42572f774f2b822feff5e189ab6c3374.ppt
- Количество слайдов: 56

PERITONEAL DIALYSIS: PRESENT AND FUTURE Bari, March 2010 Simon Davies University Hospital of North Staffordshire, Stoke-on-Trent Institute for Science and Technology in Medicine Keele University, UK

Looking into the future. . . The next president. . Of ISPD?

What are the challenges for PD? • Developing therapy to enable an aging, increasingly multimorbid dialysis population to have the opportunity for home-based treatment • Creating the clinical evidence for best practice – infection – Fluid management – Membrane injury
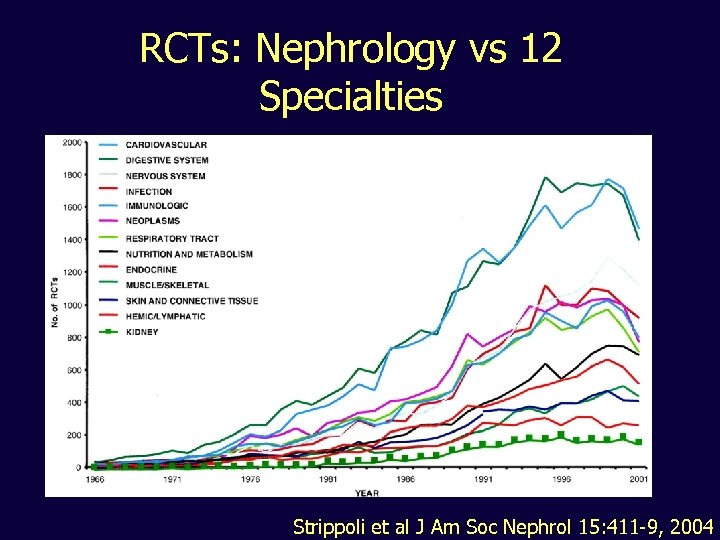
RCTs: Nephrology vs 12 Specialties Strippoli et al J Am Soc Nephrol 15: 411 -9, 2004
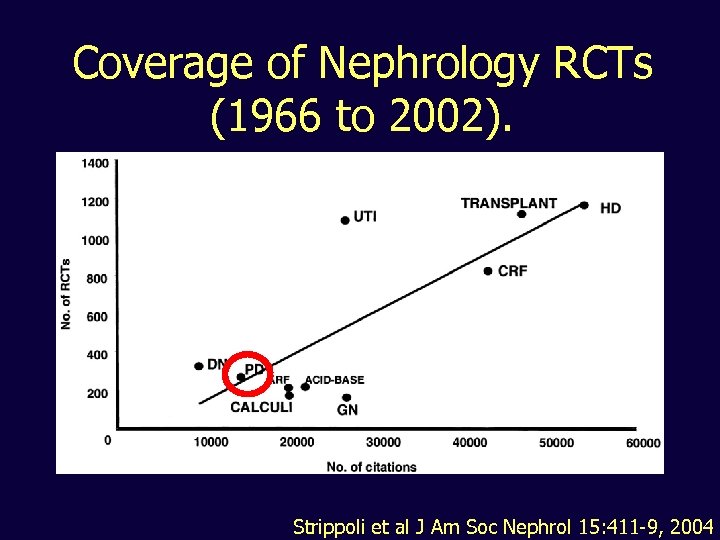
Coverage of Nephrology RCTs (1966 to 2002). Strippoli et al J Am Soc Nephrol 15: 411 -9, 2004
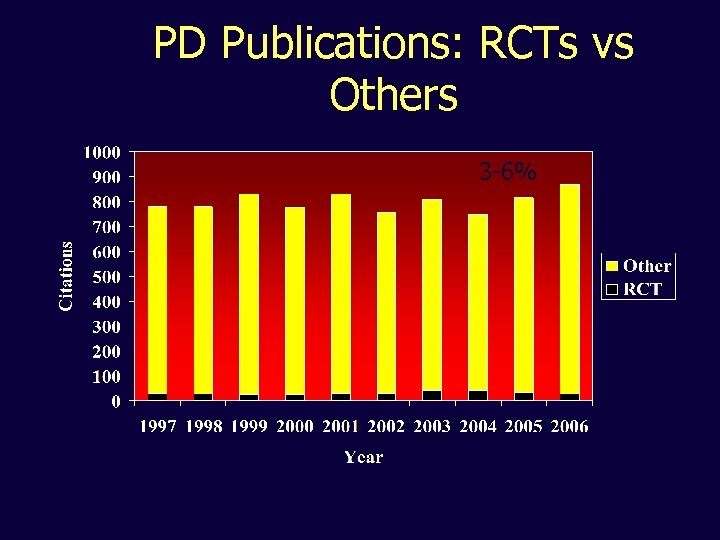
PD Publications: RCTs vs Others 3 -6%
Scope • Extending the role of PD • High/Rapid transport – a problem solved • Residual concerns over salt and water balance in PD? • Monitoring and preserving the membrane

Scope • Extending the role of PD – Part of a central line avoidance strategy – Extending choice - Assisted PD • High/Rapid transport – a problem solved • Residual concerns over salt and water balance in PD • Monitoring and preserving the membrane
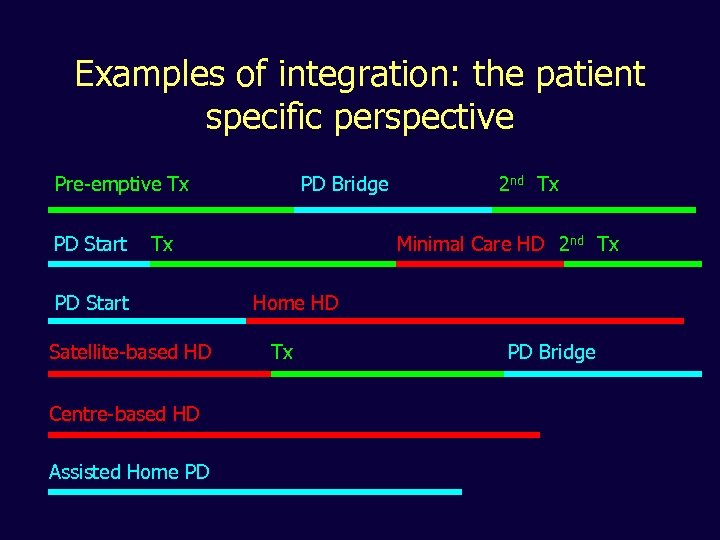
Examples of integration: the patient specific perspective Pre-emptive Tx PD Start PD Bridge Tx PD Start Satellite-based HD Centre-based HD Assisted Home PD 2 nd Tx Minimal Care HD 2 nd Tx Home HD Tx PD Bridge

1, 347 patients included Patients prefer to choose Some have no choice Given choice: Elderly less likely to choose PD Am J Kidney Dis 2004; 43: 891 -9
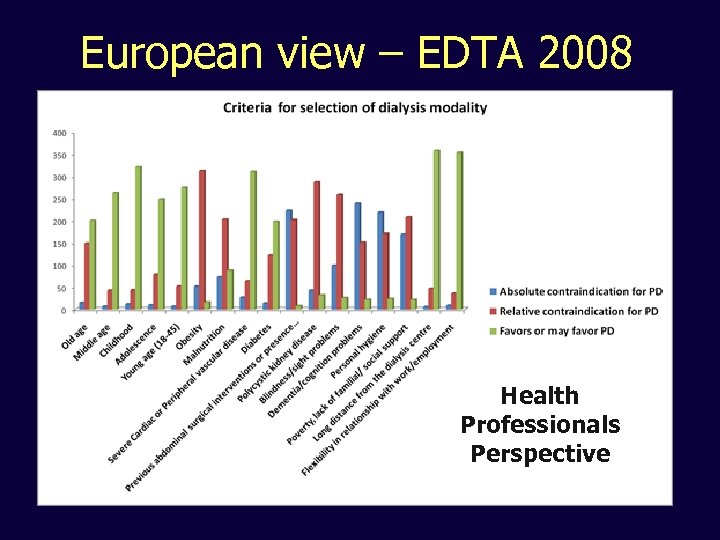
European view – EDTA 2008 Health Professionals Perspective
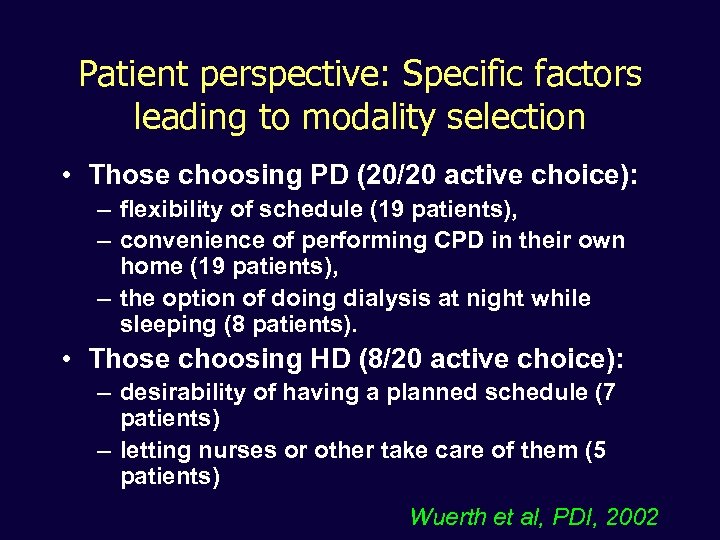
Patient perspective: Specific factors leading to modality selection • Those choosing PD (20/20 active choice): – flexibility of schedule (19 patients), – convenience of performing CPD in their own home (19 patients), – the option of doing dialysis at night while sleeping (8 patients). • Those choosing HD (8/20 active choice): – desirability of having a planned schedule (7 patients) – letting nurses or other take care of them (5 patients) Wuerth et al, PDI, 2002
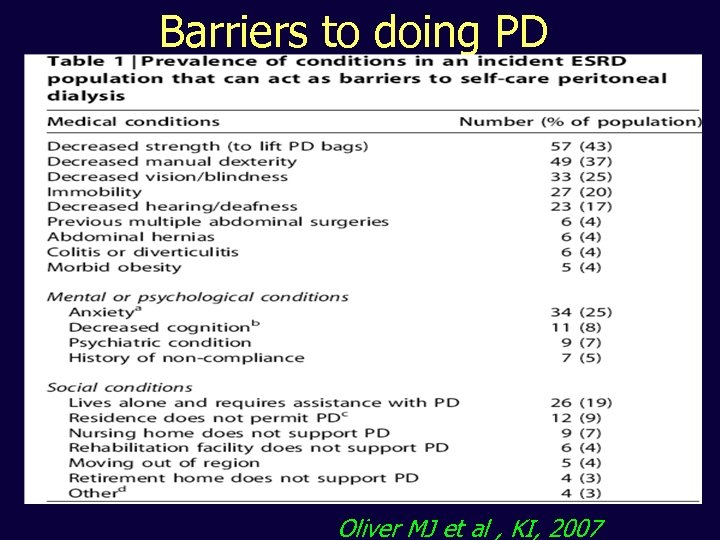
Barriers to doing PD Oliver MJ et al , KI, 2007
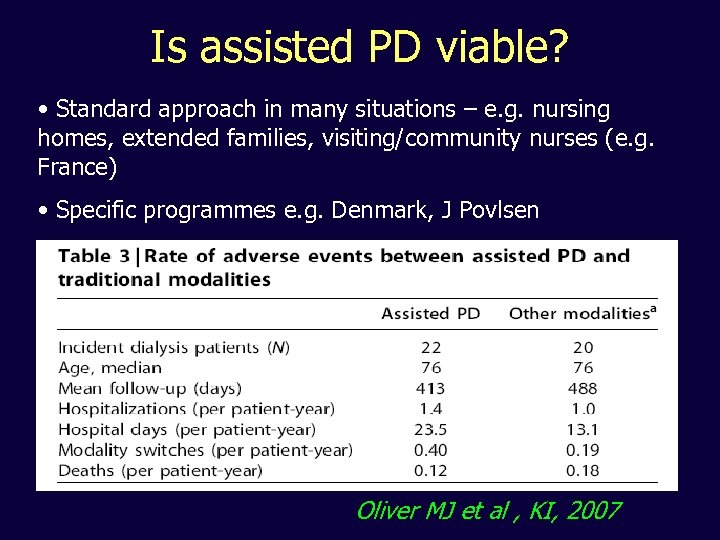
Is assisted PD viable? • Standard approach in many situations – e. g. nursing homes, extended families, visiting/community nurses (e. g. France) • Specific programmes e. g. Denmark, J Povlsen Oliver MJ et al , KI, 2007
Danish experience: retrospective review of older patients on PD • Retrospective review of new patients 2000 -4 – 100 incident patients >65 yrs old (65 -88 yrs) – survived for >2 years • 52 patients had unplanned start defined as starting PD <9 days after catheter insertion • 58 patients required assistance – Caregivers visit patients twice a day – 32 unplanned start Povlsen & Ivarson, PDI 2008
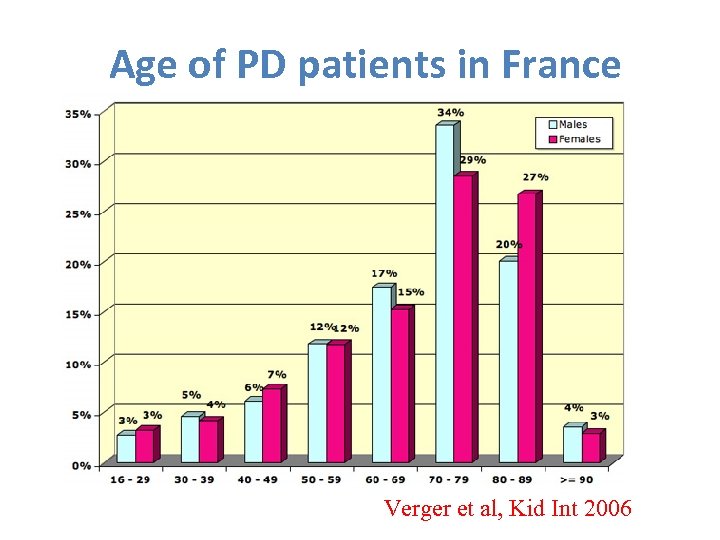
Age of PD patients in France Verger et al, Kid Int 2006
• Retrospective study of 1613 patients >75 years old who started dialysis between 1. 1. 2000 and 31. 12. 2005 • Mean age at dialysis initiation was 81. 9 ± 4. 5 years • 89% on CAPD • 81. 8% required assistance

• Flexible PD catheter insertion arrangements • Motivation and ownership by ward staff (not just the PD team) • Access to continued care with a. APD
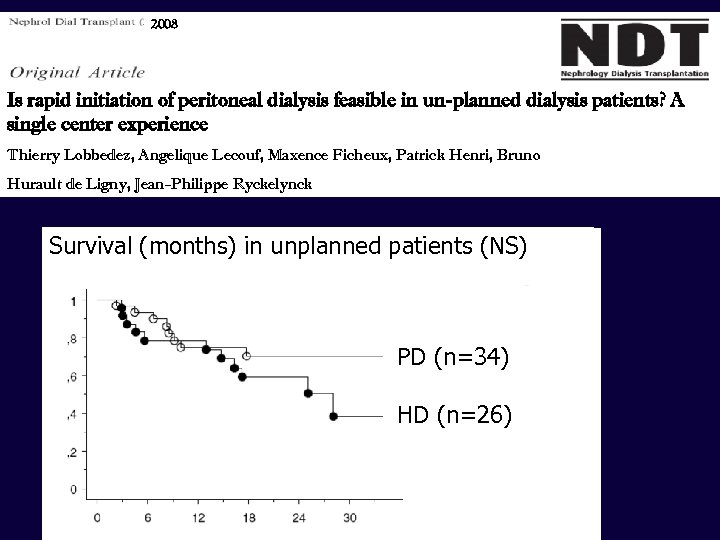
2008 Is rapid initiation of peritoneal dialysis feasible in un-planned dialysis patients? A single center experience Thierry Lobbedez, Angelique Lecouf, Maxence Ficheux, Patrick Henri, Bruno Hurault de Ligny, Jean-Philippe Ryckelynck Survival (months) in unplanned patients (NS) PD (n=34) HD (n=26)

In the future. . . • We will break down the barriers to doing PD, including age, professional bias, patient bias, social, financial. . . • There will be an egalitarian approach to enabling all patients to benefit from what PD has to offer • Evidence, evidence. . .

Scope • Extending the role of PD • High/Rapid transport – a problem solved – Identification as a risk factor – Understanding the mechanism – Improved outcomes • Residual concerns over salt and water balance in PD • Monitoring and preserving the membrane
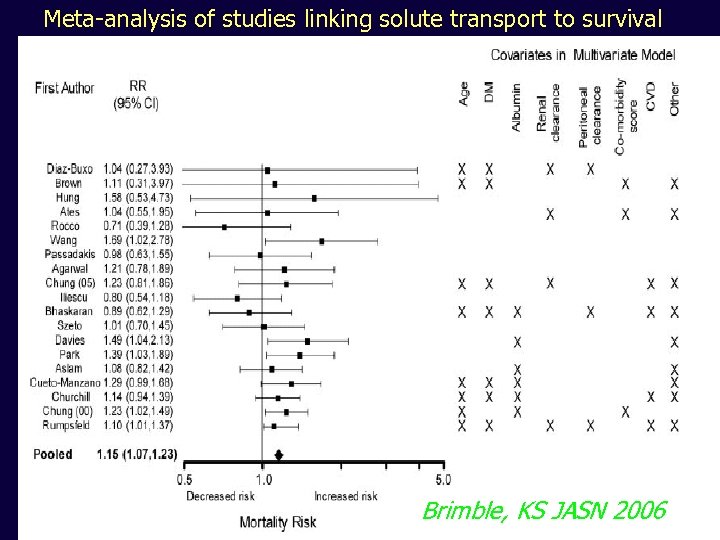
Meta-analysis of studies linking solute transport to survival Brimble, KS JASN 2006
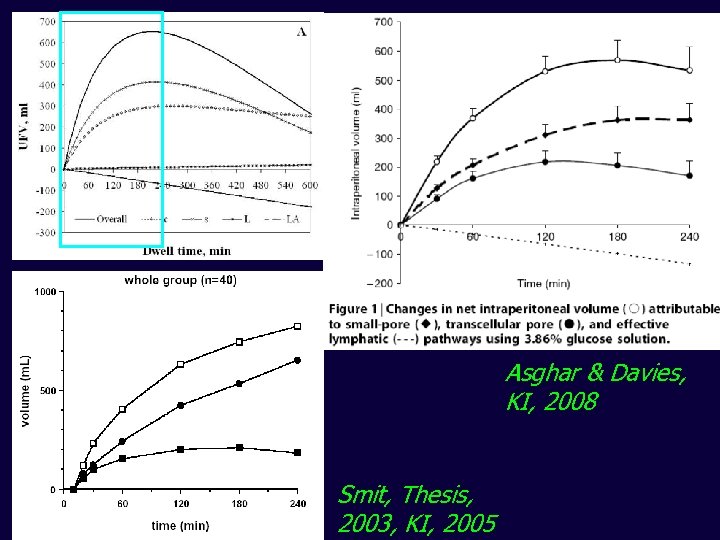
Asghar & Davies, KI, 2008 Smit, Thesis, 2003, KI, 2005
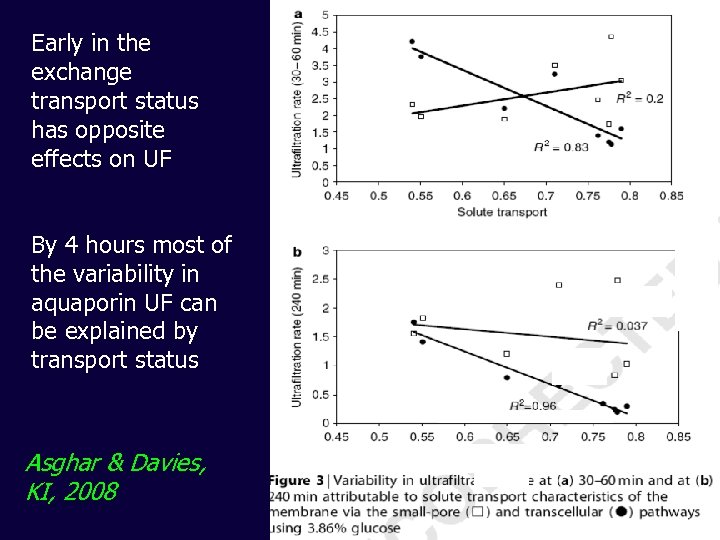
Early in the exchange transport status has opposite effects on UF By 4 hours most of the variability in aquaporin UF can be explained by transport status Asghar & Davies, KI, 2008
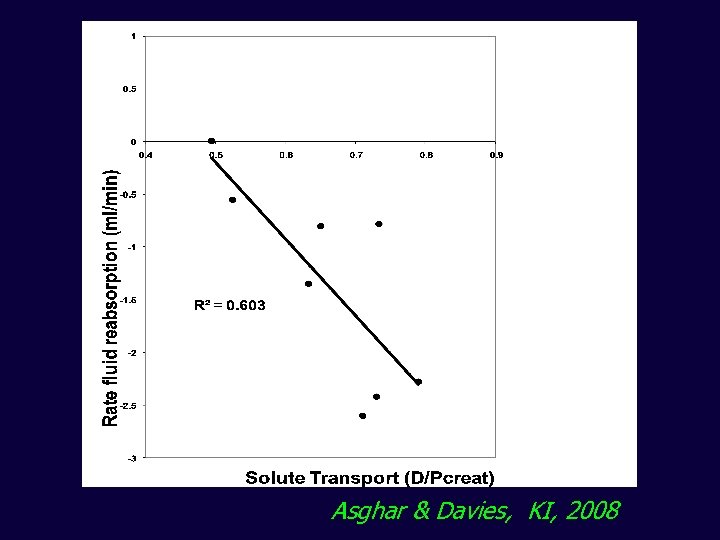
Asghar & Davies, KI, 2008

Why might high transport be associated with worse outcomes? • Worse ultrafiltration – Early loss of osmotic gradient causing less efficient aquaporin mediated UF – More rapid fluid reabsorption in long dwell via the small pores • Increased protein losses • Association with membrane inflammation
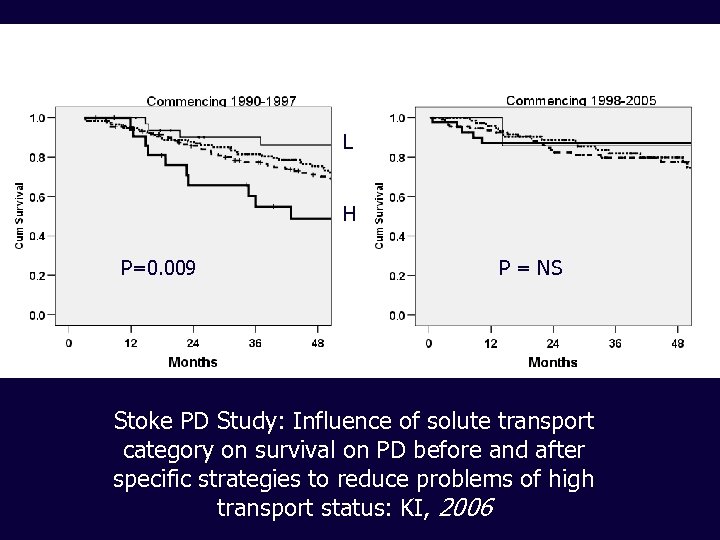
L H P=0. 009 P = NS Stoke PD Study: Influence of solute transport category on survival on PD before and after specific strategies to reduce problems of high transport status: KI, 2006
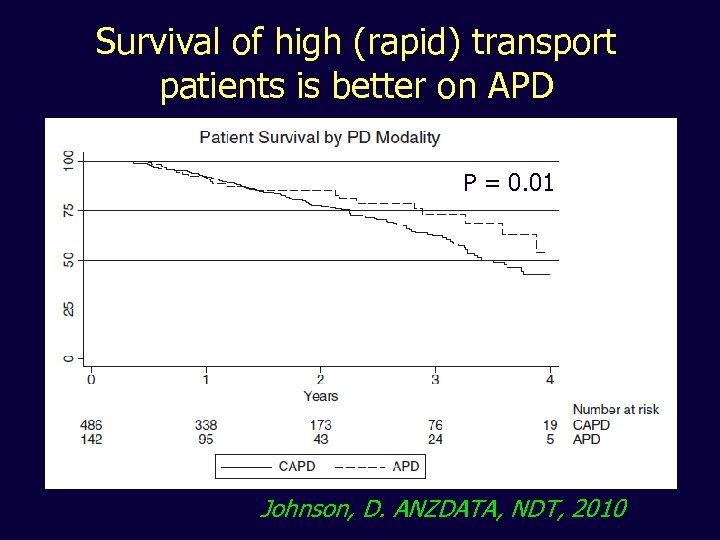
Survival of high (rapid) transport patients is better on APD P = 0. 01 Johnson, D. ANZDATA, NDT, 2010
Scope • Extending the role of PD • High/Rapid transport – a problem solved • Residual concerns over salt and water balance in PD – Are PD patients fluid loaded – How does this relate to cardiac function? • Monitoring and preserving the membrane
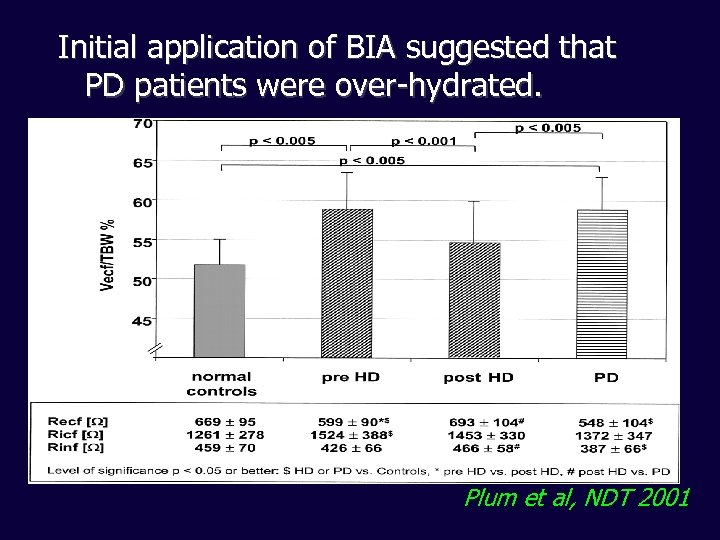
Initial application of BIA suggested that PD patients were over-hydrated. Plum et al, NDT 2001
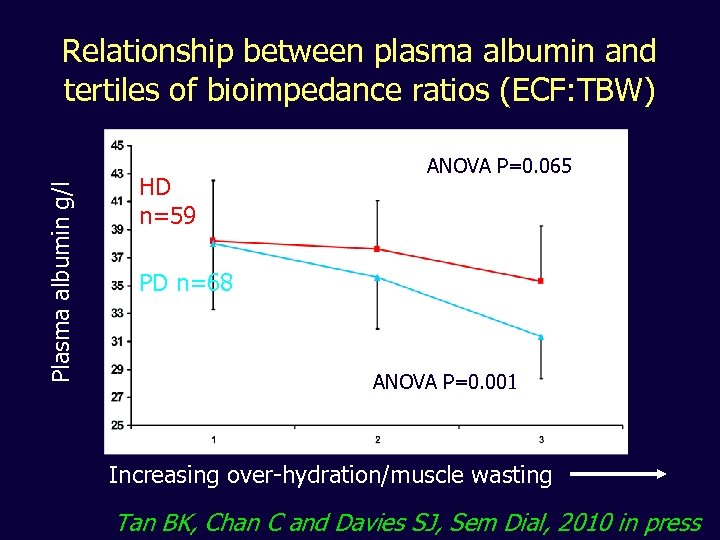
Plasma albumin g/l Relationship between plasma albumin and tertiles of bioimpedance ratios (ECF: TBW) HD n=59 ANOVA P=0. 065 PD n=68 ANOVA P=0. 001 Increasing over-hydration/muscle wasting Tan BK, Chan C and Davies SJ, Sem Dial, 2010 in press
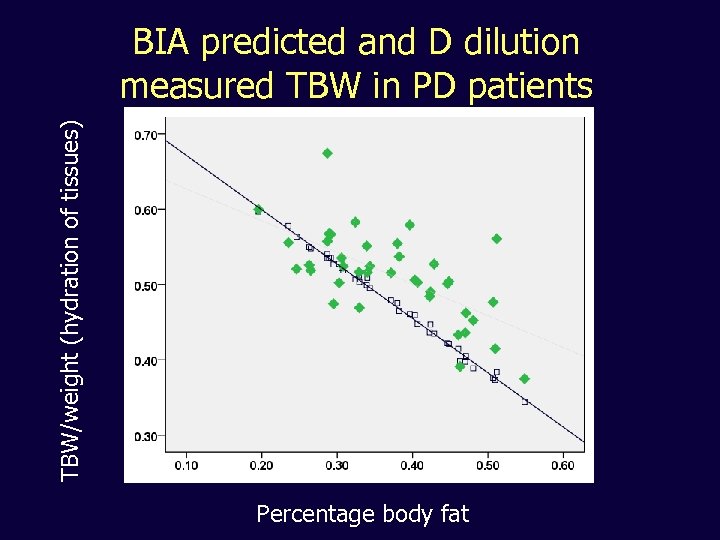
TBW/weight (hydration of tissues) BIA predicted and D dilution measured TBW in PD patients Percentage body fat
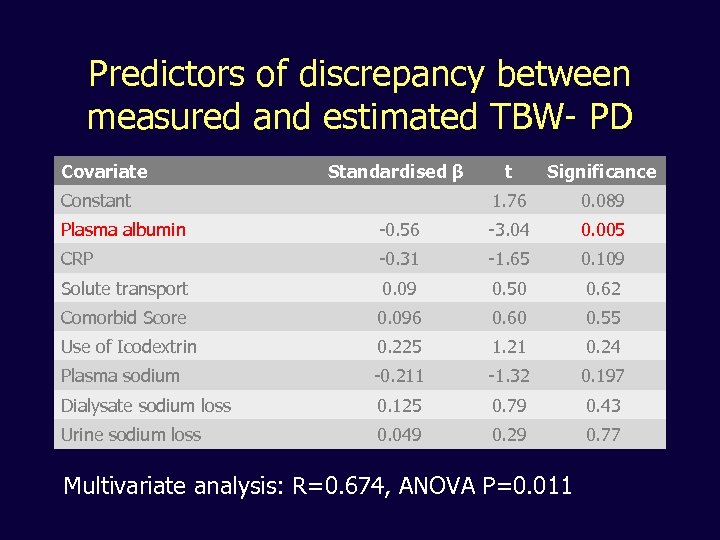
Predictors of discrepancy between measured and estimated TBW- PD Covariate Standardised β Significance 1. 76 Constant t 0. 089 Plasma albumin -0. 56 -3. 04 0. 005 CRP -0. 31 -1. 65 0. 109 Solute transport 0. 09 0. 50 0. 62 Comorbid Score 0. 096 0. 60 0. 55 Use of Icodextrin 0. 225 1. 21 0. 24 Plasma sodium -0. 211 -1. 32 0. 197 Dialysate sodium loss 0. 125 0. 79 0. 43 Urine sodium loss 0. 049 0. 29 0. 77 Multivariate analysis: R=0. 674, ANOVA P=0. 011
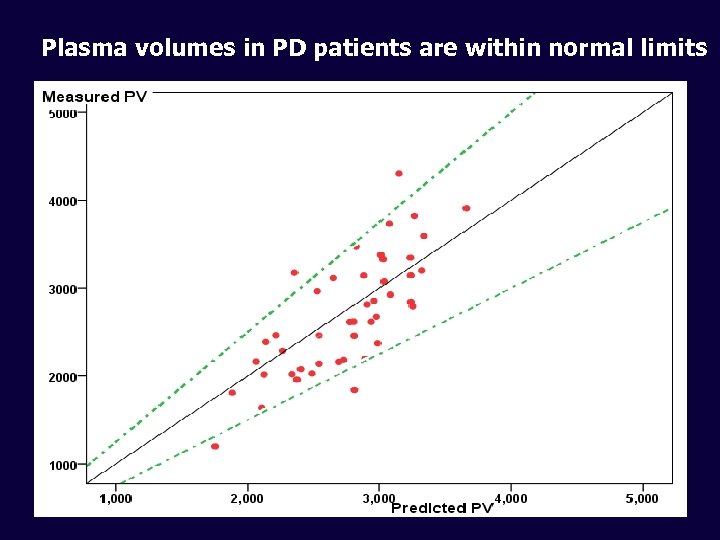
Plasma volumes in PD patients are within normal limits
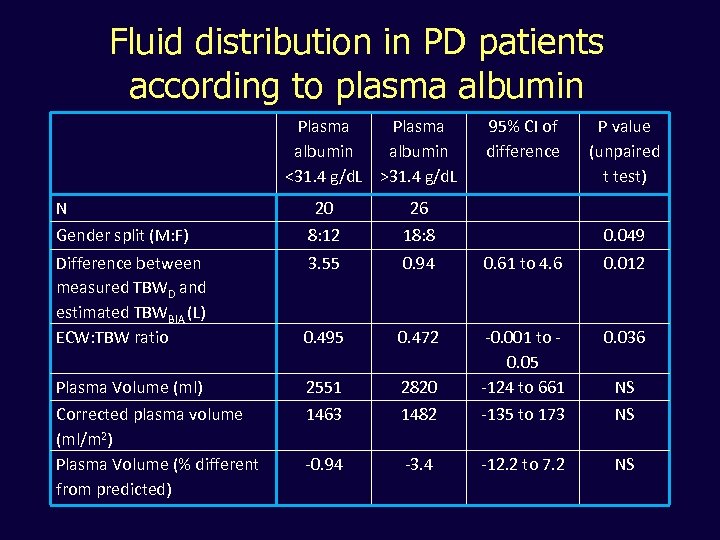
Fluid distribution in PD patients according to plasma albumin Plasma albumin <31. 4 g/d. L >31. 4 g/d. L N 95% CI of difference P value (unpaired t test) 20 26 Gender split (M: F) 8: 12 18: 8 Difference between measured TBWD and estimated TBWBIA (L) ECW: TBW ratio 3. 55 0. 94 0. 61 to 4. 6 0. 012 0. 495 0. 472 0. 036 Plasma Volume (ml) 2551 2820 -0. 001 to 0. 05 -124 to 661 Corrected plasma volume (ml/m 2) Plasma Volume (% different from predicted) 1463 1482 -135 to 173 NS -0. 94 -3. 4 -12. 2 to 7. 2 NS 0. 049 NS
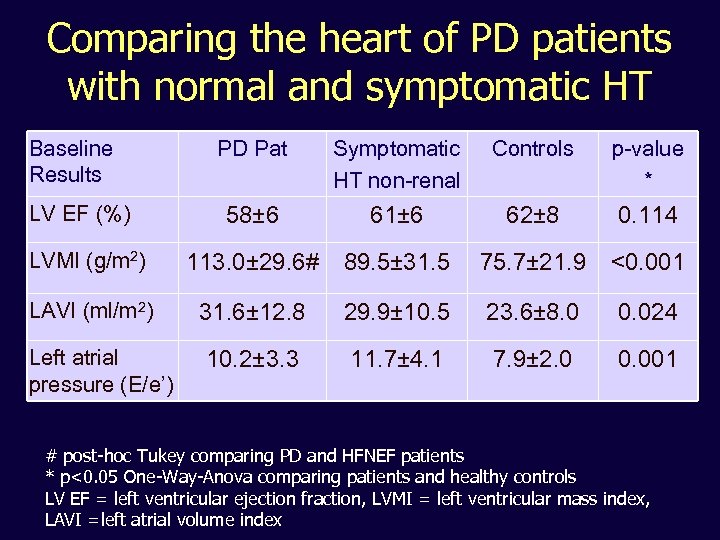
Comparing the heart of PD patients with normal and symptomatic HT Baseline Results PD Pat Symptomatic HT non-renal Controls p-value * 58± 6 61± 6 62± 8 0. 114 LVMI (g/m 2) 113. 0± 29. 6# 89. 5± 31. 5 75. 7± 21. 9 <0. 001 LAVI (ml/m 2) 31. 6± 12. 8 29. 9± 10. 5 23. 6± 8. 0 0. 024 10. 2± 3. 3 11. 7± 4. 1 7. 9± 2. 0 0. 001 LV EF (%) Left atrial pressure (E/e’) # post-hoc Tukey comparing PD and HFNEF patients * p<0. 05 One-Way-Anova comparing patients and healthy controls LV EF = left ventricular ejection fraction, LVMI = left ventricular mass index, LAVI =left atrial volume index
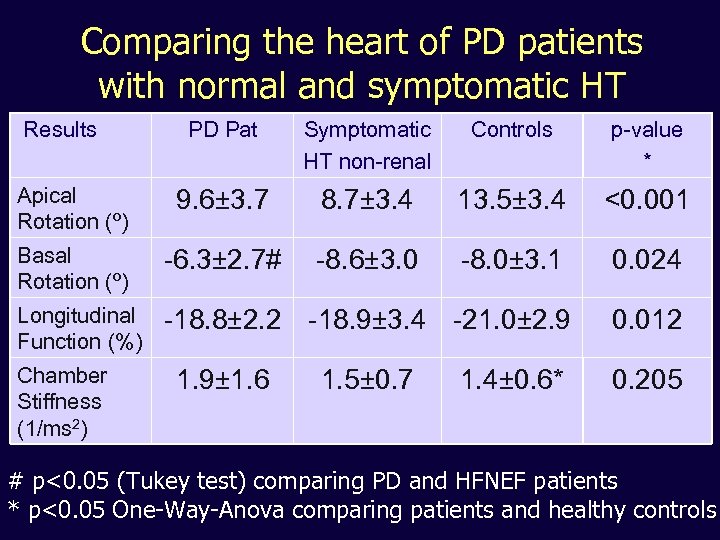
Comparing the heart of PD patients with normal and symptomatic HT Results PD Pat Symptomatic HT non-renal Controls p-value * Apical Rotation (º) 9. 6± 3. 7 8. 7± 3. 4 13. 5± 3. 4 <0. 001 Basal Rotation (º) -6. 3± 2. 7# -8. 6± 3. 0 -8. 0± 3. 1 0. 024 Longitudinal Function (%) -18. 8± 2. 2 -18. 9± 3. 4 -21. 0± 2. 9 0. 012 Chamber Stiffness (1/ms 2) 1. 9± 1. 6 1. 5± 0. 7 1. 4± 0. 6* 0. 205 # p<0. 05 (Tukey test) comparing PD and HFNEF patients * p<0. 05 One-Way-Anova comparing patients and healthy controls
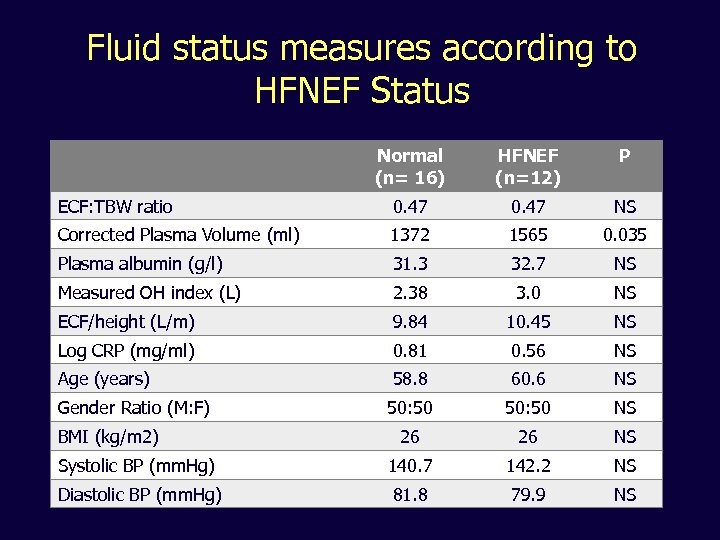
Fluid status measures according to HFNEF Status Normal (n= 16) HFNEF (n=12) P ECF: TBW ratio 0. 47 NS Corrected Plasma Volume (ml) 1372 1565 0. 035 Plasma albumin (g/l) 31. 3 32. 7 NS Measured OH index (L) 2. 38 3. 0 NS ECF/height (L/m) 9. 84 10. 45 NS Log CRP (mg/ml) 0. 81 0. 56 NS Age (years) 58. 8 60. 6 NS Gender Ratio (M: F) 50: 50 NS 26 26 NS Systolic BP (mm. Hg) 140. 7 142. 2 NS Diastolic BP (mm. Hg) 81. 8 79. 9 NS BMI (kg/m 2)
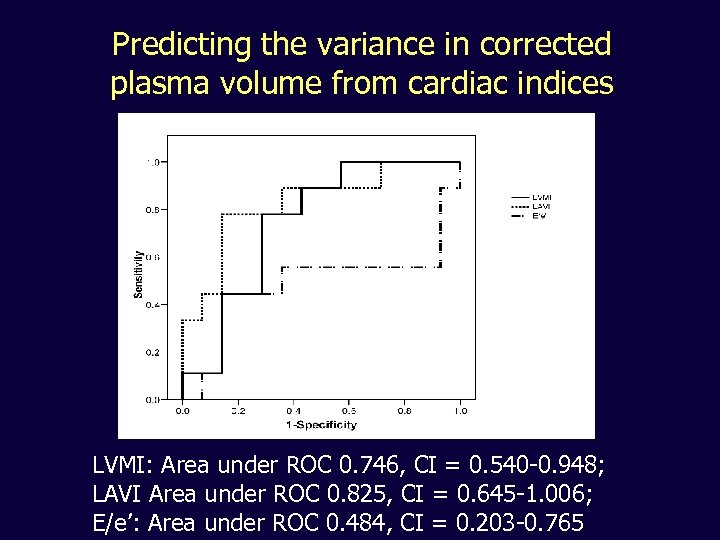
Predicting the variance in corrected plasma volume from cardiac indices LVMI: Area under ROC 0. 746, CI = 0. 540 -0. 948; LAVI Area under ROC 0. 825, CI = 0. 645 -1. 006; E/e’: Area under ROC 0. 484, CI = 0. 203 -0. 765
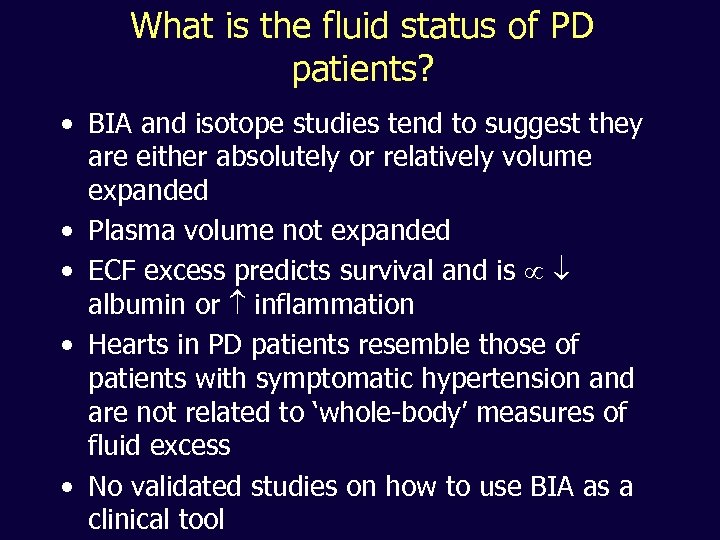
What is the fluid status of PD patients? • BIA and isotope studies tend to suggest they are either absolutely or relatively volume expanded • Plasma volume not expanded • ECF excess predicts survival and is albumin or inflammation • Hearts in PD patients resemble those of patients with symptomatic hypertension and are not related to ‘whole-body’ measures of fluid excess • No validated studies on how to use BIA as a clinical tool

In the future. . . • We will have validated tools to assess and optimally manage the fluid status of PD patients • Evidence, evidence. . .
Scope • Extending the role of PD • High/Rapid transport – a problem solved • Residual concerns over salt and water balance in PD • Monitoring and preserving the membrane – Maintain better treatment – Avoid EPS – Understand the underlying mechanisms of membrane injury and thus treatment strategies
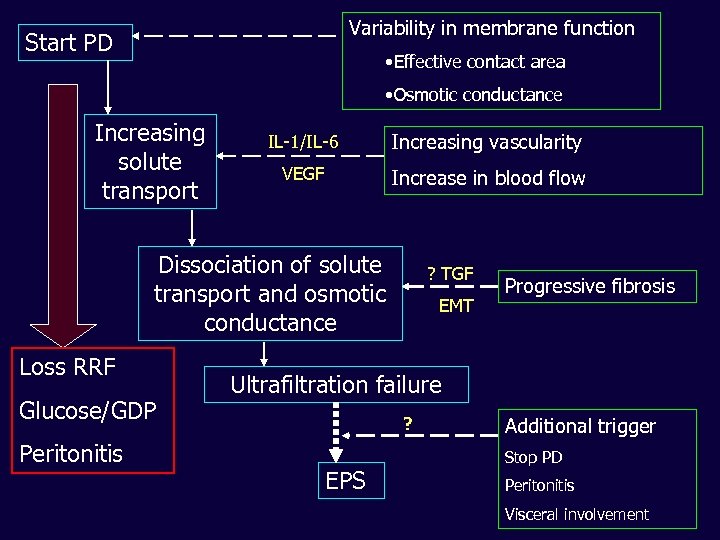
Variability in membrane function Start PD • Effective contact area • Osmotic conductance Increasing solute transport IL-1/IL-6 Increasing vascularity VEGF Increase in blood flow Dissociation of solute transport and osmotic conductance Loss RRF Glucose/GDP Peritonitis ? TGF EMT Progressive fibrosis Ultrafiltration failure ? EPS Additional trigger Stop PD Peritonitis Visceral involvement
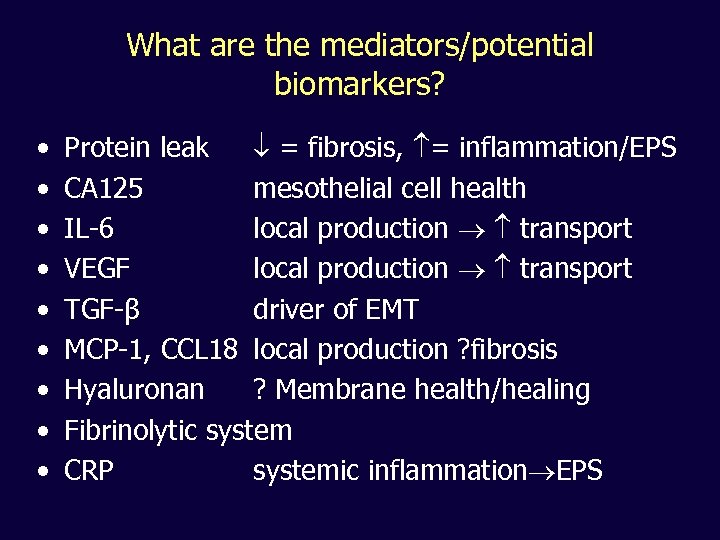
What are the mediators/potential biomarkers? • • • Protein leak = fibrosis, = inflammation/EPS CA 125 mesothelial cell health IL-6 local production transport VEGF local production transport TGF-β driver of EMT MCP-1, CCL 18 local production ? fibrosis Hyaluronan ? Membrane health/healing Fibrinolytic system CRP systemic inflammation EPS
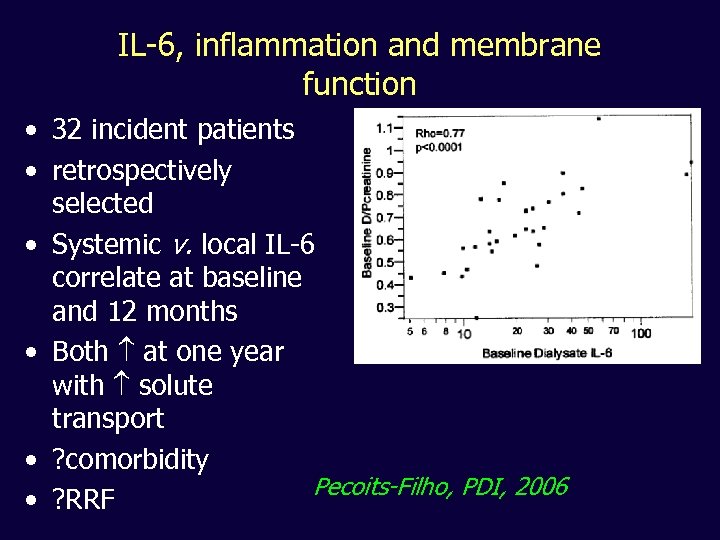
IL-6, inflammation and membrane function • 32 incident patients • retrospectively selected • Systemic v. local IL-6 correlate at baseline and 12 months • Both at one year with solute transport • ? comorbidity Pecoits-Filho, PDI, 2006 • ? RRF
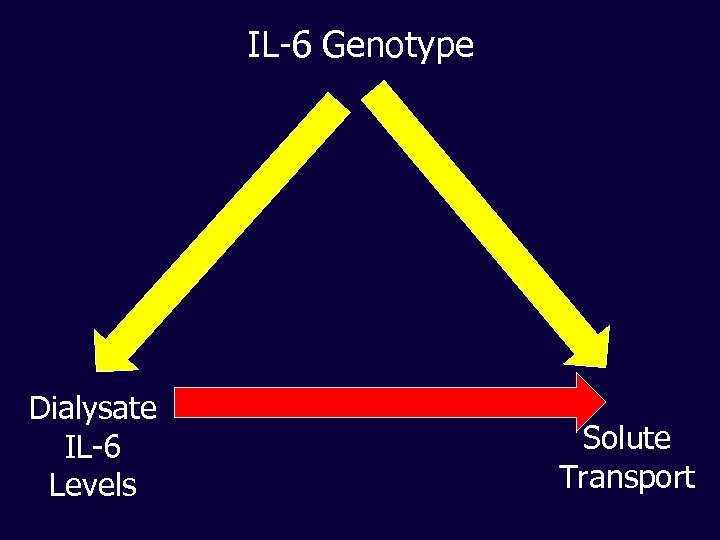
IL-6 Genotype Dialysate IL-6 Levels Solute Transport

Global Fluid Study Longitudinal evaluation of peritoneal membrane function and effluent markers of peritoneal membrane structural integrity in Peritoneal Dialysis patients

Study Design • Prospective study of membrane function, systemic and local inflammatory markers • Multi-centre (19) from Belgium, Canada, Israel, Hong Kong, Korea and UK • Incident and prevalent cohorts • Pre-defined endpoints (patient and technique survival, change in membrane function, peritonitis)
Initial analysis of biomarkers • Paired dialysate and plasma samples, IFN-γ , IL-1β, IL-6, TNF-α • Initial sample from both incident and prevalent cohorts • 10 centres selected on basis of best data integrity (5 UK, 3 Korea, 2 Canada)
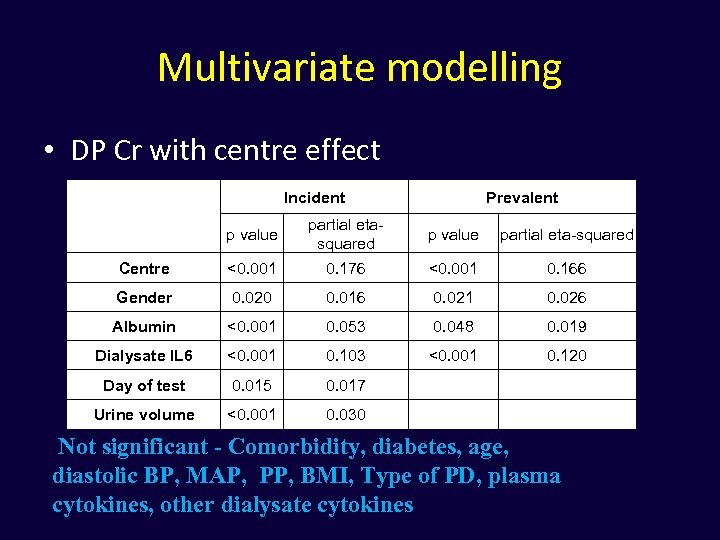
Multivariate modelling • DP Cr with centre effect Incident Prevalent p value partial etasquared p value partial eta-squared Centre <0. 001 0. 176 <0. 001 0. 166 Gender 0. 020 0. 016 0. 021 0. 026 Albumin <0. 001 0. 053 0. 048 0. 019 Dialysate IL 6 <0. 001 0. 103 <0. 001 0. 120 Day of test 0. 015 0. 017 Urine volume <0. 001 0. 030 Not significant - Comorbidity, diabetes, age, diastolic BP, MAP, PP, BMI, Type of PD, plasma cytokines, other dialysate cytokines
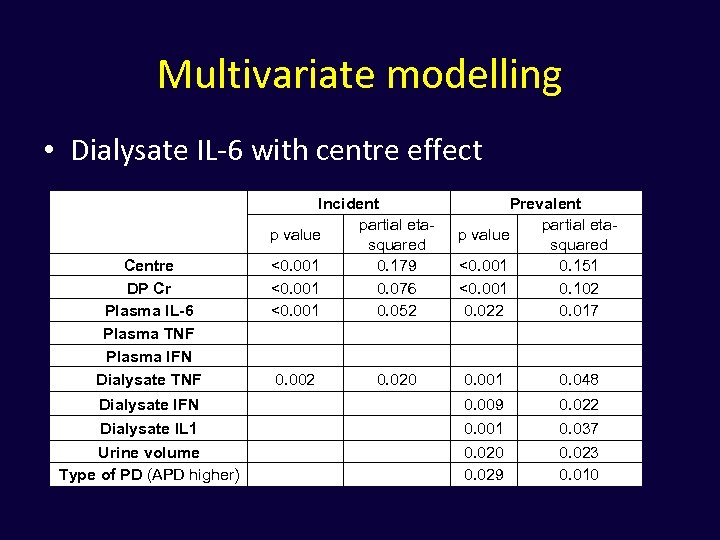
Multivariate modelling • Dialysate IL-6 with centre effect Centre DP Cr Plasma IL-6 Plasma TNF Plasma IFN Dialysate TNF Incident partial etap value squared <0. 001 0. 179 <0. 001 0. 076 <0. 001 0. 052 0. 002 0. 020 Prevalent partial etap value squared <0. 001 0. 151 <0. 001 0. 102 0. 022 0. 017 0. 001 0. 048 Dialysate IFN 0. 009 0. 022 Dialysate IL 1 Urine volume Type of PD (APD higher) 0. 001 0. 020 0. 029 0. 037 0. 023 0. 010
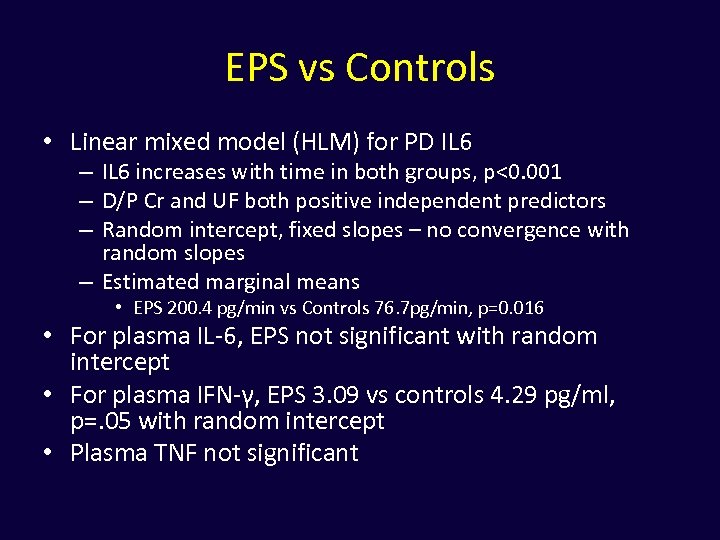
EPS vs Controls • Linear mixed model (HLM) for PD IL 6 – IL 6 increases with time in both groups, p<0. 001 – D/P Cr and UF both positive independent predictors – Random intercept, fixed slopes – no convergence with random slopes – Estimated marginal means • EPS 200. 4 pg/min vs Controls 76. 7 pg/min, p=0. 016 • For plasma IL-6, EPS not significant with random intercept • For plasma IFN-γ, EPS 3. 09 vs controls 4. 29 pg/ml, p=. 05 with random intercept • Plasma TNF not significant
Global Fluid Study Initial conclusions: • Cross sectional analysis indicates un-coupling of local (PD membrane) from systemic inflammation • Local IL-6 production is strongly associated with membrane transport characteristics • IL-6, especially in prevalent patients is associated with a more active local cytokine network • Systemic inflammation is associated with age, comorbidity, albumin • Reduced UF Capacity in prevalent patients is associated with lower IL-6 and detectable IFN independent of solute transport characteristics

In the future. . . • We will have biomarkers that help clinicians recognise peritoneal membrane injury • We will understand the mechanisms of damage and have treatments to prevent this • Evidence, evidence

Acknowledgements Cian Chan Barbara Engel Ramzana Asghar Biju John Kay Tan Frauke Wenzelburger David Smith FRS Patrik Spanel Chris Mc. Intyre John Sanderson Renal Discoveries Extramural Grant Programme

Acknowledgements Collaborators and colleagues EAPOS Group Bengt Rippe Daniele Venturoli Ray Krediet Denise Sampimon Ramzana Asghar Lily Mushahar Kit Huckvale Biju John Jeff Perl PD staff and patients Titus Augustine Paul Brenchley Biopsy Registry John Williams Nick Topley Kate Craig GLOBAL Fluid Study Nick Topley James Chess Mark Lambie Charlotte James Study Investigators
42572f774f2b822feff5e189ab6c3374.ppt