3.A._Petroleum.ppt
- Количество слайдов: 43
 PETROLEUM: WHAT IS IT? Script for car ad highlighted energy & fuelrelated features that claimed to “help conserve petroleum resources” What properties make petroleum useful for burning & building? Focus on key compounds in petroleum Their physical properties, molecular structure, & how atoms bond to make these key compounds
PETROLEUM: WHAT IS IT? Script for car ad highlighted energy & fuelrelated features that claimed to “help conserve petroleum resources” What properties make petroleum useful for burning & building? Focus on key compounds in petroleum Their physical properties, molecular structure, & how atoms bond to make these key compounds
 WHAT IS PETROLEUM? Crude oil – petroleum pumped from underground Mixture of many compounds Colorless to greenish-brown to black Fluid as water or as resistant to flow as soft tar. Crude oil is not useable Must be refined – separated into simpler mixtures
WHAT IS PETROLEUM? Crude oil – petroleum pumped from underground Mixture of many compounds Colorless to greenish-brown to black Fluid as water or as resistant to flow as soft tar. Crude oil is not useable Must be refined – separated into simpler mixtures
 WHAT IS PETROLEUM? Some mixtures are ready to use Others require more refining Refined petroleum is mainly a mixture of hydrocarbons, molecular compounds that contain only atoms of hydrogen (H) and carbon (C)
WHAT IS PETROLEUM? Some mixtures are ready to use Others require more refining Refined petroleum is mainly a mixture of hydrocarbons, molecular compounds that contain only atoms of hydrogen (H) and carbon (C)
 WHAT IS PETROLEUM? Most petroleum is used as a fuel (89%) Burning petroleum provides nearly ½ of the total U. S. energy needs Gasoline powers millions of U. S. automobiles, each traveling 14, 000 miles per year Other petroleum based fuels heat homes & office buildings, deliver energy to generate electricity, & power diesel engines & jet aircraft
WHAT IS PETROLEUM? Most petroleum is used as a fuel (89%) Burning petroleum provides nearly ½ of the total U. S. energy needs Gasoline powers millions of U. S. automobiles, each traveling 14, 000 miles per year Other petroleum based fuels heat homes & office buildings, deliver energy to generate electricity, & power diesel engines & jet aircraft
 WHAT IS PETROLEUM? Petroleum is also a raw material for many familiar & useful products New substances (e. g. , medications & plastics) (7%) Other products (e. g. , lubricants, road-paving tar) (4%) Nonrenewable resource
WHAT IS PETROLEUM? Petroleum is also a raw material for many familiar & useful products New substances (e. g. , medications & plastics) (7%) Other products (e. g. , lubricants, road-paving tar) (4%) Nonrenewable resource
 PETRO LEUM PRODU CTS
PETRO LEUM PRODU CTS
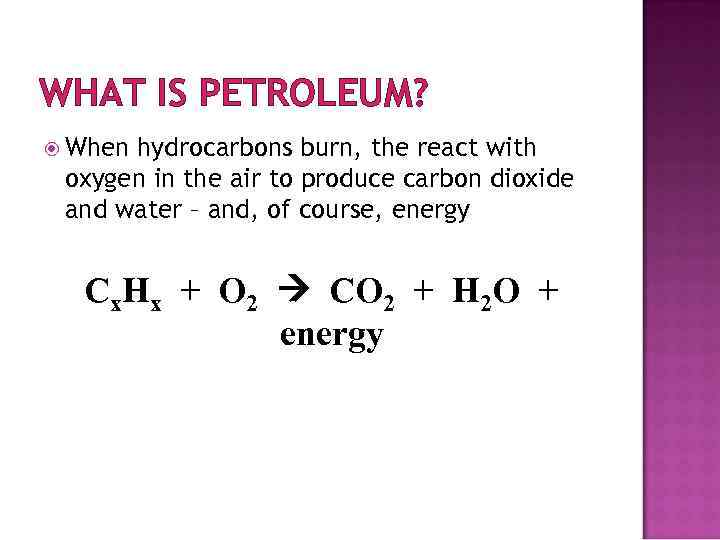 WHAT IS PETROLEUM? When hydrocarbons burn, the react with oxygen in the air to produce carbon dioxide and water – and, of course, energy Cx. Hx + O 2 CO 2 + H 2 O + energy
WHAT IS PETROLEUM? When hydrocarbons burn, the react with oxygen in the air to produce carbon dioxide and water – and, of course, energy Cx. Hx + O 2 CO 2 + H 2 O + energy
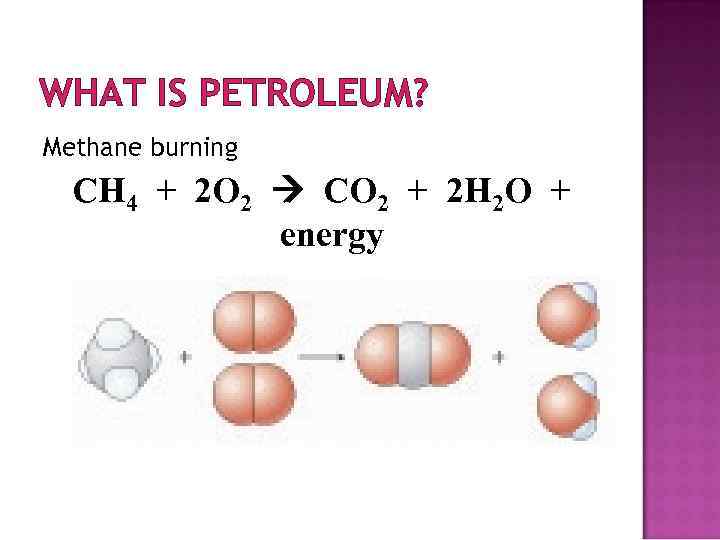 WHAT IS PETROLEUM? Methane burning CH 4 + 2 O 2 CO 2 + 2 H 2 O + energy
WHAT IS PETROLEUM? Methane burning CH 4 + 2 O 2 CO 2 + 2 H 2 O + energy
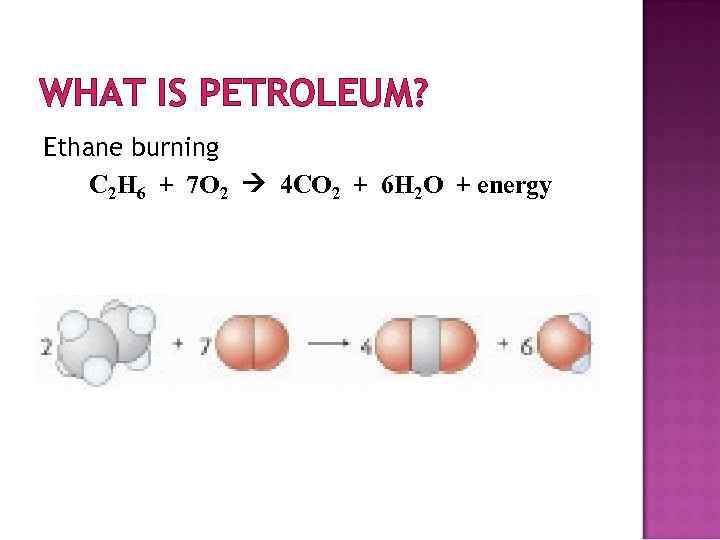 WHAT IS PETROLEUM? Ethane burning C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O + energy
WHAT IS PETROLEUM? Ethane burning C 2 H 6 + 7 O 2 4 CO 2 + 6 H 2 O + energy
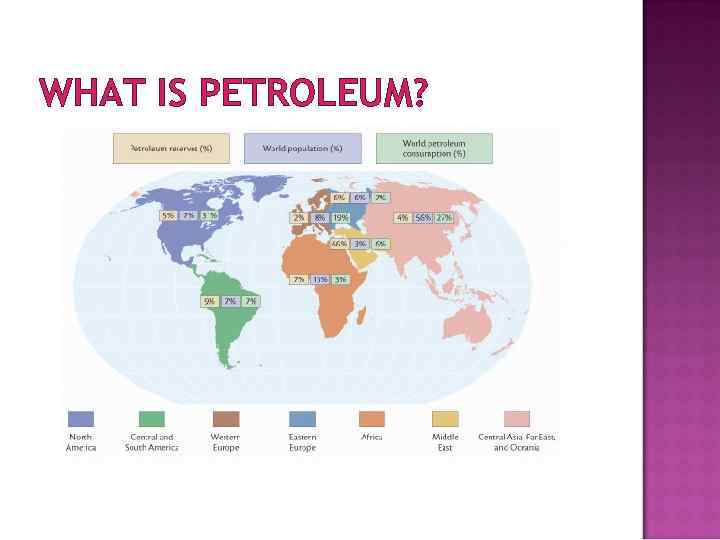
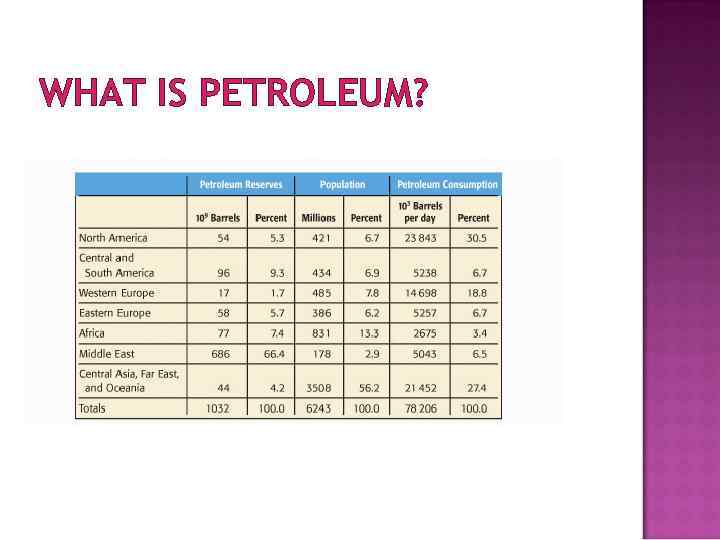
 WHAT IS PETROLEUM? Petroleum is not uniformly distributed around the world 66% Middle Eastern Nations 5% North America 4% Central Asia, Far East, Oceana Figure 3. 4, p. 214 – more details
WHAT IS PETROLEUM? Petroleum is not uniformly distributed around the world 66% Middle Eastern Nations 5% North America 4% Central Asia, Far East, Oceana Figure 3. 4, p. 214 – more details
 WHAT IS PETROLEUM?
WHAT IS PETROLEUM?
 WHAT IS PETROLEUM?
WHAT IS PETROLEUM?
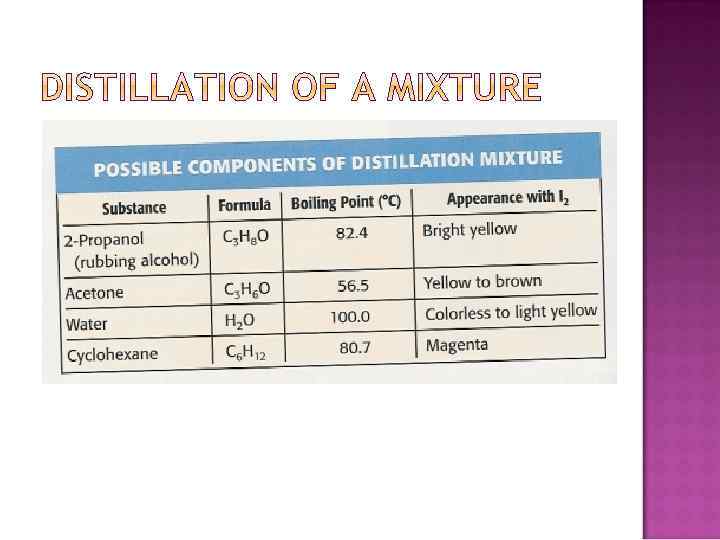
 Separation of liquid substances based on their different boiling points Distillate: Condensed liquid collected
Separation of liquid substances based on their different boiling points Distillate: Condensed liquid collected
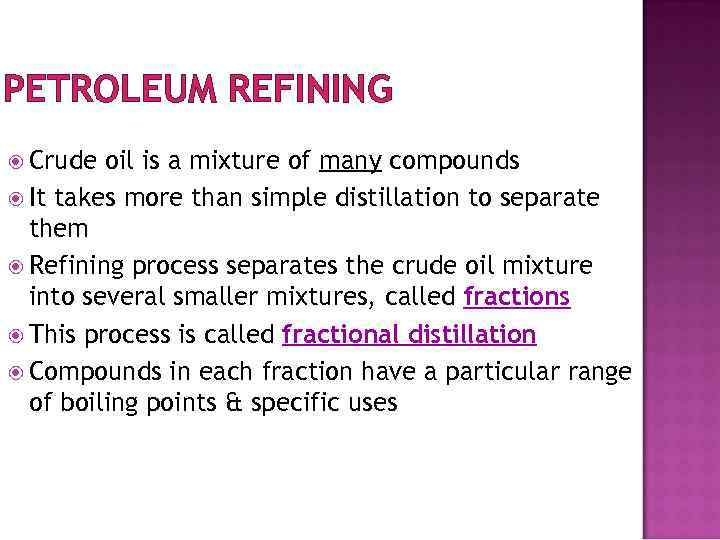 PETROLEUM REFINING Crude oil is a mixture of many compounds It takes more than simple distillation to separate them Refining process separates the crude oil mixture into several smaller mixtures, called fractions This process is called fractional distillation Compounds in each fraction have a particular range of boiling points & specific uses
PETROLEUM REFINING Crude oil is a mixture of many compounds It takes more than simple distillation to separate them Refining process separates the crude oil mixture into several smaller mixtures, called fractions This process is called fractional distillation Compounds in each fraction have a particular range of boiling points & specific uses
 REFINING CRUDE OIL These fractionating towers contain many different levels of condensers to cool the oil vapor as it rises. Temperatures range from about 400 o. C (at the base) to 40 o. C (at the top).
REFINING CRUDE OIL These fractionating towers contain many different levels of condensers to cool the oil vapor as it rises. Temperatures range from about 400 o. C (at the base) to 40 o. C (at the top).
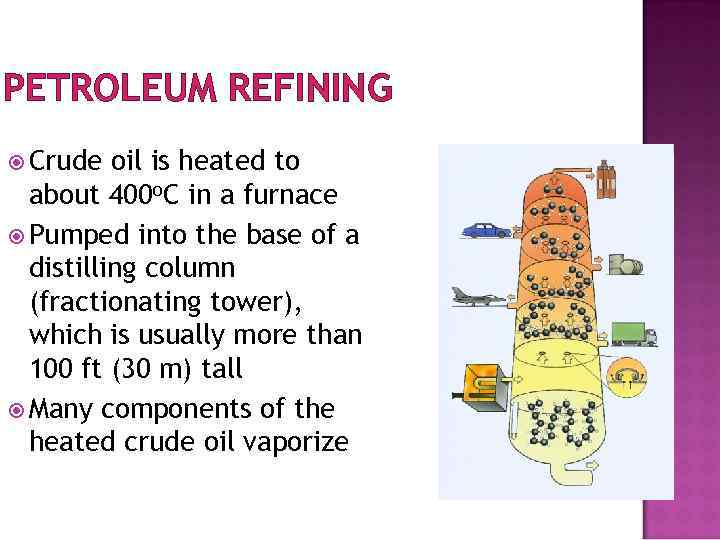 PETROLEUM REFINING Crude oil is heated to about 400 o. C in a furnace Pumped into the base of a distilling column (fractionating tower), which is usually more than 100 ft (30 m) tall Many components of the heated crude oil vaporize
PETROLEUM REFINING Crude oil is heated to about 400 o. C in a furnace Pumped into the base of a distilling column (fractionating tower), which is usually more than 100 ft (30 m) tall Many components of the heated crude oil vaporize
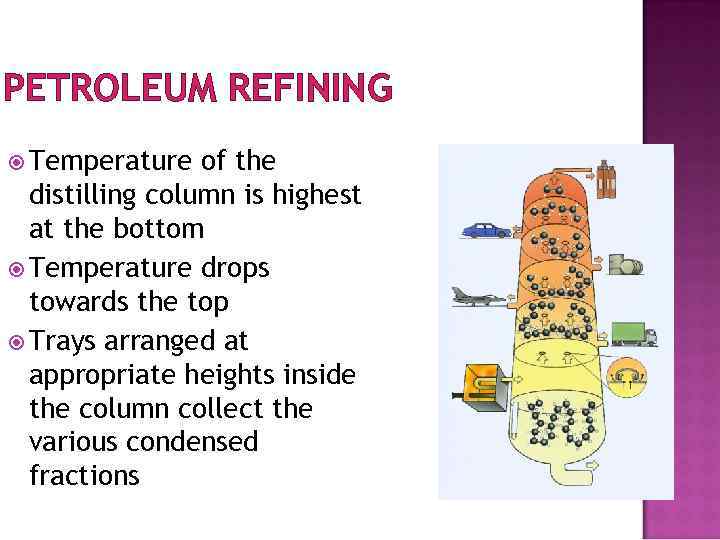 PETROLEUM REFINING Temperature of the distilling column is highest at the bottom Temperature drops towards the top Trays arranged at appropriate heights inside the column collect the various condensed fractions
PETROLEUM REFINING Temperature of the distilling column is highest at the bottom Temperature drops towards the top Trays arranged at appropriate heights inside the column collect the various condensed fractions
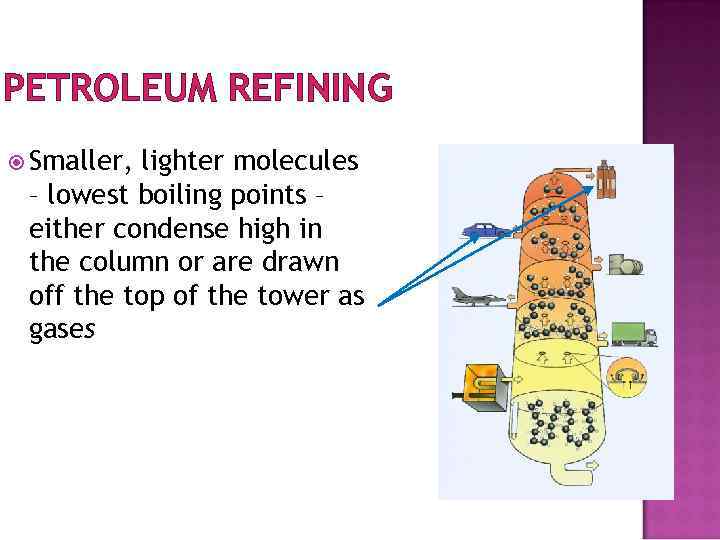 PETROLEUM REFINING Smaller, lighter molecules – lowest boiling points – either condense high in the column or are drawn off the top of the tower as gases
PETROLEUM REFINING Smaller, lighter molecules – lowest boiling points – either condense high in the column or are drawn off the top of the tower as gases
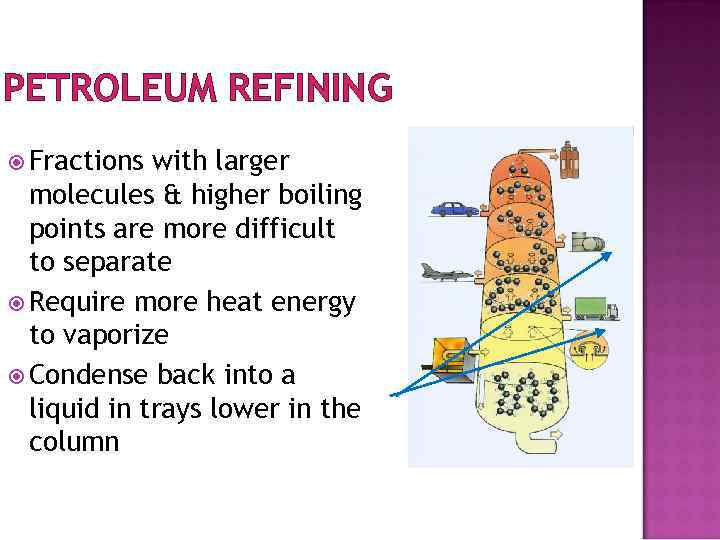 PETROLEUM REFINING Fractions with larger molecules & higher boiling points are more difficult to separate Require more heat energy to vaporize Condense back into a liquid in trays lower in the column
PETROLEUM REFINING Fractions with larger molecules & higher boiling points are more difficult to separate Require more heat energy to vaporize Condense back into a liquid in trays lower in the column
 CHEMICAL BONDING Organic Chemistry – focuses on hydrocarbons & substances that are made from them Called “organic”, because early chemists that living systems were needed to produce hydrocarbons Not so – for 150 years - reactants other than petroleum have been used to make “organic” compounds
CHEMICAL BONDING Organic Chemistry – focuses on hydrocarbons & substances that are made from them Called “organic”, because early chemists that living systems were needed to produce hydrocarbons Not so – for 150 years - reactants other than petroleum have been used to make “organic” compounds
 CHEMICAL BONDING Hydrocarbon molecules Carbon atoms join to make a carbon chain backbone Hydrogen atoms are attached to the carbon chain backbone Carbon’s ability to bond the way it does explains the abundance of different hydrocarbon compounds
CHEMICAL BONDING Hydrocarbon molecules Carbon atoms join to make a carbon chain backbone Hydrogen atoms are attached to the carbon chain backbone Carbon’s ability to bond the way it does explains the abundance of different hydrocarbon compounds
 HYDROCARBON CHAINS
HYDROCARBON CHAINS
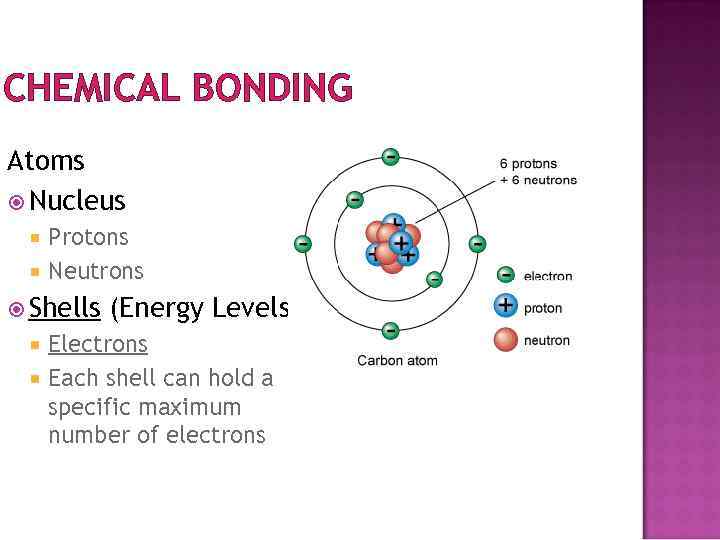 CHEMICAL BONDING Atoms Nucleus Protons Neutrons Shells (Energy Levels) Electrons Each shell can hold a specific maximum number of electrons
CHEMICAL BONDING Atoms Nucleus Protons Neutrons Shells (Energy Levels) Electrons Each shell can hold a specific maximum number of electrons
 Li Be B C N O F Ne 2 nd 3 e- - 2 in 1 st shell, 1 in 2 nd shell 4 e- - 2 in 1 st shell, 2 in 2 nd shell 5 e- - 2 in 1 st shell, 3 in 2 nd shell 6 e- - 2 in 1 st shell, 4 in 2 nd shell 7 e- - 2 in 1 st shell, 5 in 2 nd shell 8 e- - 2 in 1 st shell, 6 in 2 nd shell 9 e- - 2 in 1 st shell, 7 in 2 nd shell 10 e- - 2 in 1 st shell, 8 in 2 nd shell only holds 8 e-, so new shell starts
Li Be B C N O F Ne 2 nd 3 e- - 2 in 1 st shell, 1 in 2 nd shell 4 e- - 2 in 1 st shell, 2 in 2 nd shell 5 e- - 2 in 1 st shell, 3 in 2 nd shell 6 e- - 2 in 1 st shell, 4 in 2 nd shell 7 e- - 2 in 1 st shell, 5 in 2 nd shell 8 e- - 2 in 1 st shell, 6 in 2 nd shell 9 e- - 2 in 1 st shell, 7 in 2 nd shell 10 e- - 2 in 1 st shell, 8 in 2 nd shell only holds 8 e-, so new shell starts
 CHEMICAL BONDING
CHEMICAL BONDING
 CHEMICAL BONDING Atoms whose last (outer) shell is filled are unreactive Found on the right side of the Periodic Table – Group 18 They all have 8 e- in the outer shell (except for He, because the 1 st shell can only hold 2 e-) Called the “noble gases” or “inert gases”
CHEMICAL BONDING Atoms whose last (outer) shell is filled are unreactive Found on the right side of the Periodic Table – Group 18 They all have 8 e- in the outer shell (except for He, because the 1 st shell can only hold 2 e-) Called the “noble gases” or “inert gases”
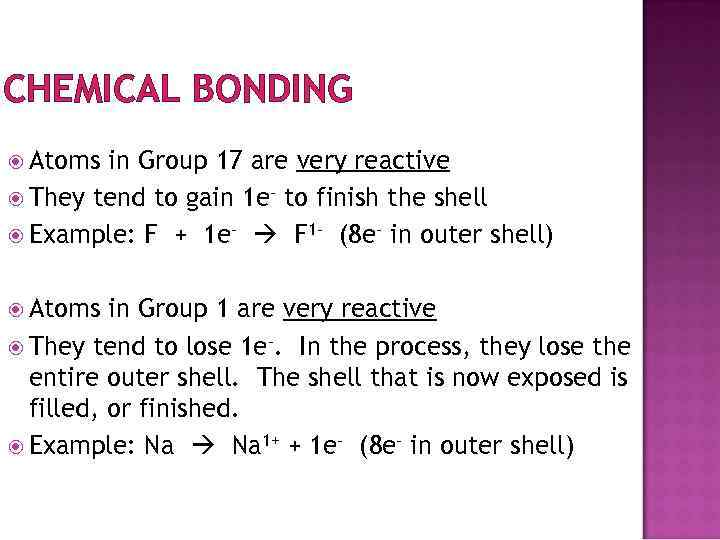 CHEMICAL BONDING Atoms in Group 17 are very reactive They tend to gain 1 e- to finish the shell Example: F + 1 e- F 1 - (8 e- in outer shell) Atoms in Group 1 are very reactive They tend to lose 1 e-. In the process, they lose the entire outer shell. The shell that is now exposed is filled, or finished. Example: Na 1+ + 1 e- (8 e- in outer shell)
CHEMICAL BONDING Atoms in Group 17 are very reactive They tend to gain 1 e- to finish the shell Example: F + 1 e- F 1 - (8 e- in outer shell) Atoms in Group 1 are very reactive They tend to lose 1 e-. In the process, they lose the entire outer shell. The shell that is now exposed is filled, or finished. Example: Na 1+ + 1 e- (8 e- in outer shell)
 CHEMICAL BONDING Compounds made of nonmetals achieve filled outer shells by sharing electrons Covalent bonding H has 1 e- in a shell that can hold 2 e-. So, it is “missing” 1 e If two H atoms come together, they can each share the 1 e- they have. Each H has the use of its e- as well as the use of the e- from the other H.
CHEMICAL BONDING Compounds made of nonmetals achieve filled outer shells by sharing electrons Covalent bonding H has 1 e- in a shell that can hold 2 e-. So, it is “missing” 1 e If two H atoms come together, they can each share the 1 e- they have. Each H has the use of its e- as well as the use of the e- from the other H.
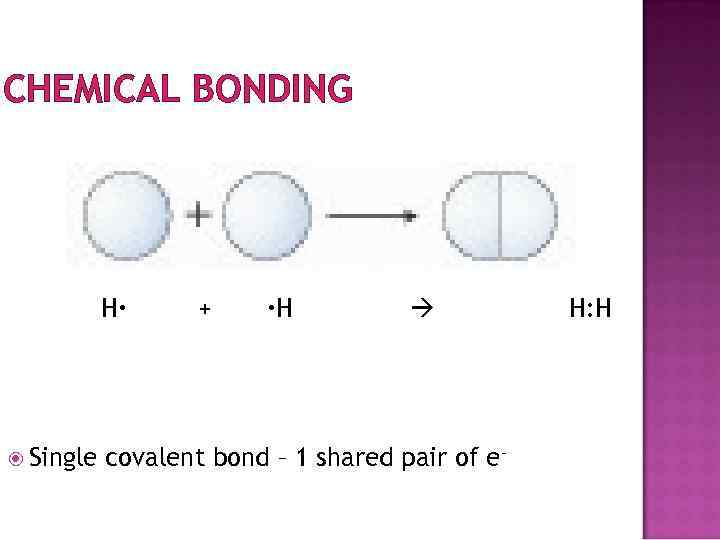 CHEMICAL BONDING H Single + H covalent bond – 1 shared pair of e- H: H
CHEMICAL BONDING H Single + H covalent bond – 1 shared pair of e- H: H
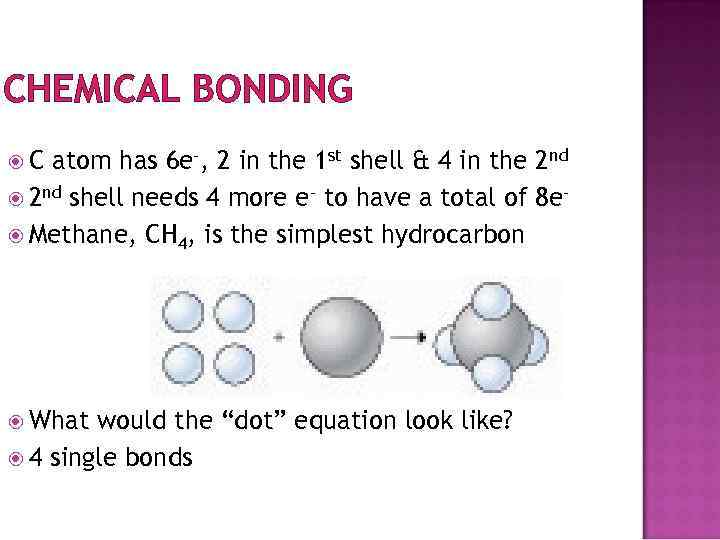 CHEMICAL BONDING C atom has 6 e-, 2 in the 1 st shell & 4 in the 2 nd shell needs 4 more e- to have a total of 8 e Methane, CH 4, is the simplest hydrocarbon What would the “dot” equation look like? 4 single bonds
CHEMICAL BONDING C atom has 6 e-, 2 in the 1 st shell & 4 in the 2 nd shell needs 4 more e- to have a total of 8 e Methane, CH 4, is the simplest hydrocarbon What would the “dot” equation look like? 4 single bonds
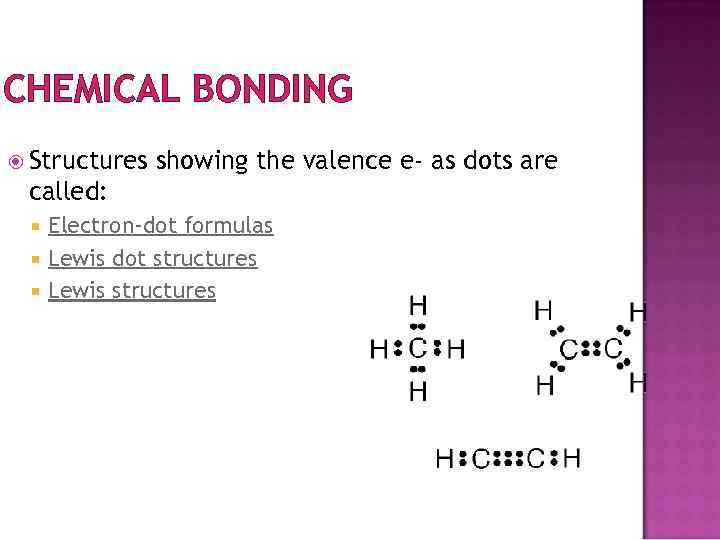 CHEMICAL BONDING Structures showing the valence e- as dots are called: Electron-dot formulas Lewis dot structures Lewis structures
CHEMICAL BONDING Structures showing the valence e- as dots are called: Electron-dot formulas Lewis dot structures Lewis structures
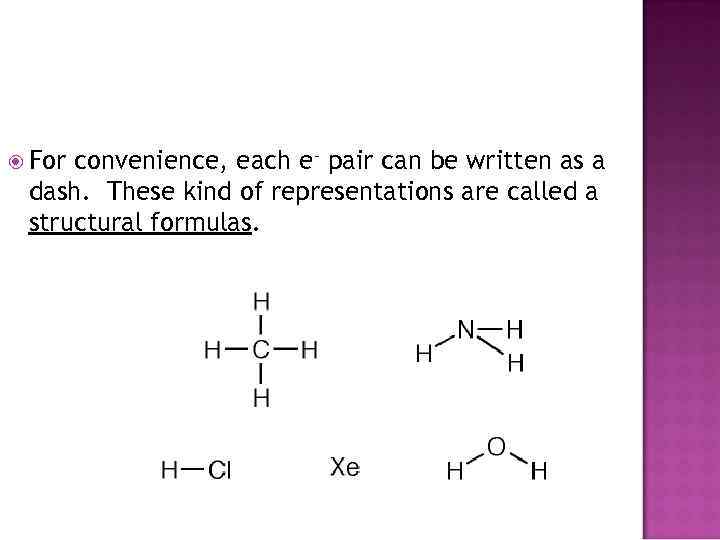 For convenience, each e- pair can be written as a dash. These kind of representations are called a structural formulas.
For convenience, each e- pair can be written as a dash. These kind of representations are called a structural formulas.
 Alkanes: hydrocarbons with single bonds Alkenes: hydrocarbons with one or more double bonds Alkynes: carbons with one or more triple bond
Alkanes: hydrocarbons with single bonds Alkenes: hydrocarbons with one or more double bonds Alkynes: carbons with one or more triple bond
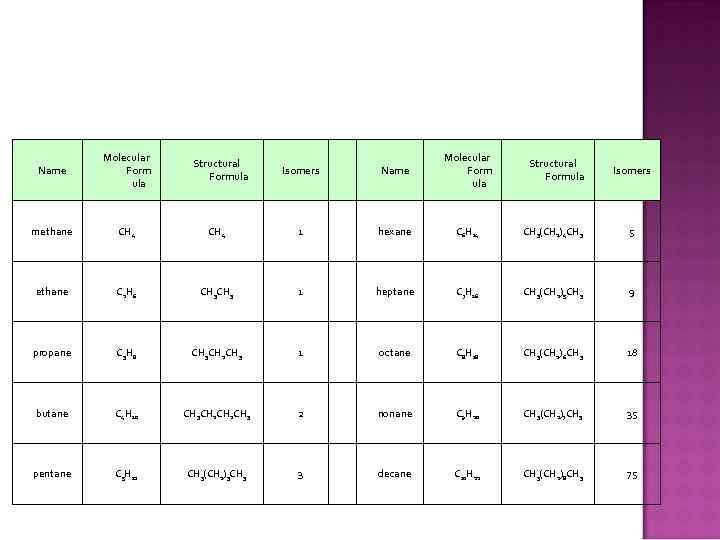 Name Molecular Form ula Isomers Name Molecular Form ula methane CH 4 1 hexane C 6 H 14 CH 3(CH 2)4 CH 3 5 ethane C 2 H 6 CH 3 1 heptane C 7 H 16 CH 3(CH 2)5 CH 3 9 propane C 3 H 8 CH 3 CH 2 CH 3 1 octane C 8 H 18 CH 3(CH 2)6 CH 3 18 butane C 4 H 10 CH 3 CH 2 CH 3 2 nonane C 9 H 20 CH 3(CH 2)7 CH 3 35 pentane C 5 H 12 CH 3(CH 2)3 CH 3 3 decane C 10 H 22 CH 3(CH 2)8 CH 3 75 Structural Formula Isomers
Name Molecular Form ula Isomers Name Molecular Form ula methane CH 4 1 hexane C 6 H 14 CH 3(CH 2)4 CH 3 5 ethane C 2 H 6 CH 3 1 heptane C 7 H 16 CH 3(CH 2)5 CH 3 9 propane C 3 H 8 CH 3 CH 2 CH 3 1 octane C 8 H 18 CH 3(CH 2)6 CH 3 18 butane C 4 H 10 CH 3 CH 2 CH 3 2 nonane C 9 H 20 CH 3(CH 2)7 CH 3 35 pentane C 5 H 12 CH 3(CH 2)3 CH 3 3 decane C 10 H 22 CH 3(CH 2)8 CH 3 75 Structural Formula Isomers
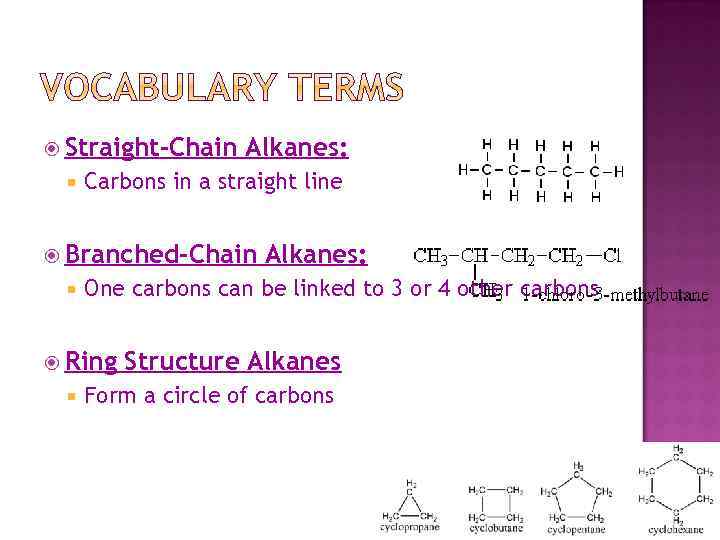 Straight-Chain Alkanes: Carbons in a straight line Branched-Chain One carbons can be linked to 3 or 4 other carbons Ring Alkanes: Structure Alkanes Form a circle of carbons
Straight-Chain Alkanes: Carbons in a straight line Branched-Chain One carbons can be linked to 3 or 4 other carbons Ring Alkanes: Structure Alkanes Form a circle of carbons
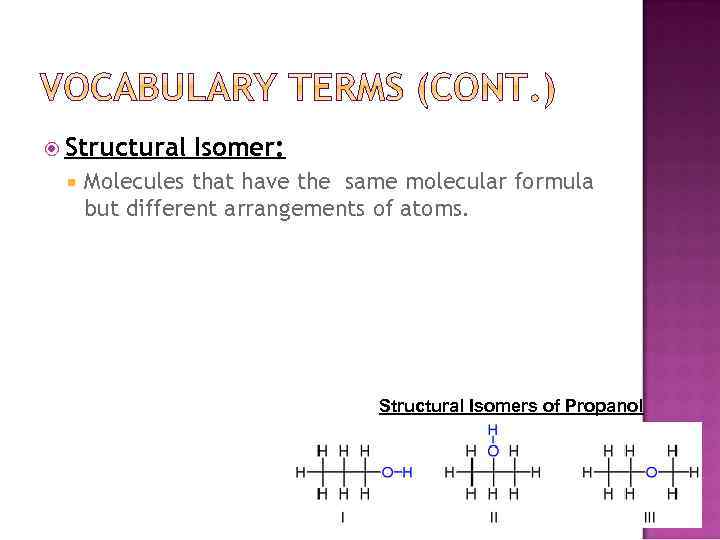 Structural Isomer: Molecules that have the same molecular formula but different arrangements of atoms. Structural Isomers of Propanol
Structural Isomer: Molecules that have the same molecular formula but different arrangements of atoms. Structural Isomers of Propanol
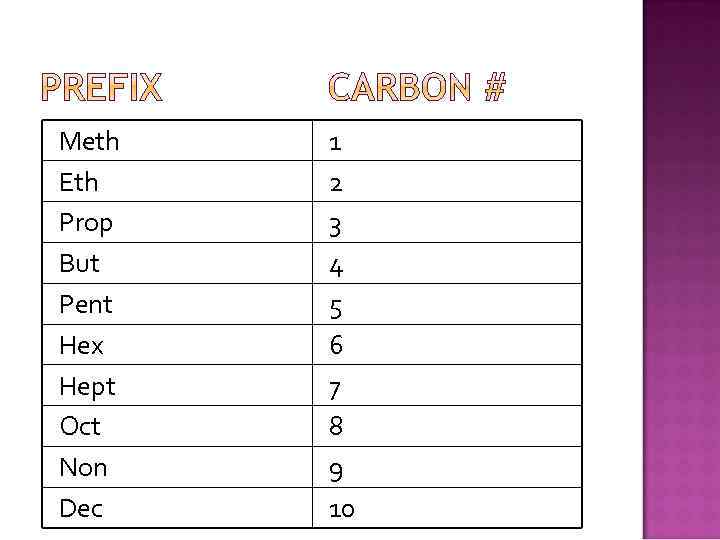 Meth Eth Prop But Pent Hex Hept Oct Non Dec 1 2 3 4 5 6 7 8 9 10
Meth Eth Prop But Pent Hex Hept Oct Non Dec 1 2 3 4 5 6 7 8 9 10
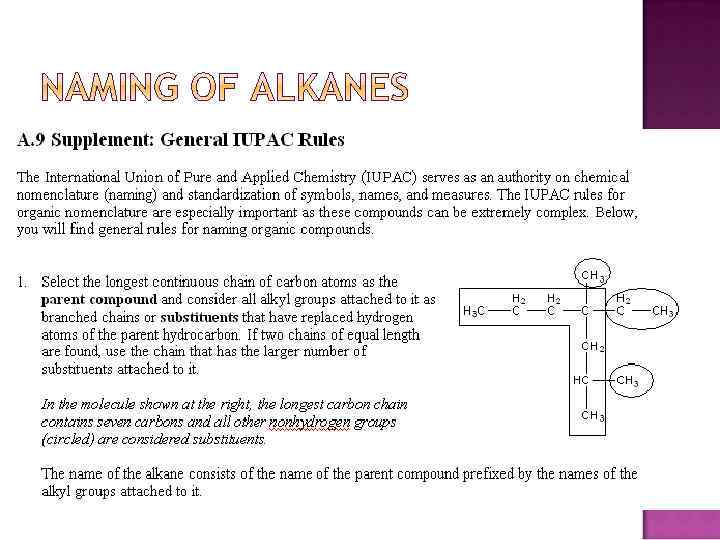

 1. The yne suffix (ending) indicates an alkyne or ene suffix indicates an alkene. 2. Select the longest chain containing the double or triple bond. 3. Number from the end nearest the double or triple bond. 4. Specify where the bond starts. EX.
1. The yne suffix (ending) indicates an alkyne or ene suffix indicates an alkene. 2. Select the longest chain containing the double or triple bond. 3. Number from the end nearest the double or triple bond. 4. Specify where the bond starts. EX.
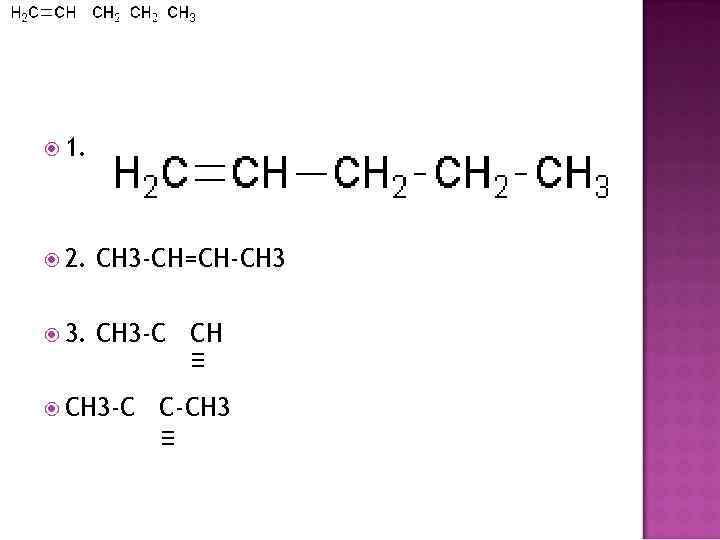 1. 2. CH 3 -CH=CH-CH 3 3. CH 3 -C C-CH 3
1. 2. CH 3 -CH=CH-CH 3 3. CH 3 -C C-CH 3
 Akhmetova Aizhan Togzhanova Bakhyttygul
Akhmetova Aizhan Togzhanova Bakhyttygul


