lecture 3-2016.ppt
- Количество слайдов: 19
 Petroleum disperse systems • The PDS classification • Nanoparticles in PDS • Lyophilic and lyophobic PDS. Shchukin-Rehbinder criterion • Some challenges of Petroleum Industry, connected with PDS exploitation, treatment, transport and refining • 14/09/16 lecture 3 1
Petroleum disperse systems • The PDS classification • Nanoparticles in PDS • Lyophilic and lyophobic PDS. Shchukin-Rehbinder criterion • Some challenges of Petroleum Industry, connected with PDS exploitation, treatment, transport and refining • 14/09/16 lecture 3 1
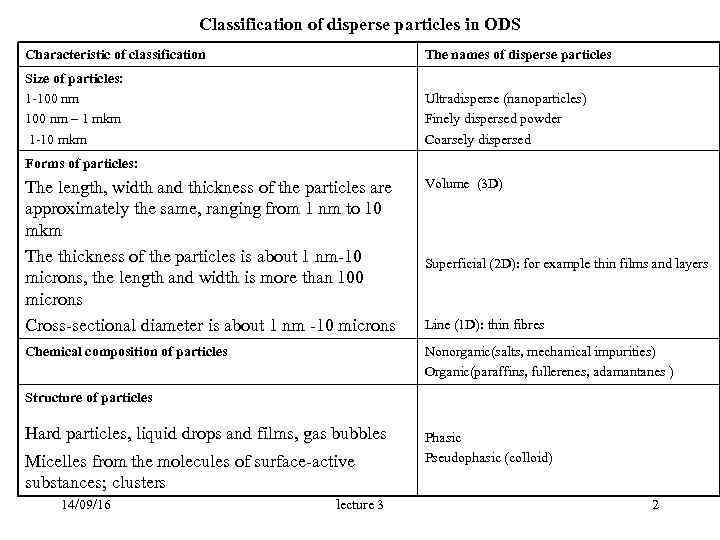 Classification of disperse particles in ODS Characteristic of classification The names of disperse particles Size of particles: 1 -100 nm Ultradisperse (nanoparticles) 100 nm – 1 mkm Finely dispersed powder 1 -10 mkm Сoarsely dispersed Forms of particles: The length, width and thickness of the particles are Volume (3 D) approximately the same, ranging from 1 nm to 10 mkm The thickness of the particles is about 1 nm-10 Superficial (2 D): for example thin films and layers microns, the length and width is more than 100 microns Cross-sectional diameter is about 1 nm -10 microns Line (1 D): thin fibres Chemical composition of particles Nonorganic(salts, mechanical impurities) Organic(paraffins, fullerenes, adamantanes ) Structure of particles Hard particles, liquid drops and films, gas bubbles Phasic Micelles from the molecules of surface-active Pseudophasic (colloid) substances; clusters 14/09/16 lecture 3 2
Classification of disperse particles in ODS Characteristic of classification The names of disperse particles Size of particles: 1 -100 nm Ultradisperse (nanoparticles) 100 nm – 1 mkm Finely dispersed powder 1 -10 mkm Сoarsely dispersed Forms of particles: The length, width and thickness of the particles are Volume (3 D) approximately the same, ranging from 1 nm to 10 mkm The thickness of the particles is about 1 nm-10 Superficial (2 D): for example thin films and layers microns, the length and width is more than 100 microns Cross-sectional diameter is about 1 nm -10 microns Line (1 D): thin fibres Chemical composition of particles Nonorganic(salts, mechanical impurities) Organic(paraffins, fullerenes, adamantanes ) Structure of particles Hard particles, liquid drops and films, gas bubbles Phasic Micelles from the molecules of surface-active Pseudophasic (colloid) substances; clusters 14/09/16 lecture 3 2
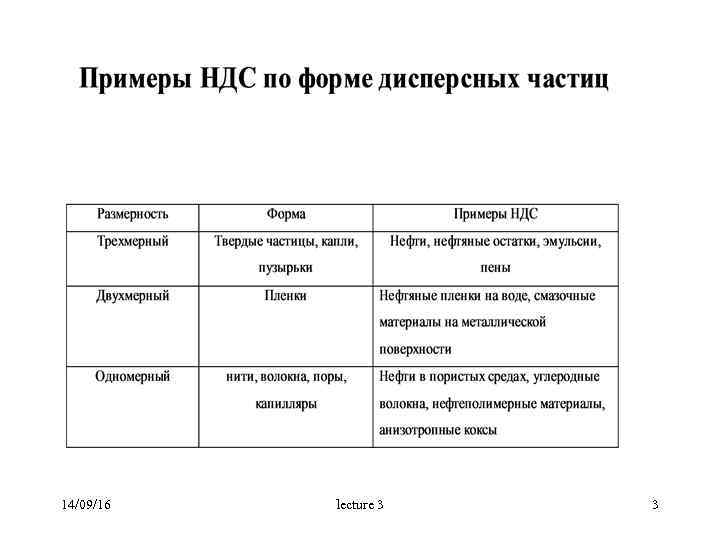 14/09/16 lecture 3 3
14/09/16 lecture 3 3
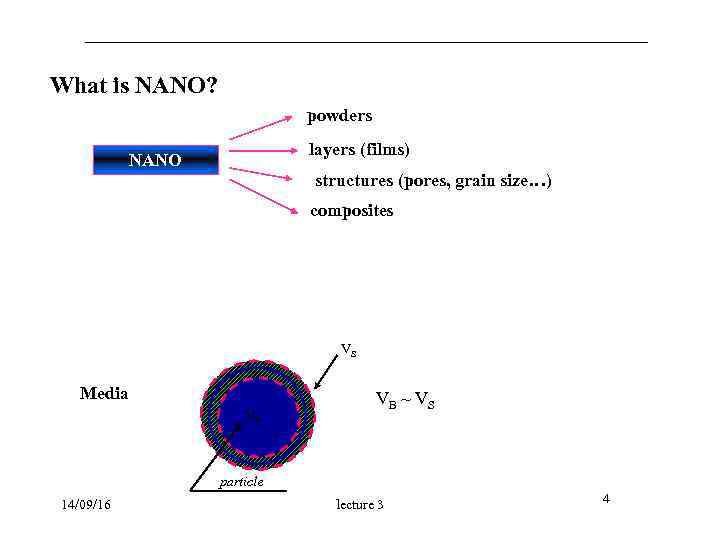 What is NANO? powders layers (films) NANO structures (pores, grain size…) composites VS Media VB ~ VS VB particle 14/09/16 lecture 3 4
What is NANO? powders layers (films) NANO structures (pores, grain size…) composites VS Media VB ~ VS VB particle 14/09/16 lecture 3 4
 Energy and power aspects of surface (interfacial)* tension The compensate of intermolecular interactions on the phase boundary leads to the fact that the phase boundary surface has an uncompensated excess energy (energy aspect), σ [erg/cm 2, J/m 2] and causes the appearance of a tangential force (power aspect), σ [din/cm, N/m] * The term "interphase boundary" is usually attributed to the boundary between two immiscible phases, the term "surface" indicates that one of the phases is a gas, usually air 14/09/16 lecture 3 5
Energy and power aspects of surface (interfacial)* tension The compensate of intermolecular interactions on the phase boundary leads to the fact that the phase boundary surface has an uncompensated excess energy (energy aspect), σ [erg/cm 2, J/m 2] and causes the appearance of a tangential force (power aspect), σ [din/cm, N/m] * The term "interphase boundary" is usually attributed to the boundary between two immiscible phases, the term "surface" indicates that one of the phases is a gas, usually air 14/09/16 lecture 3 5
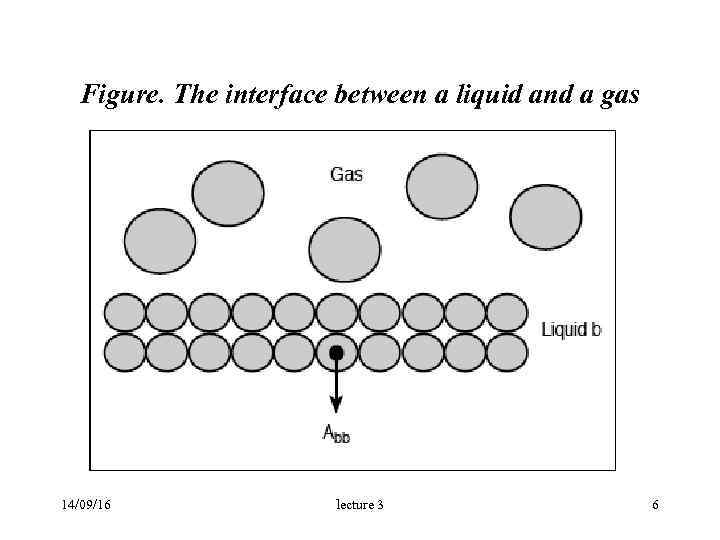 Figure. The interface between a liquid and a gas 14/09/16 lecture 3 6
Figure. The interface between a liquid and a gas 14/09/16 lecture 3 6
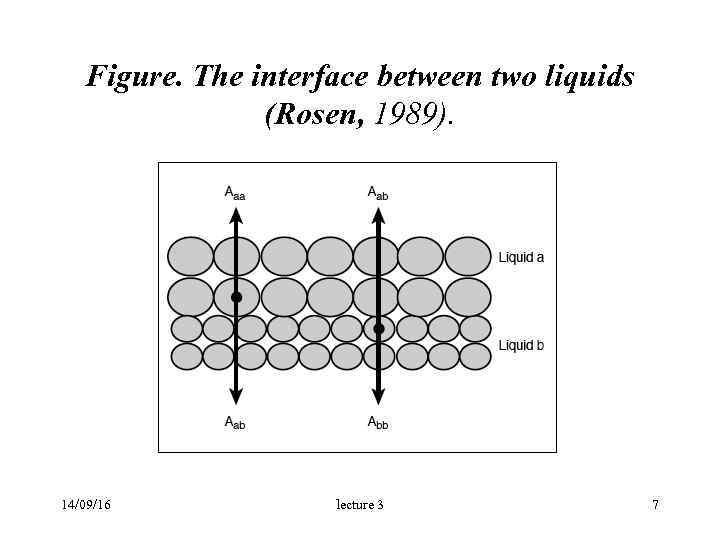 Figure. The interface between two liquids (Rosen, 1989). 14/09/16 lecture 3 7
Figure. The interface between two liquids (Rosen, 1989). 14/09/16 lecture 3 7
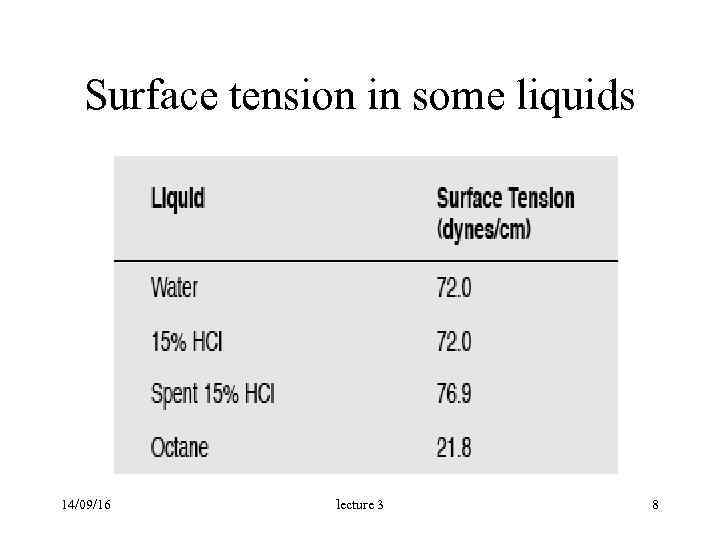 Surface tension in some liquids 14/09/16 lecture 3 8
Surface tension in some liquids 14/09/16 lecture 3 8
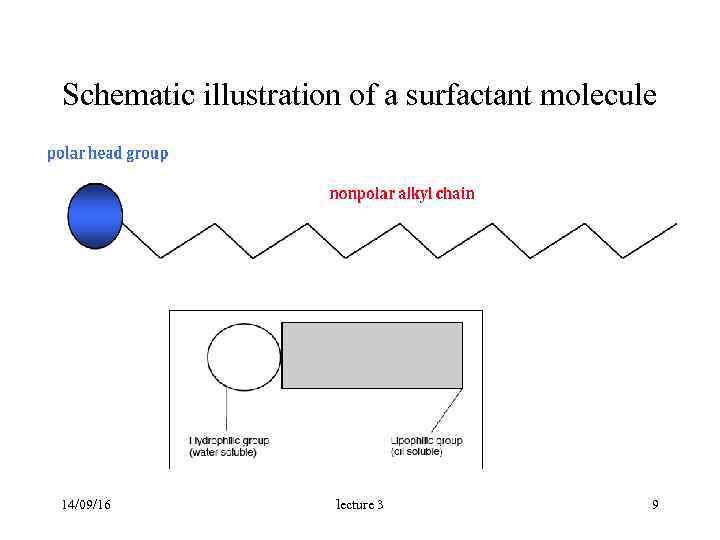 Schematic illustration of a surfactant molecule 14/09/16 lecture 3 9
Schematic illustration of a surfactant molecule 14/09/16 lecture 3 9
 Lyophilic and lyophobic ODS • ODS can be divided on lyophilic and lyophobic due to value of interfacial interactions at the interface • Shchukin-Rehbinder criterion 4πr 2σ ~ γk. T, where r - particle size, T-temperature, K - Boltzmann constant, - dimensionless coefficient Calculation shows, that at standart conditions σcrit~ 0, 01 -0, 1 m. N/m Lyophilic ODS σ < σcrit Lyophobic ODS σ > σcrit 14/09/16 lecture 3 10
Lyophilic and lyophobic ODS • ODS can be divided on lyophilic and lyophobic due to value of interfacial interactions at the interface • Shchukin-Rehbinder criterion 4πr 2σ ~ γk. T, where r - particle size, T-temperature, K - Boltzmann constant, - dimensionless coefficient Calculation shows, that at standart conditions σcrit~ 0, 01 -0, 1 m. N/m Lyophilic ODS σ < σcrit Lyophobic ODS σ > σcrit 14/09/16 lecture 3 10
 Composite ODS Five interfaces: Solid body – gas (surface) Solid body - liquid (interphase boundary) Solid body – solid body liquid – gas (surface) liquid-liquid (interphase boundary) 14/09/16 lecture 3 11
Composite ODS Five interfaces: Solid body – gas (surface) Solid body - liquid (interphase boundary) Solid body – solid body liquid – gas (surface) liquid-liquid (interphase boundary) 14/09/16 lecture 3 11
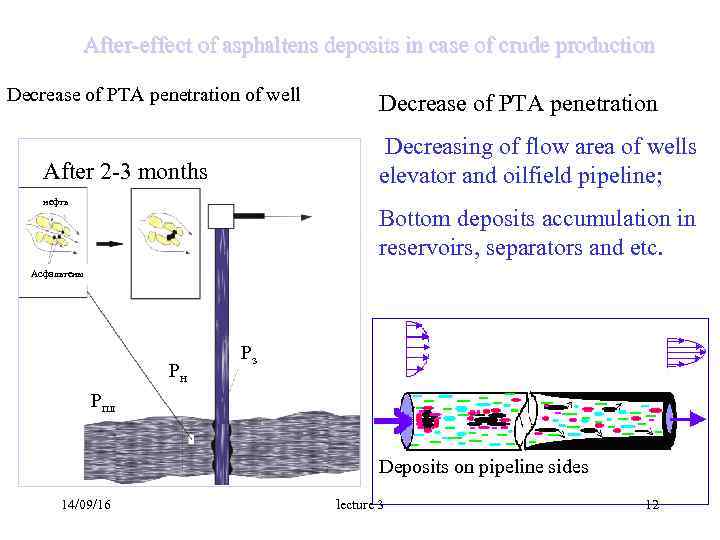 After-effect of asphaltens deposits in case of crude production Decrease of PTA penetration of well Decrease of PTA penetration Decreasing of flow area of wells After 2 -3 months elevator and oilfield pipeline; нефть Bottom deposits accumulation in reservoirs, separators and etc. Асфальтены Pз Pн Pпл Deposits on pipeline sides 14/09/16 lecture 3 12
After-effect of asphaltens deposits in case of crude production Decrease of PTA penetration of well Decrease of PTA penetration Decreasing of flow area of wells After 2 -3 months elevator and oilfield pipeline; нефть Bottom deposits accumulation in reservoirs, separators and etc. Асфальтены Pз Pн Pпл Deposits on pipeline sides 14/09/16 lecture 3 12
 Basic conditions, which determine risk of asphaltens setting out in PTA (pig trap area) • Changing of thermobaricе conditions in PTA; • Рпл – Рн > 8 – 10 МPа; • Presence of contamination; • Formation of trolly-oil; • Practice of incongruent chemicals (isopropil alcohol, methanol, propanone, solvents); • Acid treatment and other chemical methods of intensification of oil inflow from the bench 14/09/16 lecture 3 13
Basic conditions, which determine risk of asphaltens setting out in PTA (pig trap area) • Changing of thermobaricе conditions in PTA; • Рпл – Рн > 8 – 10 МPа; • Presence of contamination; • Formation of trolly-oil; • Practice of incongruent chemicals (isopropil alcohol, methanol, propanone, solvents); • Acid treatment and other chemical methods of intensification of oil inflow from the bench 14/09/16 lecture 3 13
 Deposition of asphaltenes in a pipeline wall 14/09/16 lecture 3 14
Deposition of asphaltenes in a pipeline wall 14/09/16 lecture 3 14
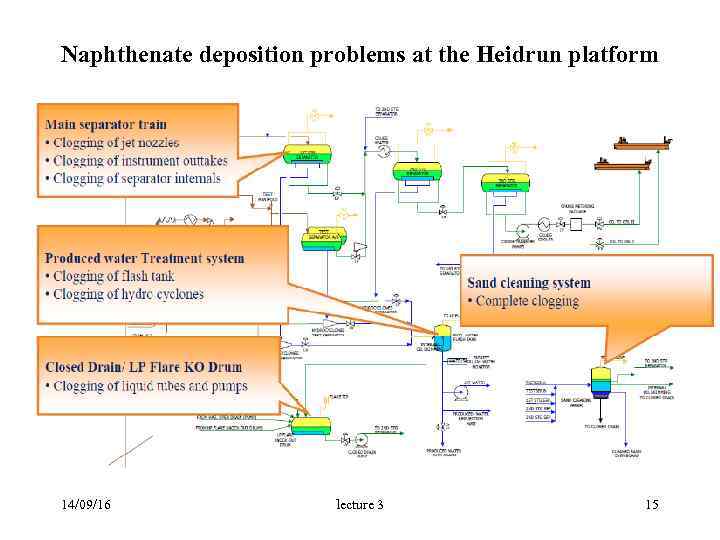 Naphthenate deposition problems at the Heidrun platform 14/09/16 lecture 3 15
Naphthenate deposition problems at the Heidrun platform 14/09/16 lecture 3 15
 Pigging operation to remove wax from the Norwegian shelf 14/09/16 lecture 3 16
Pigging operation to remove wax from the Norwegian shelf 14/09/16 lecture 3 16
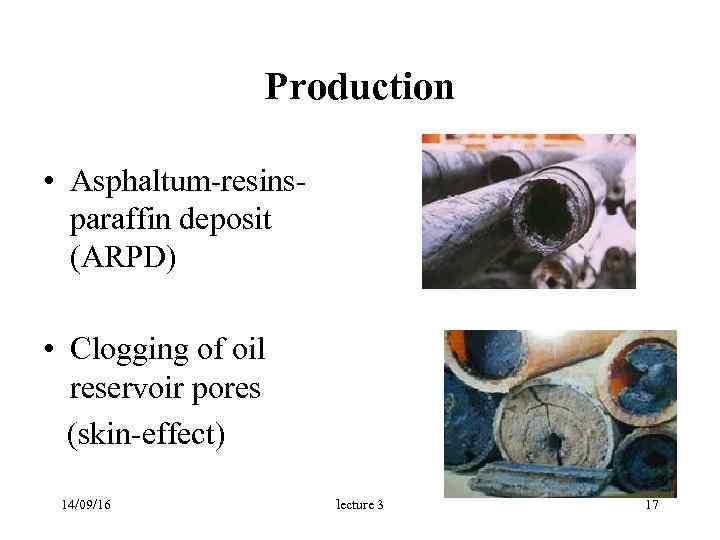 Production • Asphaltum-resins- paraffin deposit (ARPD) • Clogging of oil reservoir pores (skin-effect) 14/09/16 lecture 3 17
Production • Asphaltum-resins- paraffin deposit (ARPD) • Clogging of oil reservoir pores (skin-effect) 14/09/16 lecture 3 17
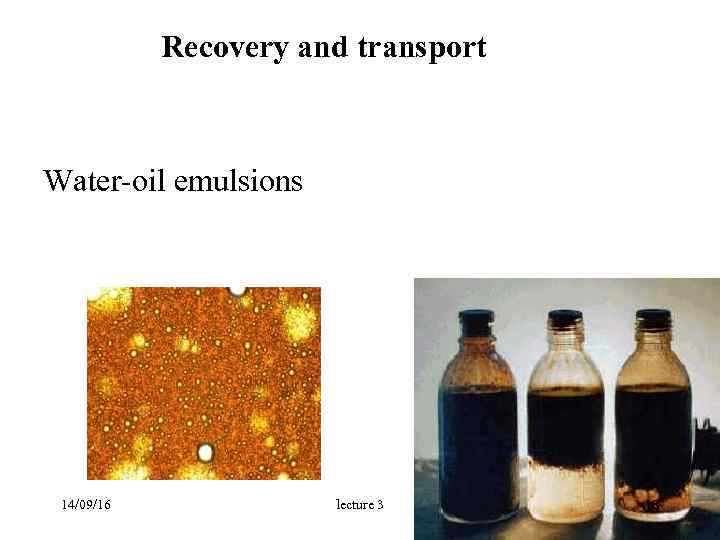 Recovery and transport Water-oil emulsions 14/09/16 lecture 3 18
Recovery and transport Water-oil emulsions 14/09/16 lecture 3 18
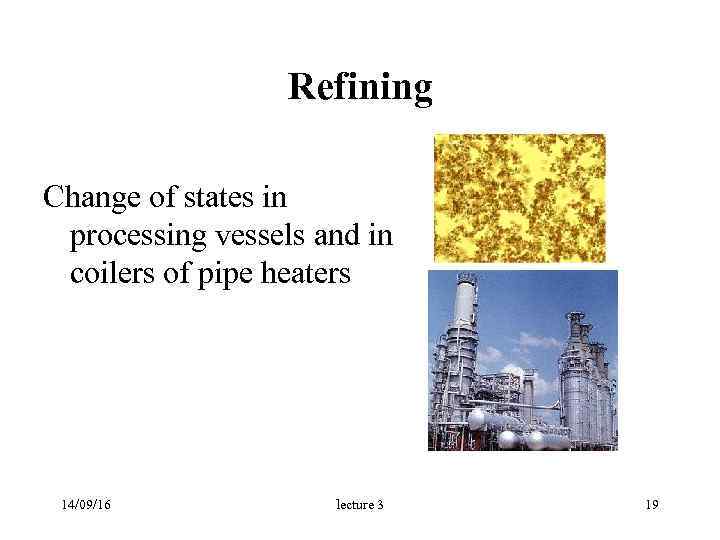 Refining Change of states in processing vessels and in coilers of pipe heaters 14/09/16 lecture 3 19
Refining Change of states in processing vessels and in coilers of pipe heaters 14/09/16 lecture 3 19


