interacted with FDA or other regulators regarding PGPK? 10 3
YES NO 19. Have scientists from your company published original PG-PK research in peer-reviewed journals? 8 6 20. Has PG-PK information been used in decision-making at your company? 11 3 21. Has your company interacted with FDA or other regulators regarding PGPK? 10 3
 Summary of Survey n n n PGx has already significantly impacted drug development and beginning to influence drug utilization PGx allows the identification, confirmation or exclusion of clearance pathways PGx analyses have utility in Explaining PK variability ¨ Ensure trial population appropriately balanced ¨ Ensure the safety of volunteers and patients ¨ Provide mechanistic information ¨ n n PGx studies may support labeling claim concerning PK dosing; ethnic variability and safety A significant effort remains in the education of the public, prescribers, ethics committees and investigators
Summary of Survey n n n PGx has already significantly impacted drug development and beginning to influence drug utilization PGx allows the identification, confirmation or exclusion of clearance pathways PGx analyses have utility in Explaining PK variability ¨ Ensure trial population appropriately balanced ¨ Ensure the safety of volunteers and patients ¨ Provide mechanistic information ¨ n n PGx studies may support labeling claim concerning PK dosing; ethnic variability and safety A significant effort remains in the education of the public, prescribers, ethics committees and investigators
 Questions or Comments?
Questions or Comments?
 Incorporation of Pharmacogenetic in Healthcare Eric Lai, Ph. D. , VP, Pharmacogenetics March 18, 2008
Incorporation of Pharmacogenetic in Healthcare Eric Lai, Ph. D. , VP, Pharmacogenetics March 18, 2008
 This presentation represents my personal views and does not necessarily reflect the policies or endorsement of Glaxo. Smith. Kline. 19
This presentation represents my personal views and does not necessarily reflect the policies or endorsement of Glaxo. Smith. Kline. 19
 What is Pharmacogenetics (PGx)? · The field of Pharmacogenetics deals with the · effect of individual gene variants on the action of a given drug. “Right Drug for the right patient at the right time” or personalized medicine. 20
What is Pharmacogenetics (PGx)? · The field of Pharmacogenetics deals with the · effect of individual gene variants on the action of a given drug. “Right Drug for the right patient at the right time” or personalized medicine. 20
 Misconceptions about PGx · There is no such things as a personalized medicine (The · · right medicine to the right patient at the right dosage at the right time). This is a perfect example of marketing talk (Drugs are not like cars or computers). Clinical trials are done on populations, effects are observed in a group of patients. PGx increase the probability whether the drug is going to be beneficial for you or may have major adverse effort(s) against you. The drug is not specifically designed for you but for a group of targeted individuals (Targeted or Informed Medicine). So it is more like clothing sizes (whether you are size 0 or 14). Not all clinical trials or drugs have PGx components 21
Misconceptions about PGx · There is no such things as a personalized medicine (The · · right medicine to the right patient at the right dosage at the right time). This is a perfect example of marketing talk (Drugs are not like cars or computers). Clinical trials are done on populations, effects are observed in a group of patients. PGx increase the probability whether the drug is going to be beneficial for you or may have major adverse effort(s) against you. The drug is not specifically designed for you but for a group of targeted individuals (Targeted or Informed Medicine). So it is more like clothing sizes (whether you are size 0 or 14). Not all clinical trials or drugs have PGx components 21
 PGx results are used to support post-marketing risk management, product differentiation in market place & asset progression · Support post-marketing risk management and product differentiation by identifying treatment and management opportunities using patient’s marker status (Phase III - IV) – Improve safety profile: Safety PGx · Support asset progression (Phase II – III) – – Improve efficacy profile (Efficacy PGx) Improve safety profile · Account for variability (Phase I) – Drug safety & efficacy varies between individuals and ethnic groups. · Explain etiology (Disease understanding) – – Genetics influences susceptibility to adverse drug reactions. Understanding these mechanisms can be used to minimize risk. Genetics influences efficacy of medicines– understanding mechanisms can be used to select back-up compounds or identify best treatment population. 22
PGx results are used to support post-marketing risk management, product differentiation in market place & asset progression · Support post-marketing risk management and product differentiation by identifying treatment and management opportunities using patient’s marker status (Phase III - IV) – Improve safety profile: Safety PGx · Support asset progression (Phase II – III) – – Improve efficacy profile (Efficacy PGx) Improve safety profile · Account for variability (Phase I) – Drug safety & efficacy varies between individuals and ethnic groups. · Explain etiology (Disease understanding) – – Genetics influences susceptibility to adverse drug reactions. Understanding these mechanisms can be used to minimize risk. Genetics influences efficacy of medicines– understanding mechanisms can be used to select back-up compounds or identify best treatment population. 22
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 23
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 23
 Safety PGx - abacavir hypersensitivity 24
Safety PGx - abacavir hypersensitivity 24
 What is abacavir hypersensitivity? · · Abacavir (ABC) commonly used in treatment of HIV-1 infection Approved products include Ziagen, Trizivir and Epzicom / Kivexa Generally well tolerated Key limitation: abacavir hypersensitivity reaction (ABC HSR) – affects ~5% of clinical trial patients · Features of ABC HSR: – Multi-organ clinical syndrome – typically fever and/or rash ± constitutional, GI and/or respiratory symptoms – Symptoms usually (>90%) occur within first 6 weeks of therapy – The symptoms worsen with continued therapy and can be lifethreatening but usually resolve upon permanent discontinuation of abacavir – Re-challenge is contraindicated and can be fatal 25
What is abacavir hypersensitivity? · · Abacavir (ABC) commonly used in treatment of HIV-1 infection Approved products include Ziagen, Trizivir and Epzicom / Kivexa Generally well tolerated Key limitation: abacavir hypersensitivity reaction (ABC HSR) – affects ~5% of clinical trial patients · Features of ABC HSR: – Multi-organ clinical syndrome – typically fever and/or rash ± constitutional, GI and/or respiratory symptoms – Symptoms usually (>90%) occur within first 6 weeks of therapy – The symptoms worsen with continued therapy and can be lifethreatening but usually resolve upon permanent discontinuation of abacavir – Re-challenge is contraindicated and can be fatal 25
 GSK research on ABC HSR · Post-approval commitments in 1999 to regulatory authorities to conduct research to understand ABC HSR and develop tests to confirm diagnosis · Pooled analysis of studies identified some risk factors for HSR: race, sex, CDC class, prior HIV drug treatment, NNRTI co-introduction · Comprehensive & effective risk management program created (educational materials, labelling, pharmacovigilance, etc. ) 26
GSK research on ABC HSR · Post-approval commitments in 1999 to regulatory authorities to conduct research to understand ABC HSR and develop tests to confirm diagnosis · Pooled analysis of studies identified some risk factors for HSR: race, sex, CDC class, prior HIV drug treatment, NNRTI co-introduction · Comprehensive & effective risk management program created (educational materials, labelling, pharmacovigilance, etc. ) 26
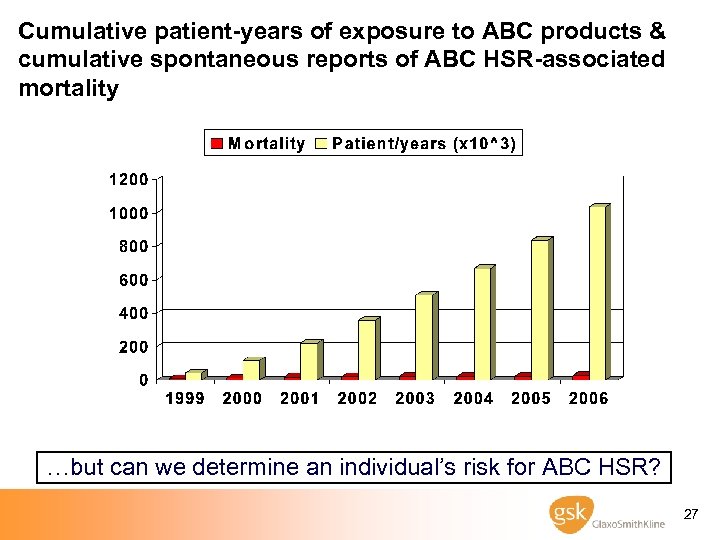 Cumulative patient-years of exposure to ABC products & cumulative spontaneous reports of ABC HSR-associated mortality …but can we determine an individual’s risk for ABC HSR? 27
Cumulative patient-years of exposure to ABC products & cumulative spontaneous reports of ABC HSR-associated mortality …but can we determine an individual’s risk for ABC HSR? 27
 GSK Abacavir-HSR project timeline · · 1994 Initiate clinical development program 1998/9 Marketing Approval for first ABC product – Ziagen 1999 Initiation of the GSK Abacavir-HSR project team 2001 Association of HLA-B*5701 with ABC HSR · Caucasian males (50 -94% sensitivity, 98% selectivity) · 2003 HLA-B*5701 extended to Caucasian females · 2004/5 Lab. Corp offers HLA-B*5701 screening assay – Response to requests by US HIV clinicians · 2006 First reports of prospective HLA-B*5701 screening on incidence of HSR · 2007 Prospective trial (Predict -1) results presented at IAS 28
GSK Abacavir-HSR project timeline · · 1994 Initiate clinical development program 1998/9 Marketing Approval for first ABC product – Ziagen 1999 Initiation of the GSK Abacavir-HSR project team 2001 Association of HLA-B*5701 with ABC HSR · Caucasian males (50 -94% sensitivity, 98% selectivity) · 2003 HLA-B*5701 extended to Caucasian females · 2004/5 Lab. Corp offers HLA-B*5701 screening assay – Response to requests by US HIV clinicians · 2006 First reports of prospective HLA-B*5701 screening on incidence of HSR · 2007 Prospective trial (Predict -1) results presented at IAS 28
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 29
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 29
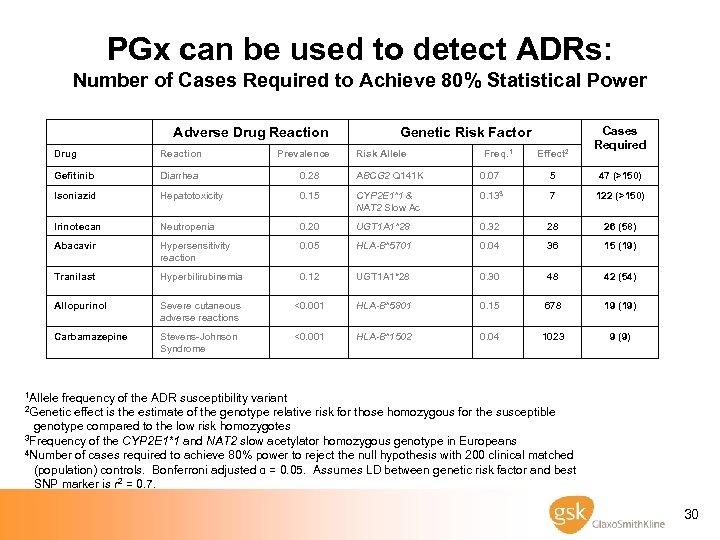 PGx can be used to detect ADRs: Number of Cases Required to Achieve 80% Statistical Power Adverse Drug Reaction Prevalence Genetic Risk Factor Risk Allele Freq. 1 Effect 2 Cases Required Drug Reaction Gefitinib Diarrhea 0. 28 ABCG 2 Q 141 K 0. 07 5 47 (>150) Isoniazid Hepatotoxicity 0. 15 CYP 2 E 1*1 & NAT 2 Slow Ac 0. 133 7 122 (>150) Irinotecan Neutropenia 0. 20 UGT 1 A 1*28 0. 32 28 26 (58) Abacavir Hypersensitivity reaction 0. 05 HLA-B*5701 0. 04 36 15 (19) Tranilast Hyperbilirubinemia 0. 12 UGT 1 A 1*28 0. 30 48 42 (54) Allopurinol Severe cutaneous adverse reactions <0. 001 HLA-B*5801 0. 15 678 19 (19) Carbamazepine Stevens-Johnson Syndrome <0. 001 HLA-B*1502 0. 04 1023 9 (9) 1 Allele frequency of the ADR susceptibility variant 2 Genetic effect is the estimate of the genotype relative risk for those homozygous for the susceptible genotype compared to the low risk homozygotes 3 Frequency of the CYP 2 E 1*1 and NAT 2 slow acetylator homozygous genotype in Europeans 4 Number of cases required to achieve 80% power to reject the null hypothesis with 200 clinical matched (population) controls. Bonferroni adjusted α = 0. 05. Assumes LD between genetic risk factor and best SNP marker is r 2 = 0. 7. 30
PGx can be used to detect ADRs: Number of Cases Required to Achieve 80% Statistical Power Adverse Drug Reaction Prevalence Genetic Risk Factor Risk Allele Freq. 1 Effect 2 Cases Required Drug Reaction Gefitinib Diarrhea 0. 28 ABCG 2 Q 141 K 0. 07 5 47 (>150) Isoniazid Hepatotoxicity 0. 15 CYP 2 E 1*1 & NAT 2 Slow Ac 0. 133 7 122 (>150) Irinotecan Neutropenia 0. 20 UGT 1 A 1*28 0. 32 28 26 (58) Abacavir Hypersensitivity reaction 0. 05 HLA-B*5701 0. 04 36 15 (19) Tranilast Hyperbilirubinemia 0. 12 UGT 1 A 1*28 0. 30 48 42 (54) Allopurinol Severe cutaneous adverse reactions <0. 001 HLA-B*5801 0. 15 678 19 (19) Carbamazepine Stevens-Johnson Syndrome <0. 001 HLA-B*1502 0. 04 1023 9 (9) 1 Allele frequency of the ADR susceptibility variant 2 Genetic effect is the estimate of the genotype relative risk for those homozygous for the susceptible genotype compared to the low risk homozygotes 3 Frequency of the CYP 2 E 1*1 and NAT 2 slow acetylator homozygous genotype in Europeans 4 Number of cases required to achieve 80% power to reject the null hypothesis with 200 clinical matched (population) controls. Bonferroni adjusted α = 0. 05. Assumes LD between genetic risk factor and best SNP marker is r 2 = 0. 7. 30
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Yes -> if they are forced to. Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 31
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Yes -> if they are forced to. Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 31
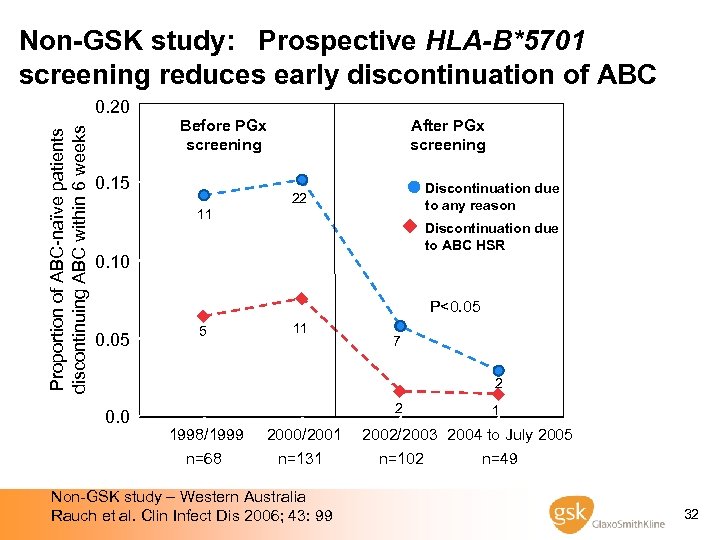 Non-GSK study: Prospective HLA-B*5701 screening reduces early discontinuation of ABC Proportion of ABC-naïve patients discontinuing ABC within 6 weeks 0. 20 Before PGx screening 0. 15 11 After PGx screening Discontinuation due to any reason 22 Discontinuation due to ABC HSR 0. 10 P<0. 05 5 11 7 2 0. 0 2 1998/1999 n=68 2000/2001 n=131 Non-GSK study – Western Australia Rauch et al. Clin Infect Dis 2006; 43: 99 1 2002/2003 2004 to July 2005 n=102 n=49 32
Non-GSK study: Prospective HLA-B*5701 screening reduces early discontinuation of ABC Proportion of ABC-naïve patients discontinuing ABC within 6 weeks 0. 20 Before PGx screening 0. 15 11 After PGx screening Discontinuation due to any reason 22 Discontinuation due to ABC HSR 0. 10 P<0. 05 5 11 7 2 0. 0 2 1998/1999 n=68 2000/2001 n=131 Non-GSK study – Western Australia Rauch et al. Clin Infect Dis 2006; 43: 99 1 2002/2003 2004 to July 2005 n=102 n=49 32
 Determining Clinical Utility: GSK’s Prospective Trial (PREDICT-1) study objectives · To determine whether prospective screening for HLA-B*5701, prior to abacavir treatment, resulted in: – a significantly lower incidence of clinically suspected ABC HSR – a significantly lower incidence of immunologically confirmed ABC HSR as determined by ABC skin patch testing Mallal S et al. NEJM, 2008 33
Determining Clinical Utility: GSK’s Prospective Trial (PREDICT-1) study objectives · To determine whether prospective screening for HLA-B*5701, prior to abacavir treatment, resulted in: – a significantly lower incidence of clinically suspected ABC HSR – a significantly lower incidence of immunologically confirmed ABC HSR as determined by ABC skin patch testing Mallal S et al. NEJM, 2008 33
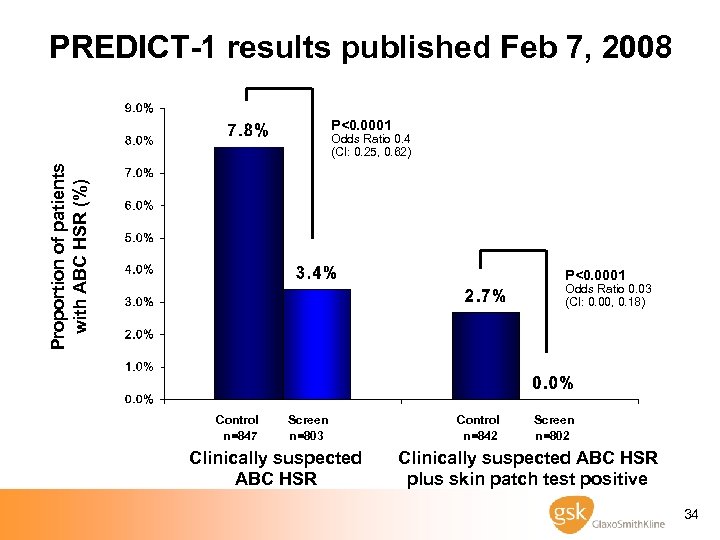 PREDICT-1 results published Feb 7, 2008 P<0. 0001 Proportion of patients with ABC HSR (%) Odds Ratio 0. 4 (CI: 0. 25, 0. 62) P<0. 0001 Odds Ratio 0. 03 (CI: 0. 00, 0. 18) Control n=847 Screen n=803 Clinically suspected ABC HSR Control n=842 Screen n=802 Clinically suspected ABC HSR plus skin patch test positive 34
PREDICT-1 results published Feb 7, 2008 P<0. 0001 Proportion of patients with ABC HSR (%) Odds Ratio 0. 4 (CI: 0. 25, 0. 62) P<0. 0001 Odds Ratio 0. 03 (CI: 0. 00, 0. 18) Control n=847 Screen n=803 Clinically suspected ABC HSR Control n=842 Screen n=802 Clinically suspected ABC HSR plus skin patch test positive 34
 PREDICT-1 conclusions · Prospective HLA-B*5701 screening and avoidance of ABC in patients who were HLA-B*5701 positive – significantly reduced the incidence of clinically suspected ABC HSR – eliminated immunologically confirmed (i. e. , patch test-positive) ABC HSR · HLA-B*5701 positive patients are at increased risk for · ABC HSR HLA-B*5701 negative patients are at reduced risk for ABC HSR Mallal S et al. NEJM, 2008 35
PREDICT-1 conclusions · Prospective HLA-B*5701 screening and avoidance of ABC in patients who were HLA-B*5701 positive – significantly reduced the incidence of clinically suspected ABC HSR – eliminated immunologically confirmed (i. e. , patch test-positive) ABC HSR · HLA-B*5701 positive patients are at increased risk for · ABC HSR HLA-B*5701 negative patients are at reduced risk for ABC HSR Mallal S et al. NEJM, 2008 35
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 36
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 36
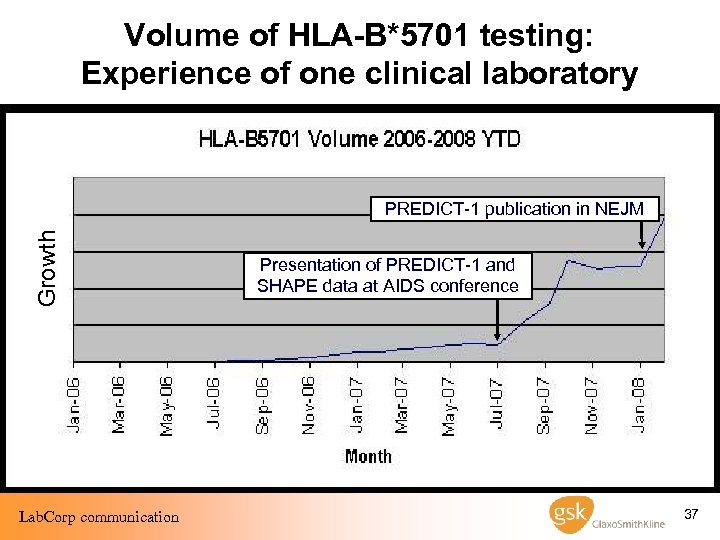 Volume of HLA-B*5701 testing: Experience of one clinical laboratory Growth PREDICT-1 publication in NEJM Lab. Corp communication Presentation of PREDICT-1 and SHAPE data at AIDS conference 37
Volume of HLA-B*5701 testing: Experience of one clinical laboratory Growth PREDICT-1 publication in NEJM Lab. Corp communication Presentation of PREDICT-1 and SHAPE data at AIDS conference 37
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 38
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 38
 Common Sense and Predictably Irrational behavior · Odds of winning in a casino. – Will you pay for a test if the result can tell you whether you can improve your odd of winning from 5% to 50%? · >80% of the people will say yes · Odds of getting an adverse drug reaction. – Will you let your physician order for a test if the result can tell you whether you have a higher chance of getting an adverse drug reaction (from 5% to 50%)? · Only about 50% of the people will say yes 39
Common Sense and Predictably Irrational behavior · Odds of winning in a casino. – Will you pay for a test if the result can tell you whether you can improve your odd of winning from 5% to 50%? · >80% of the people will say yes · Odds of getting an adverse drug reaction. – Will you let your physician order for a test if the result can tell you whether you have a higher chance of getting an adverse drug reaction (from 5% to 50%)? · Only about 50% of the people will say yes 39
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 40
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 40
 Efficacy PGx 41
Efficacy PGx 41
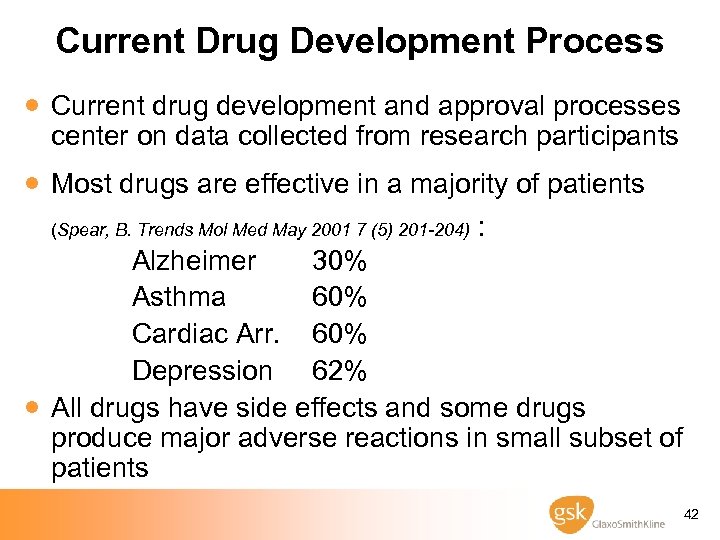 Current Drug Development Process · Current drug development and approval processes center on data collected from research participants · Most drugs are effective in a majority of patients (Spear, B. Trends Mol Med May 2001 7 (5) 201 -204) : · Alzheimer 30% Asthma 60% Cardiac Arr. 60% Depression 62% All drugs have side effects and some drugs produce major adverse reactions in small subset of patients 42
Current Drug Development Process · Current drug development and approval processes center on data collected from research participants · Most drugs are effective in a majority of patients (Spear, B. Trends Mol Med May 2001 7 (5) 201 -204) : · Alzheimer 30% Asthma 60% Cardiac Arr. 60% Depression 62% All drugs have side effects and some drugs produce major adverse reactions in small subset of patients 42
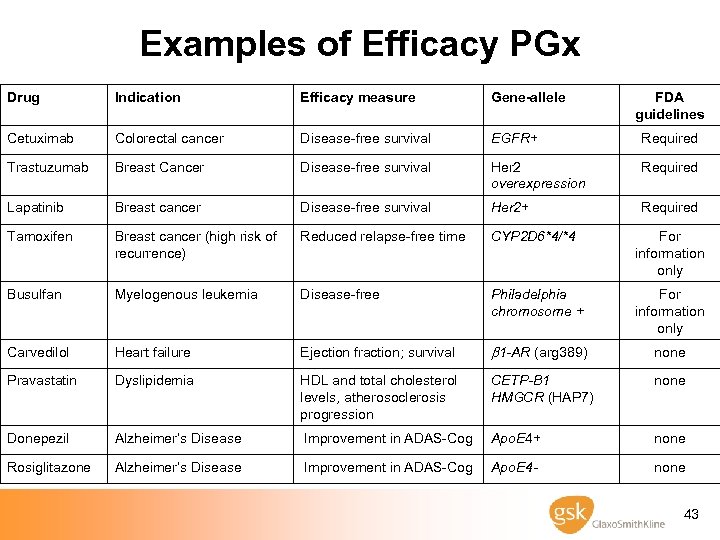 Examples of Efficacy PGx Drug Indication Efficacy measure Gene-allele FDA guidelines Cetuximab Colorectal cancer Disease-free survival EGFR+ Required Trastuzumab Breast Cancer Disease-free survival Her 2 overexpression Required Lapatinib Breast cancer Disease-free survival Her 2+ Required Tamoxifen Breast cancer (high risk of recurrence) Reduced relapse-free time CYP 2 D 6*4/*4 For information only Busulfan Myelogenous leukemia Disease-free Philadelphia chromosome + For information only Carvedilol Heart failure Ejection fraction; survival b 1 -AR (arg 389) none Pravastatin Dyslipidemia HDL and total cholesterol levels, atherosoclerosis progression CETP-B 1 HMGCR (HAP 7) none Donepezil Alzheimer’s Disease Improvement in ADAS-Cog Apo. E 4+ none Rosiglitazone Alzheimer’s Disease Improvement in ADAS-Cog Apo. E 4 - none 43
Examples of Efficacy PGx Drug Indication Efficacy measure Gene-allele FDA guidelines Cetuximab Colorectal cancer Disease-free survival EGFR+ Required Trastuzumab Breast Cancer Disease-free survival Her 2 overexpression Required Lapatinib Breast cancer Disease-free survival Her 2+ Required Tamoxifen Breast cancer (high risk of recurrence) Reduced relapse-free time CYP 2 D 6*4/*4 For information only Busulfan Myelogenous leukemia Disease-free Philadelphia chromosome + For information only Carvedilol Heart failure Ejection fraction; survival b 1 -AR (arg 389) none Pravastatin Dyslipidemia HDL and total cholesterol levels, atherosoclerosis progression CETP-B 1 HMGCR (HAP 7) none Donepezil Alzheimer’s Disease Improvement in ADAS-Cog Apo. E 4+ none Rosiglitazone Alzheimer’s Disease Improvement in ADAS-Cog Apo. E 4 - none 43
 Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 44
Is PGX ready for prime time? · Is the Science of PGx robust enough for routine · · · application? Is the Pharmaceutical industry ready to incorporate PGx in post-market drug safety management and/or to improve the probability of success in drug development? Are the Physicians ready to order PGx testing? Are the Patients ready for PGx testing? Are the payers (e. g. Government, insurance industry, etc) ready to pay for PGx testing? Are the regulatory agencies up to the task of implementing PGx in drug development and drug safety? 44
 Are current drug development process and regulatory thinking ready for Efficacy PGx? 45
Are current drug development process and regulatory thinking ready for Efficacy PGx? 45
 Last thought: what is most important? 46
Last thought: what is most important? 46
 History of mobile phone development · Importance of the regulatory agency: – The basic concept of cellular phones began in 1947, when researchers looked at crude mobile (car) phones and realized that by using small cells (range of service area) with frequency reuse they could increase the traffic capacity of mobile phones substantially. However at that time, the technology to do so was nonexistent. – Anything to do with broadcasting and sending a radio or television message out over the airwaves comes under Federal Communications Commission (FCC) regulation. A cell phone is a type of two-way radio. In 1947, AT&T proposed that the FCC allocate a large number of radiospectrum frequencies so that widespread mobile telephone service would become feasible and AT&T would have a incentive to research the new technology. We can partially blame the FCC for the gap between the initial concept of cellular service and its availability to the public. The FCC decided to limit the amount of frequencies available in 1947, the limits made only twenty-three phone conversations possible simultaneously in the same service area - not a market incentive for research. 47
History of mobile phone development · Importance of the regulatory agency: – The basic concept of cellular phones began in 1947, when researchers looked at crude mobile (car) phones and realized that by using small cells (range of service area) with frequency reuse they could increase the traffic capacity of mobile phones substantially. However at that time, the technology to do so was nonexistent. – Anything to do with broadcasting and sending a radio or television message out over the airwaves comes under Federal Communications Commission (FCC) regulation. A cell phone is a type of two-way radio. In 1947, AT&T proposed that the FCC allocate a large number of radiospectrum frequencies so that widespread mobile telephone service would become feasible and AT&T would have a incentive to research the new technology. We can partially blame the FCC for the gap between the initial concept of cellular service and its availability to the public. The FCC decided to limit the amount of frequencies available in 1947, the limits made only twenty-three phone conversations possible simultaneously in the same service area - not a market incentive for research. 47
 1968: Change in regulatory landscape · The FCC reconsidered its position in 1968. · AT&T and Bell Labs proposed a cellular system to the · FCC of many small, low-powered, broadcast towers, each covering a 'cell' a few miles in radius and collectively covering a larger area. Dr Martin Cooper, a former general manager for the systems division at Motorola, is considered the inventor of the first modern portable handset. Cooper made the first call on a portable cell phone in April 1973. 48
1968: Change in regulatory landscape · The FCC reconsidered its position in 1968. · AT&T and Bell Labs proposed a cellular system to the · FCC of many small, low-powered, broadcast towers, each covering a 'cell' a few miles in radius and collectively covering a larger area. Dr Martin Cooper, a former general manager for the systems division at Motorola, is considered the inventor of the first modern portable handset. Cooper made the first call on a portable cell phone in April 1973. 48
 1983: First commercial cellular phone Dr. Cooper Motorola is first to ship a commercial portable cellular phone, the Dyna. TAC, with a suggested retail price of $3, 995 The Dyna. Tac phone weighs 28 ounces, is 13 x 1. 75 x 3. 5 inches in dimension, boasts one hour of talk time and eight hours of standby time and has the nickname of “brick phone”. 49
1983: First commercial cellular phone Dr. Cooper Motorola is first to ship a commercial portable cellular phone, the Dyna. TAC, with a suggested retail price of $3, 995 The Dyna. Tac phone weighs 28 ounces, is 13 x 1. 75 x 3. 5 inches in dimension, boasts one hour of talk time and eight hours of standby time and has the nickname of “brick phone”. 49
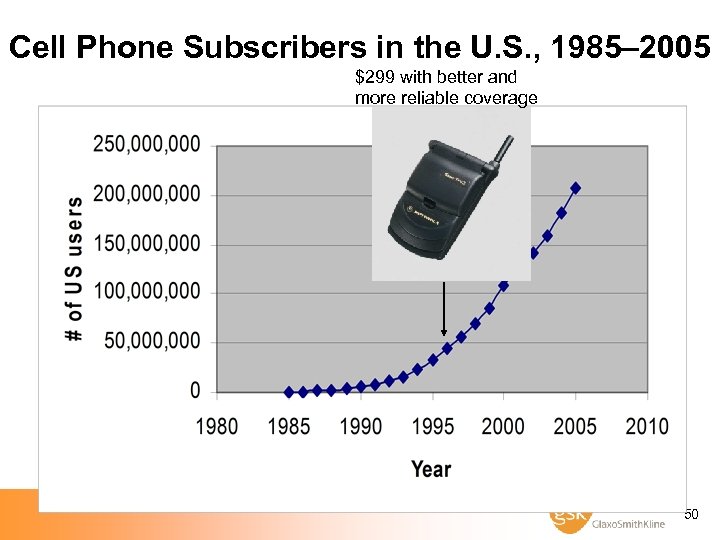 Cell Phone Subscribers in the U. S. , 1985– 2005 $299 with better and more reliable coverage 50
Cell Phone Subscribers in the U. S. , 1985– 2005 $299 with better and more reliable coverage 50
 So what did the cell phone industry teach us? · · Regulatory decisions are critical A common standard (cellular transmission) Reasonable cost for the general public Reliable and good coverage of services (US cell phone companies still have a lot of work to improve on this issue). 51
So what did the cell phone industry teach us? · · Regulatory decisions are critical A common standard (cellular transmission) Reasonable cost for the general public Reliable and good coverage of services (US cell phone companies still have a lot of work to improve on this issue). 51
 PGx results are used to support post-marketing risk management, product differentiation in market place & asset progression · Support post-marketing risk management and product differentiation by identifying treatment and management opportunities using patient’s marker status (Phase III - IV) – Improve safety profile: Safety PGx · Support asset progression (Phase II – III) – – Improve efficacy profile (Efficacy PGx) Improve safety profile · Account for variability (Phase I) – Drug safety & efficacy varies between individuals and ethnic groups. · Explain etiology (Disease understanding) – – Genetics influences susceptibility to adverse drug reactions. Understanding these mechanisms can be used to minimize risk. Genetics influences efficacy of medicines– understanding mechanisms can be used to select back-up compounds or identify best treatment population. 52
PGx results are used to support post-marketing risk management, product differentiation in market place & asset progression · Support post-marketing risk management and product differentiation by identifying treatment and management opportunities using patient’s marker status (Phase III - IV) – Improve safety profile: Safety PGx · Support asset progression (Phase II – III) – – Improve efficacy profile (Efficacy PGx) Improve safety profile · Account for variability (Phase I) – Drug safety & efficacy varies between individuals and ethnic groups. · Explain etiology (Disease understanding) – – Genetics influences susceptibility to adverse drug reactions. Understanding these mechanisms can be used to minimize risk. Genetics influences efficacy of medicines– understanding mechanisms can be used to select back-up compounds or identify best treatment population. 52
 The Long Road to P 450 testing · Cytochrome P 450 proteins with well established common · polymorphisms that affect drug metabolism have been described since 1950 s and molecular basis for the polymorphisms have been known since 1980 s. Potential predictors of optimum dose, drug choice and side effect response – Eg. CYP 2 D 6 & codeine activation, CYP 2 C 9 & warfarin inactivation · Why have they not been taken up into clinical practice? – – – Complicated gene families and difficult assays Lack of a standard and “agreed” panel Lack of regulatory input and guidance Limited awareness Feasibility · Access to test · Genetic information required at point of prescribing decision? 53
The Long Road to P 450 testing · Cytochrome P 450 proteins with well established common · polymorphisms that affect drug metabolism have been described since 1950 s and molecular basis for the polymorphisms have been known since 1980 s. Potential predictors of optimum dose, drug choice and side effect response – Eg. CYP 2 D 6 & codeine activation, CYP 2 C 9 & warfarin inactivation · Why have they not been taken up into clinical practice? – – – Complicated gene families and difficult assays Lack of a standard and “agreed” panel Lack of regulatory input and guidance Limited awareness Feasibility · Access to test · Genetic information required at point of prescribing decision? 53
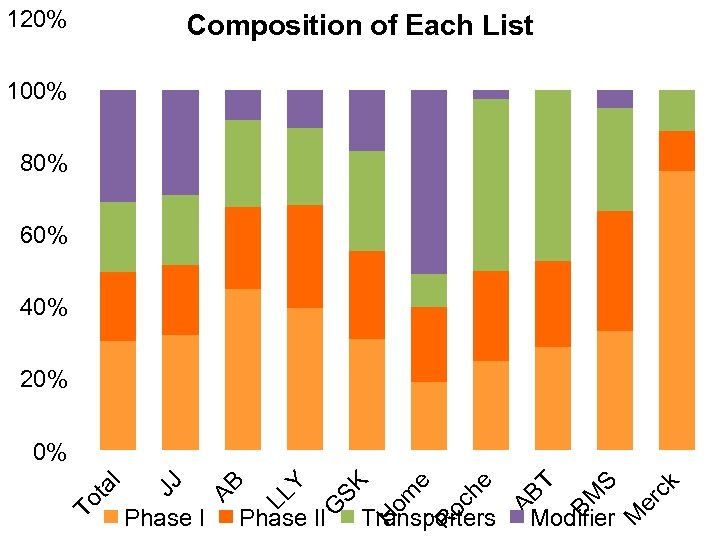
 Agreements among 9 Pharma Companies on ADME genes · Core List - “Must Have Genes” - FDA validated - Significant burden of proof - DMET scientist guided - 33 genes (213 markers) · Extended List - “Need to Have Genes” - Probable involvement - Lacking burden of proof of Core - Mostly addition of Transporters - 143 genes (~2, 500 markers) · Investigative List - “Like to Have Genes” - Unknown but possible involvement - Very little literature - Modifiers of metabolism - 333 genes (~9, 500 markers) 54
Agreements among 9 Pharma Companies on ADME genes · Core List - “Must Have Genes” - FDA validated - Significant burden of proof - DMET scientist guided - 33 genes (213 markers) · Extended List - “Need to Have Genes” - Probable involvement - Lacking burden of proof of Core - Mostly addition of Transporters - 143 genes (~2, 500 markers) · Investigative List - “Like to Have Genes” - Unknown but possible involvement - Very little literature - Modifiers of metabolism - 333 genes (~9, 500 markers) 54
 120% Composition of Each List 100% 80% 60% 40% 20% Transporters AB T BM S M er ck om e R oc he Phase II H Y G SK Phase I LL JJ AB To ta l 0% Modifier 55
120% Composition of Each List 100% 80% 60% 40% 20% Transporters AB T BM S M er ck om e R oc he Phase II H Y G SK Phase I LL JJ AB To ta l 0% Modifier 55
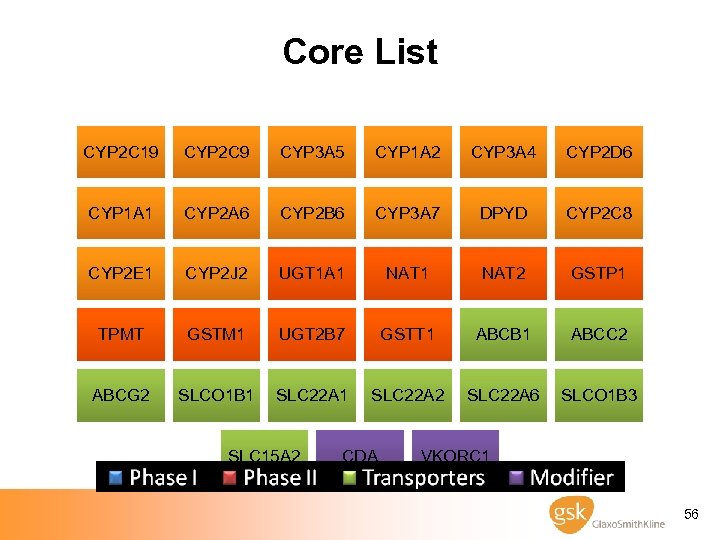 Core List CYP 2 C 19 CYP 2 C 9 CYP 3 A 5 CYP 1 A 2 CYP 3 A 4 CYP 2 D 6 CYP 1 A 1 CYP 2 A 6 CYP 2 B 6 CYP 3 A 7 DPYD CYP 2 C 8 CYP 2 E 1 CYP 2 J 2 UGT 1 A 1 NAT 2 GSTP 1 TPMT GSTM 1 UGT 2 B 7 GSTT 1 ABCB 1 ABCC 2 ABCG 2 SLCO 1 B 1 SLC 22 A 2 SLC 22 A 6 SLCO 1 B 3 SLC 15 A 2 CDA VKORC 1 56
Core List CYP 2 C 19 CYP 2 C 9 CYP 3 A 5 CYP 1 A 2 CYP 3 A 4 CYP 2 D 6 CYP 1 A 1 CYP 2 A 6 CYP 2 B 6 CYP 3 A 7 DPYD CYP 2 C 8 CYP 2 E 1 CYP 2 J 2 UGT 1 A 1 NAT 2 GSTP 1 TPMT GSTM 1 UGT 2 B 7 GSTT 1 ABCB 1 ABCC 2 ABCG 2 SLCO 1 B 1 SLC 22 A 2 SLC 22 A 6 SLCO 1 B 3 SLC 15 A 2 CDA VKORC 1 56
 PGx: Key Stakeholders Regulators Pharma Patients Healthcare Providers Central Testing Laboratories Government Payers Diagnostics and biotech industry Bioethics & Policy Organizations Drug safety and efficacy are shared responsibilities 57
PGx: Key Stakeholders Regulators Pharma Patients Healthcare Providers Central Testing Laboratories Government Payers Diagnostics and biotech industry Bioethics & Policy Organizations Drug safety and efficacy are shared responsibilities 57
 On the Use and Value of Drug-Independent Survival Models to Support Clinical Drug Development in Oncology Rene Bruno, Laurent Claret Pharsight corporation FDA Clinical Pharmacology Advisory Committee Meeting: Quantitative Clinical Pharmacology: Critical Path Opportunities Washington DC, March 18, 2008 © Pharsight Corporation All Rights Reserved Feb. 1, 2008
On the Use and Value of Drug-Independent Survival Models to Support Clinical Drug Development in Oncology Rene Bruno, Laurent Claret Pharsight corporation FDA Clinical Pharmacology Advisory Committee Meeting: Quantitative Clinical Pharmacology: Critical Path Opportunities Washington DC, March 18, 2008 © Pharsight Corporation All Rights Reserved Feb. 1, 2008
 Outline Drug development in oncology A drug-disease modeling framework to support drug development in Oncology ● Tumor growth model ● Survival model Support to end-of-Phase II development decisions: A retrospective project with capecitabine (Roche) On the use the FDA NSCLC survival model ● A case study based on erlotinib data Value of the survival simulations Conclusions © Pharsight Corporation All Rights Reserved March, 2008
Outline Drug development in oncology A drug-disease modeling framework to support drug development in Oncology ● Tumor growth model ● Survival model Support to end-of-Phase II development decisions: A retrospective project with capecitabine (Roche) On the use the FDA NSCLC survival model ● A case study based on erlotinib data Value of the survival simulations Conclusions © Pharsight Corporation All Rights Reserved March, 2008
 Drug development in oncology Lots of new drug candidates with new mechanisms of action ● Major advances in understanding the molecular biology and genetics of the disease have led to the so-called “targeted therapies” ● Highly competitive market Empirical selection of dose and dosing schedules ● MTD vs. biologically active dose paradigm in Phase I ● Phase II studies not designed to assess dose-response • Typical randomized Phase IIb dose-ranging studies are not conducted in oncology ● Analysis of clinical trial data poorly informative (e. g. response rate, neutropenia grade…) Limit the ability to learn from early clinical trials High failure rate in Phase III 60 March, 2008 © Pharsight Corporation All Rights Reserved
Drug development in oncology Lots of new drug candidates with new mechanisms of action ● Major advances in understanding the molecular biology and genetics of the disease have led to the so-called “targeted therapies” ● Highly competitive market Empirical selection of dose and dosing schedules ● MTD vs. biologically active dose paradigm in Phase I ● Phase II studies not designed to assess dose-response • Typical randomized Phase IIb dose-ranging studies are not conducted in oncology ● Analysis of clinical trial data poorly informative (e. g. response rate, neutropenia grade…) Limit the ability to learn from early clinical trials High failure rate in Phase III 60 March, 2008 © Pharsight Corporation All Rights Reserved
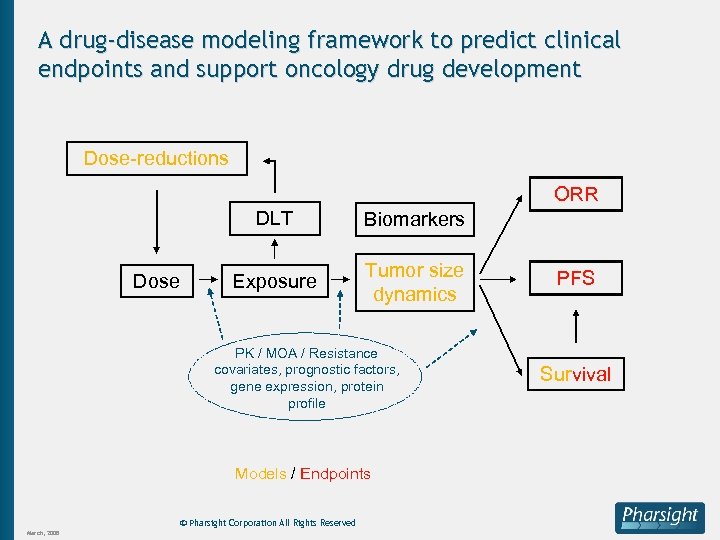 A drug-disease modeling framework to predict clinical endpoints and support oncology drug development Dose-reductions ORR DLT Dose Biomarkers Exposure Tumor size dynamics PK / MOA / Resistance covariates, prognostic factors, gene expression, protein profile Models / Endpoints © Pharsight Corporation All Rights Reserved March, 2008 PFS Survival
A drug-disease modeling framework to predict clinical endpoints and support oncology drug development Dose-reductions ORR DLT Dose Biomarkers Exposure Tumor size dynamics PK / MOA / Resistance covariates, prognostic factors, gene expression, protein profile Models / Endpoints © Pharsight Corporation All Rights Reserved March, 2008 PFS Survival
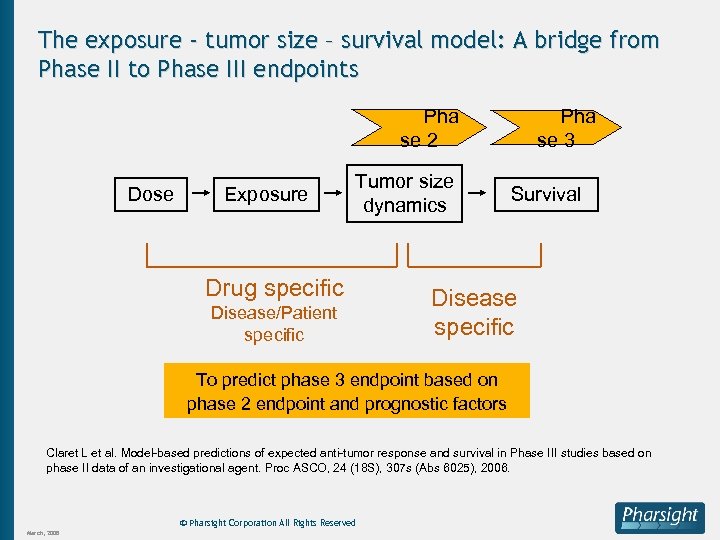 The exposure - tumor size – survival model: A bridge from Phase II to Phase III endpoints Pha se 2 Dose Exposure Tumor size dynamics Drug specific Disease/Patient specific Pha se 3 Survival Disease specific To predict phase 3 endpoint based on phase 2 endpoint and prognostic factors Claret L et al. Model-based predictions of expected anti-tumor response and survival in Phase III studies based on phase II data of an investigational agent. Proc ASCO, 24 (18 S), 307 s (Abs 6025), 2006. © Pharsight Corporation All Rights Reserved March, 2008
The exposure - tumor size – survival model: A bridge from Phase II to Phase III endpoints Pha se 2 Dose Exposure Tumor size dynamics Drug specific Disease/Patient specific Pha se 3 Survival Disease specific To predict phase 3 endpoint based on phase 2 endpoint and prognostic factors Claret L et al. Model-based predictions of expected anti-tumor response and survival in Phase III studies based on phase II data of an investigational agent. Proc ASCO, 24 (18 S), 307 s (Abs 6025), 2006. © Pharsight Corporation All Rights Reserved March, 2008
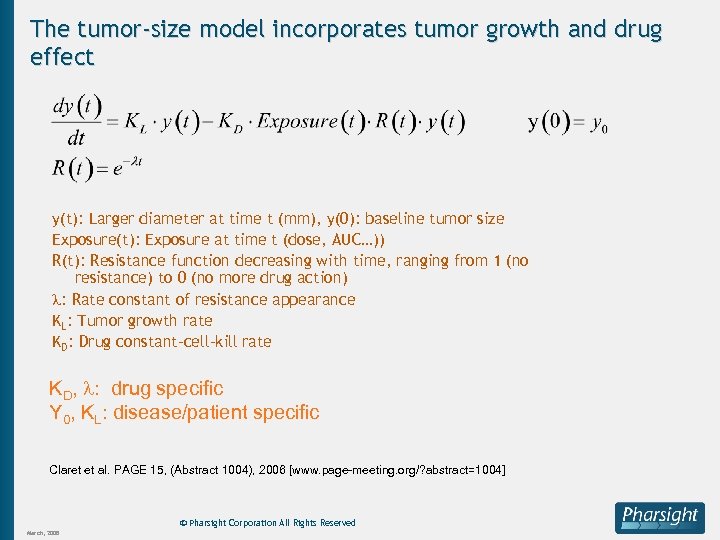 The tumor-size model incorporates tumor growth and drug effect y(t): Larger diameter at time t (mm), y(0): baseline tumor size Exposure(t): Exposure at time t (dose, AUC…)) R(t): Resistance function decreasing with time, ranging from 1 (no resistance) to 0 (no more drug action) : Rate constant of resistance appearance KL: Tumor growth rate KD: Drug constant-cell-kill rate KD, : drug specific Y 0, KL: disease/patient specific Claret et al. PAGE 15, (Abstract 1004), 2006 [www. page-meeting. org/? abstract=1004] © Pharsight Corporation All Rights Reserved March, 2008
The tumor-size model incorporates tumor growth and drug effect y(t): Larger diameter at time t (mm), y(0): baseline tumor size Exposure(t): Exposure at time t (dose, AUC…)) R(t): Resistance function decreasing with time, ranging from 1 (no resistance) to 0 (no more drug action) : Rate constant of resistance appearance KL: Tumor growth rate KD: Drug constant-cell-kill rate KD, : drug specific Y 0, KL: disease/patient specific Claret et al. PAGE 15, (Abstract 1004), 2006 [www. page-meeting. org/? abstract=1004] © Pharsight Corporation All Rights Reserved March, 2008
 Support to end-of-Phase II development decisions: A retrospective project with capecitabine (Roche) Goal: To support early drug development decisions ● Go/No go ● Design of Phase III studies Simulate expected survival difference in Phase III ● Comparing a new drug (X) to a reference drug (R) ● Based on Phase II data of X and historical data of R Retrospective project: ● To simulate: • Phase III of capecitabine (X) + docetaxel (R) vs. docetaxel in MBC • Phase III of capecitabine (X) vs. 5 -Fu (R) in CRC Claret L et al. Model-based predictions of expected anti-tumor response and survival in Phase III studies based on phase II data of an investigational agent. Proc ASCO, 24 (18 S), 307 s (Abs 6025), 2006. 64 March, 2008 © Pharsight Corporation All Rights Reserved
Support to end-of-Phase II development decisions: A retrospective project with capecitabine (Roche) Goal: To support early drug development decisions ● Go/No go ● Design of Phase III studies Simulate expected survival difference in Phase III ● Comparing a new drug (X) to a reference drug (R) ● Based on Phase II data of X and historical data of R Retrospective project: ● To simulate: • Phase III of capecitabine (X) + docetaxel (R) vs. docetaxel in MBC • Phase III of capecitabine (X) vs. 5 -Fu (R) in CRC Claret L et al. Model-based predictions of expected anti-tumor response and survival in Phase III studies based on phase II data of an investigational agent. Proc ASCO, 24 (18 S), 307 s (Abs 6025), 2006. 64 March, 2008 © Pharsight Corporation All Rights Reserved
 Simulation of a Phase III study comparing docetaxel to docetaxel + capecitabine in MBC Model parameter estimation ● Capecitabine data • Phase II (2 studies, 170 patients) ● Docetaxel data • Phase III (docetaxel arm, 223 patients) Simulation ● Phase III study of capecitabine + docetaxel vs. docetaxel (443 patients, 1000 replicates) • Assumes additive effect for the combination • Capecitabine scaled from Phase II to Phase III using disease specific parameters Focus on efficacy, no model for dose-limiting side-effects ● Simulations conditioned on observed dose intensity (dosing history) ● Drug effect driven by dose 65 March, 2008 © Pharsight Corporation All Rights Reserved
Simulation of a Phase III study comparing docetaxel to docetaxel + capecitabine in MBC Model parameter estimation ● Capecitabine data • Phase II (2 studies, 170 patients) ● Docetaxel data • Phase III (docetaxel arm, 223 patients) Simulation ● Phase III study of capecitabine + docetaxel vs. docetaxel (443 patients, 1000 replicates) • Assumes additive effect for the combination • Capecitabine scaled from Phase II to Phase III using disease specific parameters Focus on efficacy, no model for dose-limiting side-effects ● Simulations conditioned on observed dose intensity (dosing history) ● Drug effect driven by dose 65 March, 2008 © Pharsight Corporation All Rights Reserved
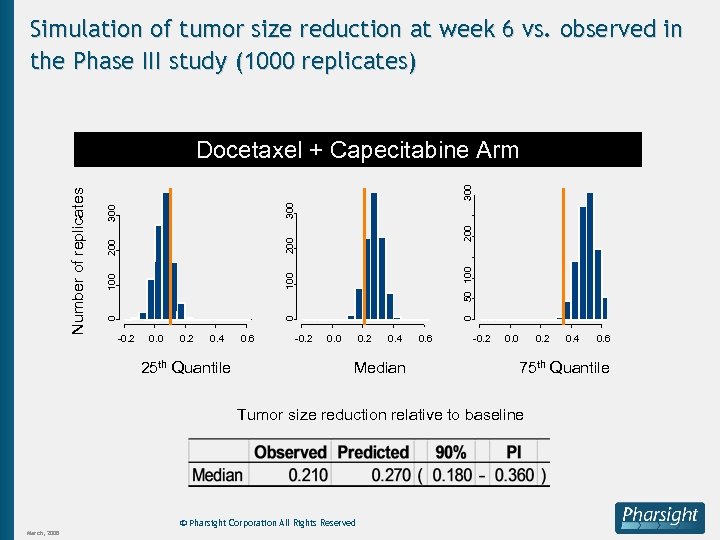 Simulation of tumor size reduction at week 6 vs. observed in the Phase III study (1000 replicates) 300 200 300 0 0 100 50 100 200 300 200 100 0 Number of replicates Docetaxel + Capecitabine Arm -0. 2 0. 0 0. 2 0. 4 25 th Quantile 0. 6 -0. 2 0. 0 0. 2 0. 4 Median 0. 6 -0. 2 0. 0 0. 2 © Pharsight Corporation All Rights Reserved 0. 6 75 th Quantile Tumor size reduction relative to baseline March, 2008 0. 4
Simulation of tumor size reduction at week 6 vs. observed in the Phase III study (1000 replicates) 300 200 300 0 0 100 50 100 200 300 200 100 0 Number of replicates Docetaxel + Capecitabine Arm -0. 2 0. 0 0. 2 0. 4 25 th Quantile 0. 6 -0. 2 0. 0 0. 2 0. 4 Median 0. 6 -0. 2 0. 0 0. 2 © Pharsight Corporation All Rights Reserved 0. 6 75 th Quantile Tumor size reduction relative to baseline March, 2008 0. 4
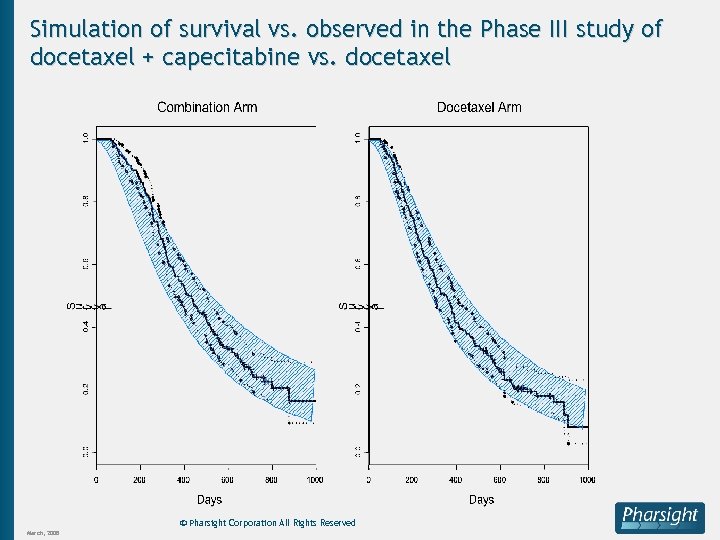 Simulation of survival vs. observed in the Phase III study of docetaxel + capecitabine vs. docetaxel © Pharsight Corporation All Rights Reserved March, 2008
Simulation of survival vs. observed in the Phase III study of docetaxel + capecitabine vs. docetaxel © Pharsight Corporation All Rights Reserved March, 2008
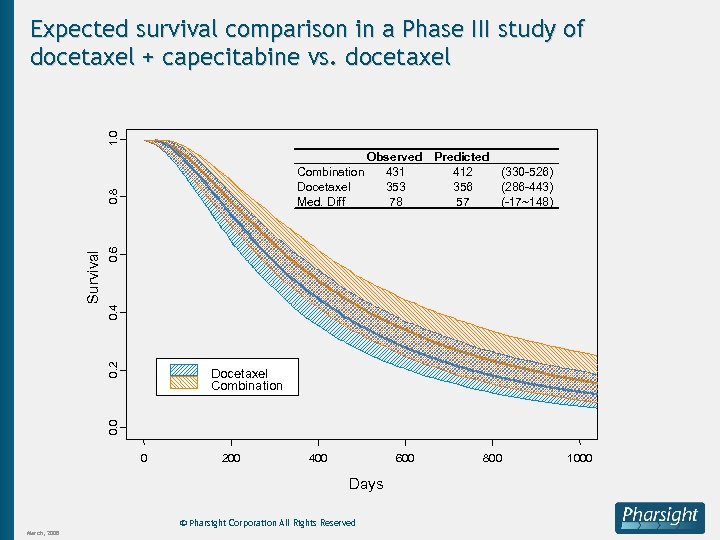 1. 0 Expected survival comparison in a Phase III study of docetaxel + capecitabine vs. docetaxel Predicted 412 (330 -526) 356 (286 -443) 57 (-17~148) 0. 6 Observed 431 353 78 0. 2 0. 4 Survival 0. 8 Combination Docetaxel Med. Diff 0. 0 Docetaxel Combination 0 200 400 600 Days © Pharsight Corporation All Rights Reserved March, 2008 800 1000
1. 0 Expected survival comparison in a Phase III study of docetaxel + capecitabine vs. docetaxel Predicted 412 (330 -526) 356 (286 -443) 57 (-17~148) 0. 6 Observed 431 353 78 0. 2 0. 4 Survival 0. 8 Combination Docetaxel Med. Diff 0. 0 Docetaxel Combination 0 200 400 600 Days © Pharsight Corporation All Rights Reserved March, 2008 800 1000
 Capecitabine project conclusions The structure of the tumor size model was robust to predict tumor growth and anti-tumor effect of: ● Three cytotoxic drugs in two tumor types Change in tumor size was a good predictor of survival ● Modeling of longitudinal tumor size data is much more informative than response rate determination ● Poor predictor of survival (primary endpoint in Phase III) • Study-level correlations (Buyse Lancet 2000, Shanafelt JCO 2004) • Even more problematic with new targeted therapies (cytostatic rather than cytotoxic) The combined tumor size and survival models: ● Successfully predicted expected treatment differences ● Is a useful approach to support early development decisions: • Does the expected survival benefit of the new drug warrant further development? • If yes, which Phase III study need be designed to show non-inferiority, superiority? 69 March, 2008 © Pharsight Corporation All Rights Reserved
Capecitabine project conclusions The structure of the tumor size model was robust to predict tumor growth and anti-tumor effect of: ● Three cytotoxic drugs in two tumor types Change in tumor size was a good predictor of survival ● Modeling of longitudinal tumor size data is much more informative than response rate determination ● Poor predictor of survival (primary endpoint in Phase III) • Study-level correlations (Buyse Lancet 2000, Shanafelt JCO 2004) • Even more problematic with new targeted therapies (cytostatic rather than cytotoxic) The combined tumor size and survival models: ● Successfully predicted expected treatment differences ● Is a useful approach to support early development decisions: • Does the expected survival benefit of the new drug warrant further development? • If yes, which Phase III study need be designed to show non-inferiority, superiority? 69 March, 2008 © Pharsight Corporation All Rights Reserved
 Pharsight uses the FDA NSCLC model Availability of data to develop the survival model is problematic in many companies The availability of generic public-domain models is critical We used the FDA NSCLC model (Wang et al, DIA, 2007) in one of our projects ● Pharsight Uses FDA Disease Model to Support Oncology Drug Development: http: //media. corporate-ir. net/media_files/irol/12/121504/Release 112007. pdf The company was interested in getting expectations of survival for a NCE in combination ● To support decision to start a large Phase III study ● They had a Phase Ib combination study in NSCLC (less than 30 patients) • We used the FDA model and simulated expected survival based on: ◦ Observed tumor shrinkage ◦ Patient’s prognostic factors ● The Pharmacometry team (Drs. Wang and Gobburu) provided us the information we needed The model will be used in several other projects soon 70 March, 2008 © Pharsight Corporation All Rights Reserved
Pharsight uses the FDA NSCLC model Availability of data to develop the survival model is problematic in many companies The availability of generic public-domain models is critical We used the FDA NSCLC model (Wang et al, DIA, 2007) in one of our projects ● Pharsight Uses FDA Disease Model to Support Oncology Drug Development: http: //media. corporate-ir. net/media_files/irol/12/121504/Release 112007. pdf The company was interested in getting expectations of survival for a NCE in combination ● To support decision to start a large Phase III study ● They had a Phase Ib combination study in NSCLC (less than 30 patients) • We used the FDA model and simulated expected survival based on: ◦ Observed tumor shrinkage ◦ Patient’s prognostic factors ● The Pharmacometry team (Drs. Wang and Gobburu) provided us the information we needed The model will be used in several other projects soon 70 March, 2008 © Pharsight Corporation All Rights Reserved
 A case study to illustrate the use of the FDA NSCLC model with erlotinib data Tumor shrinkage data are generally not reported in papers Karrison, Maitland, Stadler and Ratain recently proposed to use change in tumor size as the primary endpoint in randomized Phase II trials (J Natl Cancer Inst, 99, 1455 -1461, 2007) ● In table 1 they report data for change in tumor size from 4 trials ● We used data from the pivotal erlotinib trial in 2 nd line patients (Shepherd et al. New Engl J Med, 353, 123 -132) • 488 patients in the erlotinib arm • ORR: 8. 9% • Survival: 6. 7 months 71 March, 2008 © Pharsight Corporation All Rights Reserved
A case study to illustrate the use of the FDA NSCLC model with erlotinib data Tumor shrinkage data are generally not reported in papers Karrison, Maitland, Stadler and Ratain recently proposed to use change in tumor size as the primary endpoint in randomized Phase II trials (J Natl Cancer Inst, 99, 1455 -1461, 2007) ● In table 1 they report data for change in tumor size from 4 trials ● We used data from the pivotal erlotinib trial in 2 nd line patients (Shepherd et al. New Engl J Med, 353, 123 -132) • 488 patients in the erlotinib arm • ORR: 8. 9% • Survival: 6. 7 months 71 March, 2008 © Pharsight Corporation All Rights Reserved
 A case study to illustrate the use of the FDA NSCLC model with erlotinib data (cont. ) Data used for the simulation ● Week 8 fractional change data based on Karrison reported log ratio • • • The ratio of tumor size at week 8 to baseline size is normally distributed Log ratio was sampled from normal distribution (Table 1): Mean 0. 048, SD: 0. 340 Shrinkage = exp(logratio)-1 (distribution given in backup) ● Baseline tumor size distribution • • Sampled from lognormal distribution Mean: 100 mm, SD: 57 mm (distribution in backup) ● EGOG 0, 1 proportion (80/20%) based on observed in Shepherd • • Shepherd trial included 66 % ECOG 0, 1 and 34 % ECOG 2, 3 patients (Table 1) Among ECOG 0, 1 patients, 20% had ECOG 1 and 80% ECOG 2 We simulated 1000 replicates of a virtual treatment arm of 300 patients (second–line, ECOG 0 or 1) ● C and D models (assumed to be second line treatments) were used to simulate 1000 replicates • • 25% of the replicates with C 1, C 2, D 1 and D 2 Parameters for each of the replicates were sampled in uncertainty of estimates ● Adjusted with early dropouts 72 March, 2008 © Pharsight Corporation All Rights Reserved
A case study to illustrate the use of the FDA NSCLC model with erlotinib data (cont. ) Data used for the simulation ● Week 8 fractional change data based on Karrison reported log ratio • • • The ratio of tumor size at week 8 to baseline size is normally distributed Log ratio was sampled from normal distribution (Table 1): Mean 0. 048, SD: 0. 340 Shrinkage = exp(logratio)-1 (distribution given in backup) ● Baseline tumor size distribution • • Sampled from lognormal distribution Mean: 100 mm, SD: 57 mm (distribution in backup) ● EGOG 0, 1 proportion (80/20%) based on observed in Shepherd • • Shepherd trial included 66 % ECOG 0, 1 and 34 % ECOG 2, 3 patients (Table 1) Among ECOG 0, 1 patients, 20% had ECOG 1 and 80% ECOG 2 We simulated 1000 replicates of a virtual treatment arm of 300 patients (second–line, ECOG 0 or 1) ● C and D models (assumed to be second line treatments) were used to simulate 1000 replicates • • 25% of the replicates with C 1, C 2, D 1 and D 2 Parameters for each of the replicates were sampled in uncertainty of estimates ● Adjusted with early dropouts 72 March, 2008 © Pharsight Corporation All Rights Reserved
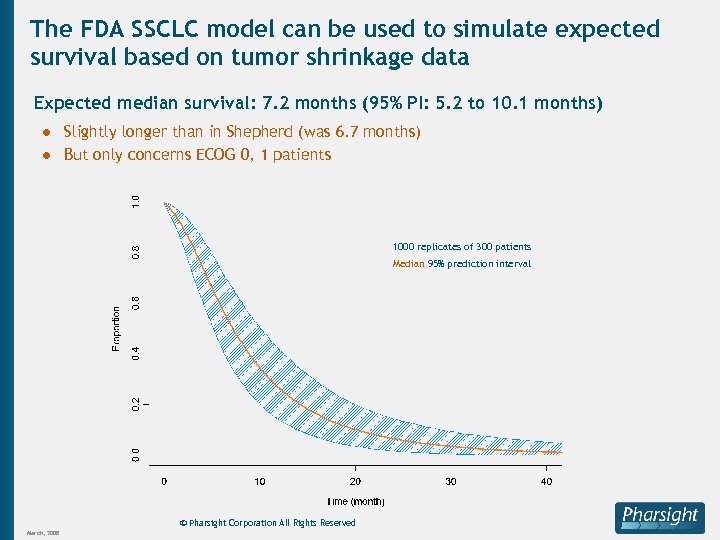 The FDA SSCLC model can be used to simulate expected survival based on tumor shrinkage data Expected median survival: 7. 2 months (95% PI: 5. 2 to 10. 1 months) ● Slightly longer than in Shepherd (was 6. 7 months) ● But only concerns ECOG 0, 1 patients 1000 replicates of 300 patients Median 95% prediction interval © Pharsight Corporation All Rights Reserved March, 2008
The FDA SSCLC model can be used to simulate expected survival based on tumor shrinkage data Expected median survival: 7. 2 months (95% PI: 5. 2 to 10. 1 months) ● Slightly longer than in Shepherd (was 6. 7 months) ● But only concerns ECOG 0, 1 patients 1000 replicates of 300 patients Median 95% prediction interval © Pharsight Corporation All Rights Reserved March, 2008
 Value of the survival simulations The survival probability distribution of an investigational treatment can be quantified based on early tumor shrinkage clinical data (typically available in Phase Ib or II) ● Can be a new NCE ● Can be a new combination treatment An arm of the investigational treatment can be simulated conditional on a sample size ● To mimic a clinical trial arm These simulations can be compared to a survival distribution from a reference treatment ● Expected treatment arm difference can support • • Go/no go decision Phase III clinical trial design Phase III clinical trials can be simulated to assess probability of success 74 March, 2008 © Pharsight Corporation All Rights Reserved
Value of the survival simulations The survival probability distribution of an investigational treatment can be quantified based on early tumor shrinkage clinical data (typically available in Phase Ib or II) ● Can be a new NCE ● Can be a new combination treatment An arm of the investigational treatment can be simulated conditional on a sample size ● To mimic a clinical trial arm These simulations can be compared to a survival distribution from a reference treatment ● Expected treatment arm difference can support • • Go/no go decision Phase III clinical trial design Phase III clinical trials can be simulated to assess probability of success 74 March, 2008 © Pharsight Corporation All Rights Reserved
 Conclusions Change in tumor size is a good predictor of survival ● Response rate (the primary endpoint in Phase II) is a poor predictor of survival • Study-level correlations (Buyse Lancet 2000, Shanafelt JCO 2004) ● Modeling of longitudinal tumor size data is much more informative than response rate determination to predict clinical benefit ● Supports use of change in tumor size as a primary endpoint in Phase II studies Drug-independent survival models allow to predict survival expectations or simulate Phase III trials based on early Phase Ib or Phase II data ● Availability of these models is limited ● FDA is in a unique position to develop such models without disclosing proprietary information ● Models for other endpoints (e. g. PFS) might be needed A modeling framework combining longitudinal tumor size models and drug independent survival models can be used ● To predict expected treatment efficacy (ORR, Survival, PFS) ● To simulate clinical trials ● To support • • • 75 March, 2008 Drug development decisions Clinical trial design Drug registration © Pharsight Corporation All Rights Reserved
Conclusions Change in tumor size is a good predictor of survival ● Response rate (the primary endpoint in Phase II) is a poor predictor of survival • Study-level correlations (Buyse Lancet 2000, Shanafelt JCO 2004) ● Modeling of longitudinal tumor size data is much more informative than response rate determination to predict clinical benefit ● Supports use of change in tumor size as a primary endpoint in Phase II studies Drug-independent survival models allow to predict survival expectations or simulate Phase III trials based on early Phase Ib or Phase II data ● Availability of these models is limited ● FDA is in a unique position to develop such models without disclosing proprietary information ● Models for other endpoints (e. g. PFS) might be needed A modeling framework combining longitudinal tumor size models and drug independent survival models can be used ● To predict expected treatment efficacy (ORR, Survival, PFS) ● To simulate clinical trials ● To support • • • 75 March, 2008 Drug development decisions Clinical trial design Drug registration © Pharsight Corporation All Rights Reserved
 Acknowledgements Contributors to the Roche project ● P. Girard, Pharsight corporation, now with INSERM, University of Lyon, France ● K. Zuideveld, K. Jorga, J. Fagerberg, F. Sirzen, M. Abt, F. Hoffmann-La Roche J. O’Shaughnessy, Baylor-Sammons Cancer Center ● P. Hoff, MD Anderson Cancer Center ● E. Van Cutsem, University Hospital Gasthuisberg ● J. Blum, US Oncology Dallas FDA pharmacometrics team ● Y. Wang and J. Gobburu 76 March, 2008 © Pharsight Corporation All Rights Reserved
Acknowledgements Contributors to the Roche project ● P. Girard, Pharsight corporation, now with INSERM, University of Lyon, France ● K. Zuideveld, K. Jorga, J. Fagerberg, F. Sirzen, M. Abt, F. Hoffmann-La Roche J. O’Shaughnessy, Baylor-Sammons Cancer Center ● P. Hoff, MD Anderson Cancer Center ● E. Van Cutsem, University Hospital Gasthuisberg ● J. Blum, US Oncology Dallas FDA pharmacometrics team ● Y. Wang and J. Gobburu 76 March, 2008 © Pharsight Corporation All Rights Reserved


