dfd3bfbb45a115d9540e01ce62dfae4f.ppt
- Количество слайдов: 39
 Peritoneal dialysis Jana Fialová Martina Peiskerová Klinika nefrologie 1. LF a VFN Praha 10/2007
Peritoneal dialysis Jana Fialová Martina Peiskerová Klinika nefrologie 1. LF a VFN Praha 10/2007
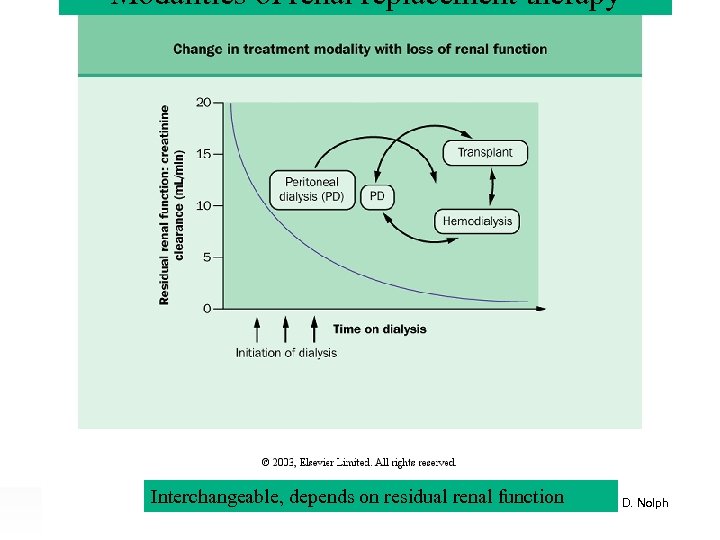 Modalities of renal replacement therapy Interchangeable, depends on residual renal. Ramesh Khanna & Karl D. Nolph function
Modalities of renal replacement therapy Interchangeable, depends on residual renal. Ramesh Khanna & Karl D. Nolph function
 Peritoneal dialysis - outline • • • Principles of PD PD solutions PD catheter Indication / contraindication of PD PD schemes : CAPD, CCPD Assessement of PD adequacy, ultrafiltration • Assessement of peritoneal function • Complications • Perspectives – new dialysis solutions
Peritoneal dialysis - outline • • • Principles of PD PD solutions PD catheter Indication / contraindication of PD PD schemes : CAPD, CCPD Assessement of PD adequacy, ultrafiltration • Assessement of peritoneal function • Complications • Perspectives – new dialysis solutions
 Peritoneal dialysis – introduction • method of RRT for 100. 000 patients worldwide • complementary to hemodialysis Principles: • peritoneum (capillary endothelium, matrix, mesothelium) = semipermeable dialysis membrane through which fluid and solute move from blood to dialysis solution via diffusion and convection • effective peritoneal surface area = perfused capillaries closed to peritoneum (↓ in peritonitis) • ultrafiltration (movement of water) enabled by osmotic gradient generated by glucose or glucose polymers (isodextrin)
Peritoneal dialysis – introduction • method of RRT for 100. 000 patients worldwide • complementary to hemodialysis Principles: • peritoneum (capillary endothelium, matrix, mesothelium) = semipermeable dialysis membrane through which fluid and solute move from blood to dialysis solution via diffusion and convection • effective peritoneal surface area = perfused capillaries closed to peritoneum (↓ in peritonitis) • ultrafiltration (movement of water) enabled by osmotic gradient generated by glucose or glucose polymers (isodextrin)
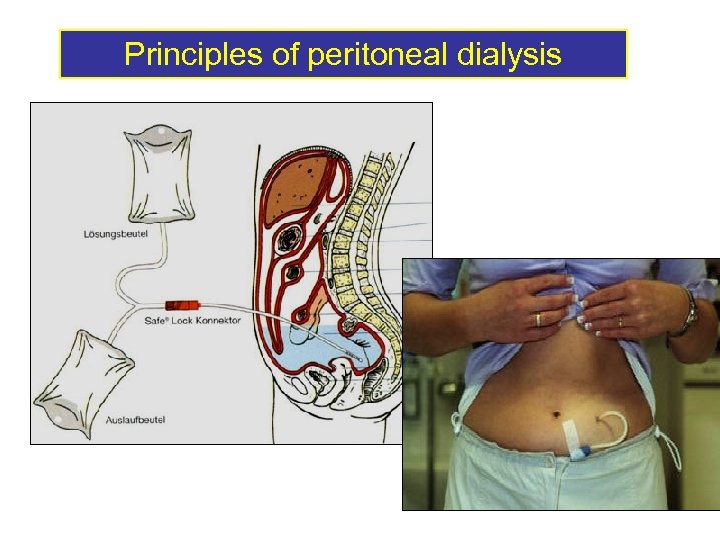 Principles of peritoneal dialysis
Principles of peritoneal dialysis
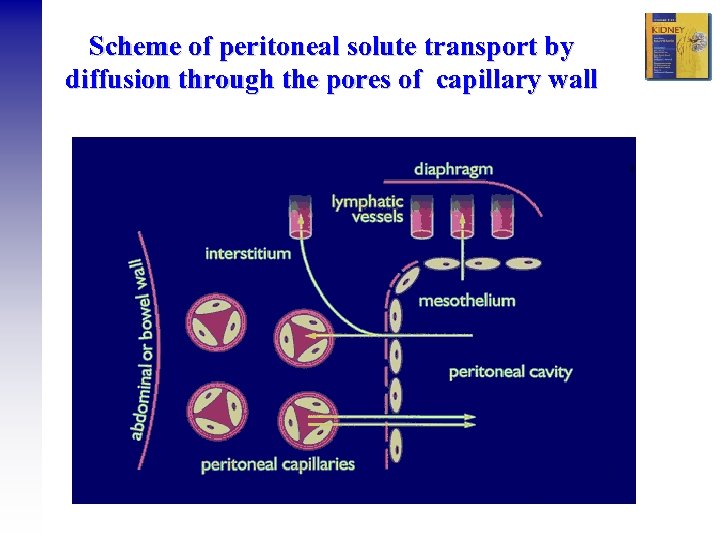 Scheme of peritoneal solute transport by diffusion through the pores of capillary wall
Scheme of peritoneal solute transport by diffusion through the pores of capillary wall
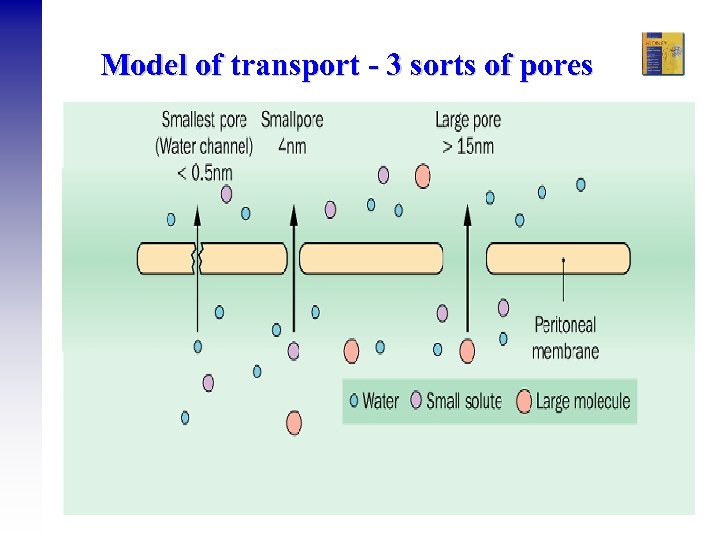 Model of transport - 3 sorts of pores Ramesh Khanna & Karl D. Nolph
Model of transport - 3 sorts of pores Ramesh Khanna & Karl D. Nolph
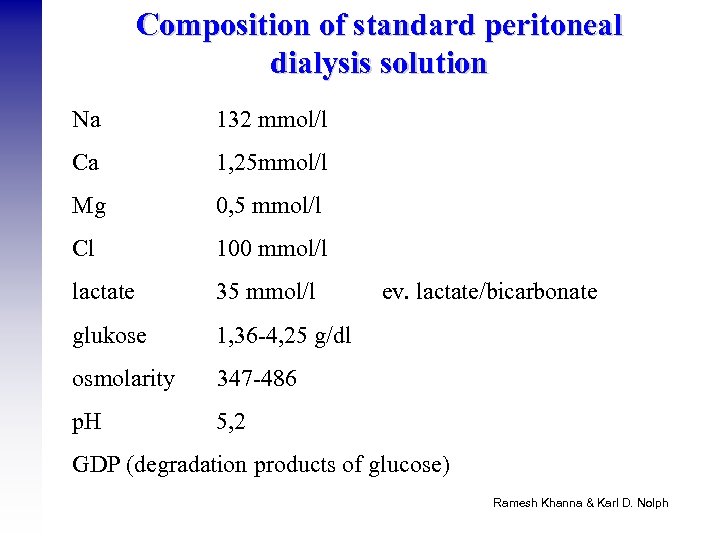 Composition of standard peritoneal dialysis solution Na 132 mmol/l Ca 1, 25 mmol/l Mg 0, 5 mmol/l Cl 100 mmol/l lactate 35 mmol/l glukose 1, 36 -4, 25 g/dl osmolarity 347 -486 p. H 5, 2 ev. lactate/bicarbonate GDP (degradation products of glucose) Ramesh Khanna & Karl D. Nolph
Composition of standard peritoneal dialysis solution Na 132 mmol/l Ca 1, 25 mmol/l Mg 0, 5 mmol/l Cl 100 mmol/l lactate 35 mmol/l glukose 1, 36 -4, 25 g/dl osmolarity 347 -486 p. H 5, 2 ev. lactate/bicarbonate GDP (degradation products of glucose) Ramesh Khanna & Karl D. Nolph
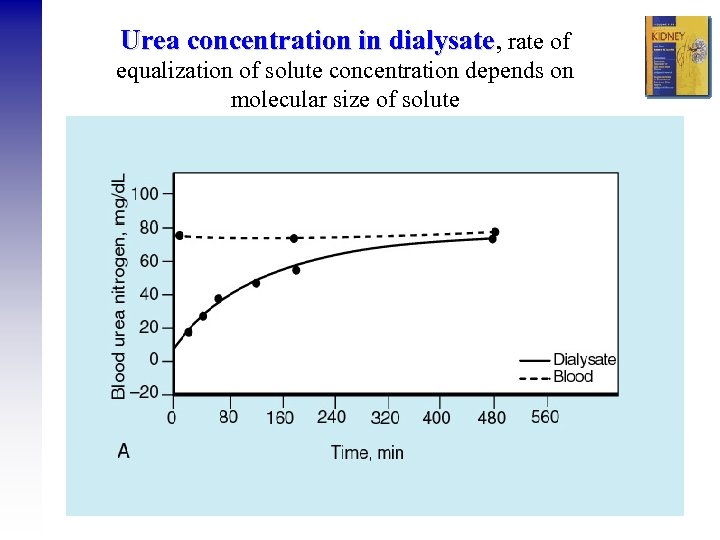 Urea concentration in dialysate, rate of dialysate equalization of solute concentration depends on molecular size of solute
Urea concentration in dialysate, rate of dialysate equalization of solute concentration depends on molecular size of solute
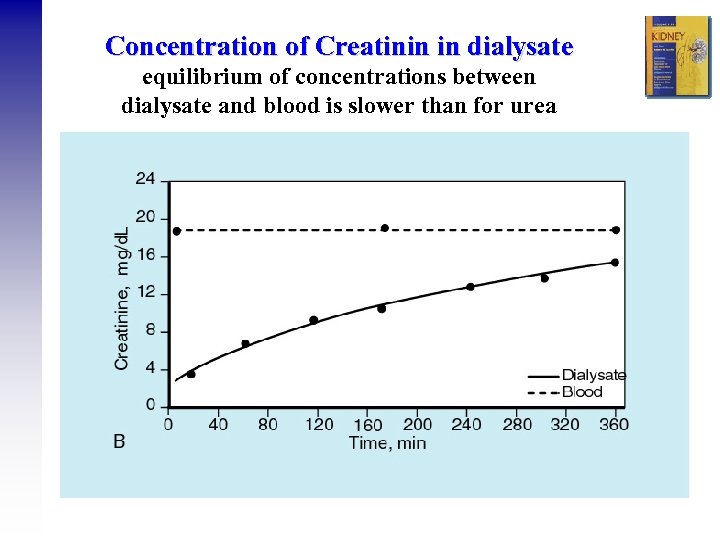 Concentration of Creatinin in dialysate equilibrium of concentrations between dialysate and blood is slower than for urea
Concentration of Creatinin in dialysate equilibrium of concentrations between dialysate and blood is slower than for urea
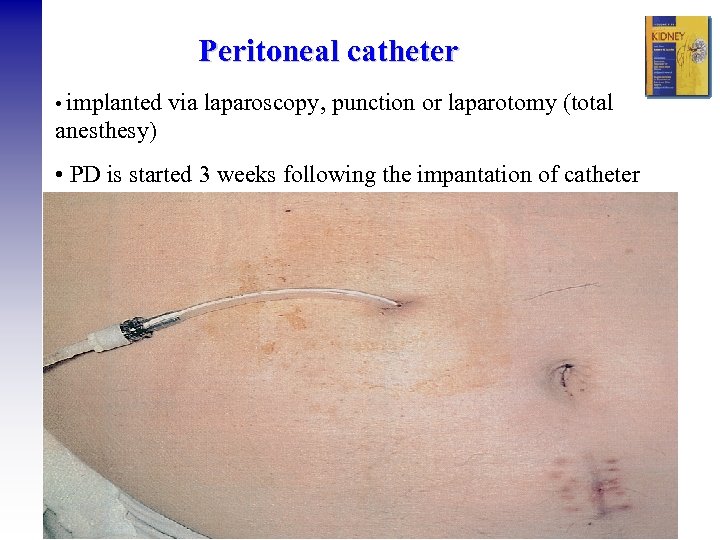 Peritoneal catheter • implanted via laparoscopy, punction or laparotomy (total anesthesy) • PD is started 3 weeks following the impantation of catheter
Peritoneal catheter • implanted via laparoscopy, punction or laparotomy (total anesthesy) • PD is started 3 weeks following the impantation of catheter
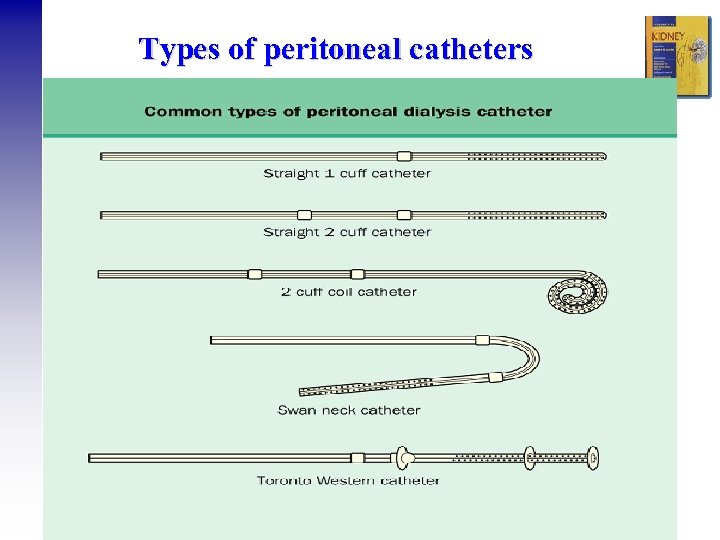 Types of peritoneal catheters
Types of peritoneal catheters
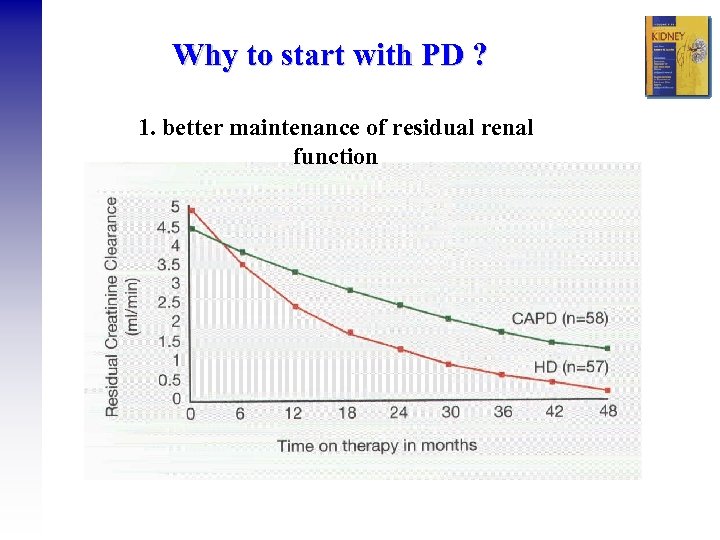 Why to start with PD ? 1. better maintenance of residual renal function
Why to start with PD ? 1. better maintenance of residual renal function
 Why to start with PD ? • clinical outcomes comparable to HD, no difference in 2 year and 5 year mortality vs. HD (study NECOSAD) • saves vascular access • preferred for children (APD) • modality choice is a lifestyle issue
Why to start with PD ? • clinical outcomes comparable to HD, no difference in 2 year and 5 year mortality vs. HD (study NECOSAD) • saves vascular access • preferred for children (APD) • modality choice is a lifestyle issue
 Indication / Contraindications of PD 80% of patients have no contra-indication to any of the dialysis methods and may choose according to their life style between HD a PD Absolute contra-indications of PD: 1. peritoneal fibrosis and adhesions following intraabdominal operations 2. inflammatory gut diseases Ramesh Khanna & Karl D. Nolph
Indication / Contraindications of PD 80% of patients have no contra-indication to any of the dialysis methods and may choose according to their life style between HD a PD Absolute contra-indications of PD: 1. peritoneal fibrosis and adhesions following intraabdominal operations 2. inflammatory gut diseases Ramesh Khanna & Karl D. Nolph
 Relative contraindications of PD • pleuro-peritoneal leakage • hernias • significant loin pain • big polycystic kidneys diverticulosis • colostomy • obesity • blindness * • severe deformant arthritis • psychosis • significant decrease of lung functions
Relative contraindications of PD • pleuro-peritoneal leakage • hernias • significant loin pain • big polycystic kidneys diverticulosis • colostomy • obesity • blindness * • severe deformant arthritis • psychosis • significant decrease of lung functions
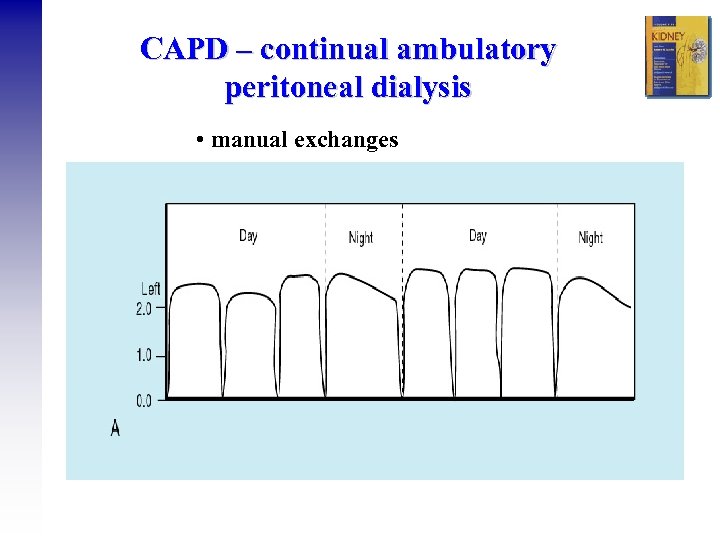 CAPD – continual ambulatory peritoneal dialysis • manual exchanges
CAPD – continual ambulatory peritoneal dialysis • manual exchanges
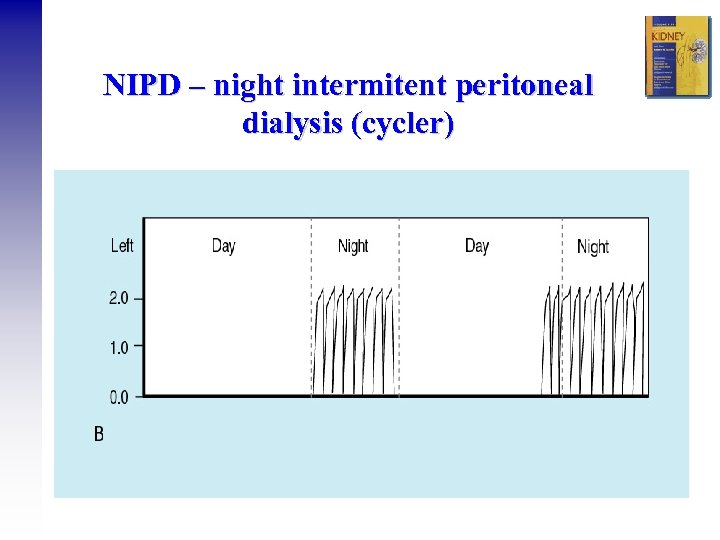 NIPD – night intermitent peritoneal dialysis (cycler)
NIPD – night intermitent peritoneal dialysis (cycler)
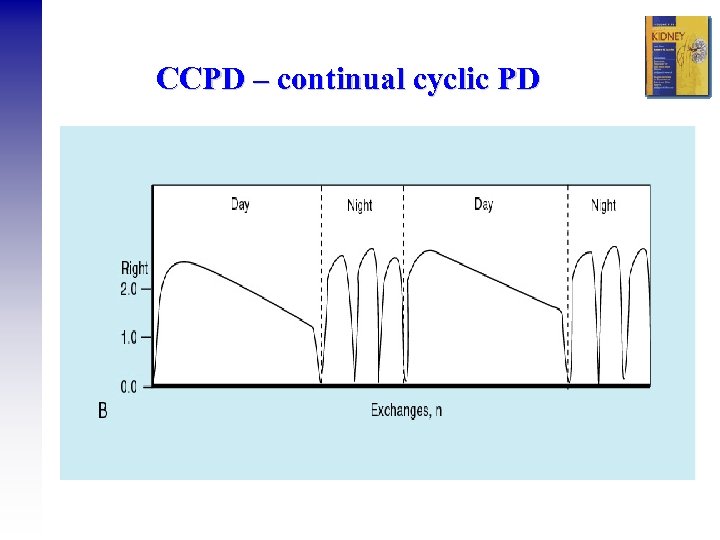 CCPD – continual cyclic PD
CCPD – continual cyclic PD
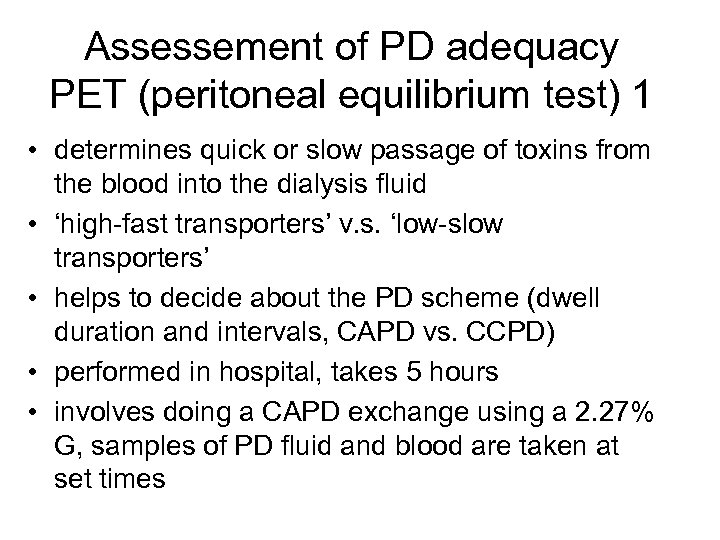 Assessement of PD adequacy PET (peritoneal equilibrium test) 1 • determines quick or slow passage of toxins from the blood into the dialysis fluid • ‘high-fast transporters’ v. s. ‘low-slow transporters’ • helps to decide about the PD scheme (dwell duration and intervals, CAPD vs. CCPD) • performed in hospital, takes 5 hours • involves doing a CAPD exchange using a 2. 27% G, samples of PD fluid and blood are taken at set times
Assessement of PD adequacy PET (peritoneal equilibrium test) 1 • determines quick or slow passage of toxins from the blood into the dialysis fluid • ‘high-fast transporters’ v. s. ‘low-slow transporters’ • helps to decide about the PD scheme (dwell duration and intervals, CAPD vs. CCPD) • performed in hospital, takes 5 hours • involves doing a CAPD exchange using a 2. 27% G, samples of PD fluid and blood are taken at set times
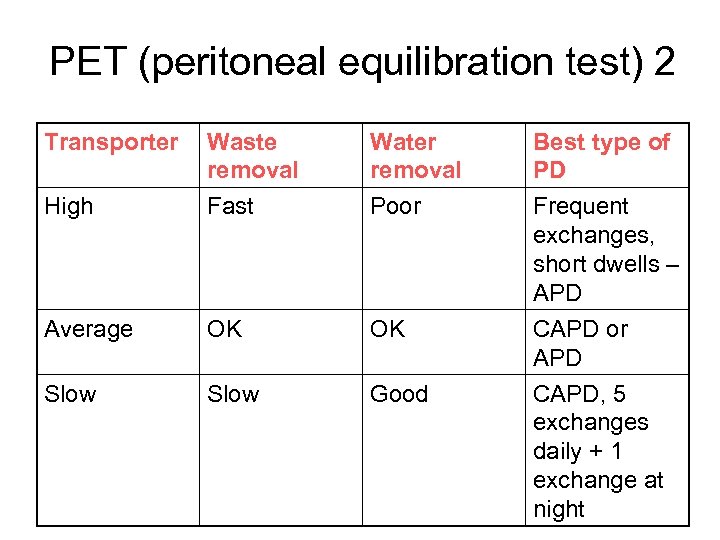 PET (peritoneal equilibration test) 2 Transporter High Waste removal Fast Water removal Poor Average OK OK Slow Good Best type of PD Frequent exchanges, short dwells – APD CAPD or APD CAPD, 5 exchanges daily + 1 exchange at night
PET (peritoneal equilibration test) 2 Transporter High Waste removal Fast Water removal Poor Average OK OK Slow Good Best type of PD Frequent exchanges, short dwells – APD CAPD or APD CAPD, 5 exchanges daily + 1 exchange at night
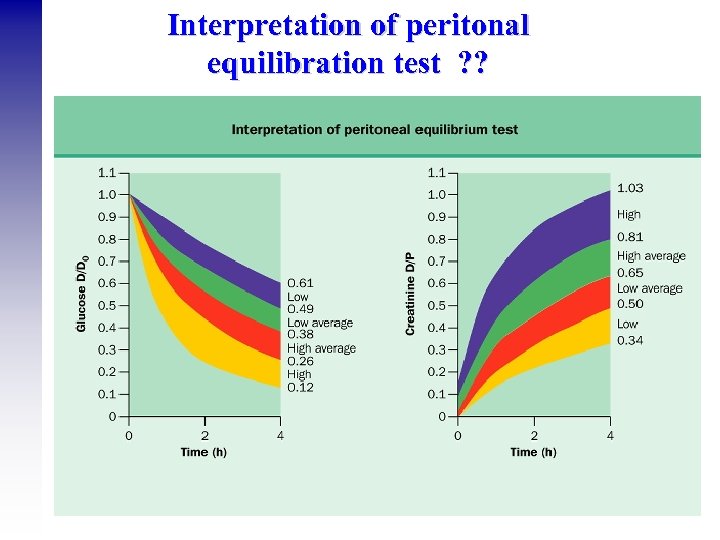 Interpretation of peritonal equilibration test ? ?
Interpretation of peritonal equilibration test ? ?
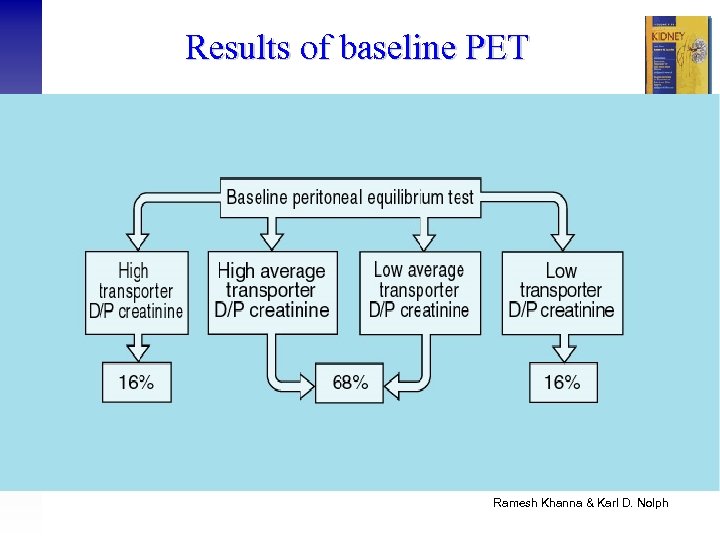 Results of baseline PET Ramesh Khanna & Karl D. Nolph
Results of baseline PET Ramesh Khanna & Karl D. Nolph
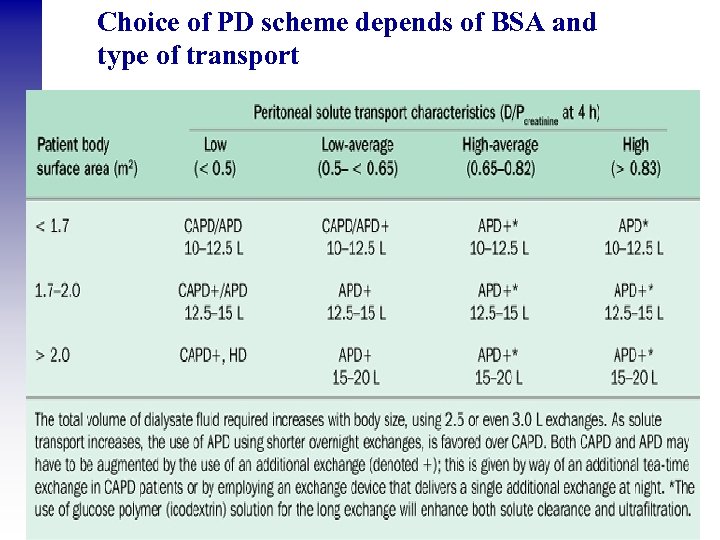 Choice of PD scheme depends of BSA and type of transport
Choice of PD scheme depends of BSA and type of transport
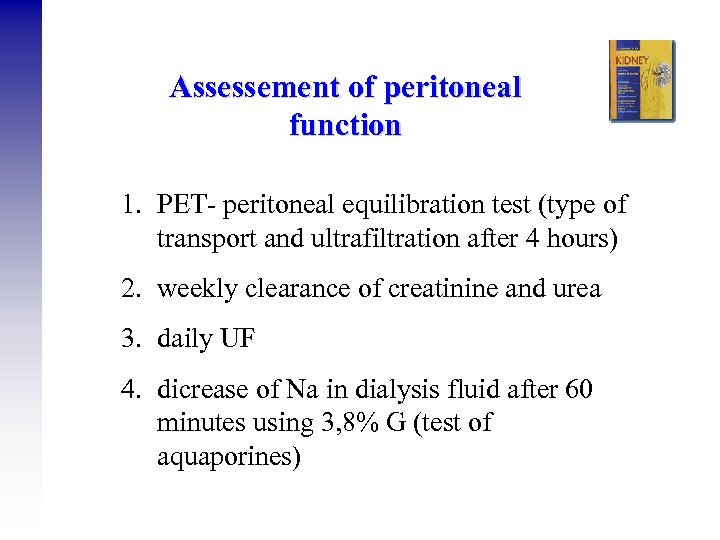 Assessement of peritoneal function 1. PET- peritoneal equilibration test (type of transport and ultrafiltration after 4 hours) 2. weekly clearance of creatinine and urea 3. daily UF 4. dicrease of Na in dialysis fluid after 60 minutes using 3, 8% G (test of aquaporines)
Assessement of peritoneal function 1. PET- peritoneal equilibration test (type of transport and ultrafiltration after 4 hours) 2. weekly clearance of creatinine and urea 3. daily UF 4. dicrease of Na in dialysis fluid after 60 minutes using 3, 8% G (test of aquaporines)
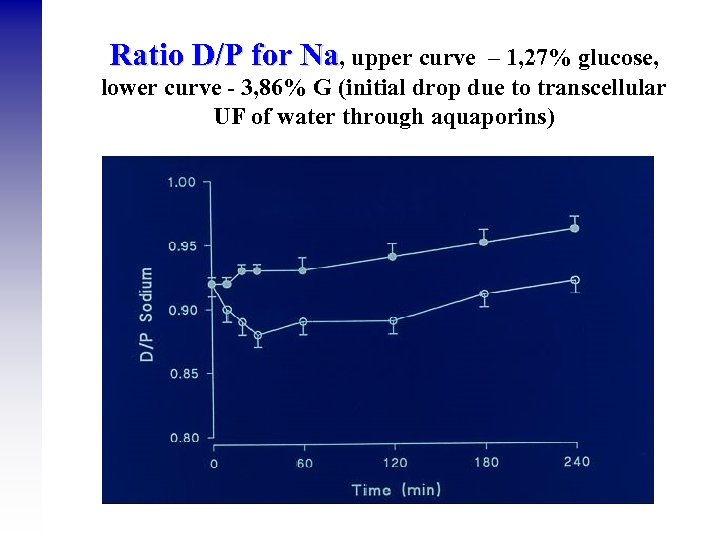 Ratio D/P for Na, upper curve – 1, 27% glucose, lower curve - 3, 86% G (initial drop due to transcellular UF of water through aquaporins)
Ratio D/P for Na, upper curve – 1, 27% glucose, lower curve - 3, 86% G (initial drop due to transcellular UF of water through aquaporins)
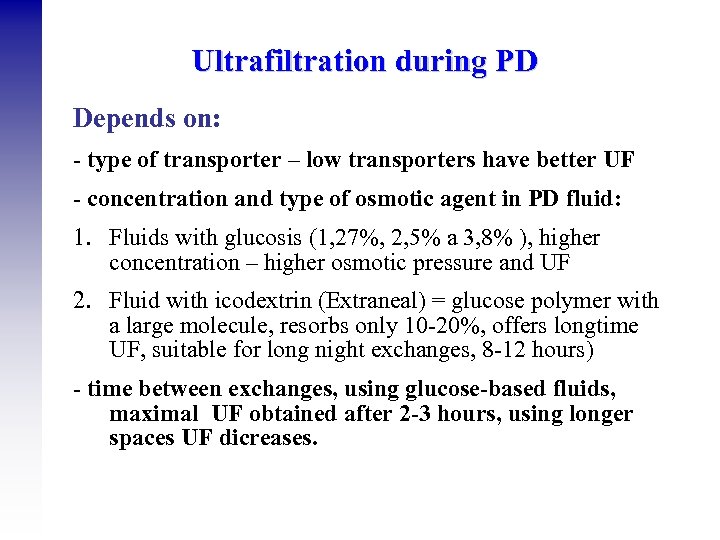 Ultrafiltration during PD Depends on: - type of transporter – low transporters have better UF - concentration and type of osmotic agent in PD fluid: 1. Fluids with glucosis (1, 27%, 2, 5% a 3, 8% ), higher concentration – higher osmotic pressure and UF 2. Fluid with icodextrin (Extraneal) = glucose polymer with a large molecule, resorbs only 10 -20%, offers longtime UF, suitable for long night exchanges, 8 -12 hours) - time between exchanges, using glucose-based fluids, maximal UF obtained after 2 -3 hours, using longer spaces UF dicreases.
Ultrafiltration during PD Depends on: - type of transporter – low transporters have better UF - concentration and type of osmotic agent in PD fluid: 1. Fluids with glucosis (1, 27%, 2, 5% a 3, 8% ), higher concentration – higher osmotic pressure and UF 2. Fluid with icodextrin (Extraneal) = glucose polymer with a large molecule, resorbs only 10 -20%, offers longtime UF, suitable for long night exchanges, 8 -12 hours) - time between exchanges, using glucose-based fluids, maximal UF obtained after 2 -3 hours, using longer spaces UF dicreases.
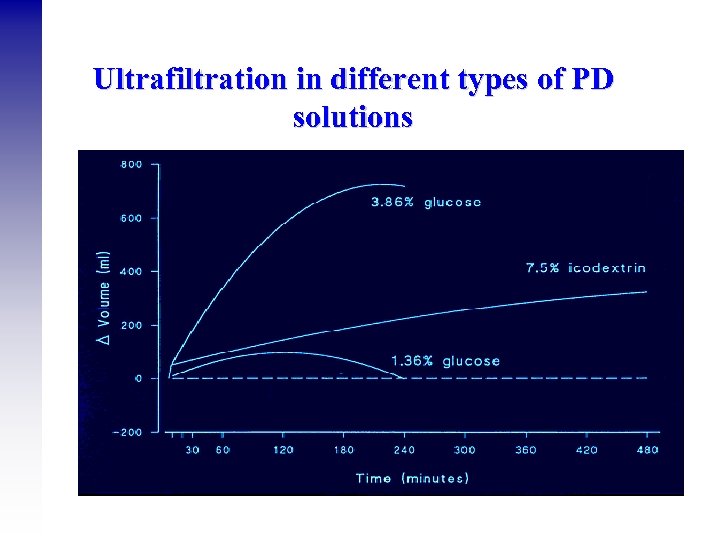 Ultrafiltration in different types of PD solutions
Ultrafiltration in different types of PD solutions
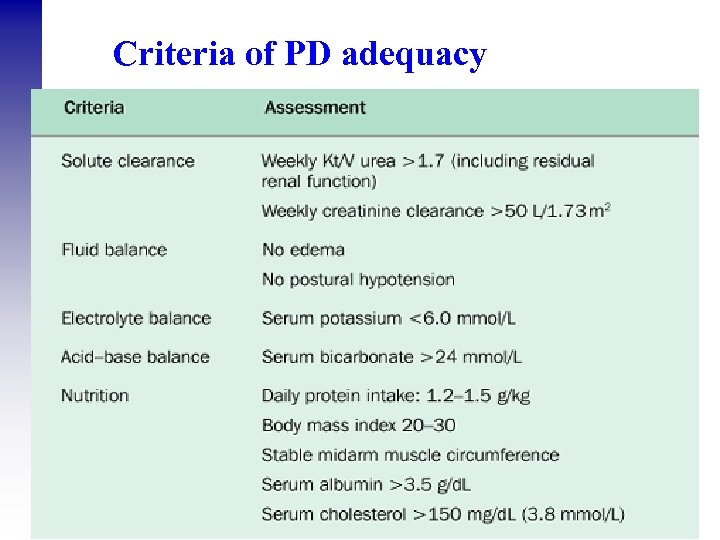 Criteria of PD adequacy
Criteria of PD adequacy
 Complications of PD 1 Infectious: • exit-site inflammation (flare, suppurative secretion, granulation) • peritonitis (turbid dialysate, abdominal pain, fever) Non-infectious: • hernias • hydrothorax • sclerosing encapsulating peritonitis (rare, life threatening complication, mostly after ≥ 6 years on PD, peritoneum is massively thickened and calcificated, leading to intestinal obstruction)
Complications of PD 1 Infectious: • exit-site inflammation (flare, suppurative secretion, granulation) • peritonitis (turbid dialysate, abdominal pain, fever) Non-infectious: • hernias • hydrothorax • sclerosing encapsulating peritonitis (rare, life threatening complication, mostly after ≥ 6 years on PD, peritoneum is massively thickened and calcificated, leading to intestinal obstruction)
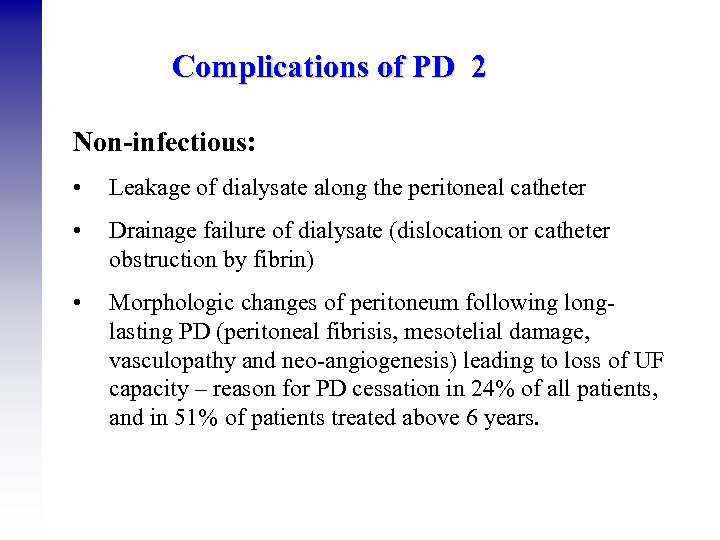 Complications of PD 2 Non-infectious: • Leakage of dialysate along the peritoneal catheter • Drainage failure of dialysate (dislocation or catheter obstruction by fibrin) • Morphologic changes of peritoneum following longlasting PD (peritoneal fibrisis, mesotelial damage, vasculopathy and neo-angiogenesis) leading to loss of UF capacity – reason for PD cessation in 24% of all patients, and in 51% of patients treated above 6 years.
Complications of PD 2 Non-infectious: • Leakage of dialysate along the peritoneal catheter • Drainage failure of dialysate (dislocation or catheter obstruction by fibrin) • Morphologic changes of peritoneum following longlasting PD (peritoneal fibrisis, mesotelial damage, vasculopathy and neo-angiogenesis) leading to loss of UF capacity – reason for PD cessation in 24% of all patients, and in 51% of patients treated above 6 years.
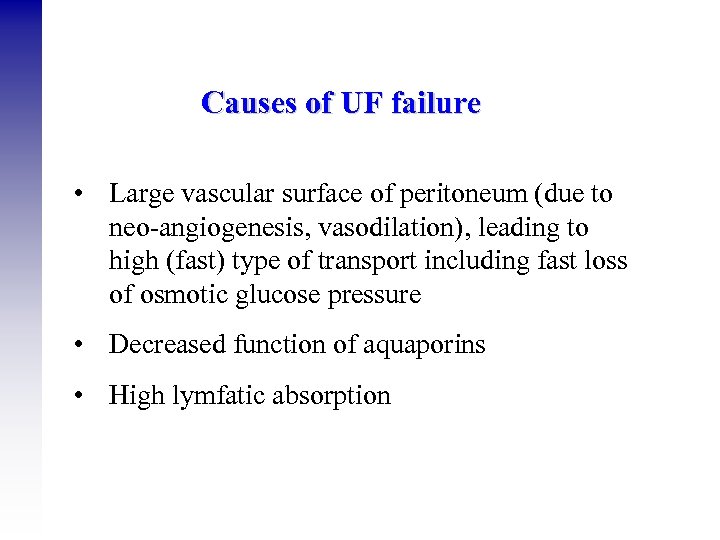 Causes of UF failure • Large vascular surface of peritoneum (due to neo-angiogenesis, vasodilation), leading to high (fast) type of transport including fast loss of osmotic glucose pressure • Decreased function of aquaporins • High lymfatic absorption
Causes of UF failure • Large vascular surface of peritoneum (due to neo-angiogenesis, vasodilation), leading to high (fast) type of transport including fast loss of osmotic glucose pressure • Decreased function of aquaporins • High lymfatic absorption
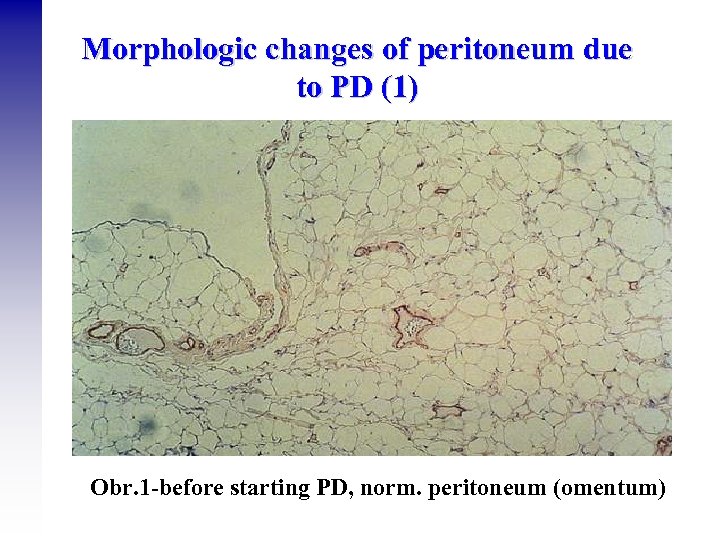 Morphologic changes of peritoneum due to PD (1) Obr. 1 -before starting PD, norm. peritoneum (omentum)
Morphologic changes of peritoneum due to PD (1) Obr. 1 -before starting PD, norm. peritoneum (omentum)
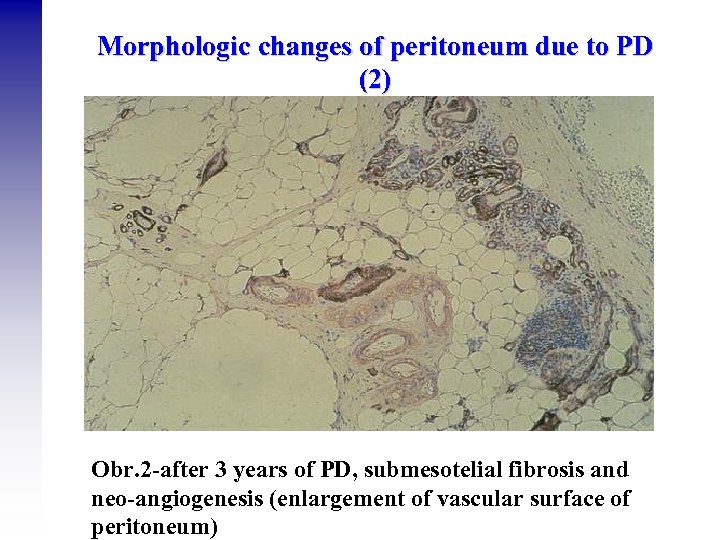 Morphologic changes of peritoneum due to PD (2) Obr. 2 -after 3 years of PD, submesotelial fibrosis and neo-angiogenesis (enlargement of vascular surface of peritoneum)
Morphologic changes of peritoneum due to PD (2) Obr. 2 -after 3 years of PD, submesotelial fibrosis and neo-angiogenesis (enlargement of vascular surface of peritoneum)
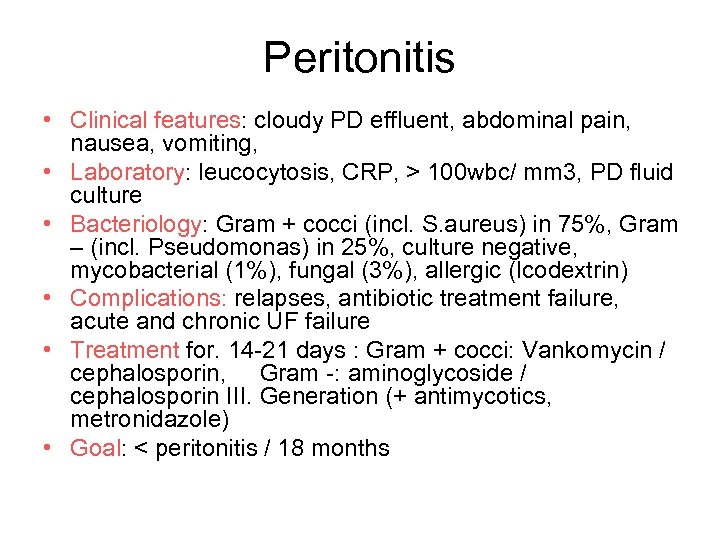 Peritonitis • Clinical features: cloudy PD effluent, abdominal pain, nausea, vomiting, • Laboratory: leucocytosis, CRP, > 100 wbc/ mm 3, PD fluid culture • Bacteriology: Gram + cocci (incl. S. aureus) in 75%, Gram – (incl. Pseudomonas) in 25%, culture negative, mycobacterial (1%), fungal (3%), allergic (Icodextrin) • Complications: relapses, antibiotic treatment failure, acute and chronic UF failure • Treatment for. 14 -21 days : Gram + cocci: Vankomycin / cephalosporin, Gram -: aminoglycoside / cephalosporin III. Generation (+ antimycotics, metronidazole) • Goal: < peritonitis / 18 months
Peritonitis • Clinical features: cloudy PD effluent, abdominal pain, nausea, vomiting, • Laboratory: leucocytosis, CRP, > 100 wbc/ mm 3, PD fluid culture • Bacteriology: Gram + cocci (incl. S. aureus) in 75%, Gram – (incl. Pseudomonas) in 25%, culture negative, mycobacterial (1%), fungal (3%), allergic (Icodextrin) • Complications: relapses, antibiotic treatment failure, acute and chronic UF failure • Treatment for. 14 -21 days : Gram + cocci: Vankomycin / cephalosporin, Gram -: aminoglycoside / cephalosporin III. Generation (+ antimycotics, metronidazole) • Goal: < peritonitis / 18 months
 From PD gudelines (ISPD) • biocompatible PD solutions - normal p. H, low concentration of glucose • insertion of PD catheter – 10 days-6 weeks before RRT • urea / creatinine clearance measured every 6 months • PET: 6 weeks after commencing treatment + annually • avoid routine use of high glucose concentrations )use of icodextrin, aminoacids instead) • preserve residual diuresis, obtain UF above 750 ml/day • peritonitis and exit-site infection rates, regular revision of technique • invasive procedures cover by ATB prophylaxis • topical ATB administration if needed (S. aureus, Ps. aeruginosa) • beware central obesity
From PD gudelines (ISPD) • biocompatible PD solutions - normal p. H, low concentration of glucose • insertion of PD catheter – 10 days-6 weeks before RRT • urea / creatinine clearance measured every 6 months • PET: 6 weeks after commencing treatment + annually • avoid routine use of high glucose concentrations )use of icodextrin, aminoacids instead) • preserve residual diuresis, obtain UF above 750 ml/day • peritonitis and exit-site infection rates, regular revision of technique • invasive procedures cover by ATB prophylaxis • topical ATB administration if needed (S. aureus, Ps. aeruginosa) • beware central obesity
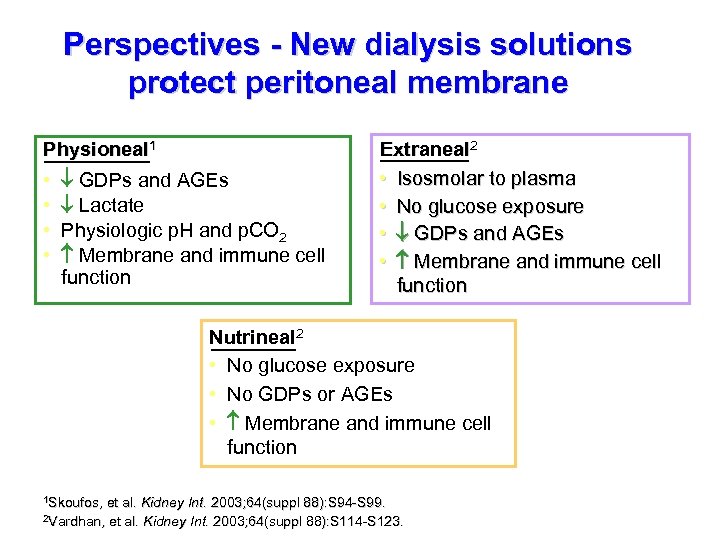 Perspectives - New dialysis solutions protect peritoneal membrane Physioneal 1 • • GDPs and AGEs Lactate Physiologic p. H and p. CO 2 Membrane and immune cell function Extraneal 2 • Isosmolar to plasma • No glucose exposure • GDPs and AGEs • Membrane and immune cell function Nutrineal 2 • No glucose exposure • No GDPs or AGEs • Membrane and immune cell function 1 Skoufos, et al. Kidney Int. 2003; 64(suppl 88): S 94 -S 99. 2 Vardhan, et al. Kidney Int. 2003; 64(suppl 88): S 114 -S 123.
Perspectives - New dialysis solutions protect peritoneal membrane Physioneal 1 • • GDPs and AGEs Lactate Physiologic p. H and p. CO 2 Membrane and immune cell function Extraneal 2 • Isosmolar to plasma • No glucose exposure • GDPs and AGEs • Membrane and immune cell function Nutrineal 2 • No glucose exposure • No GDPs or AGEs • Membrane and immune cell function 1 Skoufos, et al. Kidney Int. 2003; 64(suppl 88): S 94 -S 99. 2 Vardhan, et al. Kidney Int. 2003; 64(suppl 88): S 114 -S 123.
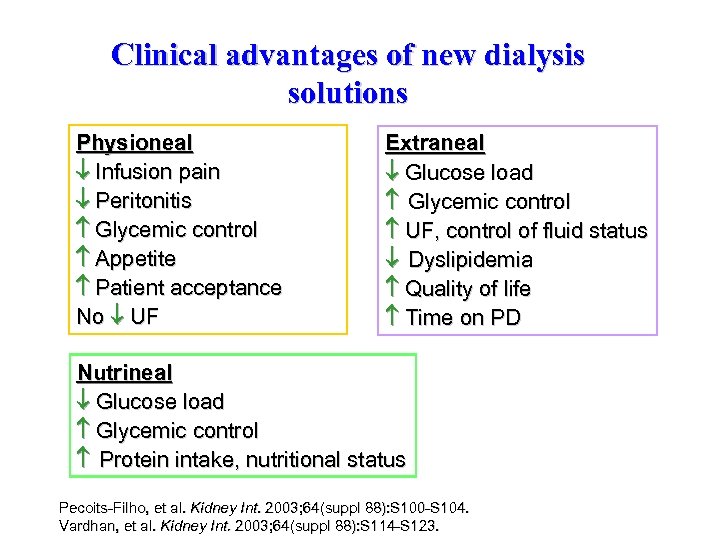 Clinical advantages of new dialysis solutions Physioneal Infusion pain Peritonitis Glycemic control Appetite Patient acceptance No UF Extraneal Glucose load Glycemic control UF, control of fluid status Dyslipidemia Quality of life Time on PD Nutrineal Glucose load Glycemic control Protein intake, nutritional status Pecoits-Filho, et al. Kidney Int. 2003; 64(suppl 88): S 100 -S 104. Vardhan, et al. Kidney Int. 2003; 64(suppl 88): S 114 -S 123.
Clinical advantages of new dialysis solutions Physioneal Infusion pain Peritonitis Glycemic control Appetite Patient acceptance No UF Extraneal Glucose load Glycemic control UF, control of fluid status Dyslipidemia Quality of life Time on PD Nutrineal Glucose load Glycemic control Protein intake, nutritional status Pecoits-Filho, et al. Kidney Int. 2003; 64(suppl 88): S 100 -S 104. Vardhan, et al. Kidney Int. 2003; 64(suppl 88): S 114 -S 123.
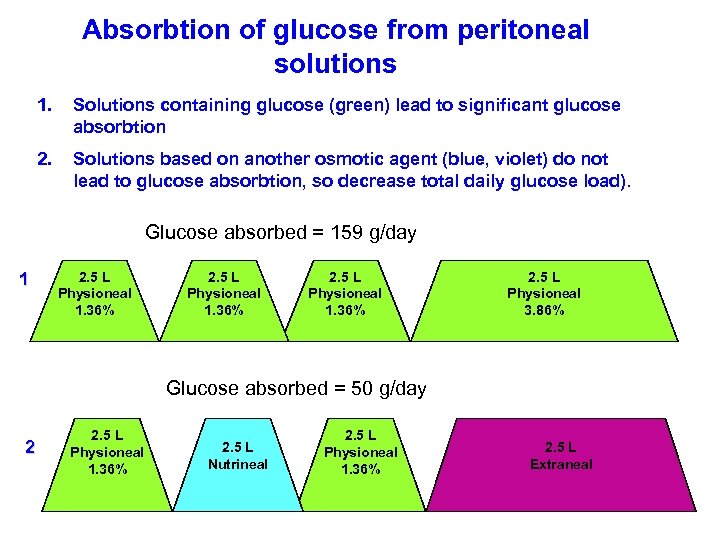 Absorbtion of glucose from peritoneal solutions 1. Solutions containing glucose (green) lead to significant glucose absorbtion 2. Solutions based on another osmotic agent (blue, violet) do not lead to glucose absorbtion, so decrease total daily glucose load). Glucose absorbed = 159 g/day 1 2. 5 L Physioneal 1. 36% 2. 5 L Physioneal 3. 86% Glucose absorbed = 50 g/day 2 2. 5 L Physioneal 1. 36% 2. 5 L Nutrineal 2. 5 L Physioneal 1. 36% 2. 5 L Extraneal
Absorbtion of glucose from peritoneal solutions 1. Solutions containing glucose (green) lead to significant glucose absorbtion 2. Solutions based on another osmotic agent (blue, violet) do not lead to glucose absorbtion, so decrease total daily glucose load). Glucose absorbed = 159 g/day 1 2. 5 L Physioneal 1. 36% 2. 5 L Physioneal 3. 86% Glucose absorbed = 50 g/day 2 2. 5 L Physioneal 1. 36% 2. 5 L Nutrineal 2. 5 L Physioneal 1. 36% 2. 5 L Extraneal


