 PFR design. Accounting for pressure drop Chemical Reaction Engineering I Aug 2011 - Dec 2011 Dept. Chem. Engg. , IIT-Madras
PFR design. Accounting for pressure drop Chemical Reaction Engineering I Aug 2011 - Dec 2011 Dept. Chem. Engg. , IIT-Madras
 Overview • Notation • PFR design equation (mass balance) • Pressure drop equation • Accounts for change in number of moles (due to reaction) • Does not consider phase change • Methodology, with examples • Liquid phase reaction: Calculations are simple • Gas phase reaction: Calculations are more involved
Overview • Notation • PFR design equation (mass balance) • Pressure drop equation • Accounts for change in number of moles (due to reaction) • Does not consider phase change • Methodology, with examples • Liquid phase reaction: Calculations are simple • Gas phase reaction: Calculations are more involved
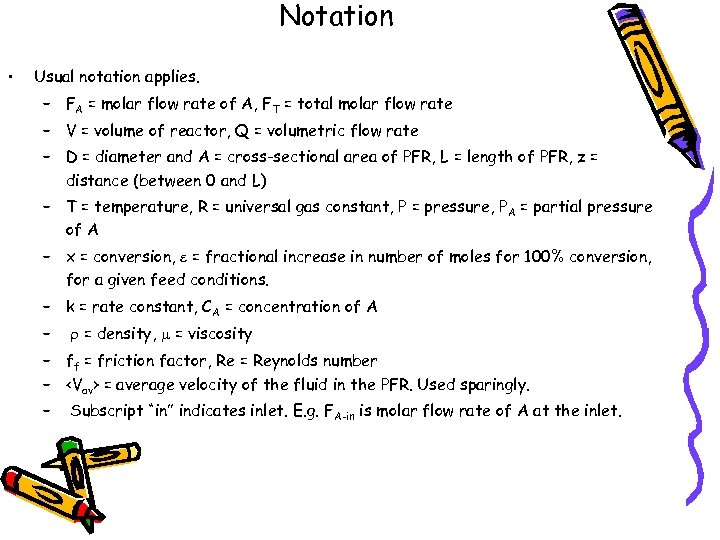 Notation • Usual notation applies. – FA = molar flow rate of A, FT = total molar flow rate – V = volume of reactor, Q = volumetric flow rate – D = diameter and A = cross-sectional area of PFR, L = length of PFR, z = distance (between 0 and L) – T = temperature, R = universal gas constant, P = pressure, P A = partial pressure of A – x = conversion, e = fractional increase in number of moles for 100% conversion, for a given feed conditions. – k = rate constant, CA = concentration of A – r = density, m = viscosity – ff = friction factor, Re = Reynolds number – = average velocity of the fluid in the PFR. Used sparingly. – Subscript “in” indicates inlet. E. g. FA-in is molar flow rate of A at the inlet.
Notation • Usual notation applies. – FA = molar flow rate of A, FT = total molar flow rate – V = volume of reactor, Q = volumetric flow rate – D = diameter and A = cross-sectional area of PFR, L = length of PFR, z = distance (between 0 and L) – T = temperature, R = universal gas constant, P = pressure, P A = partial pressure of A – x = conversion, e = fractional increase in number of moles for 100% conversion, for a given feed conditions. – k = rate constant, CA = concentration of A – r = density, m = viscosity – ff = friction factor, Re = Reynolds number – = average velocity of the fluid in the PFR. Used sparingly. – Subscript “in” indicates inlet. E. g. FA-in is molar flow rate of A at the inlet.
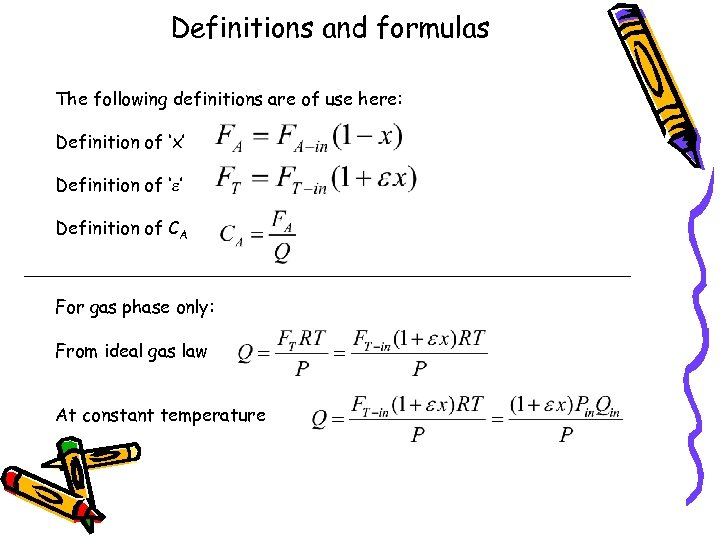 Definitions and formulas The following definitions are of use here: Definition of ‘x’ Definition of ‘e’ Definition of CA For gas phase only: From ideal gas law At constant temperature
Definitions and formulas The following definitions are of use here: Definition of ‘x’ Definition of ‘e’ Definition of CA For gas phase only: From ideal gas law At constant temperature
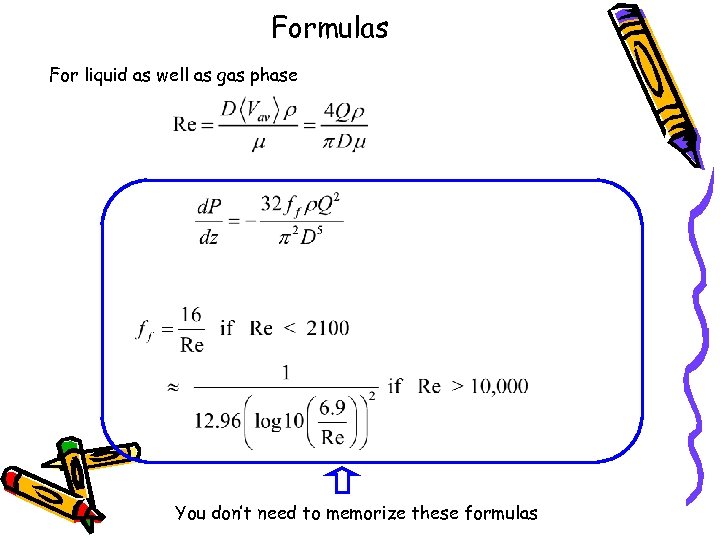 Formulas For liquid as well as gas phase You don’t need to memorize these formulas
Formulas For liquid as well as gas phase You don’t need to memorize these formulas
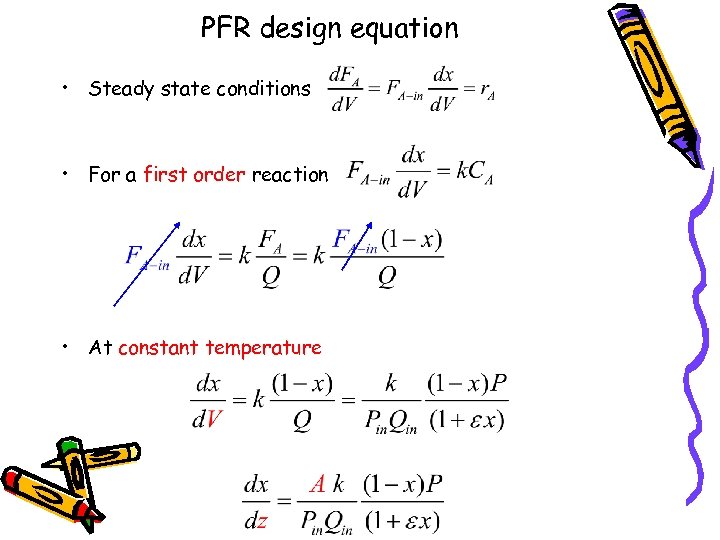 PFR design equation • Steady state conditions • For a first order reaction • At constant temperature
PFR design equation • Steady state conditions • For a first order reaction • At constant temperature
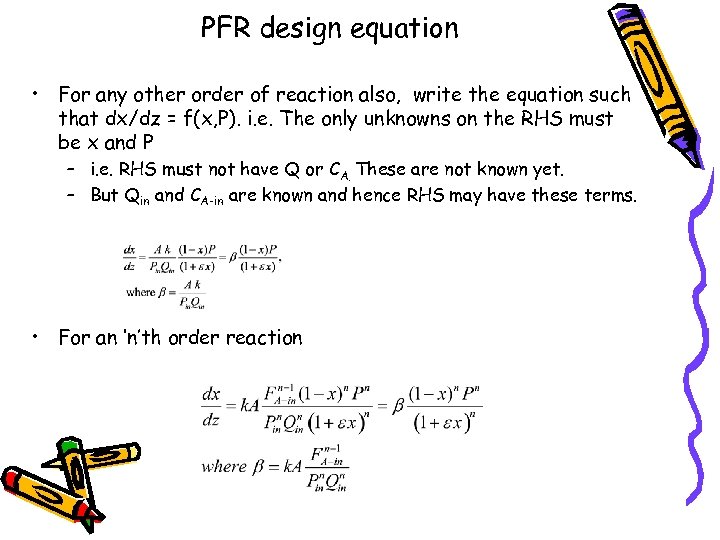 PFR design equation • For any other order of reaction also, write the equation such that dx/dz = f(x, P). i. e. The only unknowns on the RHS must be x and P – i. e. RHS must not have Q or CA. These are not known yet. – But Qin and CA-in are known and hence RHS may have these terms. • For an ‘n’th order reaction
PFR design equation • For any other order of reaction also, write the equation such that dx/dz = f(x, P). i. e. The only unknowns on the RHS must be x and P – i. e. RHS must not have Q or CA. These are not known yet. – But Qin and CA-in are known and hence RHS may have these terms. • For an ‘n’th order reaction
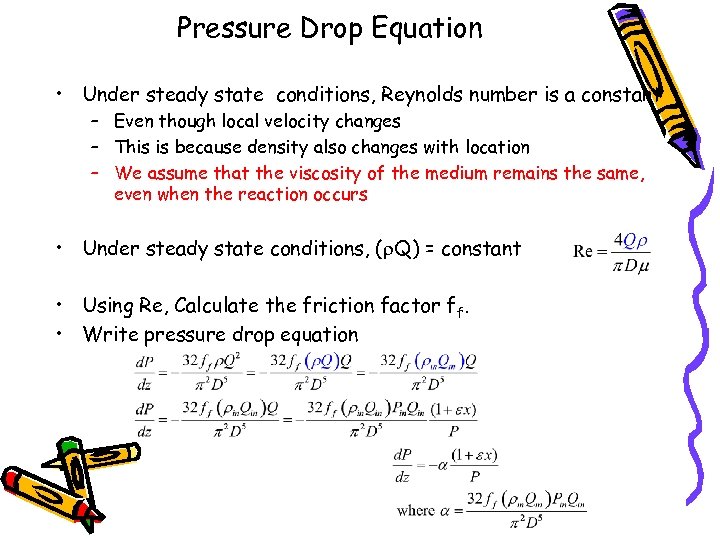 Pressure Drop Equation • Under steady state conditions, Reynolds number is a constant – Even though local velocity changes – This is because density also changes with location – We assume that the viscosity of the medium remains the same, even when the reaction occurs • Under steady state conditions, (r. Q) = constant • Using Re, Calculate the friction factor ff. • Write pressure drop equation
Pressure Drop Equation • Under steady state conditions, Reynolds number is a constant – Even though local velocity changes – This is because density also changes with location – We assume that the viscosity of the medium remains the same, even when the reaction occurs • Under steady state conditions, (r. Q) = constant • Using Re, Calculate the friction factor ff. • Write pressure drop equation
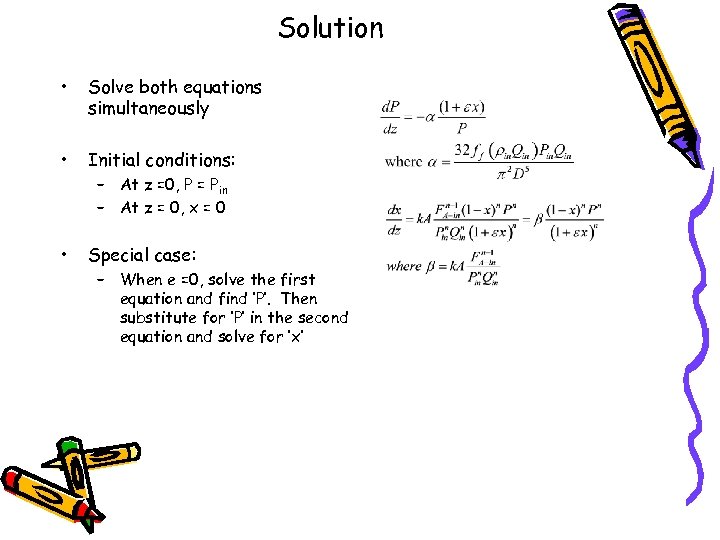 Solution • Solve both equations simultaneously • Initial conditions: • Special case: – At z =0, P = Pin – At z = 0, x = 0 – When e =0, solve the first equation and find ‘P’. Then substitute for ‘P’ in the second equation and solve for ‘x’
Solution • Solve both equations simultaneously • Initial conditions: • Special case: – At z =0, P = Pin – At z = 0, x = 0 – When e =0, solve the first equation and find ‘P’. Then substitute for ‘P’ in the second equation and solve for ‘x’
 Example • Consider a gas phase reaction under isothermal conditions. (Isomerization) • A B • Pin = 10 atm, Q = 0. 005 m 3/s, T = 300 K, Pure A is fed, k = 0. 1 lit/s, Molecular weight M = 60 g/gmol, Viscosity of the gas = 10 -5 Pa-s • What should be the length of the PFR if it is constructed (a) using a 2 cm pipe and the conversion desired is 10%? (b) using a 1. 5 cm pipe and the conversion desired is 10% and (c) using a 1. 5 cm pipe and the conversion desired is 20%
Example • Consider a gas phase reaction under isothermal conditions. (Isomerization) • A B • Pin = 10 atm, Q = 0. 005 m 3/s, T = 300 K, Pure A is fed, k = 0. 1 lit/s, Molecular weight M = 60 g/gmol, Viscosity of the gas = 10 -5 Pa-s • What should be the length of the PFR if it is constructed (a) using a 2 cm pipe and the conversion desired is 10%? (b) using a 1. 5 cm pipe and the conversion desired is 10% and (c) using a 1. 5 cm pipe and the conversion desired is 20%
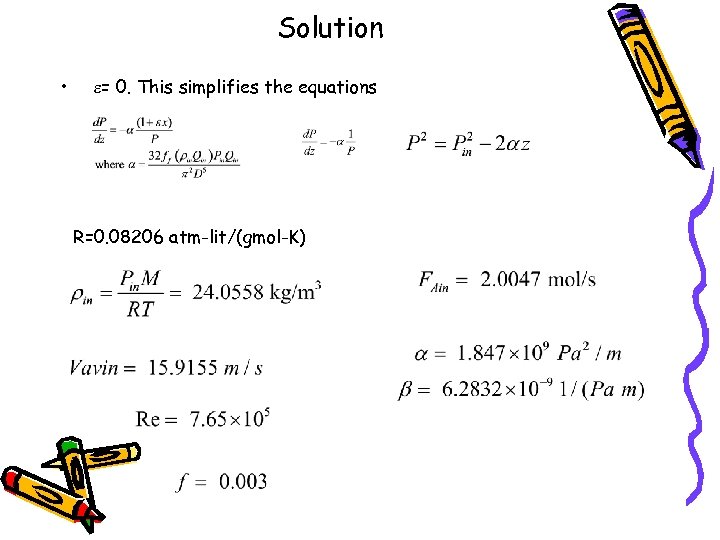 Solution • e= 0. This simplifies the equations R=0. 08206 atm-lit/(gmol-K)
Solution • e= 0. This simplifies the equations R=0. 08206 atm-lit/(gmol-K)
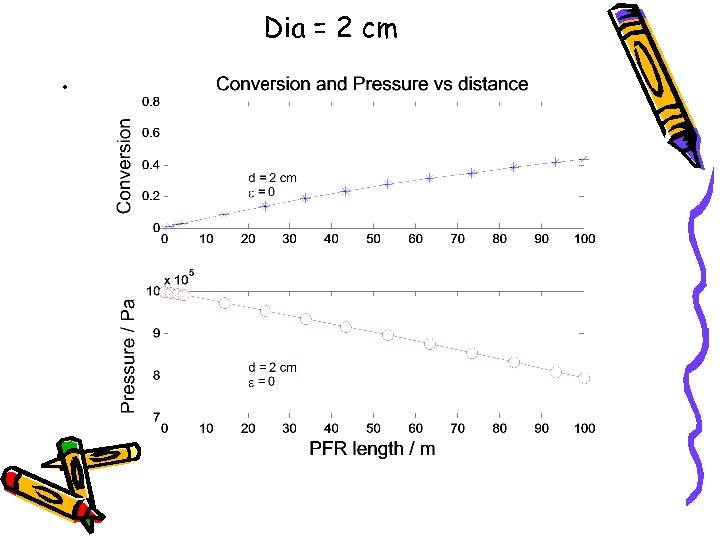 Dia = 2 cm •
Dia = 2 cm •
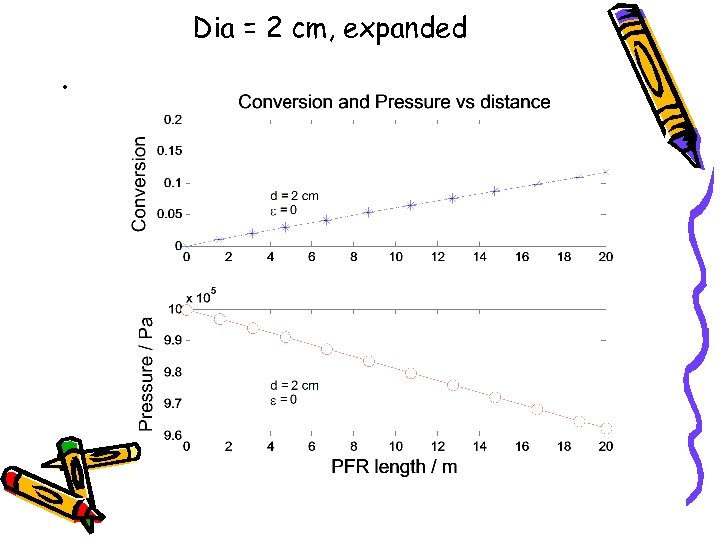 Dia = 2 cm, expanded •
Dia = 2 cm, expanded •
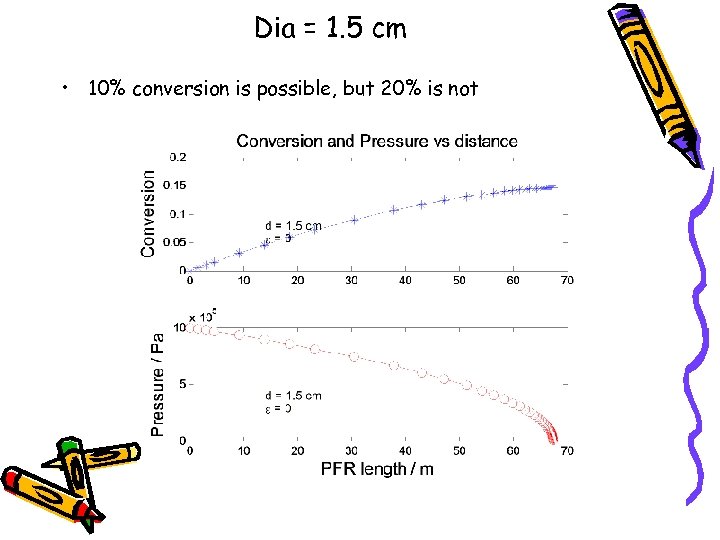 Dia = 1. 5 cm • 10% conversion is possible, but 20% is not
Dia = 1. 5 cm • 10% conversion is possible, but 20% is not