ca9b8e200738cce0c650278fa42262c8.ppt
- Количество слайдов: 34
Permeability: transporting drugs through (lipid) membranes Paula Garcia 1 st Physical Chemistry Symposium, November 30, 2005
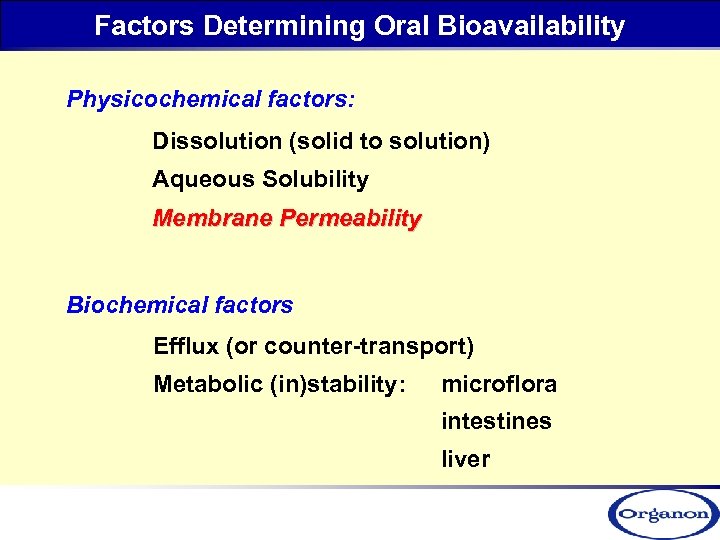
Factors Determining Oral Bioavailability Physicochemical factors: Dissolution (solid to solution) Aqueous Solubility Membrane Permeability Biochemical factors Efflux (or counter-transport) Metabolic (in)stability: microflora intestines liver
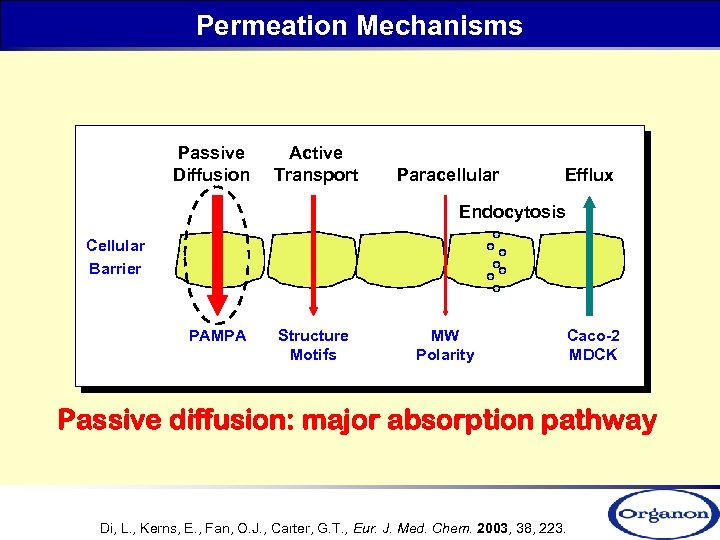
Permeation Mechanisms Passive Diffusion Active Transport Paracellular Efflux Endocytosis Cellular Barrier PAMPA Structure Motifs MW Polarity Caco-2 MDCK Passive diffusion: major absorption pathway Di, L. , Kerns, E. , Fan, O. J. , Carter, G. T. , Eur. J. Med. Chem. 2003, 38, 223.
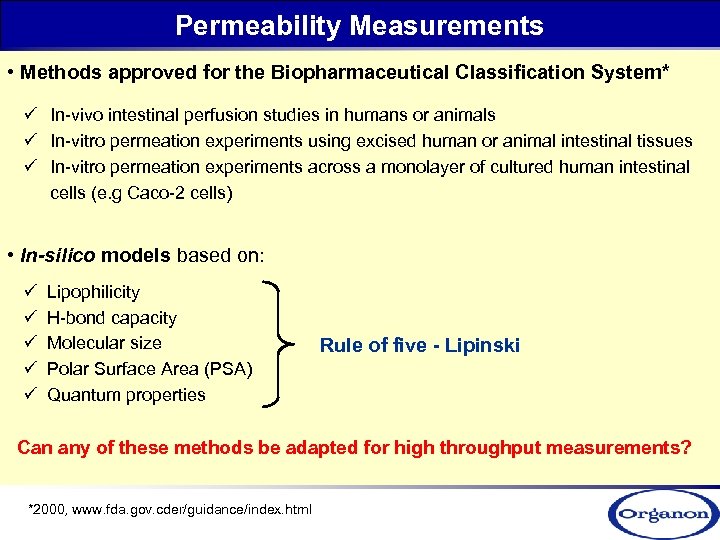
Permeability Measurements • Methods approved for the Biopharmaceutical Classification System* ü In-vivo intestinal perfusion studies in humans or animals ü In-vitro permeation experiments using excised human or animal intestinal tissues ü In-vitro permeation experiments across a monolayer of cultured human intestinal cells (e. g Caco-2 cells) • In-silico models based on: ü ü ü Lipophilicity H-bond capacity Molecular size Polar Surface Area (PSA) Quantum properties Rule of five - Lipinski Can any of these methods be adapted for high throughput measurements? *2000, www. fda. gov. cder/guidance/index. html
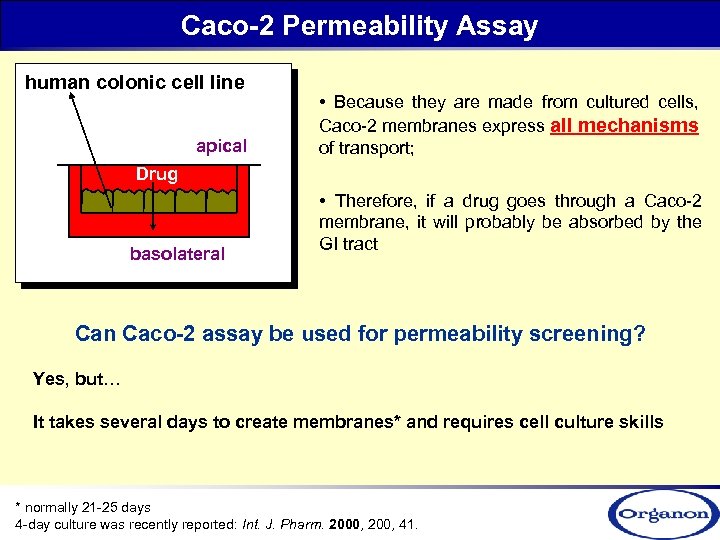
Caco-2 Permeability Assay human colonic cell line apical • Because they are made from cultured cells, Caco-2 membranes express all mechanisms of transport; Drug basolateral • Therefore, if a drug goes through a Caco-2 membrane, it will probably be absorbed by the GI tract Can Caco-2 assay be used for permeability screening? Yes, but… It takes several days to create membranes* and requires cell culture skills * normally 21 -25 days 4 -day culture was recently reported: Int. J. Pharm. 2000, 200, 41.
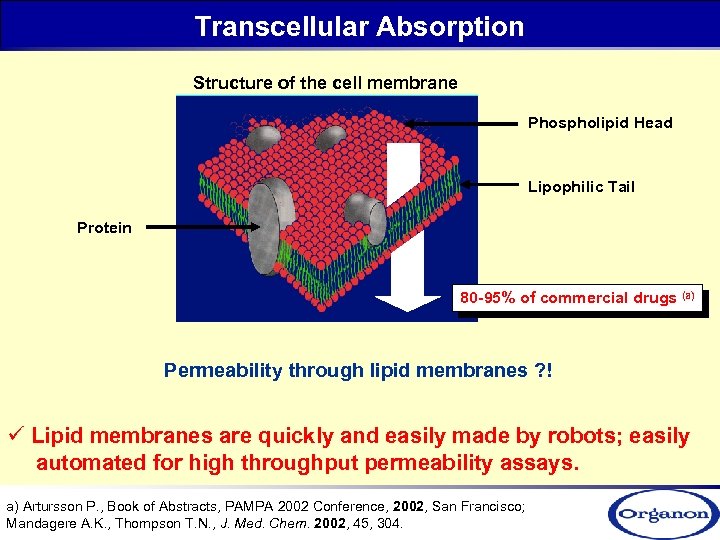
Transcellular Absorption Structure of the cell membrane Phospholipid Head Lipophilic Tail Protein 80 -95% of commercial drugs (a) Permeability through lipid membranes ? ! ü Lipid membranes are quickly and easily made by robots; easily automated for high throughput permeability assays. a) Artursson P. , Book of Abstracts, PAMPA 2002 Conference, 2002, San Francisco; Mandagere A. K. , Thompson T. N. , J. Med. Chem. 2002, 45, 304.
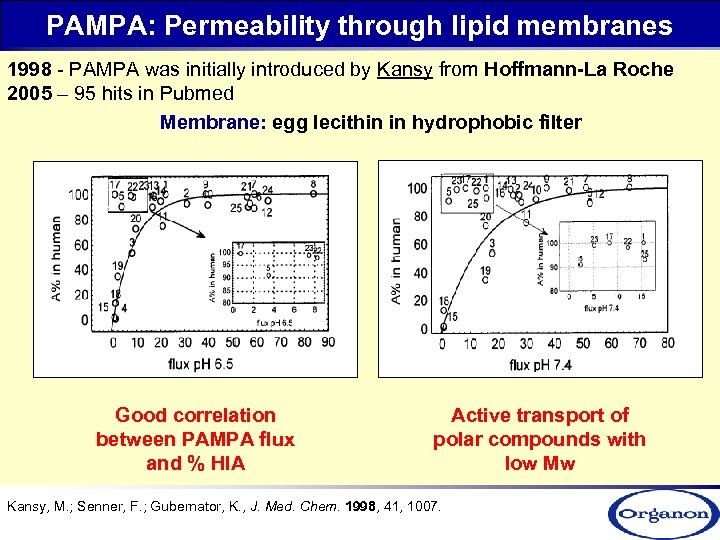
PAMPA: Permeability through lipid membranes 1998 - PAMPA was initially introduced by Kansy from Hoffmann-La Roche 2005 – 95 hits in Pubmed Membrane: egg lecithin in hydrophobic filter Good correlation between PAMPA flux and % HIA Active transport of polar compounds with low Mw Kansy, M. ; Senner, F. ; Gubernator, K. , J. Med. Chem. 1998, 41, 1007.
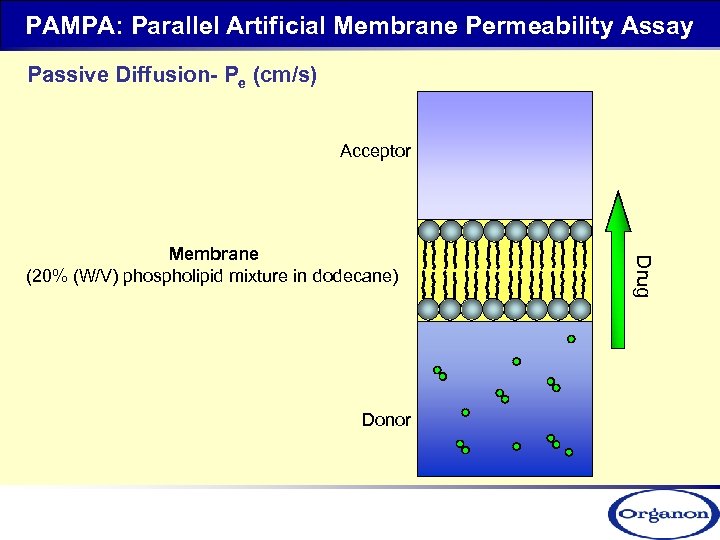
PAMPA: Parallel Artificial Membrane Permeability Assay Passive Diffusion- Pe (cm/s) Acceptor Donor Drug Membrane (20% (W/V) phospholipid mixture in dodecane)

PAMPA: Parallel Artificial Membrane Permeability Assay Passive Diffusion- Pe (cm/s) Acceptor (p. H=7. 4) SDS micelles Gastro. Intestinal Tract (GIT) Double-Sink Conditions Unstirred Water Layer Donor (p. H= 5 -8) Drug Membrane
PAMPA: workstation
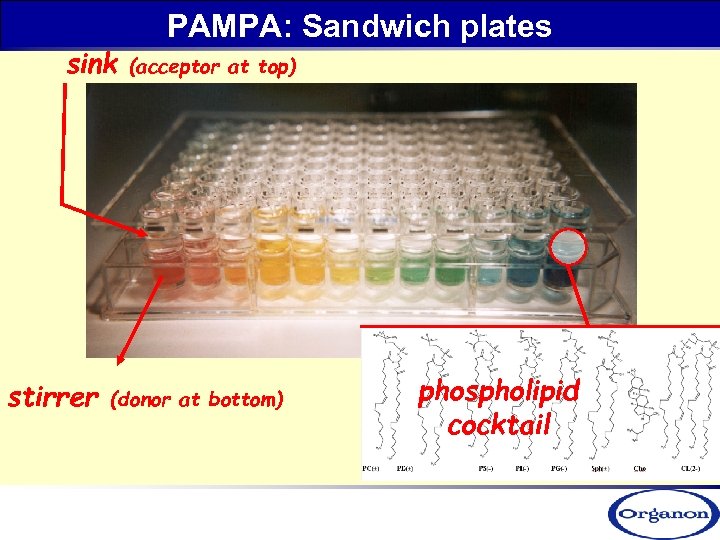
sink stirrer PAMPA: Sandwich plates (acceptor at top) (donor at bottom) phospholipid cocktail
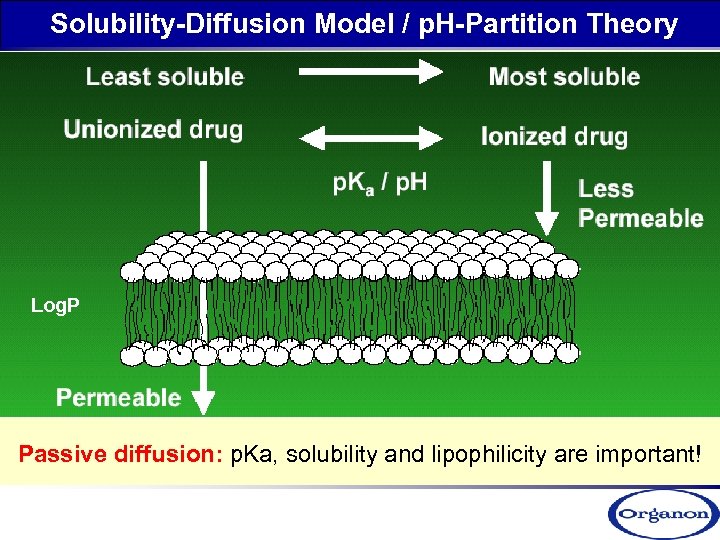
Solubility-Diffusion Model / p. H-Partition Theory Log. P Passive diffusion: p. Ka, solubility and lipophilicity are important!
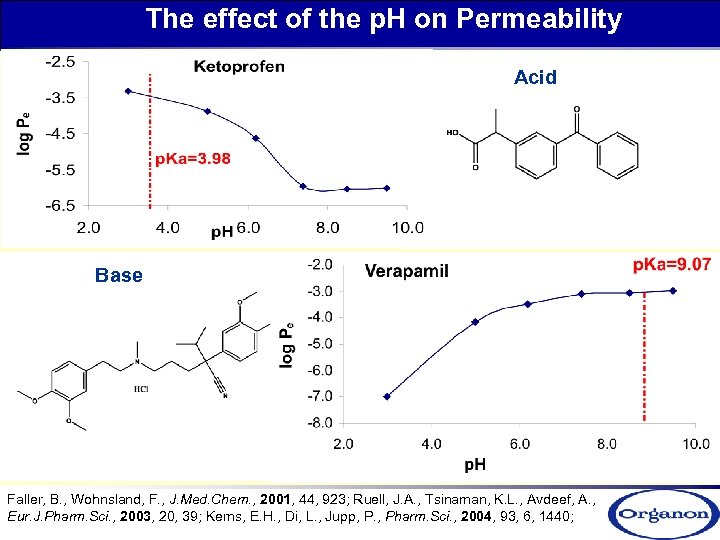
The effect of the p. H on Permeability Acid Base Faller, B. , Wohnsland, F. , J. Med. Chem. , 2001, 44, 923; Ruell, J. A. , Tsinaman, K. L. , Avdeef, A. , Eur. J. Pharm. Sci. , 2003, 20, 39; Kerns, E. H. , Di, L. , Jupp, P. , Pharm. Sci. , 2004, 93, 6, 1440;
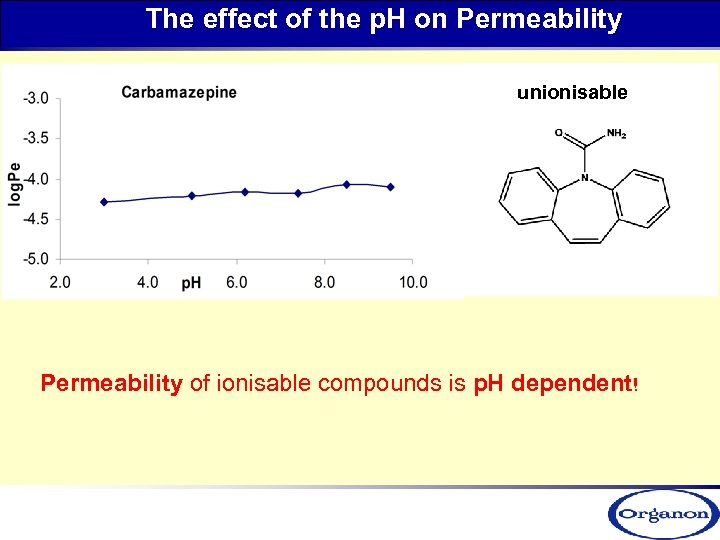
The effect of the p. H on Permeability unionisable Permeability of ionisable compounds is p. H dependent!
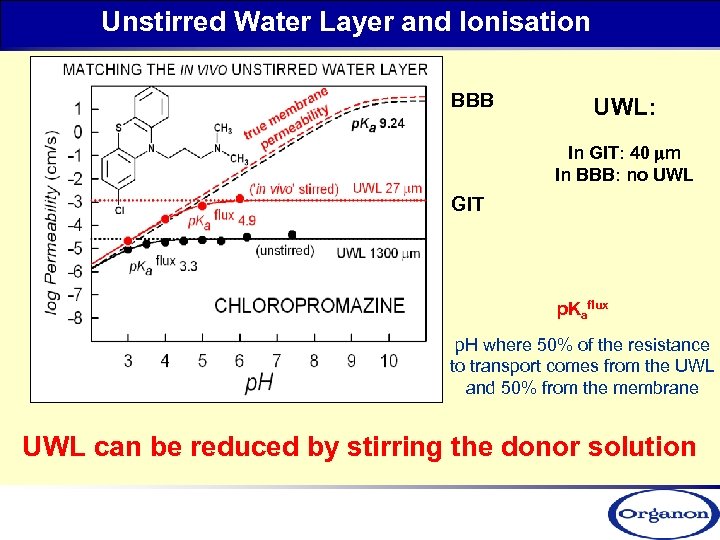
Unstirred Water Layer and Ionisation BBB UWL: In GIT: 40 mm In BBB: no UWL GIT p. Kaflux p. H where 50% of the resistance to transport comes from the UWL and 50% from the membrane UWL can be reduced by stirring the donor solution
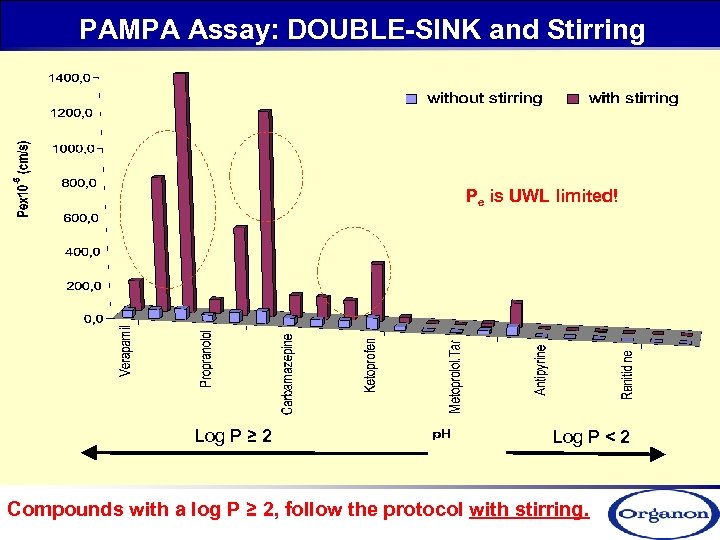
PAMPA Assay: DOUBLE-SINK and Stirring Pe is UWL limited! Log P ≥ 2 Log P < 2 Compounds with a log P ≥ 2, follow the protocol with stirring.
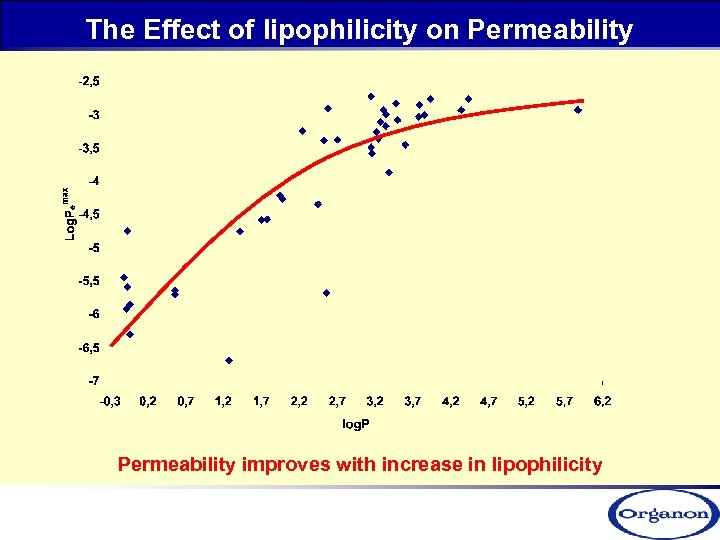
The Effect of lipophilicity on Permeability improves with increase in lipophilicity
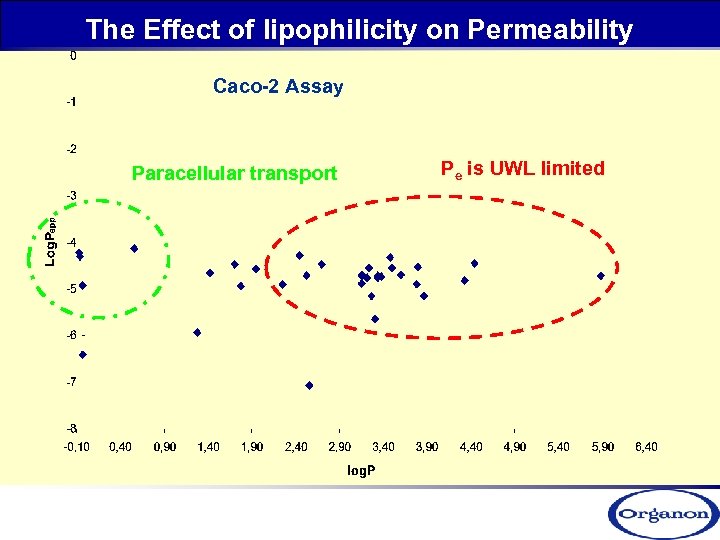
The Effect of lipophilicity on Permeability Caco-2 Assay Paracellular transport Pe is UWL limited
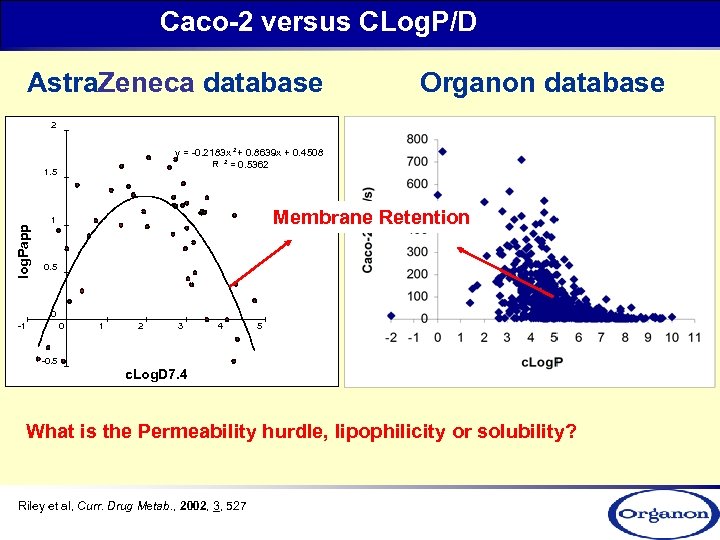
Caco-2 versus CLog. P/D Astra. Zeneca database Organon database 2 y = -0. 2183 x 2 + 0. 8639 x + 0. 4508 R 2 = 0. 5362 log. Papp 1. 5 Membrane Retention 1 0. 5 0 -1 0 1 2 3 4 5 -0. 5 c. Log. D 7. 4 What is the Permeability hurdle, lipophilicity or solubility? Riley et al, Curr. Drug Metab. , 2002, 3, 527
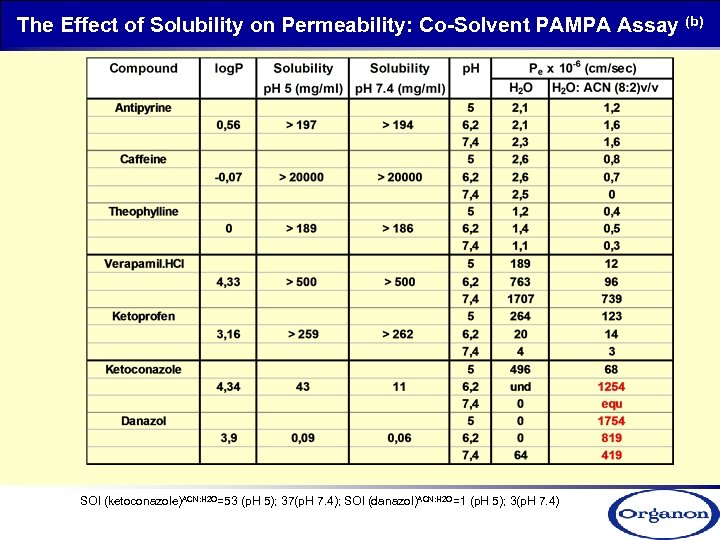
The Effect of Solubility on Permeability: Co-Solvent PAMPA Assay (b) SOl (ketoconazole)ACN: H 2 O=53 (p. H 5); 37(p. H 7. 4); SOl (danazol)ACN: H 2 O=1 (p. H 5); 3(p. H 7. 4)
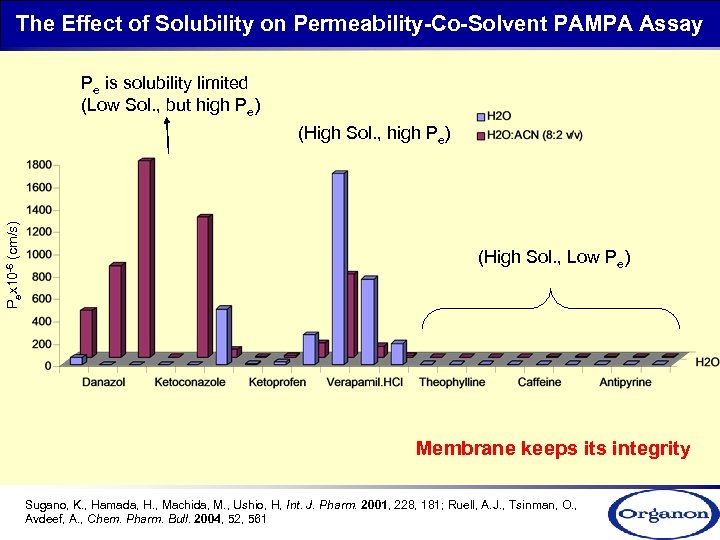
The Effect of Solubility on Permeability-Co-Solvent PAMPA Assay Pe is solubility limited (Low Sol. , but high Pe) Pex 10 -6 (cm/s) (High Sol. , high Pe) (High Sol. , Low Pe) Membrane keeps its integrity Sugano, K. , Hamada, H. , Machida, M. , Ushio, H, Int. J. Pharm. 2001, 228, 181; Ruell, A. J. , Tsinman, O. , Avdeef, A. , Chem. Pharm. Bull. 2004, 52, 561
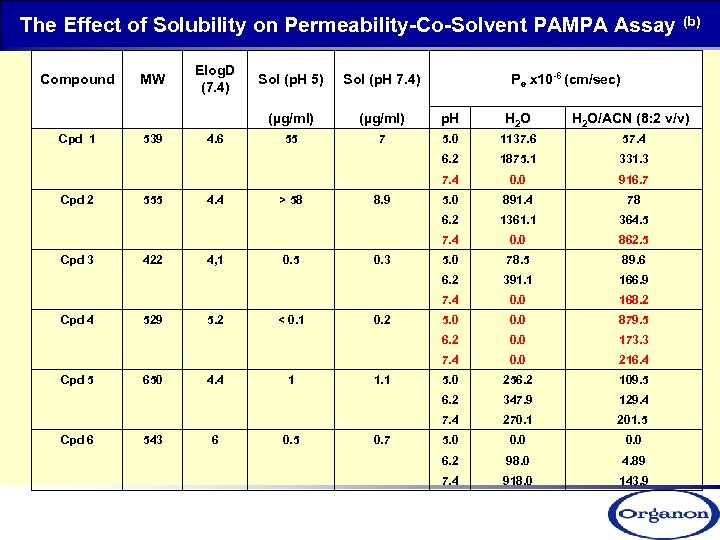
The Effect of Solubility on Permeability-Co-Solvent PAMPA Assay (b) Compound MW Elog. D (7. 4) Sol (p. H 5) Pe x 10 -6 (cm/sec) Sol (p. H 7. 4) (µg/ml) p. H H 2 O/ACN (8: 2 v/v) Cpd 1 539 4. 6 55 7 5. 0 1137. 6 57. 4 6. 2 1875. 1 331. 3 7. 4 0. 0 916. 7 Cpd 2 555 4. 4 > 58 8. 9 5. 0 891. 4 78 6. 2 1361. 1 364. 5 7. 4 0. 0 862. 5 Cpd 3 422 4, 1 0. 5 0. 3 5. 0 78. 5 89. 6 6. 2 391. 1 166. 9 7. 4 0. 0 168. 2 Cpd 4 529 5. 2 < 0. 1 0. 2 5. 0 0. 0 879. 5 6. 2 0. 0 173. 3 7. 4 0. 0 216. 4 Cpd 5 650 4. 4 1 1. 1 5. 0 256. 2 109. 5 6. 2 347. 9 129. 4 7. 4 270. 1 201. 5 Cpd 6 543 6 0. 5 0. 7 5. 0 0. 0 6. 2 98. 0 4. 89 7. 4 918. 0 143. 9
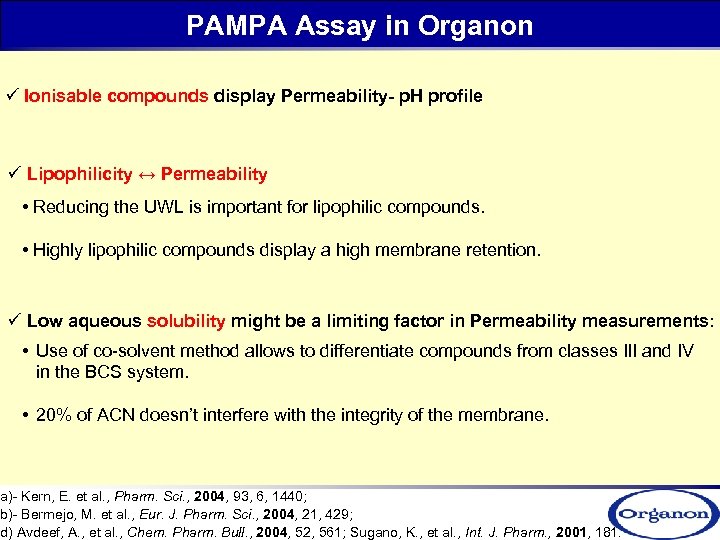
PAMPA Assay in Organon ü Ionisable compounds display Permeability- p. H profile ü Lipophilicity ↔ Permeability • Reducing the UWL is important for lipophilic compounds. • Highly lipophilic compounds display a high membrane retention. ü Low aqueous solubility might be a limiting factor in Permeability measurements: • Use of co-solvent method allows to differentiate compounds from classes III and IV in the BCS system. • 20% of ACN doesn’t interfere with the integrity of the membrane. a)- Kern, E. et al. , Pharm. Sci. , 2004, 93, 6, 1440; b)- Bermejo, M. et al. , Eur. J. Pharm. Sci. , 2004, 21, 429; d) Avdeef, A. , et al. , Chem. Pharm. Bull. , 2004, 52, 561; Sugano, K. , et al. , Int. J. Pharm. , 2001, 181.
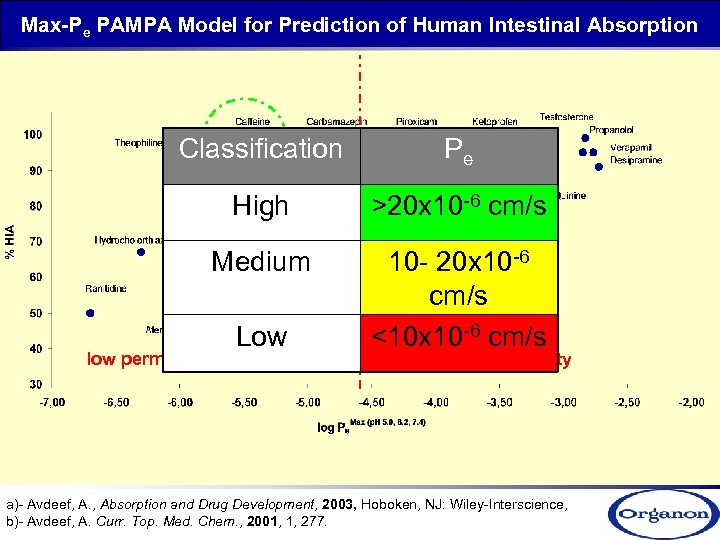
Max-Pe PAMPA Model for Prediction of Human Intestinal Absorption Classification Pe High >20 x 10 -6 cm/s Medium 10 - 20 x 10 -6 cm/s <10 x 10 -6 cm/s low permeability Low high permeability a)- Avdeef, A. , Absorption and Drug Development, 2003, Hoboken, NJ: Wiley-Interscience, b)- Avdeef, A. Curr. Top. Med. Chem. , 2001, 1, 277.
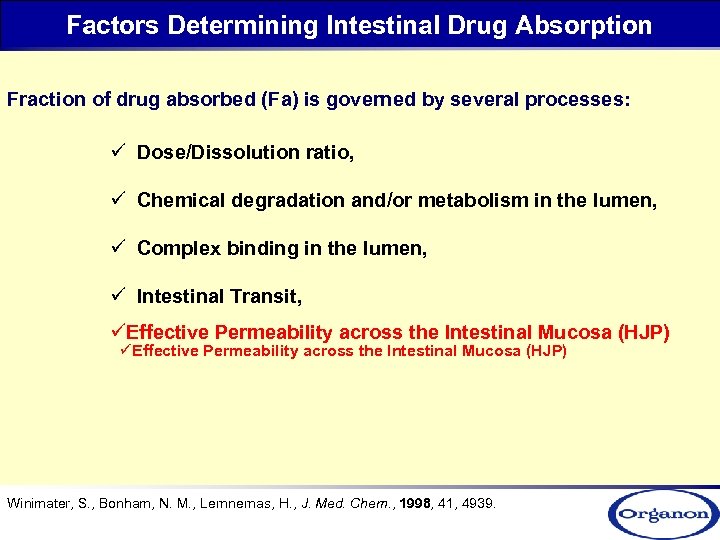
Factors Determining Intestinal Drug Absorption Fraction of drug absorbed (Fa) is governed by several processes: ü Dose/Dissolution ratio, ü Chemical degradation and/or metabolism in the lumen, ü Complex binding in the lumen, ü Intestinal Transit, üEffective Permeability across the Intestinal Mucosa (HJP) Winimater, S. , Bonham, N. M. , Lernnernas, H. , J. Med. Chem. , 1998, 41, 4939.
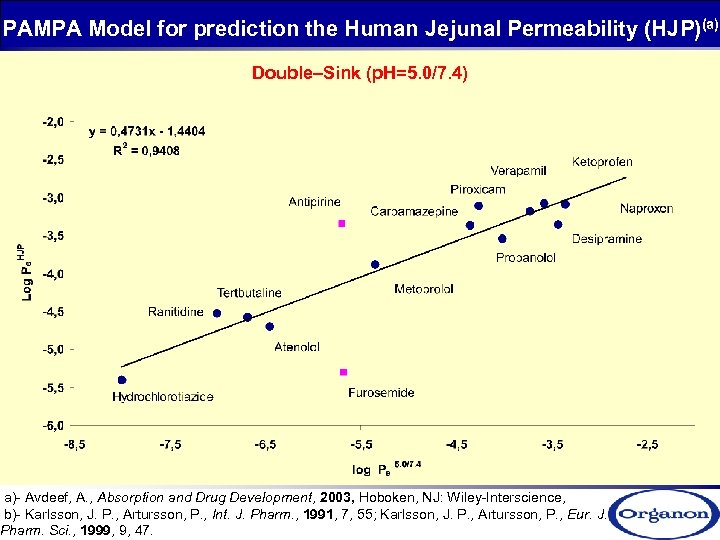
PAMPA Model for prediction the Human Jejunal Permeability (HJP)(a) Double–Sink (p. H=5. 0/7. 4) a)- Avdeef, A. , Absorption and Drug Development, 2003, Hoboken, NJ: Wiley-Interscience, b)- Karlsson, J. P. , Artursson, P. , Int. J. Pharm. , 1991, 7, 55; Karlsson, J. P. , Artursson, P. , Eur. J. Pharm. Sci. , 1999, 9, 47.
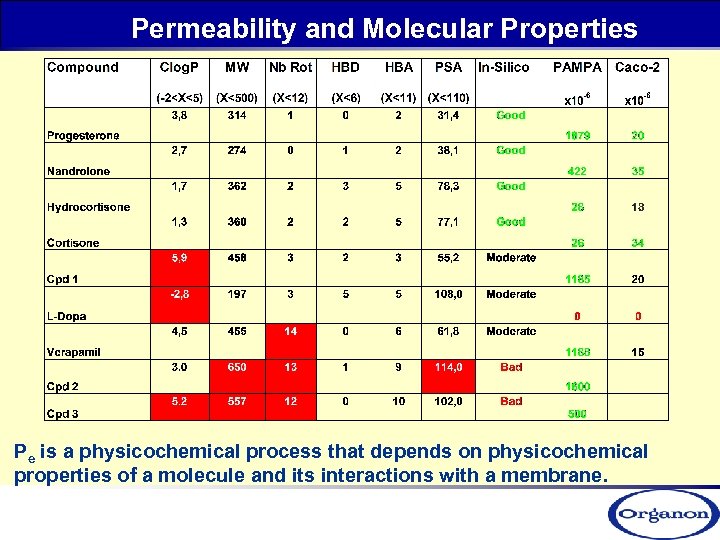
Permeability and Molecular Properties Pe is a physicochemical process that depends on physicochemical properties of a molecule and its interactions with a membrane.
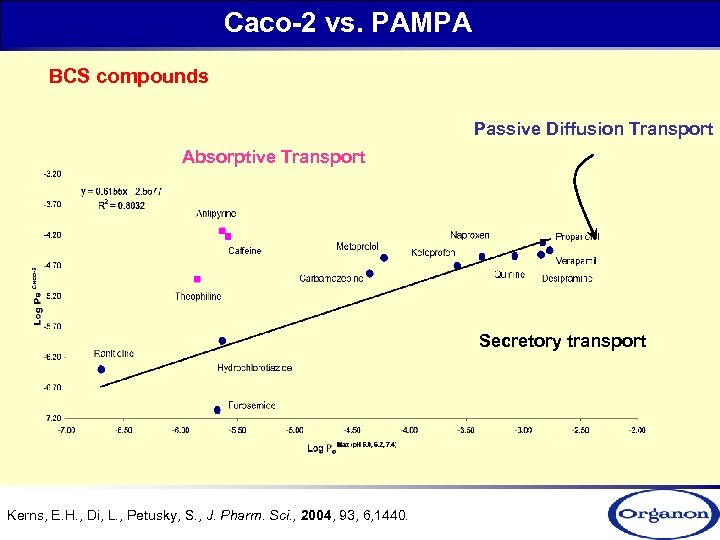
Caco-2 vs. PAMPA BCS compounds Passive Diffusion Transport Absorptive Transport Secretory transport Kerns, E. H. , Di, L. , Petusky, S. , J. Pharm. Sci. , 2004, 93, 6, 1440.
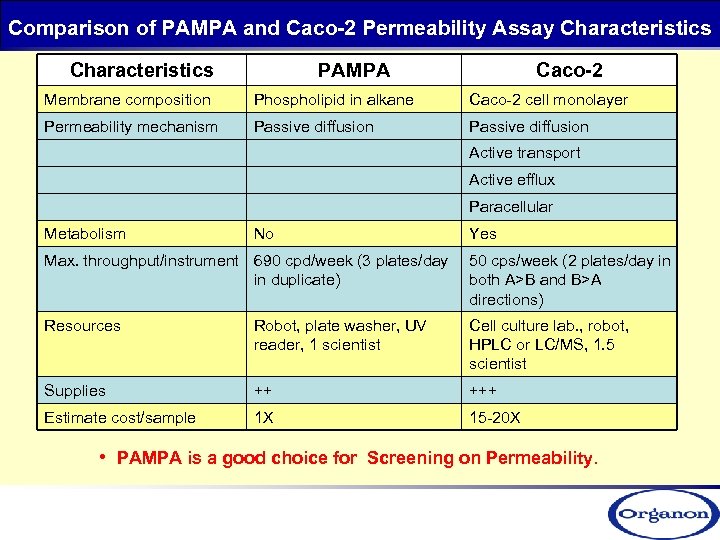
Comparison of PAMPA and Caco-2 Permeability Assay Characteristics PAMPA Caco-2 Membrane composition Phospholipid in alkane Caco-2 cell monolayer Permeability mechanism Passive diffusion Active transport Active efflux Paracellular Metabolism No Yes Max. throughput/instrument 690 cpd/week (3 plates/day in duplicate) 50 cps/week (2 plates/day in both A>B and B>A directions) Resources Robot, plate washer, UV reader, 1 scientist Cell culture lab. , robot, HPLC or LC/MS, 1. 5 scientist Supplies ++ +++ Estimate cost/sample 1 X 15 -20 X • PAMPA is a good choice for Screening on Permeability.
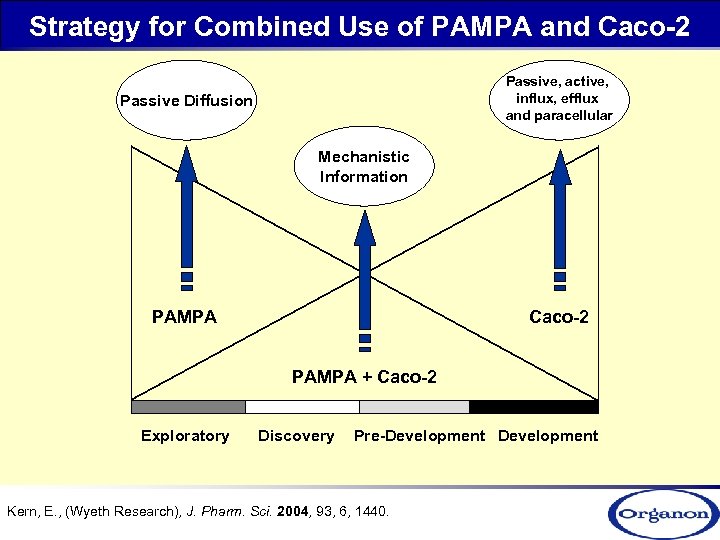
Strategy for Combined Use of PAMPA and Caco-2 Passive, active, influx, efflux and paracellular Passive Diffusion Mechanistic Information PAMPA Caco-2 PAMPA + Caco-2 Exploratory Discovery Pre-Development Kern, E. , (Wyeth Research), J. Pharm. Sci. 2004, 93, 6, 1440.
Acknowledgements Medicinal Chemistry • Maarten Honing • Marcel Hermkens • Michiel Scheffer • Department of Medicinal Chemistry
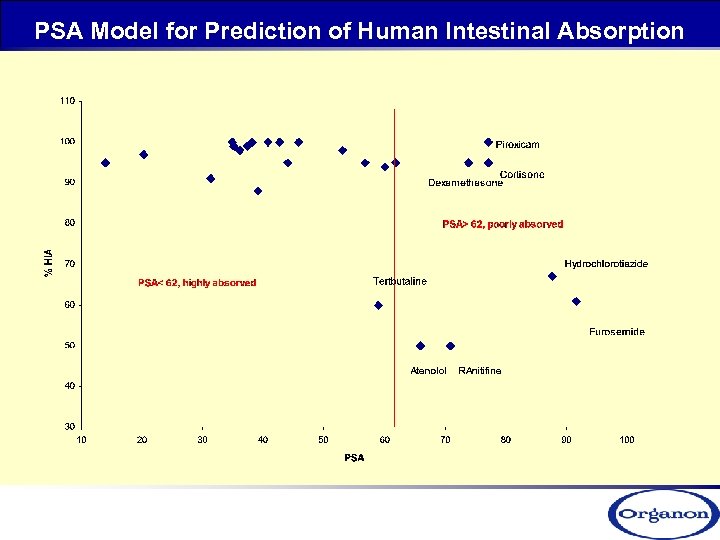
PSA Model for Prediction of Human Intestinal Absorption
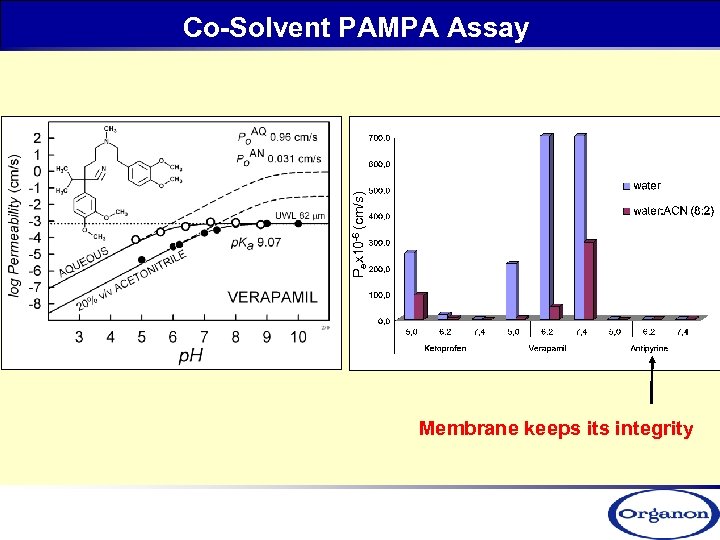
Pex 10 -6 (cm/s) Co-Solvent PAMPA Assay Membrane keeps its integrity
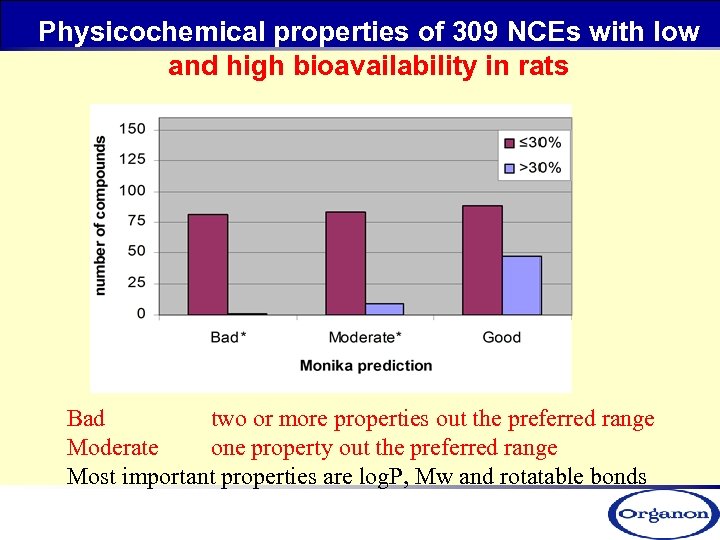
Physicochemical properties of 309 NCEs with low and high bioavailability in rats Bad two or more properties out the preferred range Moderate one property out the preferred range Most important properties are log. P, Mw and rotatable bonds
ca9b8e200738cce0c650278fa42262c8.ppt