a005c9e61398e56b94c6434d88e1dd16.ppt
- Количество слайдов: 20
 PH 103 Dr. Cecilia Vogel Lecture 21
PH 103 Dr. Cecilia Vogel Lecture 21
 Review a. Spectra aof hydrogen aof multi-electron atoms Outline a. Fluorescence a. Nuclei aproperties acomposition, N, Z, A aenergy
Review a. Spectra aof hydrogen aof multi-electron atoms Outline a. Fluorescence a. Nuclei aproperties acomposition, N, Z, A aenergy
 Transition Up a. Electron absorbs energy aperhaps from a photon agoes to a higher energy level eephoton
Transition Up a. Electron absorbs energy aperhaps from a photon agoes to a higher energy level eephoton
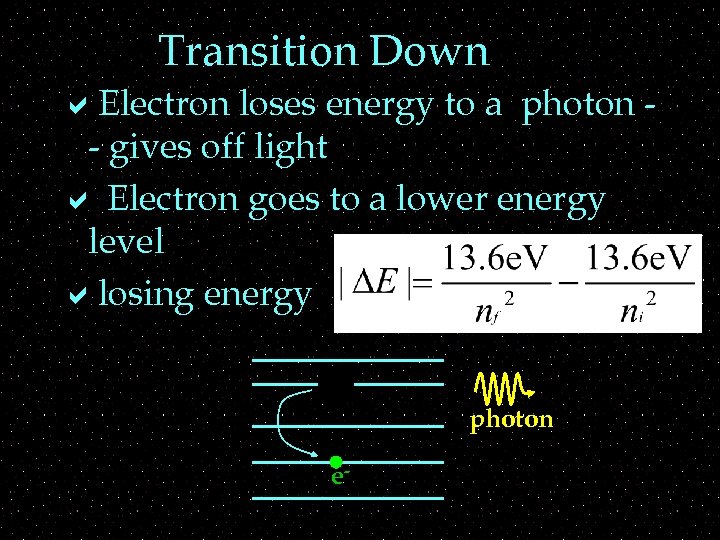 Transition Down a. Electron loses energy to a photon - gives off light a Electron goes to a lower energy level alosing energy ee- photon
Transition Down a. Electron loses energy to a photon - gives off light a Electron goes to a lower energy level alosing energy ee- photon
 Fluorescence a. Fluorescent material excited by aabsorbing light of short wavelength, high frequency, like UV a. Fluorescent material then de-excites by a emitting longer wavelength light a twice a. Two photons are emitted atotal energy of the two photons = energy of the absorbed UV photon
Fluorescence a. Fluorescent material excited by aabsorbing light of short wavelength, high frequency, like UV a. Fluorescent material then de-excites by a emitting longer wavelength light a twice a. Two photons are emitted atotal energy of the two photons = energy of the absorbed UV photon
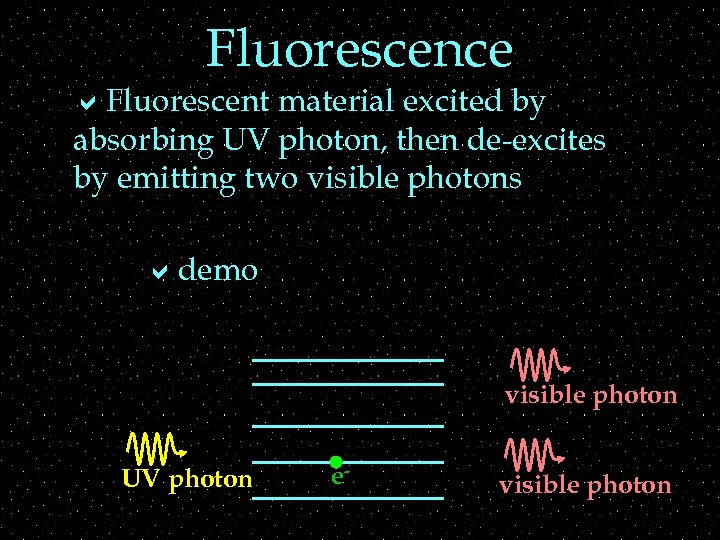 Fluorescence a. Fluorescent material excited by absorbing UV photon, then de-excites by emitting two visible photons ademo visible photon UV photon e- visible photon
Fluorescence a. Fluorescent material excited by absorbing UV photon, then de-excites by emitting two visible photons ademo visible photon UV photon e- visible photon
 Fluorescent lights a. Mercury discharge tube is basis a. Hg excited by electric discharge a. Hg gives off many wavelengths aincluding UV a. The UV given off by Hg aexcites the fluorescent material on the tube’s surface awhich in turn gives off visible light (fluoresces) athat’s what we see
Fluorescent lights a. Mercury discharge tube is basis a. Hg excited by electric discharge a. Hg gives off many wavelengths aincluding UV a. The UV given off by Hg aexcites the fluorescent material on the tube’s surface awhich in turn gives off visible light (fluoresces) athat’s what we see
 a. Very small Nucleus asize is several Fermi a= femtometer = fm = 10 -15 m acompare to size of atom = several Å = 10 -10 m a. If the atom is scaled up to the size of the Earth, the nucleus would scale up to the size of a house. a. Very heavy a. About 99. 98% of the mass of the atom is nucleus. a. Nucleus about 4000 times as massive as electrons.
a. Very small Nucleus asize is several Fermi a= femtometer = fm = 10 -15 m acompare to size of atom = several Å = 10 -10 m a. If the atom is scaled up to the size of the Earth, the nucleus would scale up to the size of a house. a. Very heavy a. About 99. 98% of the mass of the atom is nucleus. a. Nucleus about 4000 times as massive as electrons.
 a. Very dense Nucleus a. If this building were as dense as nucleus, it would have as much mass as the whole Earth! a. Positively charged athe charge of a nucleus is +Ze a. Z= atomic number of element aso with Z electrons (-Ze) athe atom is neutral a. Made up of protons and neutrons atogether protons and neutrons are called nucleons
a. Very dense Nucleus a. If this building were as dense as nucleus, it would have as much mass as the whole Earth! a. Positively charged athe charge of a nucleus is +Ze a. Z= atomic number of element aso with Z electrons (-Ze) athe atom is neutral a. Made up of protons and neutrons atogether protons and neutrons are called nucleons
 Protons and Neutrons a. Proton apositive charge +e a. Neutron azero charge, neutral a. Both proton and neutron ahave mass almost 2000 times the electron’s mass
Protons and Neutrons a. Proton apositive charge +e a. Neutron azero charge, neutral a. Both proton and neutron ahave mass almost 2000 times the electron’s mass
 Counting Protons a. How many protons in nucleus acharge of nucleus is +Ze, so a. The number of protons must be a=Z a= atomic number adepends only on element aex – all carbon atoms have 6 protons ano matter what isotope ano matter what ion
Counting Protons a. How many protons in nucleus acharge of nucleus is +Ze, so a. The number of protons must be a=Z a= atomic number adepends only on element aex – all carbon atoms have 6 protons ano matter what isotope ano matter what ion
 Counting Neutrons a. How many neutrons in nucleus amany possibilities for each element. a. Different isotopes of same element have different numbers of neutrons a. N = neutron number aex: A nucleus with 6 protons and 6 neutrons is different from a nucleus with 6 protons and 7 neutrons a. They are different isotopes of carbon adiffer in # of neutrons
Counting Neutrons a. How many neutrons in nucleus amany possibilities for each element. a. Different isotopes of same element have different numbers of neutrons a. N = neutron number aex: A nucleus with 6 protons and 6 neutrons is different from a nucleus with 6 protons and 7 neutrons a. They are different isotopes of carbon adiffer in # of neutrons
 Counting Nucleons a. How many nucleons in the nucleus a. Let A = Z + N. a. A = “mass number” a. This is not the mass of the nucleus!!! a. This is NOT the mass of the nucleus!!!
Counting Nucleons a. How many nucleons in the nucleus a. Let A = Z + N. a. A = “mass number” a. This is not the mass of the nucleus!!! a. This is NOT the mass of the nucleus!!!
 Notation a. C is atomic symbol for carbon apre-subscript is the atomic number a 6 protons asubscript is the neutron number a 7 neutrons apre-superscript is the mass number a 13 total nucleons a. Don’t need to give N, can find it from a. N = A - Z (N = 13 - 6 = 7) a. Don’t need to give Z, can find it ain the periodic table (Carbon is Z=6)
Notation a. C is atomic symbol for carbon apre-subscript is the atomic number a 6 protons asubscript is the neutron number a 7 neutrons apre-superscript is the mass number a 13 total nucleons a. Don’t need to give N, can find it from a. N = A - Z (N = 13 - 6 = 7) a. Don’t need to give Z, can find it ain the periodic table (Carbon is Z=6)
 Strong Nuclear Force a. Strong nuclear force is what holds the nucleus together, a“Nuclear” because it aacts between nucleons, aprotons and neutrons alike; aelectrons unaffected
Strong Nuclear Force a. Strong nuclear force is what holds the nucleus together, a“Nuclear” because it aacts between nucleons, aprotons and neutrons alike; aelectrons unaffected
 Strong Nuclear Force a. Strong amust overcome electric repulsion, aand still hold the protons and neutrons in nucleus a million times stronger than electrons are held to atom. a. Compare aa few e. V to ionize an atom aseveral Me. V to get a neutron or proton out of nucleus
Strong Nuclear Force a. Strong amust overcome electric repulsion, aand still hold the protons and neutrons in nucleus a million times stronger than electrons are held to atom. a. Compare aa few e. V to ionize an atom aseveral Me. V to get a neutron or proton out of nucleus
 Energetics of Nucleus a. The protons and neutrons in the nucleus have less energy than free protons and neutrons ait requires an input of energy to make them free a. E(nucleus) < Z*E(free proton) + N* E(free neutron) amnucleusc 2 < Zmpc 2 + Nmnc 2 amnucleus < Zmp + Nmn amass of nucleus is less than the mass of its components!
Energetics of Nucleus a. The protons and neutrons in the nucleus have less energy than free protons and neutrons ait requires an input of energy to make them free a. E(nucleus) < Z*E(free proton) + N* E(free neutron) amnucleusc 2 < Zmpc 2 + Nmnc 2 amnucleus < Zmp + Nmn amass of nucleus is less than the mass of its components!
 Binding Energy a. The binding energy of atom a= energy it requires to break it apart into constituents a. BE =[Z(mp+me)c 2 + Nmnc 2] – matomc 2 a. BE =[Z(m 1 H)c 2 + Nmnc 2] – matomc 2
Binding Energy a. The binding energy of atom a= energy it requires to break it apart into constituents a. BE =[Z(mp+me)c 2 + Nmnc 2] – matomc 2 a. BE =[Z(m 1 H)c 2 + Nmnc 2] – matomc 2
 Where to Find the Info a. The only quantity of importance in nuclear physics that can be found in the periodic table is athe atomic number, Z aotherwise leave periodic table alone! a. Most of the info we need is in Appendix B a. Find the correct element a. Within that element, find the correct isotope a. Z and A both important a. Can find mass in Appendix B athis is mass of atom with Z electrons
Where to Find the Info a. The only quantity of importance in nuclear physics that can be found in the periodic table is athe atomic number, Z aotherwise leave periodic table alone! a. Most of the info we need is in Appendix B a. Find the correct element a. Within that element, find the correct isotope a. Z and A both important a. Can find mass in Appendix B athis is mass of atom with Z electrons
 Nuclear Units a. Often use atomic mass units for mass (abbrev. amu or u) a. Example mass of 107 Ag is 106. 905 u a. What units do we get when we do mc 2? a. Example mass energy of 107 Ag a= mc 2= (106. 905 u) c 2. a= 106. 905 uc 2 a. Do not leave these units in answers. a. Convert: 1 uc 2=931. 5 Me. V a 106. 905 uc 2= 99582 Me. V
Nuclear Units a. Often use atomic mass units for mass (abbrev. amu or u) a. Example mass of 107 Ag is 106. 905 u a. What units do we get when we do mc 2? a. Example mass energy of 107 Ag a= mc 2= (106. 905 u) c 2. a= 106. 905 uc 2 a. Do not leave these units in answers. a. Convert: 1 uc 2=931. 5 Me. V a 106. 905 uc 2= 99582 Me. V


