12ffdefcedd0f9c5a67017ba830ea7c2.ppt
- Количество слайдов: 17
 Peripheral Vascular Intervention East and West ~PMDA View~ Mami Ho, M. D. , Ph. D Pharmaceuticals and Medical Devices Agency, Japan
Peripheral Vascular Intervention East and West ~PMDA View~ Mami Ho, M. D. , Ph. D Pharmaceuticals and Medical Devices Agency, Japan
 Mami Ho, M. D. , Ph. D I have no real or apparent conflicts of interest to report.
Mami Ho, M. D. , Ph. D I have no real or apparent conflicts of interest to report.
 Today’s Agenda • Current situation of PAD • Global clinical trial in SFA • Issues in Reviewing other countries’ trial for SFA • Points of designing clinical trial for BTK • Points of designing clinical trial for PAD • Summary • Future Plan
Today’s Agenda • Current situation of PAD • Global clinical trial in SFA • Issues in Reviewing other countries’ trial for SFA • Points of designing clinical trial for BTK • Points of designing clinical trial for PAD • Summary • Future Plan
 Current Situation of PAD Treatments in Japan • • • Increasing number of patient in Japan, too Progressive disease, Poor prognosis Increasing the importance of endovascular treatment ü Increasing ‘high-risk’ patient with aging ü Approved device; ordinary balloon catheter, (drug eluting stent) There is an urgent need of endovascular devices for PAD.
Current Situation of PAD Treatments in Japan • • • Increasing number of patient in Japan, too Progressive disease, Poor prognosis Increasing the importance of endovascular treatment ü Increasing ‘high-risk’ patient with aging ü Approved device; ordinary balloon catheter, (drug eluting stent) There is an urgent need of endovascular devices for PAD.
 Global clinical trial in SFA • Efficacy and Safety are similar both countries • Stent fracture rate was different 0. 9%(4/457) in global v. s. 8. 3%(3/36) in Japanese patients. ü No differences of stent fracture rate between both countries that reported some references. ü No serious adverse event from stent fracture →’stent thrombosis’ is not classified clearly. • Which reason causes stent fracture? ü Differences of stent strut design? ü Differences of life style? ü Differences of procedure technique? We need more post market data for evaluation
Global clinical trial in SFA • Efficacy and Safety are similar both countries • Stent fracture rate was different 0. 9%(4/457) in global v. s. 8. 3%(3/36) in Japanese patients. ü No differences of stent fracture rate between both countries that reported some references. ü No serious adverse event from stent fracture →’stent thrombosis’ is not classified clearly. • Which reason causes stent fracture? ü Differences of stent strut design? ü Differences of life style? ü Differences of procedure technique? We need more post market data for evaluation
 Issues in Reviewing Other Countries’ Trials for SFA Region Previous Current • Evidence of standard of care Insufficient Some references reported Japanese data Some trials are going on • Extrapolability of other countries’ trials Influencing factors to efficacy and safety are not clear • Stent fracture Efficacy and safety are almost similar Different but the influencing to efficacy is acceptable Japanese clinical data is required , Acceptable global trial
Issues in Reviewing Other Countries’ Trials for SFA Region Previous Current • Evidence of standard of care Insufficient Some references reported Japanese data Some trials are going on • Extrapolability of other countries’ trials Influencing factors to efficacy and safety are not clear • Stent fracture Efficacy and safety are almost similar Different but the influencing to efficacy is acceptable Japanese clinical data is required , Acceptable global trial
 Points of Designing Trials for BTK • Targeted Patients – High population of hemodialysis (HD) patients – High population of elderly patients – Criteria for amputation is different: Japanese population includes patients who are applied amputation – Rutherford 4 and 5+ can be evaluated by the same endpoints? • Primary endpoint – – – Patency may not reflect Amputation free survival Clinical endpont should be evaluated There are so many factors about success treatment Composite endpoint is required?
Points of Designing Trials for BTK • Targeted Patients – High population of hemodialysis (HD) patients – High population of elderly patients – Criteria for amputation is different: Japanese population includes patients who are applied amputation – Rutherford 4 and 5+ can be evaluated by the same endpoints? • Primary endpoint – – – Patency may not reflect Amputation free survival Clinical endpont should be evaluated There are so many factors about success treatment Composite endpoint is required?
 Points of Designing Trials for BTK • Insufficient evidence of Japanese population with SOC (both in distal bypass&intravascular treatment) • Difference in targeted patient population • Need to re-examine endpoints • Limitation of extrapolability - different patient population - care setting (drugs, devices, SOC) Limitation of extrapolability → Japanese clinical data is required
Points of Designing Trials for BTK • Insufficient evidence of Japanese population with SOC (both in distal bypass&intravascular treatment) • Difference in targeted patient population • Need to re-examine endpoints • Limitation of extrapolability - different patient population - care setting (drugs, devices, SOC) Limitation of extrapolability → Japanese clinical data is required
 Points of Designing Clinical Trial for PAD SFA BTK Targeted Pts of Intravascular Treatment TASC II, Type A and B Fontaine II - Legion length ? *Criteria is not clear Objective of Therapy Remission of intermittent claudication Rutherford 4? : pain relief Rutherford 5 ≦? :ulcer healing? , avoidance of amputation? Control in Clinical Trial Balloon angioplasty (DES? ) Balloon angioplasty Primary Endpoint Primary patency? Revascularization? Clinical endpoint? Evidence for SOC in Japan Some clinical trial are going on insufficient Relatively-simple in SFA, but many obstacles for BTK
Points of Designing Clinical Trial for PAD SFA BTK Targeted Pts of Intravascular Treatment TASC II, Type A and B Fontaine II - Legion length ? *Criteria is not clear Objective of Therapy Remission of intermittent claudication Rutherford 4? : pain relief Rutherford 5 ≦? :ulcer healing? , avoidance of amputation? Control in Clinical Trial Balloon angioplasty (DES? ) Balloon angioplasty Primary Endpoint Primary patency? Revascularization? Clinical endpoint? Evidence for SOC in Japan Some clinical trial are going on insufficient Relatively-simple in SFA, but many obstacles for BTK
 Summary • Japanese clinical results is being accumulated from clinical trails for SFA devices → Raising the possibility of standardization with trials in other countries • For BTK devices, clear distinction with targeted patient population. Need to reexamine endpoints. → Uncertain possibility of standardization
Summary • Japanese clinical results is being accumulated from clinical trails for SFA devices → Raising the possibility of standardization with trials in other countries • For BTK devices, clear distinction with targeted patient population. Need to reexamine endpoints. → Uncertain possibility of standardization
 Future Plan The effective devices for CLI patients in the world is urgent! Need more discussion in expert meetings with industry/academia/government with transparency To facilitate mutual understanding of the distinction and stimulate developments are important
Future Plan The effective devices for CLI patients in the world is urgent! Need more discussion in expert meetings with industry/academia/government with transparency To facilitate mutual understanding of the distinction and stimulate developments are important
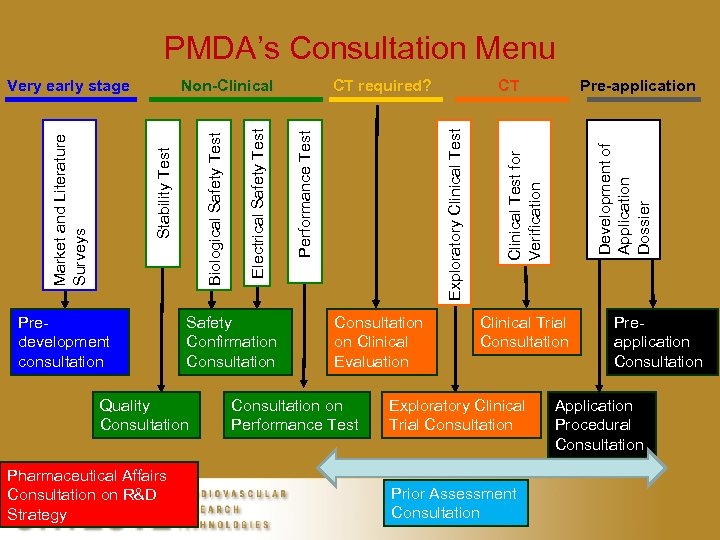 PMDA’s Consultation Menu CT required? Predevelopment consultation Safety Confirmation Consultation Quality Consultation Pharmaceutical Affairs Consultation on R&D Strategy Consultation on Clinical Evaluation Consultation on Performance Test Pre-application Clinical Test for Verification Development of Application Dossier CT Exploratory Clinical Test Performance Test Electrical Safety Test Biological Safety Test Non-Clinical Stability Test Market and Literature Surveys Very early stage Clinical Trial Consultation Exploratory Clinical Trial Consultation Prior Assessment Consultation Preapplication Consultation Application Procedural Consultation
PMDA’s Consultation Menu CT required? Predevelopment consultation Safety Confirmation Consultation Quality Consultation Pharmaceutical Affairs Consultation on R&D Strategy Consultation on Clinical Evaluation Consultation on Performance Test Pre-application Clinical Test for Verification Development of Application Dossier CT Exploratory Clinical Test Performance Test Electrical Safety Test Biological Safety Test Non-Clinical Stability Test Market and Literature Surveys Very early stage Clinical Trial Consultation Exploratory Clinical Trial Consultation Prior Assessment Consultation Preapplication Consultation Application Procedural Consultation
 PMDA, Japan PMDA will keep our effort to continue valuable communication with FDA and the Industry through HBD activities and the Collaborative Review Scheme project. Thanks for your understanding and corporation. For more information, see our website: URL : http: //www. pmda. go. jp/ 13
PMDA, Japan PMDA will keep our effort to continue valuable communication with FDA and the Industry through HBD activities and the Collaborative Review Scheme project. Thanks for your understanding and corporation. For more information, see our website: URL : http: //www. pmda. go. jp/ 13
 Back up
Back up
 PMDA, Japan • Mami Ho, M. D. , Ph. D Deputy Review Director, Office of Medical Device I ho-mami@pmda. go. jp For more information, see our website: URL : http: //www. pmda. go. jp/ Thank you! 15
PMDA, Japan • Mami Ho, M. D. , Ph. D Deputy Review Director, Office of Medical Device I ho-mami@pmda. go. jp For more information, see our website: URL : http: //www. pmda. go. jp/ Thank you! 15
 Action Program for Acceleration of Medical Device Reviews (issued in Dec. 2008) MHLW / PMDA will accelerate the Medical Device review processes and reduce total review time* to approval, l l on the premise of ensuring quality, efficacy, and safety of medical devices paying due consideration to minimize burdens to applicants under combined efforts by both the regulatory side and the applicants side by taking scientific and reasonable measures (* Total elapsed time from submission to approval)
Action Program for Acceleration of Medical Device Reviews (issued in Dec. 2008) MHLW / PMDA will accelerate the Medical Device review processes and reduce total review time* to approval, l l on the premise of ensuring quality, efficacy, and safety of medical devices paying due consideration to minimize burdens to applicants under combined efforts by both the regulatory side and the applicants side by taking scientific and reasonable measures (* Total elapsed time from submission to approval)
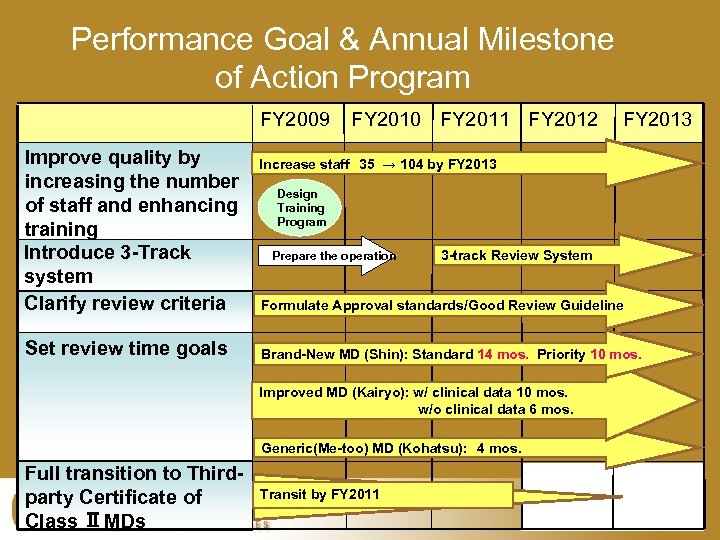 Performance Goal & Annual Milestone of Action Program FY 2009 Improve quality by increasing the number of staff and enhancing training Introduce 3 -Track system Clarify review criteria Set review time goals FY 2010 FY 2011 FY 2012 Increase staff 35 → 104 by FY 2013 Design Training Program Prepare the operation 3 -track Review System Formulate Approval standards/Good Review Guideline Brand-New MD (Shin): Standard 14 mos. Priority 10 mos. Improved MD (Kairyo): w/ clinical data 10 mos. w/o clinical data 6 mos. Generic(Me-too) MD (Kohatsu): 4 mos. Full transition to Thirdparty Certificate of Class ⅡMDs FY 2013 Transit by FY 2011
Performance Goal & Annual Milestone of Action Program FY 2009 Improve quality by increasing the number of staff and enhancing training Introduce 3 -Track system Clarify review criteria Set review time goals FY 2010 FY 2011 FY 2012 Increase staff 35 → 104 by FY 2013 Design Training Program Prepare the operation 3 -track Review System Formulate Approval standards/Good Review Guideline Brand-New MD (Shin): Standard 14 mos. Priority 10 mos. Improved MD (Kairyo): w/ clinical data 10 mos. w/o clinical data 6 mos. Generic(Me-too) MD (Kohatsu): 4 mos. Full transition to Thirdparty Certificate of Class ⅡMDs FY 2013 Transit by FY 2011


