d987137db32aa2b73d094bde60dc2f9e.ppt
- Количество слайдов: 115
 Peripheral & Central Nervous System Drugs Advisory Committee January 28, 2000 Mitoxantrone for Multiple Sclerosis (Novantrone®) Immunex Corporation Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-1
Peripheral & Central Nervous System Drugs Advisory Committee January 28, 2000 Mitoxantrone for Multiple Sclerosis (Novantrone®) Immunex Corporation Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-1
 Mitoxantrone Approved Indications • Adult acute myeloid leukemia (1987) – 12 mg/m 2/day x 3 days every 4 -6 weeks • Symptomatic hormone-refractory prostate cancer (1996) – 12 -14 mg/m 2 every 3 weeks Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-2
Mitoxantrone Approved Indications • Adult acute myeloid leukemia (1987) – 12 mg/m 2/day x 3 days every 4 -6 weeks • Symptomatic hormone-refractory prostate cancer (1996) – 12 -14 mg/m 2 every 3 weeks Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-2
 Mitoxantrone Use in Cancer Patients • Since approval • • • – Over 180, 000 patients treated in U. S. – Over 400, 000 patients treated worldwide Range of dose and schedule – 12 mg/m 2/day x 3 days every 4 -6 weeks – 8 -14 mg/m 2 repeated every 3 -4 weeks – 30 -80 mg/m 2 single dose Alone or in combination with other drugs Well characterized safety profile Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-3
Mitoxantrone Use in Cancer Patients • Since approval • • • – Over 180, 000 patients treated in U. S. – Over 400, 000 patients treated worldwide Range of dose and schedule – 12 mg/m 2/day x 3 days every 4 -6 weeks – 8 -14 mg/m 2 repeated every 3 -4 weeks – 30 -80 mg/m 2 single dose Alone or in combination with other drugs Well characterized safety profile Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-3
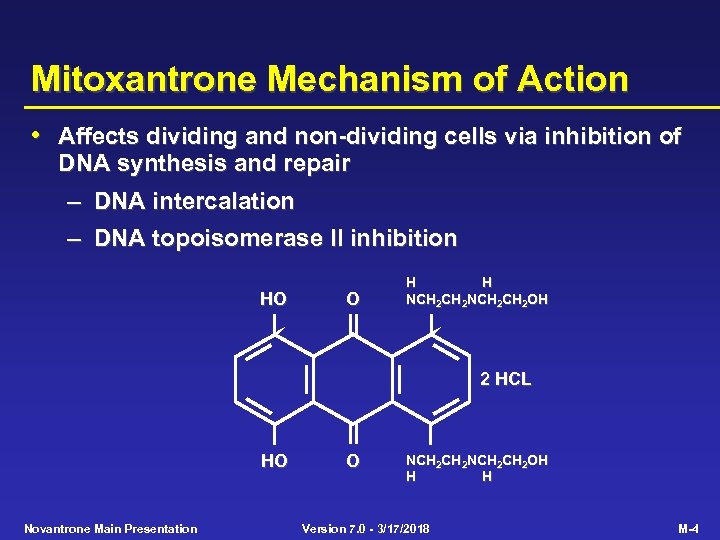 Mitoxantrone Mechanism of Action • Affects dividing and non-dividing cells via inhibition of DNA synthesis and repair – DNA intercalation – DNA topoisomerase II inhibition HO O H H NCH 2 CH 2 OH 2 HCL HO Novantrone Main Presentation O NCH 2 CH 2 OH H H Version 7. 0 - 3/17/2018 M-4
Mitoxantrone Mechanism of Action • Affects dividing and non-dividing cells via inhibition of DNA synthesis and repair – DNA intercalation – DNA topoisomerase II inhibition HO O H H NCH 2 CH 2 OH 2 HCL HO Novantrone Main Presentation O NCH 2 CH 2 OH H H Version 7. 0 - 3/17/2018 M-4
 Proposed Mechanism of Action in MS • Antiproliferative effects -- reduces – B-lymphocytes – T-lymphocytes – Macrophages • Immunomodulatory effects – Decreases antigen presentation – Decreases cytokine production (IL-2, TNF , IFN ) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-5
Proposed Mechanism of Action in MS • Antiproliferative effects -- reduces – B-lymphocytes – T-lymphocytes – Macrophages • Immunomodulatory effects – Decreases antigen presentation – Decreases cytokine production (IL-2, TNF , IFN ) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-5
 Multiple Sclerosis • MS is a debilitating disease • Afflicts 350, 000+ Americans • 140, 000+ with secondary progressive MS – No currently approved treatment options Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-6
Multiple Sclerosis • MS is a debilitating disease • Afflicts 350, 000+ Americans • 140, 000+ with secondary progressive MS – No currently approved treatment options Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-6
 Mitoxantrone for MS Regulatory History • • • End of Phase III meeting 11/2/98 Pre-NDA meeting 4/15/99 NDA submitted 6/4/99 Priority review status 7/26/99 Orphan designation granted Novantrone Main Presentation Version 7. 0 - 3/17/2018 8/13/99 M-7
Mitoxantrone for MS Regulatory History • • • End of Phase III meeting 11/2/98 Pre-NDA meeting 4/15/99 NDA submitted 6/4/99 Priority review status 7/26/99 Orphan designation granted Novantrone Main Presentation Version 7. 0 - 3/17/2018 8/13/99 M-7
 Requested Approval “To slow progression of neurological disability and reduce the relapse rate in patients with progressive forms of multiple sclerosis excluding primary progressive MS. ” Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-8
Requested Approval “To slow progression of neurological disability and reduce the relapse rate in patients with progressive forms of multiple sclerosis excluding primary progressive MS. ” Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-8
 Presentation Agenda • Introduction Ann Hayes, M. D. Senior Vice President Immunex Corporation • Efficacy & Safety Richard Ghalie, M. D. Senior Director Immunex Corporation • Clinician’s Fred Lublin, M. D. Professor of Neurology MCP Hahnemann University Perspective Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-9
Presentation Agenda • Introduction Ann Hayes, M. D. Senior Vice President Immunex Corporation • Efficacy & Safety Richard Ghalie, M. D. Senior Director Immunex Corporation • Clinician’s Fred Lublin, M. D. Professor of Neurology MCP Hahnemann University Perspective Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-9
 Study Investigators • H. Peter Hartung, MD Chairman, Department of Neurology Graz University, Austria Chairman, Study 901 • Gilles Edan, MD Chairman, Department of Neurology CHU Rennes, France Chairman, Study 902 • Erich Mauch, MD Medical Director, Neurology Clinic Academic Hospital of Ulm University, Germany Chairman, Study 903 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-10
Study Investigators • H. Peter Hartung, MD Chairman, Department of Neurology Graz University, Austria Chairman, Study 901 • Gilles Edan, MD Chairman, Department of Neurology CHU Rennes, France Chairman, Study 902 • Erich Mauch, MD Medical Director, Neurology Clinic Academic Hospital of Ulm University, Germany Chairman, Study 903 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-10
 Immunex Advisors • Hillel Panitch, MD Professor, Department of Neurology University of Maryland Baltimore, MD • Craig Smith, MD Clinical Professor of Neurology, Medicine and Ophthalmology University of Washington Seattle, WA • David Alberts, MD Professor of Medicine, Pharmacology and Public Health Associate Dean for Research University of Arizona Tucson, AZ Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-11
Immunex Advisors • Hillel Panitch, MD Professor, Department of Neurology University of Maryland Baltimore, MD • Craig Smith, MD Clinical Professor of Neurology, Medicine and Ophthalmology University of Washington Seattle, WA • David Alberts, MD Professor of Medicine, Pharmacology and Public Health Associate Dean for Research University of Arizona Tucson, AZ Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-11
 Mitoxantrone in Multiple Sclerosis (MS) Efficacy and Safety Review Novantrone Main Presentation Version 7. 0 - M-
Mitoxantrone in Multiple Sclerosis (MS) Efficacy and Safety Review Novantrone Main Presentation Version 7. 0 - M-
 Mitoxantrone in Multiple Sclerosis Data Presentation and Discussion • Efficacy data from 2 randomized trials • Safety data – Two randomized trials (901 -902) – One retrospective study (903) – 12+ years post marketing experience • Benefit and risk assessment • Discussion of questions raised by Dr. Katz Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-13
Mitoxantrone in Multiple Sclerosis Data Presentation and Discussion • Efficacy data from 2 randomized trials • Safety data – Two randomized trials (901 -902) – One retrospective study (903) – 12+ years post marketing experience • Benefit and risk assessment • Discussion of questions raised by Dr. Katz Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-13
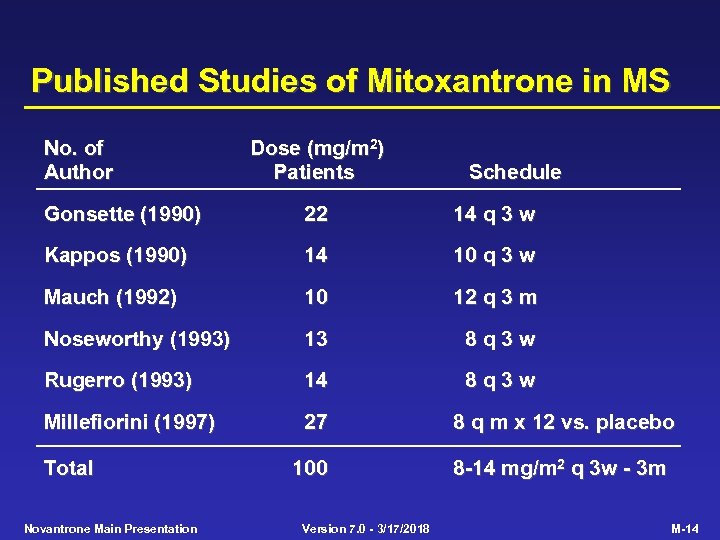 Published Studies of Mitoxantrone in MS No. of Author Dose (mg/m 2) Patients Schedule Gonsette (1990) 22 14 q 3 w Kappos (1990) 14 10 q 3 w Mauch (1992) 10 12 q 3 m Noseworthy (1993) 13 8 q 3 w Rugerro (1993) 14 8 q 3 w Millefiorini (1997) 27 8 q m x 12 vs. placebo 100 8 -14 mg/m 2 q 3 w - 3 m Total Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-14
Published Studies of Mitoxantrone in MS No. of Author Dose (mg/m 2) Patients Schedule Gonsette (1990) 22 14 q 3 w Kappos (1990) 14 10 q 3 w Mauch (1992) 10 12 q 3 m Noseworthy (1993) 13 8 q 3 w Rugerro (1993) 14 8 q 3 w Millefiorini (1997) 27 8 q m x 12 vs. placebo 100 8 -14 mg/m 2 q 3 w - 3 m Total Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-14
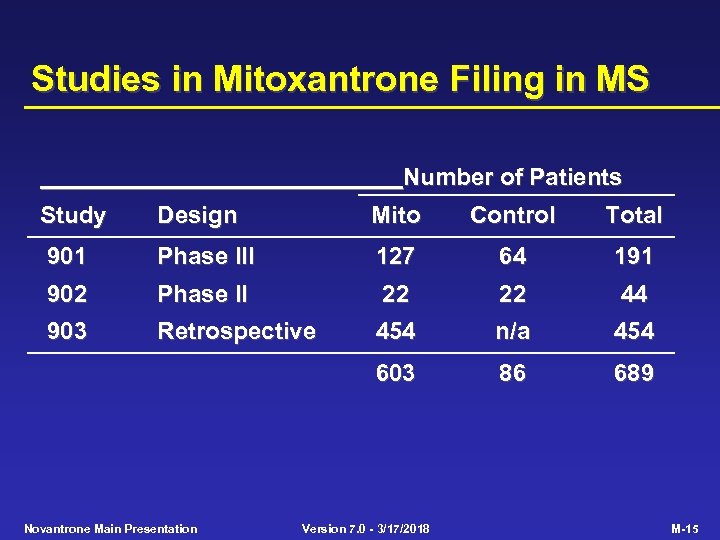 Studies in Mitoxantrone Filing in MS Number of Patients Study Design Mito Control Total 901 Phase III 127 64 191 902 Phase II 22 22 44 903 Retrospective 454 n/a 454 603 86 689 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-15
Studies in Mitoxantrone Filing in MS Number of Patients Study Design Mito Control Total 901 Phase III 127 64 191 902 Phase II 22 22 44 903 Retrospective 454 n/a 454 603 86 689 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-15
 Study 901 Design and Efficacy Results Novantrone Main Presentation Version 7. 0 - M-
Study 901 Design and Efficacy Results Novantrone Main Presentation Version 7. 0 - M-
 Study 901 - MIMS Trial • • • Phase III, randomized, placebo-controlled study 17 centers in 4 European countries 194 patients Chairs - Professors H-P. Hartung and R. Gonsette Study approved by Bf. Ar. M/Germany June 1993 - July 1997 ECTRIMS - 1998, 1999 and AAN - 1999 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-17
Study 901 - MIMS Trial • • • Phase III, randomized, placebo-controlled study 17 centers in 4 European countries 194 patients Chairs - Professors H-P. Hartung and R. Gonsette Study approved by Bf. Ar. M/Germany June 1993 - July 1997 ECTRIMS - 1998, 1999 and AAN - 1999 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-17
 Study 901 - Inclusion Criteria • Age 18 to 55 • MS according to Poser’s criteria • Secondary progressive or remitting progressive* MS • EDSS progression 1 point in preceding 18 months • Baseline EDSS from 3 to 6 * Relapsing remitting MS with residual deficit after relapse Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-18
Study 901 - Inclusion Criteria • Age 18 to 55 • MS according to Poser’s criteria • Secondary progressive or remitting progressive* MS • EDSS progression 1 point in preceding 18 months • Baseline EDSS from 3 to 6 * Relapsing remitting MS with residual deficit after relapse Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-18
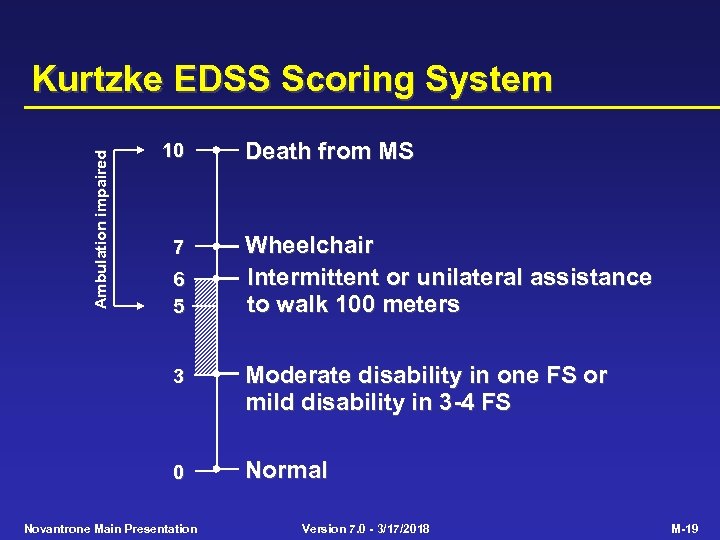 Ambulation impaired Kurtzke EDSS Scoring System 10 7 6 5 Death from MS Wheelchair Intermittent or unilateral assistance to walk 100 meters 3 Moderate disability in one FS or mild disability in 3 -4 FS 0 Normal Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-19
Ambulation impaired Kurtzke EDSS Scoring System 10 7 6 5 Death from MS Wheelchair Intermittent or unilateral assistance to walk 100 meters 3 Moderate disability in one FS or mild disability in 3 -4 FS 0 Normal Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-19
 Study 901 - Exclusion Criteria • Benign or primary progressive MS • Relapse or treatment with corticosteroids in preceding 8 weeks • Prior treatment with mitoxantrone • Immunosuppressive therapy in preceding 9 months • Cardiac risk factors • Major medical illness Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-20
Study 901 - Exclusion Criteria • Benign or primary progressive MS • Relapse or treatment with corticosteroids in preceding 8 weeks • Prior treatment with mitoxantrone • Immunosuppressive therapy in preceding 9 months • Cardiac risk factors • Major medical illness Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-20
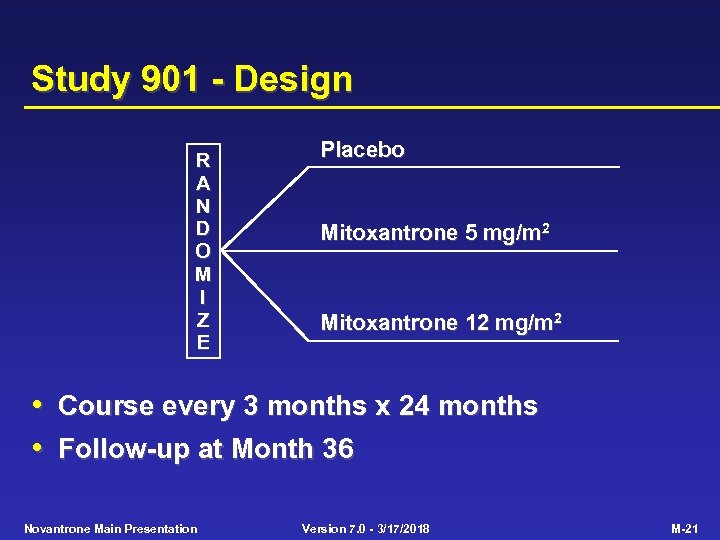 Study 901 - Design R A N D O M I Z E Placebo Mitoxantrone 5 mg/m 2 Mitoxantrone 12 mg/m 2 • Course every 3 months x 24 months • Follow-up at Month 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-21
Study 901 - Design R A N D O M I Z E Placebo Mitoxantrone 5 mg/m 2 Mitoxantrone 12 mg/m 2 • Course every 3 months x 24 months • Follow-up at Month 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-21
 Study 901 - Masking Study Drug • Placebo (methylene blue) to mask patients • Evaluators of neurologic disability – Trained prior to study initiation – Masked to study drug – Not involved in patient management • MRI evaluators masked to study drug and outcomes • Treating physicians not masked to study drug – Drug administration and patient management – Evaluation of adverse events – Assessment and treatment of relapses Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-22
Study 901 - Masking Study Drug • Placebo (methylene blue) to mask patients • Evaluators of neurologic disability – Trained prior to study initiation – Masked to study drug – Not involved in patient management • MRI evaluators masked to study drug and outcomes • Treating physicians not masked to study drug – Drug administration and patient management – Evaluation of adverse events – Assessment and treatment of relapses Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-22
 Study 901 - Primary Efficacy Criterion • Single multivariate test* of 5 variables: – EDSS change from baseline – AI change from baseline – Number of relapses requiring treatment – Time to first treated relapse – SNS change from baseline • Mitoxantrone 12 mg/m 2 vs placebo at = 0. 05 * Wei-Lachin procedure (1992) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-23
Study 901 - Primary Efficacy Criterion • Single multivariate test* of 5 variables: – EDSS change from baseline – AI change from baseline – Number of relapses requiring treatment – Time to first treated relapse – SNS change from baseline • Mitoxantrone 12 mg/m 2 vs placebo at = 0. 05 * Wei-Lachin procedure (1992) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-23
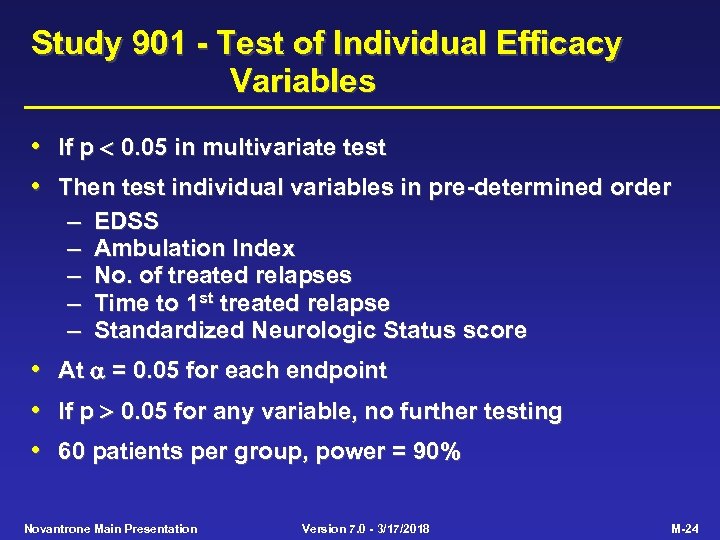 Study 901 - Test of Individual Efficacy Variables • • If p 0. 05 in multivariate test Then test individual variables in pre-determined order – EDSS – Ambulation Index – No. of treated relapses – Time to 1 st treated relapse – Standardized Neurologic Status score • At = 0. 05 for each endpoint • If p 0. 05 for any variable, no further testing • 60 patients per group, power = 90% Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-24
Study 901 - Test of Individual Efficacy Variables • • If p 0. 05 in multivariate test Then test individual variables in pre-determined order – EDSS – Ambulation Index – No. of treated relapses – Time to 1 st treated relapse – Standardized Neurologic Status score • At = 0. 05 for each endpoint • If p 0. 05 for any variable, no further testing • 60 patients per group, power = 90% Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-24
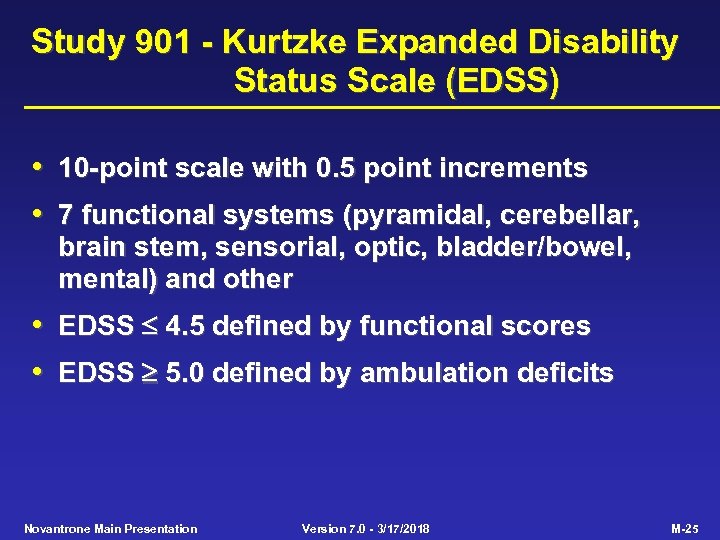 Study 901 - Kurtzke Expanded Disability Status Scale (EDSS) • 10 -point scale with 0. 5 point increments • 7 functional systems (pyramidal, cerebellar, brain stem, sensorial, optic, bladder/bowel, mental) and other • EDSS 4. 5 defined by functional scores • EDSS 5. 0 defined by ambulation deficits Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-25
Study 901 - Kurtzke Expanded Disability Status Scale (EDSS) • 10 -point scale with 0. 5 point increments • 7 functional systems (pyramidal, cerebellar, brain stem, sensorial, optic, bladder/bowel, mental) and other • EDSS 4. 5 defined by functional scores • EDSS 5. 0 defined by ambulation deficits Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-25
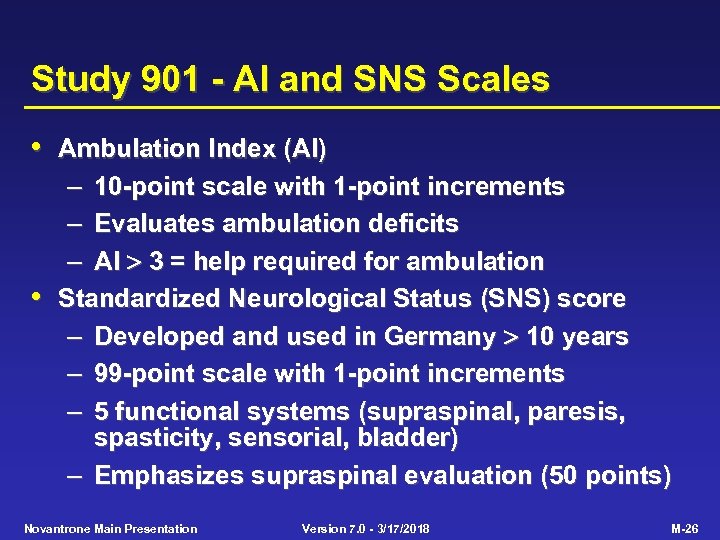 Study 901 - AI and SNS Scales • Ambulation Index (AI) – 10 -point scale with 1 -point increments – Evaluates ambulation deficits – AI 3 = help required for ambulation • Standardized Neurological Status (SNS) score – Developed and used in Germany 10 years – 99 -point scale with 1 -point increments – 5 functional systems (supraspinal, paresis, spasticity, sensorial, bladder) – Emphasizes supraspinal evaluation (50 points) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-26
Study 901 - AI and SNS Scales • Ambulation Index (AI) – 10 -point scale with 1 -point increments – Evaluates ambulation deficits – AI 3 = help required for ambulation • Standardized Neurological Status (SNS) score – Developed and used in Germany 10 years – 99 -point scale with 1 -point increments – 5 functional systems (supraspinal, paresis, spasticity, sensorial, bladder) – Emphasizes supraspinal evaluation (50 points) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-26
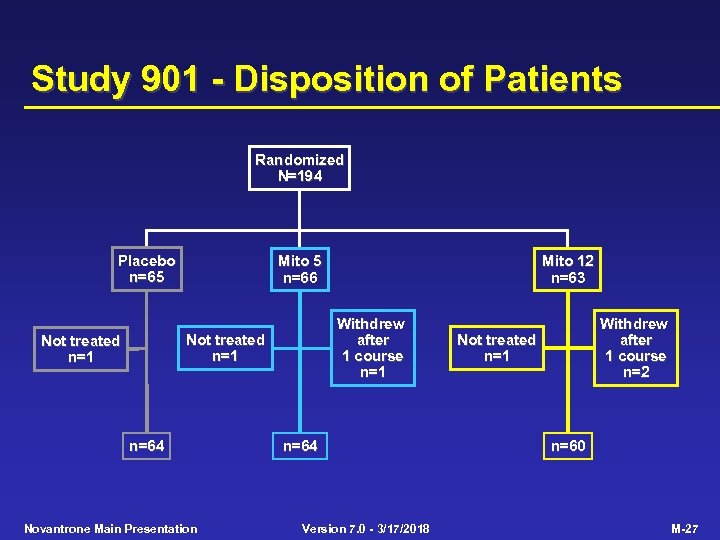 Study 901 - Disposition of Patients Randomized N=194 Placebo n=65 Mito 5 n=66 Withdrew after 1 course n=1 Not treated n=1 n=64 Novantrone Main Presentation Mito 12 n=63 n=64 Version 7. 0 - 3/17/2018 Withdrew after 1 course n=2 Not treated n=1 n=60 M-27
Study 901 - Disposition of Patients Randomized N=194 Placebo n=65 Mito 5 n=66 Withdrew after 1 course n=1 Not treated n=1 n=64 Novantrone Main Presentation Mito 12 n=63 n=64 Version 7. 0 - 3/17/2018 Withdrew after 1 course n=2 Not treated n=1 n=60 M-27
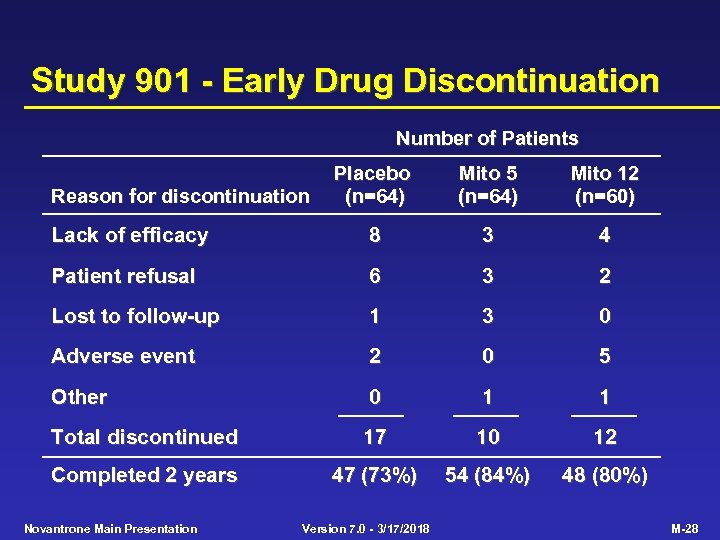 Study 901 - Early Drug Discontinuation Number of Patients Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Lack of efficacy 8 3 4 Patient refusal 6 3 2 Lost to follow-up 1 3 0 Adverse event 2 0 5 Other 0 1 1 Total discontinued 17 10 12 Completed 2 years 47 (73%) 54 (84%) 48 (80%) Reason for discontinuation Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-28
Study 901 - Early Drug Discontinuation Number of Patients Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Lack of efficacy 8 3 4 Patient refusal 6 3 2 Lost to follow-up 1 3 0 Adverse event 2 0 5 Other 0 1 1 Total discontinued 17 10 12 Completed 2 years 47 (73%) 54 (84%) 48 (80%) Reason for discontinuation Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-28
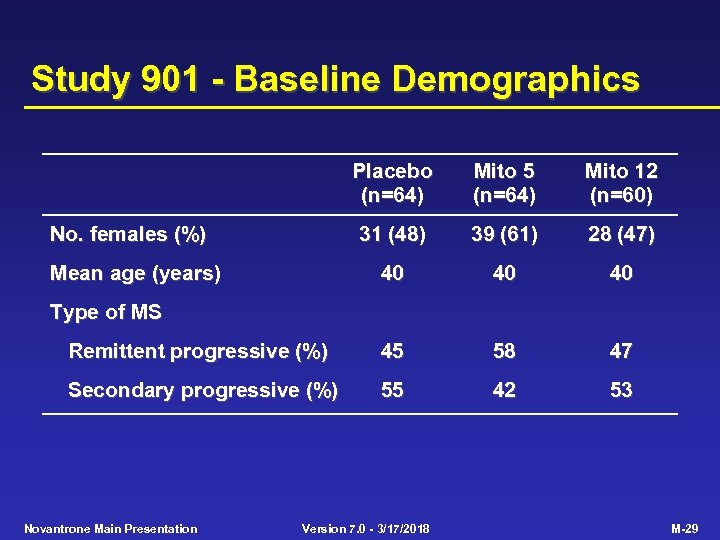 Study 901 - Baseline Demographics Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) 31 (48) 39 (61) 28 (47) 40 40 40 Remittent progressive (%) 45 58 47 Secondary progressive (%) 55 42 53 No. females (%) Mean age (years) Type of MS Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-29
Study 901 - Baseline Demographics Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) 31 (48) 39 (61) 28 (47) 40 40 40 Remittent progressive (%) 45 58 47 Secondary progressive (%) 55 42 53 No. females (%) Mean age (years) Type of MS Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-29
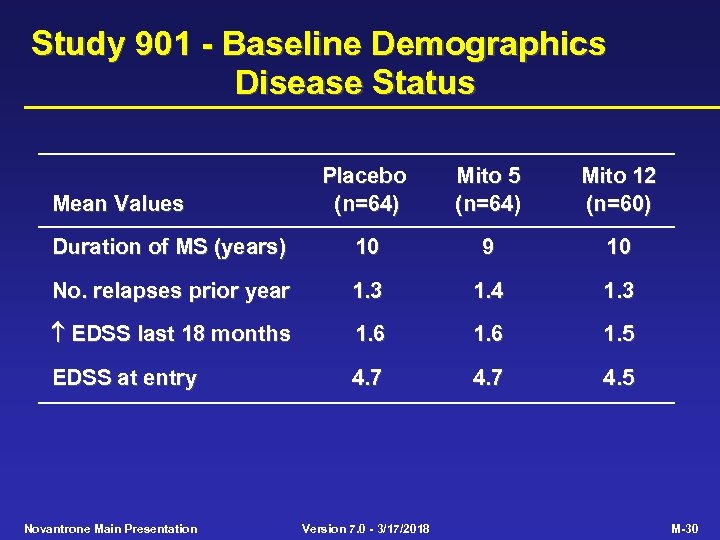 Study 901 - Baseline Demographics Disease Status Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Duration of MS (years) 10 9 10 No. relapses prior year 1. 3 1. 4 1. 3 EDSS last 18 months 1. 6 1. 5 EDSS at entry 4. 7 4. 5 Mean Values Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-30
Study 901 - Baseline Demographics Disease Status Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Duration of MS (years) 10 9 10 No. relapses prior year 1. 3 1. 4 1. 3 EDSS last 18 months 1. 6 1. 5 EDSS at entry 4. 7 4. 5 Mean Values Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-30
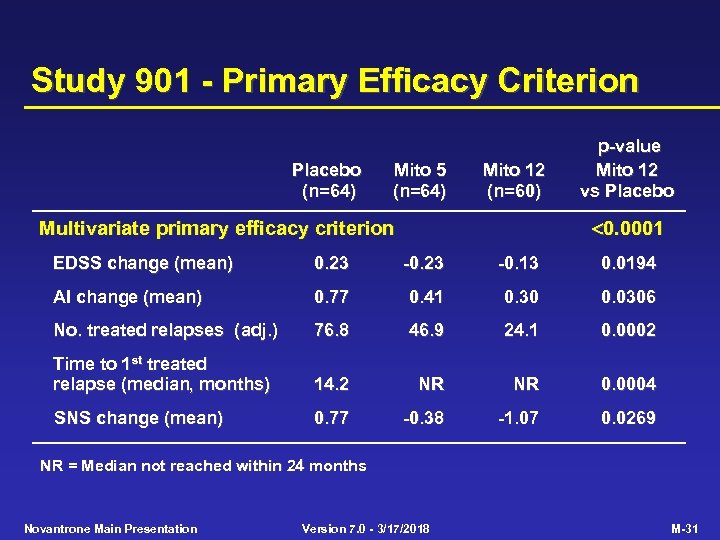 Study 901 - Primary Efficacy Criterion Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) p-value Mito 12 vs Placebo 0. 0001 Multivariate primary efficacy criterion EDSS change (mean) 0. 23 -0. 13 0. 0194 AI change (mean) 0. 77 0. 41 0. 30 0. 0306 No. treated relapses (adj. ) 76. 8 46. 9 24. 1 0. 0002 Time to 1 st treated relapse (median, months) 14. 2 NR NR 0. 0004 SNS change (mean) 0. 77 -0. 38 -1. 07 0. 0269 NR = Median not reached within 24 months Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-31
Study 901 - Primary Efficacy Criterion Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) p-value Mito 12 vs Placebo 0. 0001 Multivariate primary efficacy criterion EDSS change (mean) 0. 23 -0. 13 0. 0194 AI change (mean) 0. 77 0. 41 0. 30 0. 0306 No. treated relapses (adj. ) 76. 8 46. 9 24. 1 0. 0002 Time to 1 st treated relapse (median, months) 14. 2 NR NR 0. 0004 SNS change (mean) 0. 77 -0. 38 -1. 07 0. 0269 NR = Median not reached within 24 months Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-31
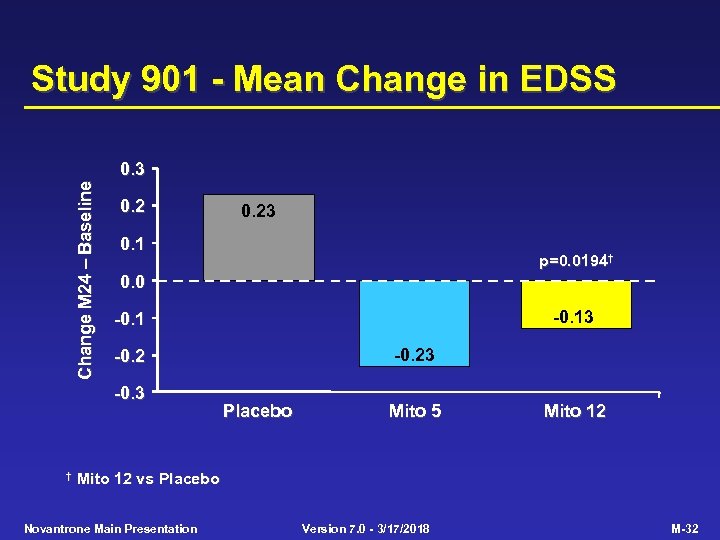 Study 901 - Mean Change in EDSS Change M 24 Baseline 0. 3 0. 2 0. 1 p=0. 0194† 0. 0 -0. 13 -0. 1 -0. 23 -0. 2 -0. 3 † 0. 23 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-32
Study 901 - Mean Change in EDSS Change M 24 Baseline 0. 3 0. 2 0. 1 p=0. 0194† 0. 0 -0. 13 -0. 1 -0. 23 -0. 2 -0. 3 † 0. 23 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-32
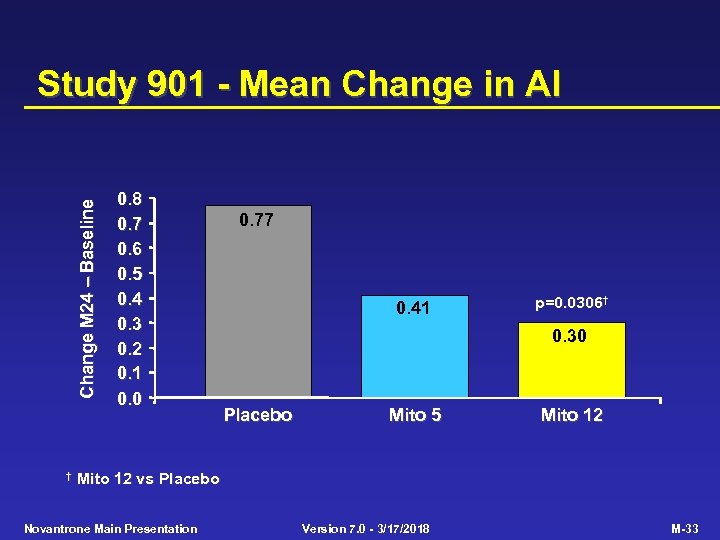 Change M 24 Baseline Study 901 - Mean Change in AI † 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0. 77 0. 41 p=0. 0306† 0. 30 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-33
Change M 24 Baseline Study 901 - Mean Change in AI † 0. 8 0. 7 0. 6 0. 5 0. 4 0. 3 0. 2 0. 1 0. 0 0. 77 0. 41 p=0. 0306† 0. 30 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-33
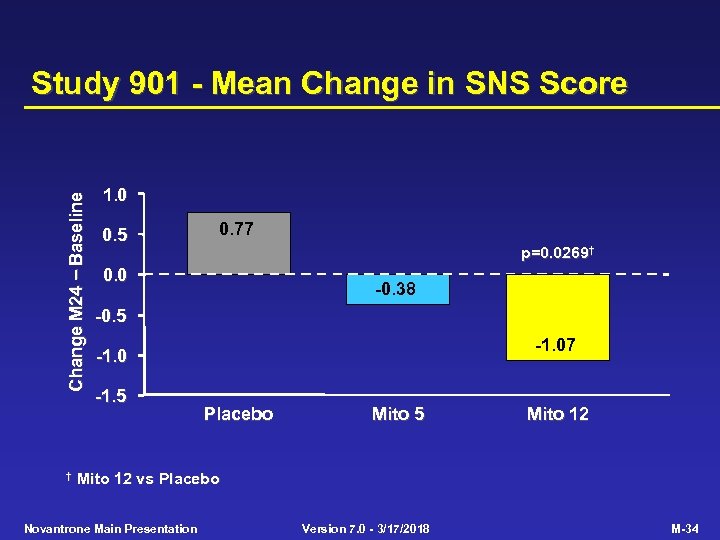 Change M 24 Baseline Study 901 - Mean Change in SNS Score † 1. 0 0. 5 0. 77 p=0. 0269† 0. 0 -0. 38 -0. 5 -1. 07 -1. 0 -1. 5 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-34
Change M 24 Baseline Study 901 - Mean Change in SNS Score † 1. 0 0. 5 0. 77 p=0. 0269† 0. 0 -0. 38 -0. 5 -1. 07 -1. 0 -1. 5 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-34
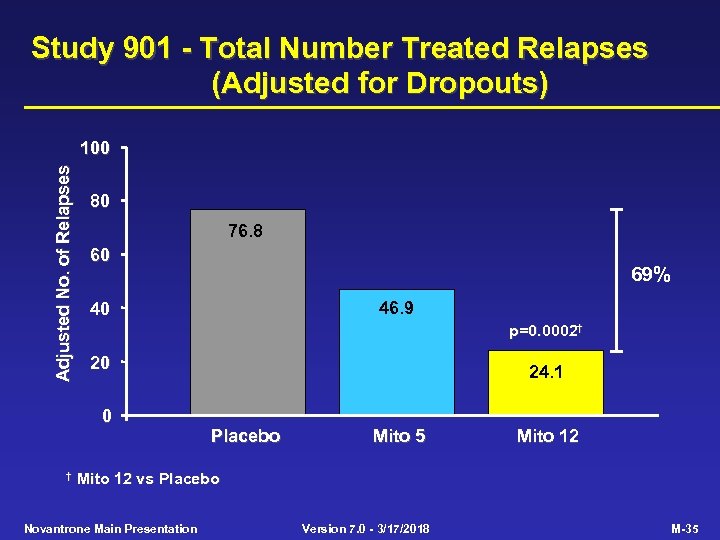 Study 901 - Total Number Treated Relapses (Adjusted for Dropouts) Adjusted No. of Relapses 100 80 76. 8 60 46. 9 40 p=0. 0002† 20 0 † 69% 24. 1 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-35
Study 901 - Total Number Treated Relapses (Adjusted for Dropouts) Adjusted No. of Relapses 100 80 76. 8 60 46. 9 40 p=0. 0002† 20 0 † 69% 24. 1 Placebo Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-35
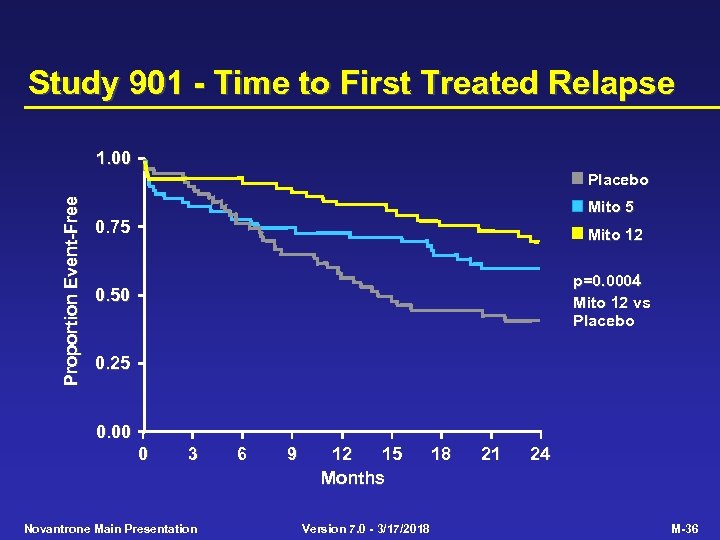 Study 901 - Time to First Treated Relapse 1. 00 Proportion Event-Free Placebo Mito 5 0. 75 Mito 12 0. 50 p=0. 0004 Mito 12 vs Placebo 0. 25 0. 00 0 3 Novantrone Main Presentation 6 9 12 15 Months Version 7. 0 - 3/17/2018 18 21 24 M-36
Study 901 - Time to First Treated Relapse 1. 00 Proportion Event-Free Placebo Mito 5 0. 75 Mito 12 0. 50 p=0. 0004 Mito 12 vs Placebo 0. 25 0. 00 0 3 Novantrone Main Presentation 6 9 12 15 Months Version 7. 0 - 3/17/2018 18 21 24 M-36
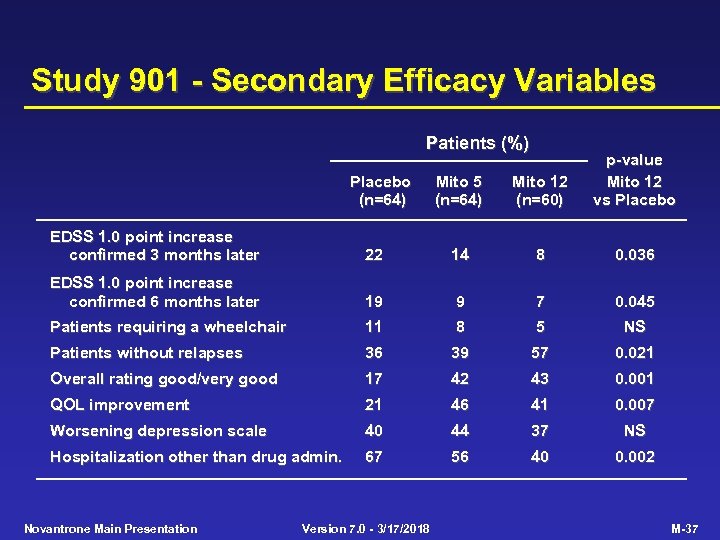 Study 901 - Secondary Efficacy Variables Patients (%) Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) p-value Mito 12 vs Placebo EDSS 1. 0 point increase confirmed 3 months later 22 14 8 0. 036 EDSS 1. 0 point increase confirmed 6 months later 19 9 7 0. 045 Patients requiring a wheelchair 11 8 5 NS Patients without relapses 36 39 57 0. 021 Overall rating good/very good 17 42 43 0. 001 QOL improvement 21 46 41 0. 007 Worsening depression scale 40 44 37 NS Hospitalization other than drug admin. 67 56 40 0. 002 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-37
Study 901 - Secondary Efficacy Variables Patients (%) Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) p-value Mito 12 vs Placebo EDSS 1. 0 point increase confirmed 3 months later 22 14 8 0. 036 EDSS 1. 0 point increase confirmed 6 months later 19 9 7 0. 045 Patients requiring a wheelchair 11 8 5 NS Patients without relapses 36 39 57 0. 021 Overall rating good/very good 17 42 43 0. 001 QOL improvement 21 46 41 0. 007 Worsening depression scale 40 44 37 NS Hospitalization other than drug admin. 67 56 40 0. 002 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-37
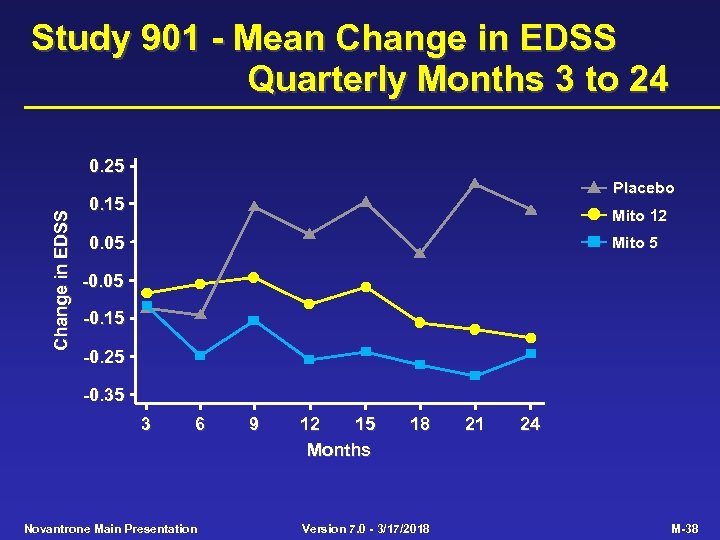 Study 901 - Mean Change in EDSS Quarterly Months 3 to 24 Change in EDSS 0. 25 Placebo 0. 15 Mito 12 0. 05 Mito 5 -0. 05 -0. 15 -0. 25 -0. 35 3 6 Novantrone Main Presentation 9 12 15 Months 18 Version 7. 0 - 3/17/2018 21 24 M-38
Study 901 - Mean Change in EDSS Quarterly Months 3 to 24 Change in EDSS 0. 25 Placebo 0. 15 Mito 12 0. 05 Mito 5 -0. 05 -0. 15 -0. 25 -0. 35 3 6 Novantrone Main Presentation 9 12 15 Months 18 Version 7. 0 - 3/17/2018 21 24 M-38
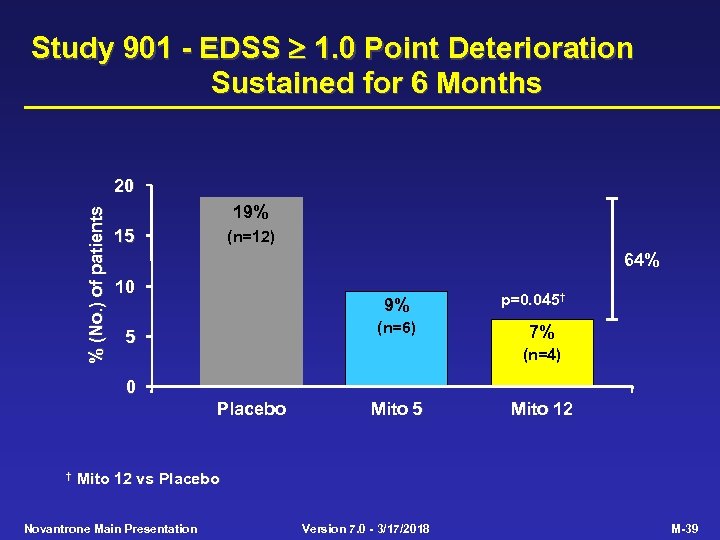 Study 901 - EDSS 1. 0 Point Deterioration Sustained for 6 Months % (No. ) of patients 20 19% 15 (n=12) 64% 10 9% (n=6) 5 p=0. 045† 7% (n=4) 0 Placebo † Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-39
Study 901 - EDSS 1. 0 Point Deterioration Sustained for 6 Months % (No. ) of patients 20 19% 15 (n=12) 64% 10 9% (n=6) 5 p=0. 045† 7% (n=4) 0 Placebo † Mito 5 Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-39
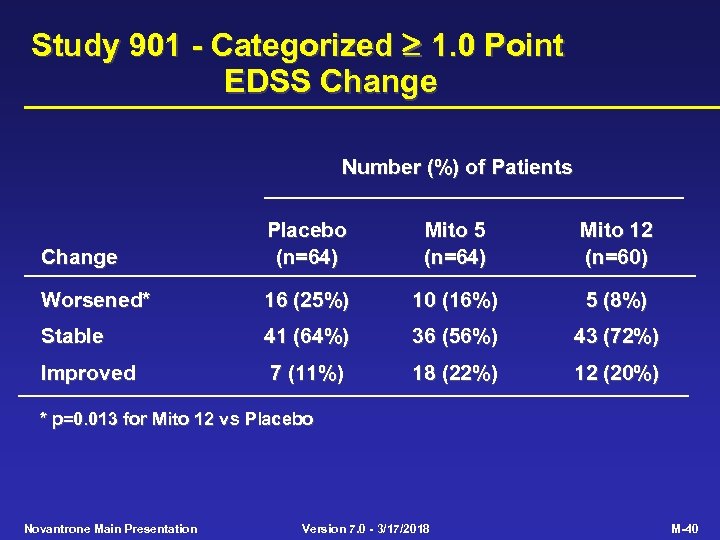 Study 901 - Categorized 1. 0 Point EDSS Change Number (%) of Patients Change Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Worsened* 16 (25%) 10 (16%) 5 (8%) Stable 41 (64%) 36 (56%) 43 (72%) Improved 7 (11%) 18 (22%) 12 (20%) * p=0. 013 for Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-40
Study 901 - Categorized 1. 0 Point EDSS Change Number (%) of Patients Change Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Worsened* 16 (25%) 10 (16%) 5 (8%) Stable 41 (64%) 36 (56%) 43 (72%) Improved 7 (11%) 18 (22%) 12 (20%) * p=0. 013 for Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-40
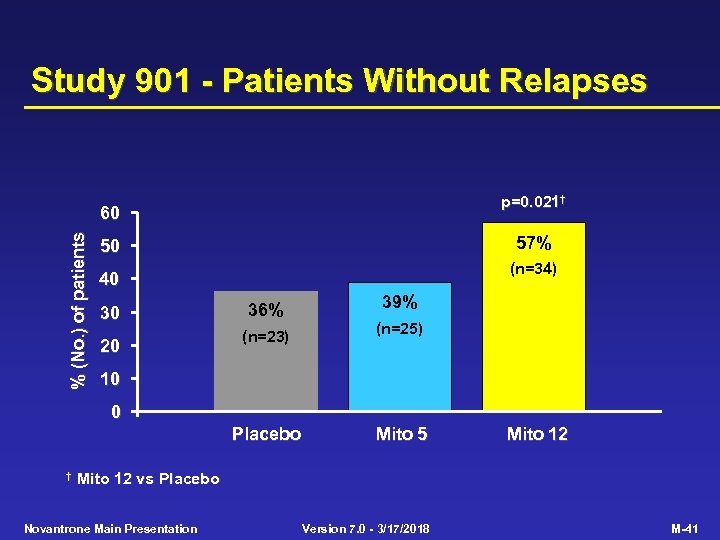 Study 901 - Patients Without Relapses p=0. 021† % (No. ) of patients 60 57% 50 (n=34) 40 30 36% 39% (n=25) Placebo 20 (n=23) Mito 5 10 0 † Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-41
Study 901 - Patients Without Relapses p=0. 021† % (No. ) of patients 60 57% 50 (n=34) 40 30 36% 39% (n=25) Placebo 20 (n=23) Mito 5 10 0 † Mito 12 vs Placebo Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-41
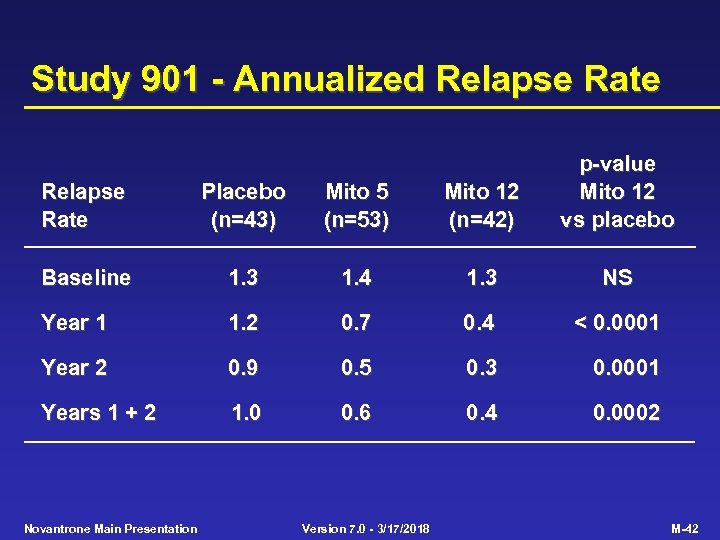 Study 901 - Annualized Relapse Rate Placebo (n=43) Mito 5 (n=53) Mito 12 (n=42) p-value Mito 12 vs placebo Baseline 1. 3 1. 4 1. 3 NS Year 1 1. 2 0. 7 0. 4 < 0. 0001 Year 2 0. 9 0. 5 0. 3 0. 0001 Years 1 + 2 1. 0 0. 6 0. 4 0. 0002 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-42
Study 901 - Annualized Relapse Rate Placebo (n=43) Mito 5 (n=53) Mito 12 (n=42) p-value Mito 12 vs placebo Baseline 1. 3 1. 4 1. 3 NS Year 1 1. 2 0. 7 0. 4 < 0. 0001 Year 2 0. 9 0. 5 0. 3 0. 0001 Years 1 + 2 1. 0 0. 6 0. 4 0. 0002 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-42
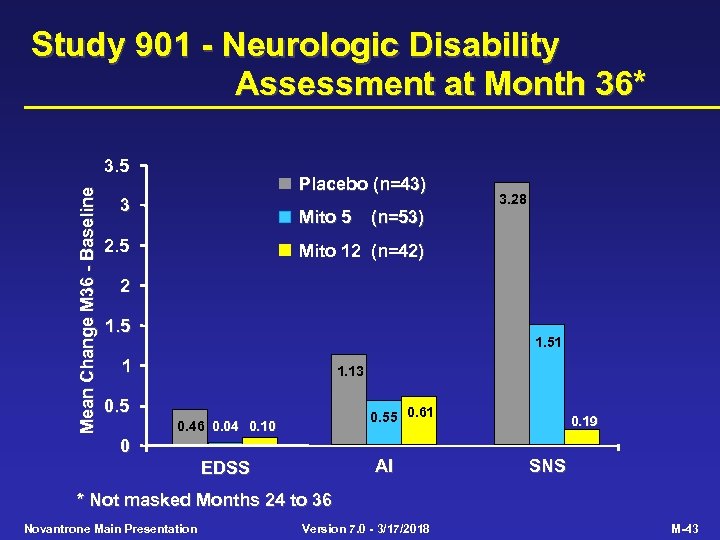 Study 901 - Neurologic Disability Assessment at Month 36* Mean Change M 36 - Baseline 3. 5 Placebo (n=43) 3 Mito 5 2. 5 (n=53) 3. 28 Mito 12 (n=42) 2 1. 51 1 1. 13 0. 55 0. 61 0. 46 0. 04 0. 10 0 AI EDSS 0. 19 SNS * Not masked Months 24 to 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-43
Study 901 - Neurologic Disability Assessment at Month 36* Mean Change M 36 - Baseline 3. 5 Placebo (n=43) 3 Mito 5 2. 5 (n=53) 3. 28 Mito 12 (n=42) 2 1. 51 1 1. 13 0. 55 0. 61 0. 46 0. 04 0. 10 0 AI EDSS 0. 19 SNS * Not masked Months 24 to 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-43
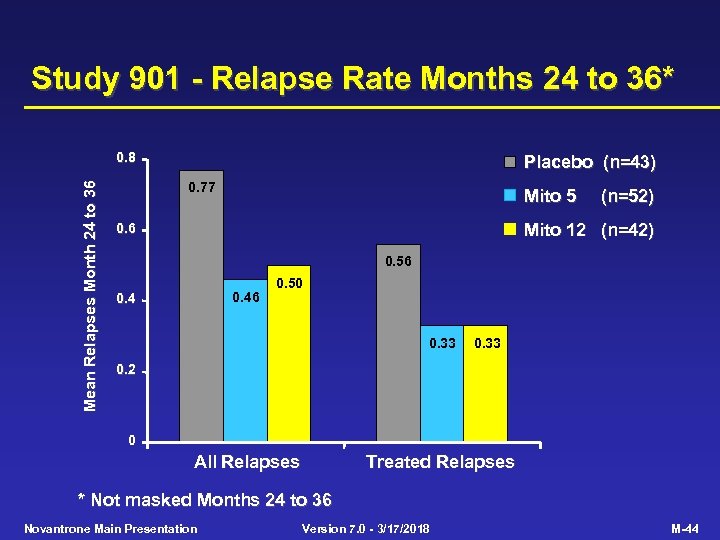 Study 901 - Relapse Rate Months 24 to 36* Mean Relapses Month 24 to 36 0. 8 Placebo (n=43) 0. 77 Mito 5 (n=52) Mito 12 (n=42) 0. 6 0. 56 0. 4 0. 50 0. 33 0. 2 0 All Relapses Treated Relapses * Not masked Months 24 to 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-44
Study 901 - Relapse Rate Months 24 to 36* Mean Relapses Month 24 to 36 0. 8 Placebo (n=43) 0. 77 Mito 5 (n=52) Mito 12 (n=42) 0. 6 0. 56 0. 4 0. 50 0. 33 0. 2 0 All Relapses Treated Relapses * Not masked Months 24 to 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-44
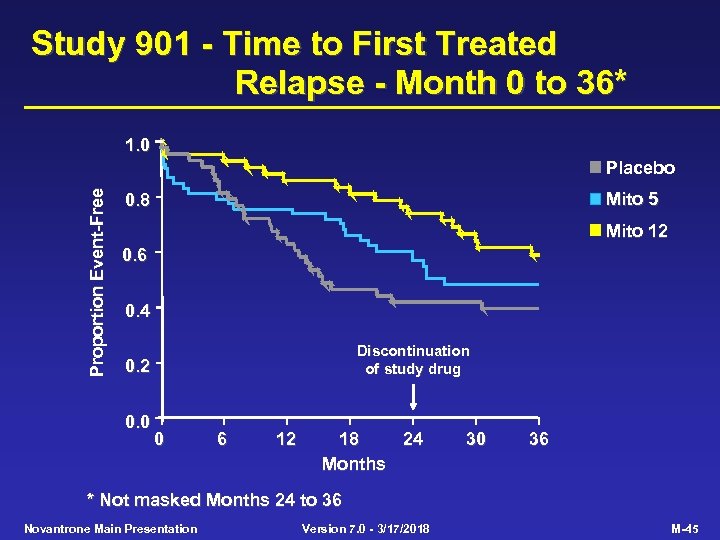 Study 901 - Time to First Treated Relapse - Month 0 to 36* 1. 0 Proportion Event-Free Placebo Mito 5 0. 8 Mito 12 0. 6 0. 4 Discontinuation of study drug 0. 2 0. 0 0 6 12 18 24 Months 30 36 * Not masked Months 24 to 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-45
Study 901 - Time to First Treated Relapse - Month 0 to 36* 1. 0 Proportion Event-Free Placebo Mito 5 0. 8 Mito 12 0. 6 0. 4 Discontinuation of study drug 0. 2 0. 0 0 6 12 18 24 Months 30 36 * Not masked Months 24 to 36 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-45
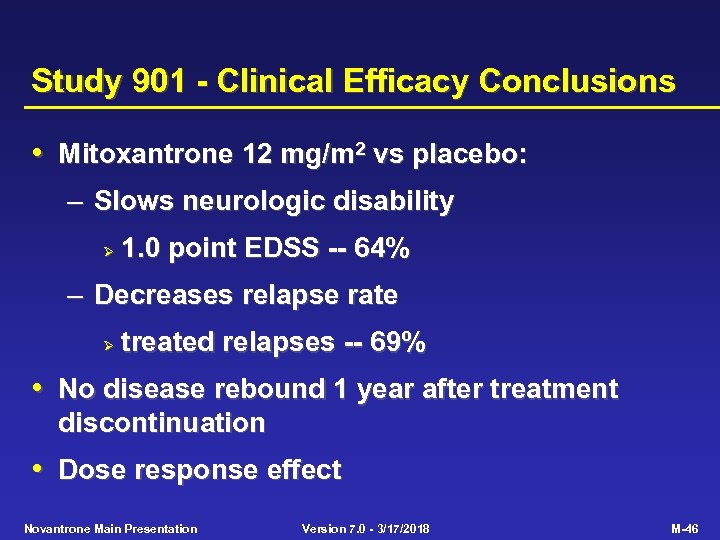 Study 901 - Clinical Efficacy Conclusions • Mitoxantrone 12 mg/m 2 vs placebo: – Slows neurologic disability Ø 1. 0 point EDSS -- 64% – Decreases relapse rate Ø treated relapses -- 69% • No disease rebound 1 year after treatment discontinuation • Dose response effect Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-46
Study 901 - Clinical Efficacy Conclusions • Mitoxantrone 12 mg/m 2 vs placebo: – Slows neurologic disability Ø 1. 0 point EDSS -- 64% – Decreases relapse rate Ø treated relapses -- 69% • No disease rebound 1 year after treatment discontinuation • Dose response effect Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-46
 Study 901 - MRI Subgroup Evaluation • T 1 -w Gd-enhanced and T 2 -w scans • Scans performed at baseline, end of Years 1 and 2 • 110 patients in specified sites • MRI review: – Centrally at the end of the study – Evaluators masked to study drug and clinical outcomes Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-47
Study 901 - MRI Subgroup Evaluation • T 1 -w Gd-enhanced and T 2 -w scans • Scans performed at baseline, end of Years 1 and 2 • 110 patients in specified sites • MRI review: – Centrally at the end of the study – Evaluators masked to study drug and clinical outcomes Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-47
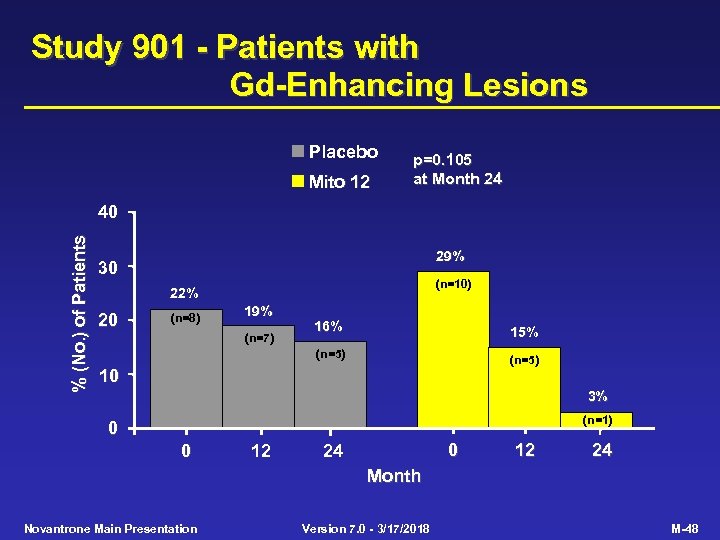 Study 901 - Patients with Gd-Enhancing Lesions Placebo Mito 12 p=0. 105 at Month 24 % (No. ) of Patients 40 29% 30 (n=10) 22% 20 (n=8) 19% 16% 15% (n=5) (n=7) (n=5) 10 3% (n=1) 0 0 12 0 24 12 24 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-48
Study 901 - Patients with Gd-Enhancing Lesions Placebo Mito 12 p=0. 105 at Month 24 % (No. ) of Patients 40 29% 30 (n=10) 22% 20 (n=8) 19% 16% 15% (n=5) (n=7) (n=5) 10 3% (n=1) 0 0 12 0 24 12 24 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-48
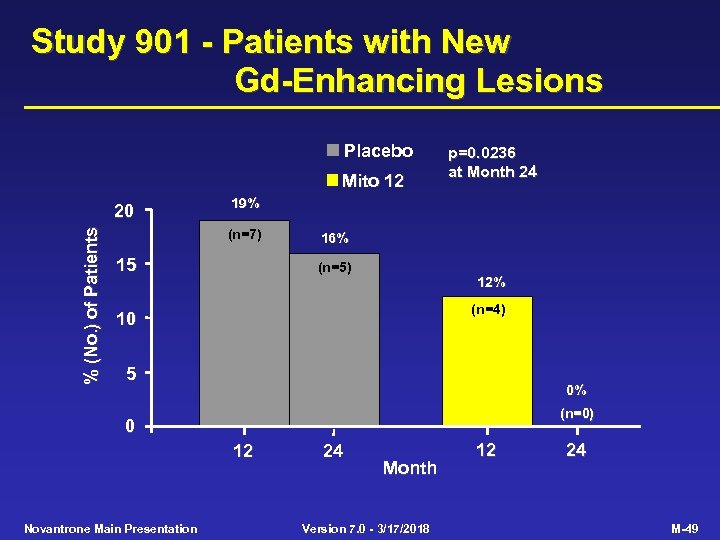 Study 901 - Patients with New Gd-Enhancing Lesions Placebo Mito 12 % (No. ) of Patients 20 p=0. 0236 at Month 24 19% (n=7) 15 16% (n=5) 12% (n=4) 10 5 0% (n=0) 0 12 Novantrone Main Presentation 24 Month Version 7. 0 - 3/17/2018 12 24 M-49
Study 901 - Patients with New Gd-Enhancing Lesions Placebo Mito 12 % (No. ) of Patients 20 p=0. 0236 at Month 24 19% (n=7) 15 16% (n=5) 12% (n=4) 10 5 0% (n=0) 0 12 Novantrone Main Presentation 24 Month Version 7. 0 - 3/17/2018 12 24 M-49
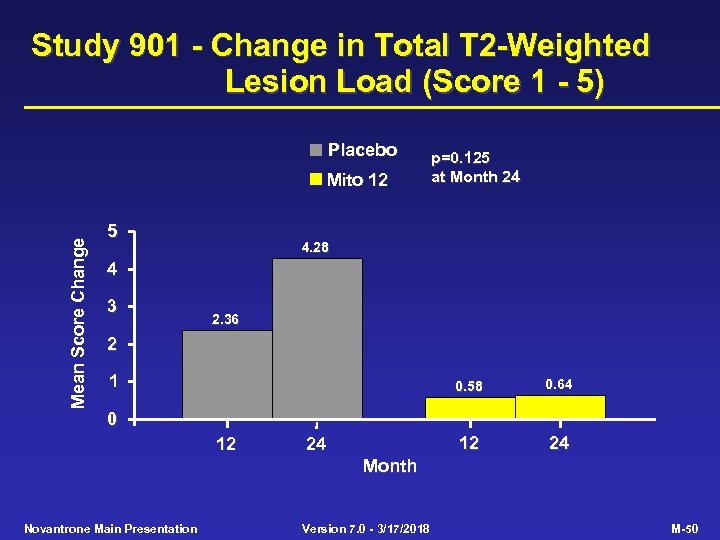 Study 901 - Change in Total T 2 -Weighted Lesion Load (Score 1 - 5) Placebo Mean Score Change Mito 12 5 p=0. 125 at Month 24 4. 28 4 3 2. 36 2 1 0. 58 0. 64 12 24 0 12 24 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-50
Study 901 - Change in Total T 2 -Weighted Lesion Load (Score 1 - 5) Placebo Mean Score Change Mito 12 5 p=0. 125 at Month 24 4. 28 4 3 2. 36 2 1 0. 58 0. 64 12 24 0 12 24 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-50
 Study 901 - MRI Efficacy Conclusions • Mitoxantrone – – Decreased Gd-enhancing lesions Slowed progression of T 2 -w lesion load • Indicates a reduction of inflammation in CNS • Support clinical findings of study Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-51
Study 901 - MRI Efficacy Conclusions • Mitoxantrone – – Decreased Gd-enhancing lesions Slowed progression of T 2 -w lesion load • Indicates a reduction of inflammation in CNS • Support clinical findings of study Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-51
 Study 902 Design and Efficacy Results Novantrone Main Presentation Version 7. 0 - M-
Study 902 Design and Efficacy Results Novantrone Main Presentation Version 7. 0 - M-
 Study 902 - Phase II Trial in Active MS • • • Randomized, corticosteroid-controlled trial 5 academic centers in France 44 patients Chair - Professor G. Edan April 1992 - March 1995 Journal of Neurology, Neurosurgery and Psychiatry 1997 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-53
Study 902 - Phase II Trial in Active MS • • • Randomized, corticosteroid-controlled trial 5 academic centers in France 44 patients Chair - Professor G. Edan April 1992 - March 1995 Journal of Neurology, Neurosurgery and Psychiatry 1997 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-53
 Study 902 - Inclusion Criteria • • • Age 18 to 45 Disease history of less than 10 years Highly active disease – EDSS progression 2 points – Or 2 relapses – In preceding 12 months • Baseline EDSS 6. 0 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-54
Study 902 - Inclusion Criteria • • • Age 18 to 45 Disease history of less than 10 years Highly active disease – EDSS progression 2 points – Or 2 relapses – In preceding 12 months • Baseline EDSS 6. 0 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-54
 Study 902 - Exclusion Criteria • Corticosteroids in previous month • Immunosuppressive therapy in previous 3 months • Cardiac risk factors or major illness • Pregnancy or breast feeding Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-55
Study 902 - Exclusion Criteria • Corticosteroids in previous month • Immunosuppressive therapy in previous 3 months • Cardiac risk factors or major illness • Pregnancy or breast feeding Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-55
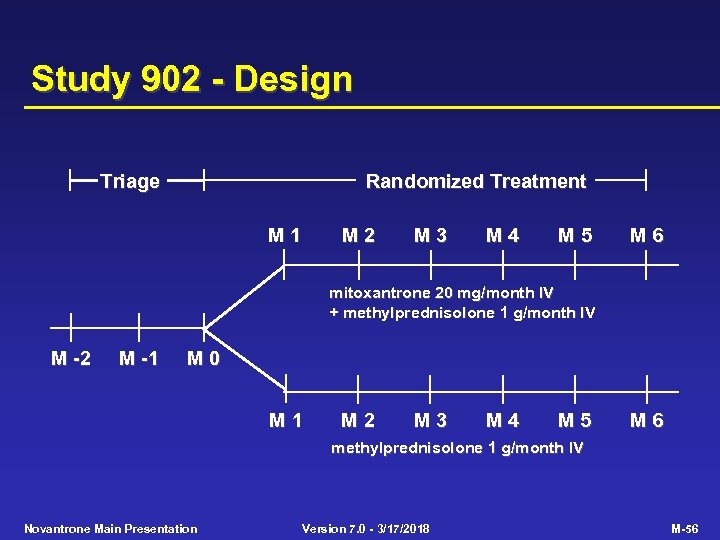 Study 902 - Design Triage Randomized Treatment M 1 M 2 M 3 M 4 M 5 M 6 mitoxantrone 20 mg/month IV + methylprednisolone 1 g/month IV M -2 M -1 M 0 M 1 M 2 M 3 M 4 M 5 M 6 methylprednisolone 1 g/month IV Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-56
Study 902 - Design Triage Randomized Treatment M 1 M 2 M 3 M 4 M 5 M 6 mitoxantrone 20 mg/month IV + methylprednisolone 1 g/month IV M -2 M -1 M 0 M 1 M 2 M 3 M 4 M 5 M 6 methylprednisolone 1 g/month IV Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-56
 Study 902 - Efficacy Evaluations • Monthly MRI – MRI evaluators masked to study drug and outcome • Monthly clinical evaluations – Treating physicians not masked to study drug – Evaluated EDSS, relapses, safety Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-57
Study 902 - Efficacy Evaluations • Monthly MRI – MRI evaluators masked to study drug and outcome • Monthly clinical evaluations – Treating physicians not masked to study drug – Evaluated EDSS, relapses, safety Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-57
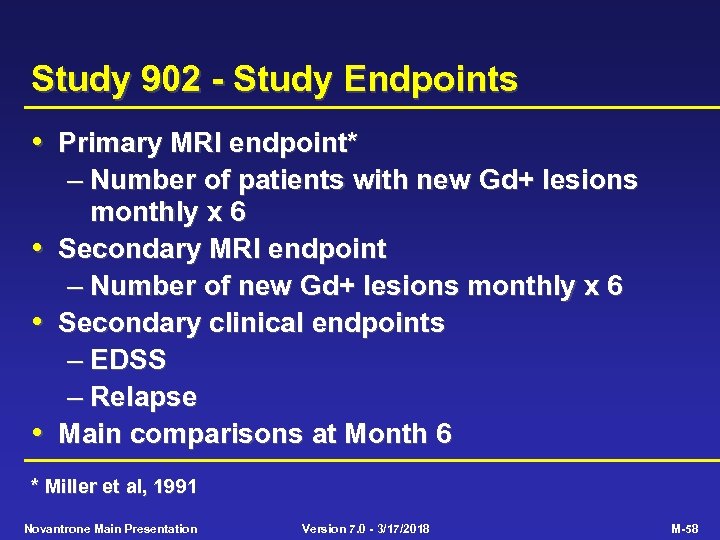 Study 902 - Study Endpoints • Primary MRI endpoint* • • • – Number of patients with new Gd+ lesions monthly x 6 Secondary MRI endpoint – Number of new Gd+ lesions monthly x 6 Secondary clinical endpoints – EDSS – Relapse Main comparisons at Month 6 * Miller et al, 1991 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-58
Study 902 - Study Endpoints • Primary MRI endpoint* • • • – Number of patients with new Gd+ lesions monthly x 6 Secondary MRI endpoint – Number of new Gd+ lesions monthly x 6 Secondary clinical endpoints – EDSS – Relapse Main comparisons at Month 6 * Miller et al, 1991 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-58
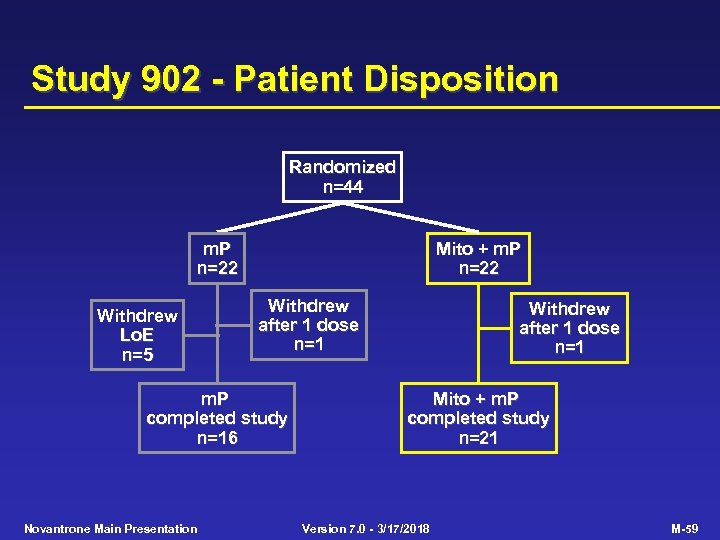 Study 902 - Patient Disposition Randomized n=44 m. P n=22 Withdrew Lo. E n=5 Mito + m. P n=22 Withdrew after 1 dose n=1 m. P completed study n=16 Novantrone Main Presentation Withdrew after 1 dose n=1 Mito + m. P completed study n=21 Version 7. 0 - 3/17/2018 M-59
Study 902 - Patient Disposition Randomized n=44 m. P n=22 Withdrew Lo. E n=5 Mito + m. P n=22 Withdrew after 1 dose n=1 m. P completed study n=16 Novantrone Main Presentation Withdrew after 1 dose n=1 Mito + m. P completed study n=21 Version 7. 0 - 3/17/2018 M-59
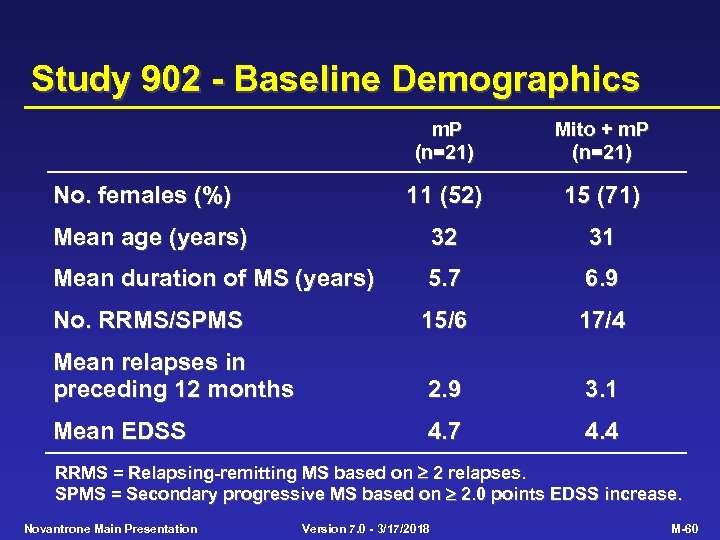 Study 902 - Baseline Demographics m. P (n=21) Mito + m. P (n=21) 11 (52) 15 (71) Mean age (years) 32 31 Mean duration of MS (years) 5. 7 6. 9 No. RRMS/SPMS 15/6 17/4 Mean relapses in preceding 12 months 2. 9 3. 1 Mean EDSS 4. 7 4. 4 No. females (%) RRMS = Relapsing-remitting MS based on 2 relapses. SPMS = Secondary progressive MS based on 2. 0 points EDSS increase. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-60
Study 902 - Baseline Demographics m. P (n=21) Mito + m. P (n=21) 11 (52) 15 (71) Mean age (years) 32 31 Mean duration of MS (years) 5. 7 6. 9 No. RRMS/SPMS 15/6 17/4 Mean relapses in preceding 12 months 2. 9 3. 1 Mean EDSS 4. 7 4. 4 No. females (%) RRMS = Relapsing-remitting MS based on 2 relapses. SPMS = Secondary progressive MS based on 2. 0 points EDSS increase. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-60
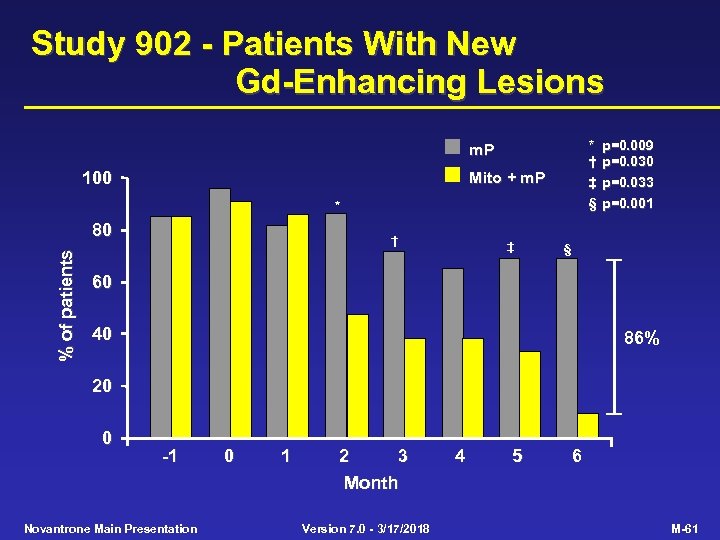 Study 902 - Patients With New Gd-Enhancing Lesions * † ‡ § m. P 100 Mito + m. P * % of patients 80 † ‡ p=0. 009 p=0. 030 p=0. 033 p=0. 001 § 60 40 86% 20 0 -1 Novantrone Main Presentation 0 1 2 3 Month Version 7. 0 - 3/17/2018 4 5 6 M-61
Study 902 - Patients With New Gd-Enhancing Lesions * † ‡ § m. P 100 Mito + m. P * % of patients 80 † ‡ p=0. 009 p=0. 030 p=0. 033 p=0. 001 § 60 40 86% 20 0 -1 Novantrone Main Presentation 0 1 2 3 Month Version 7. 0 - 3/17/2018 4 5 6 M-61
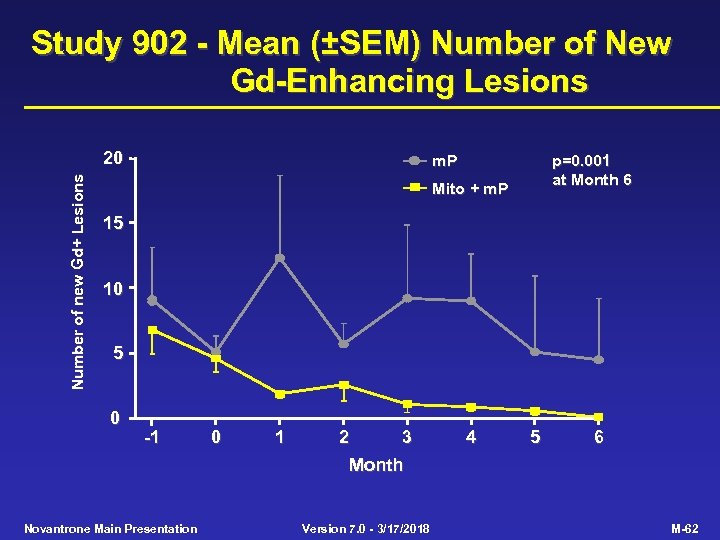 Study 902 - Mean (±SEM) Number of New Gd-Enhancing Lesions Number of new Gd+ Lesions 20 m. P p=0. 001 at Month 6 Mito + m. P 15 10 5 0 -1 0 1 2 3 4 5 6 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-62
Study 902 - Mean (±SEM) Number of New Gd-Enhancing Lesions Number of new Gd+ Lesions 20 m. P p=0. 001 at Month 6 Mito + m. P 15 10 5 0 -1 0 1 2 3 4 5 6 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-62
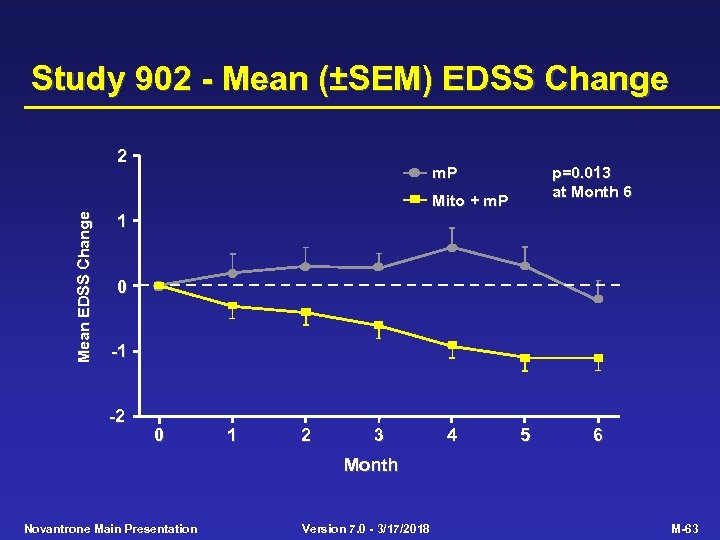 Study 902 - Mean (±SEM) EDSS Change 2 m. P p=0. 013 at Month 6 Mean EDSS Change Mito + m. P 1 0 -1 -2 0 1 2 3 4 5 6 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-63
Study 902 - Mean (±SEM) EDSS Change 2 m. P p=0. 013 at Month 6 Mean EDSS Change Mito + m. P 1 0 -1 -2 0 1 2 3 4 5 6 Month Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-63
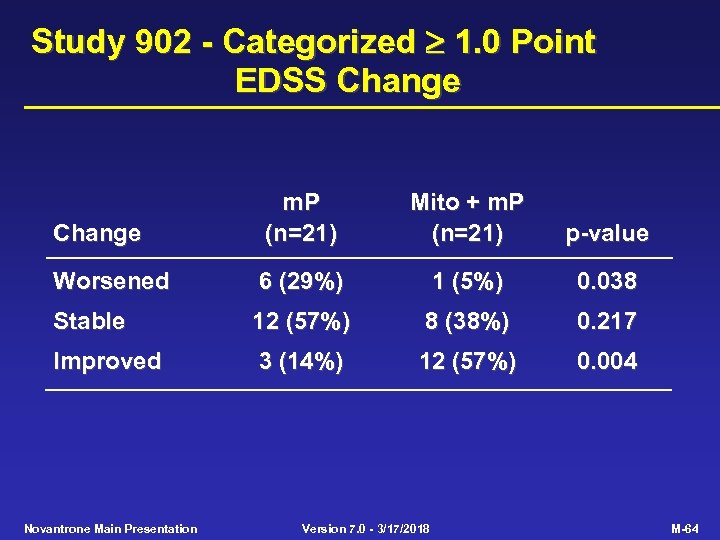 Study 902 - Categorized 1. 0 Point EDSS Change m. P (n=21) Mito + m. P (n=21) p-value Worsened 6 (29%) 1 (5%) 0. 038 Stable 12 (57%) 8 (38%) 0. 217 Improved 3 (14%) 12 (57%) 0. 004 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-64
Study 902 - Categorized 1. 0 Point EDSS Change m. P (n=21) Mito + m. P (n=21) p-value Worsened 6 (29%) 1 (5%) 0. 038 Stable 12 (57%) 8 (38%) 0. 217 Improved 3 (14%) 12 (57%) 0. 004 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-64
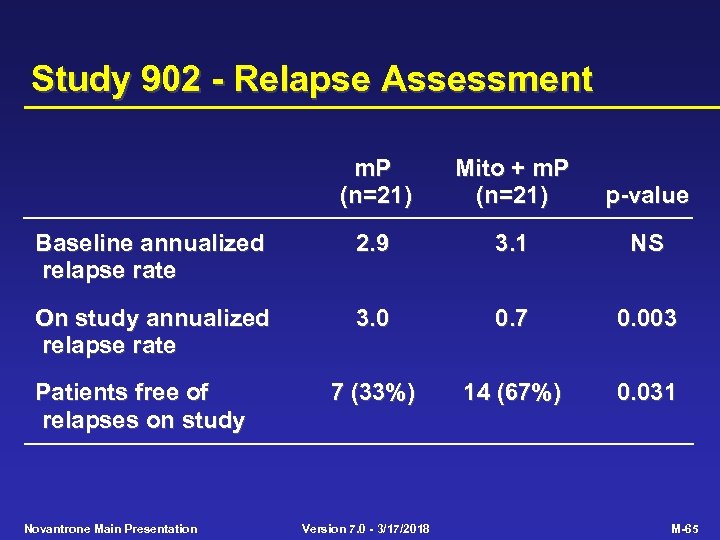 Study 902 - Relapse Assessment m. P (n=21) Mito + m. P (n=21) p-value Baseline annualized relapse rate 2. 9 3. 1 NS On study annualized relapse rate 3. 0 0. 7 0. 003 7 (33%) 14 (67%) 0. 031 Patients free of relapses on study Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-65
Study 902 - Relapse Assessment m. P (n=21) Mito + m. P (n=21) p-value Baseline annualized relapse rate 2. 9 3. 1 NS On study annualized relapse rate 3. 0 0. 7 0. 003 7 (33%) 14 (67%) 0. 031 Patients free of relapses on study Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-65
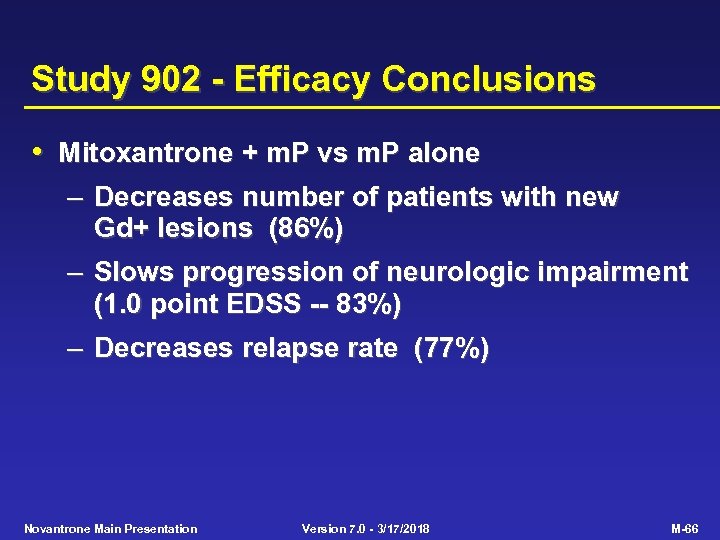 Study 902 - Efficacy Conclusions • Mitoxantrone + m. P vs m. P alone – Decreases number of patients with new Gd+ lesions (86%) – Slows progression of neurologic impairment (1. 0 point EDSS -- 83%) – Decreases relapse rate (77%) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-66
Study 902 - Efficacy Conclusions • Mitoxantrone + m. P vs m. P alone – Decreases number of patients with new Gd+ lesions (86%) – Slows progression of neurologic impairment (1. 0 point EDSS -- 83%) – Decreases relapse rate (77%) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-66
 Mitoxantrone Safety Data Novantrone Main Presentation Version 7. 0 - M-
Mitoxantrone Safety Data Novantrone Main Presentation Version 7. 0 - M-
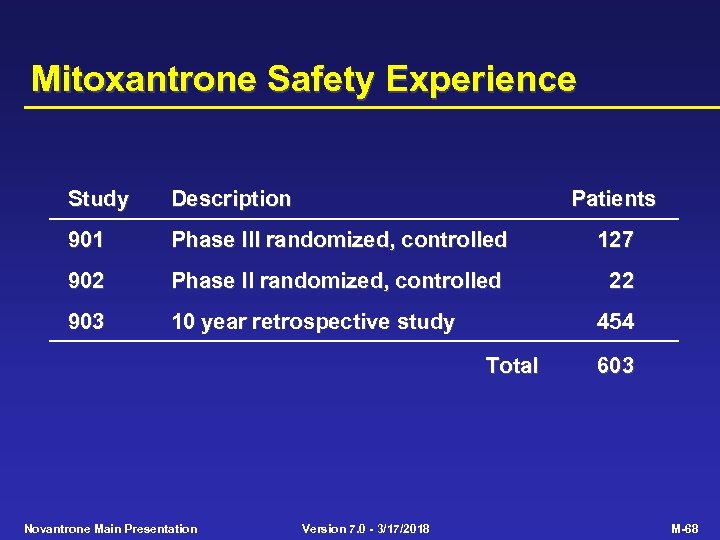 Mitoxantrone Safety Experience Study Description Patients 901 Phase III randomized, controlled 127 902 Phase II randomized, controlled 22 903 10 year retrospective study 454 Total Novantrone Main Presentation Version 7. 0 - 3/17/2018 603 M-68
Mitoxantrone Safety Experience Study Description Patients 901 Phase III randomized, controlled 127 902 Phase II randomized, controlled 22 903 10 year retrospective study 454 Total Novantrone Main Presentation Version 7. 0 - 3/17/2018 603 M-68
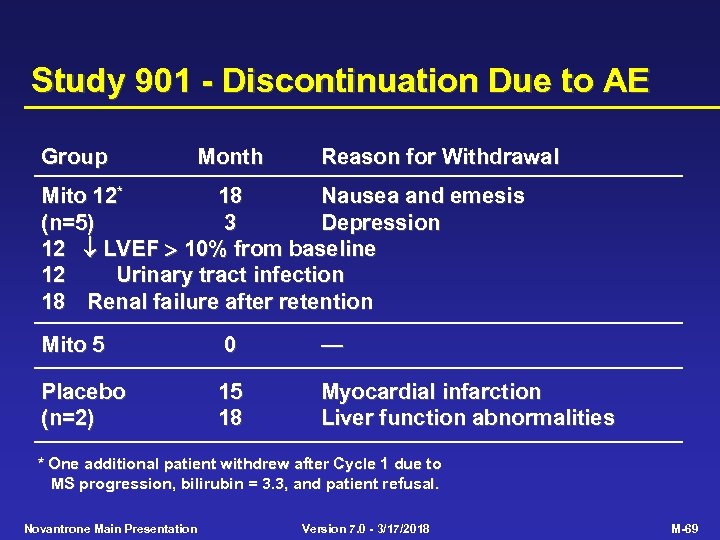 Study 901 - Discontinuation Due to AE Group Month Reason for Withdrawal Mito 12* 18 Nausea and emesis (n=5) 3 Depression 12 LVEF 10% from baseline 12 Urinary tract infection 18 Renal failure after retention Mito 5 0 — Placebo (n=2) 15 18 Myocardial infarction Liver function abnormalities * One additional patient withdrew after Cycle 1 due to MS progression, bilirubin = 3. 3, and patient refusal. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-69
Study 901 - Discontinuation Due to AE Group Month Reason for Withdrawal Mito 12* 18 Nausea and emesis (n=5) 3 Depression 12 LVEF 10% from baseline 12 Urinary tract infection 18 Renal failure after retention Mito 5 0 — Placebo (n=2) 15 18 Myocardial infarction Liver function abnormalities * One additional patient withdrew after Cycle 1 due to MS progression, bilirubin = 3. 3, and patient refusal. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-69
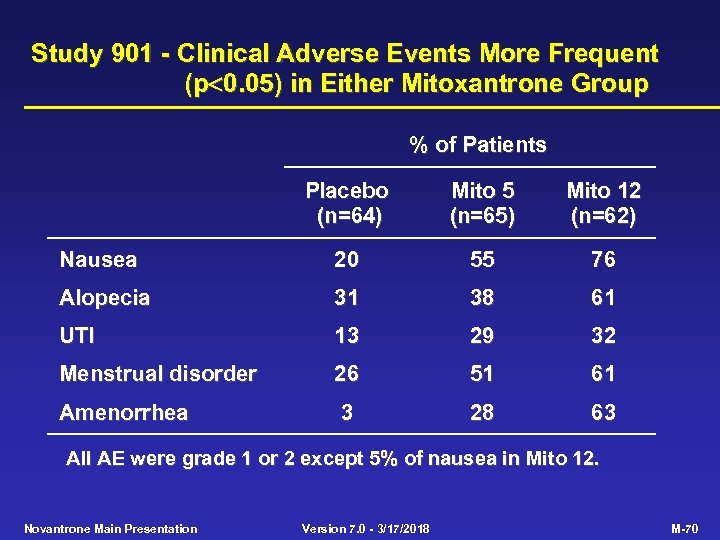 Study 901 - Clinical Adverse Events More Frequent (p 0. 05) in Either Mitoxantrone Group % of Patients Placebo (n=64) Mito 5 (n=65) Mito 12 (n=62) Nausea 20 55 76 Alopecia 31 38 61 UTI 13 29 32 Menstrual disorder 26 51 61 Amenorrhea 3 28 63 All AE were grade 1 or 2 except 5% of nausea in Mito 12. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-70
Study 901 - Clinical Adverse Events More Frequent (p 0. 05) in Either Mitoxantrone Group % of Patients Placebo (n=64) Mito 5 (n=65) Mito 12 (n=62) Nausea 20 55 76 Alopecia 31 38 61 UTI 13 29 32 Menstrual disorder 26 51 61 Amenorrhea 3 28 63 All AE were grade 1 or 2 except 5% of nausea in Mito 12. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-70
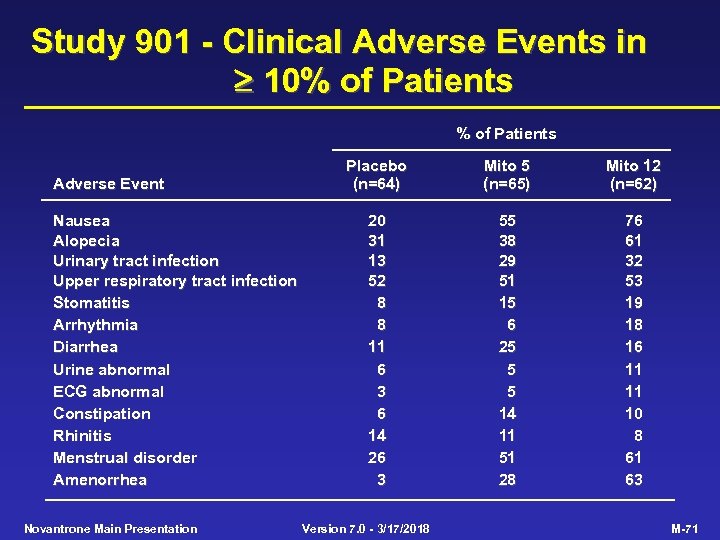 Study 901 - Clinical Adverse Events in 10% of Patients Adverse Event Nausea Alopecia Urinary tract infection Upper respiratory tract infection Stomatitis Arrhythmia Diarrhea Urine abnormal ECG abnormal Constipation Rhinitis Menstrual disorder Amenorrhea Novantrone Main Presentation Placebo (n=64) Mito 5 (n=65) Mito 12 (n=62) 20 31 13 52 8 8 11 6 3 6 14 26 3 55 38 29 51 15 6 25 5 5 14 11 51 28 76 61 32 53 19 18 16 11 11 10 8 61 63 Version 7. 0 - 3/17/2018 M-71
Study 901 - Clinical Adverse Events in 10% of Patients Adverse Event Nausea Alopecia Urinary tract infection Upper respiratory tract infection Stomatitis Arrhythmia Diarrhea Urine abnormal ECG abnormal Constipation Rhinitis Menstrual disorder Amenorrhea Novantrone Main Presentation Placebo (n=64) Mito 5 (n=65) Mito 12 (n=62) 20 31 13 52 8 8 11 6 3 6 14 26 3 55 38 29 51 15 6 25 5 5 14 11 51 28 76 61 32 53 19 18 16 11 11 10 8 61 63 Version 7. 0 - 3/17/2018 M-71
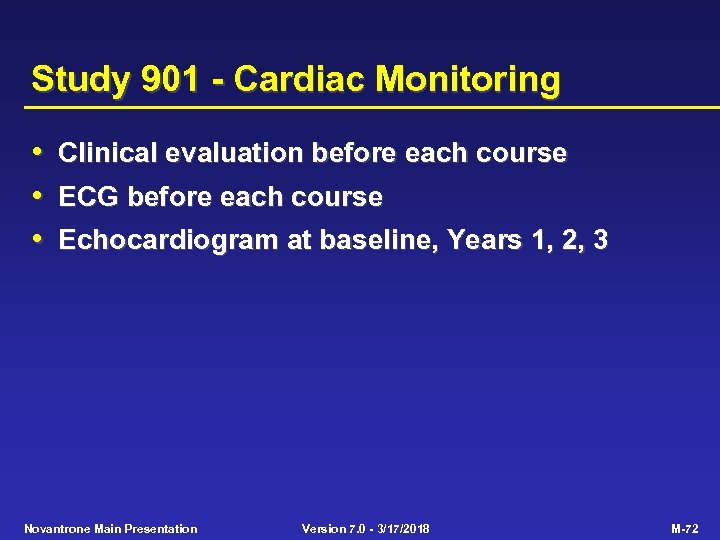 Study 901 - Cardiac Monitoring • Clinical evaluation before each course • ECG before each course • Echocardiogram at baseline, Years 1, 2, 3 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-72
Study 901 - Cardiac Monitoring • Clinical evaluation before each course • ECG before each course • Echocardiogram at baseline, Years 1, 2, 3 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-72
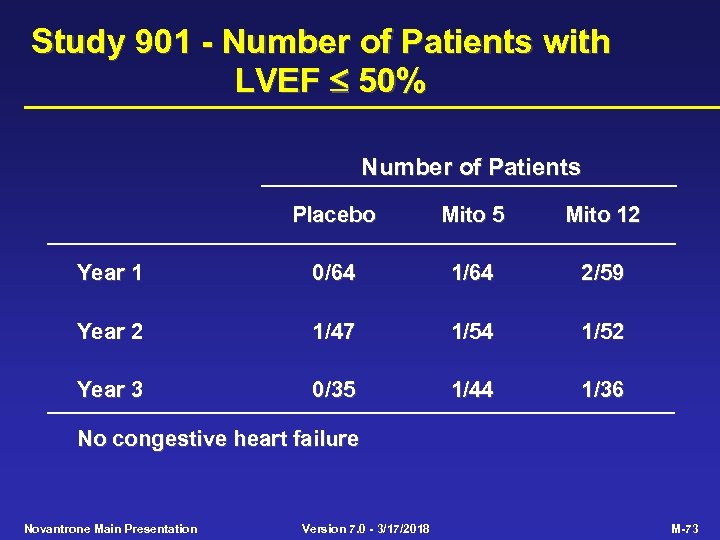 Study 901 - Number of Patients with LVEF 50% Number of Patients Placebo Mito 5 Mito 12 Year 1 0/64 1/64 2/59 Year 2 1/47 1/54 1/52 Year 3 0/35 1/44 1/36 No congestive heart failure Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-73
Study 901 - Number of Patients with LVEF 50% Number of Patients Placebo Mito 5 Mito 12 Year 1 0/64 1/64 2/59 Year 2 1/47 1/54 1/52 Year 3 0/35 1/44 1/36 No congestive heart failure Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-73
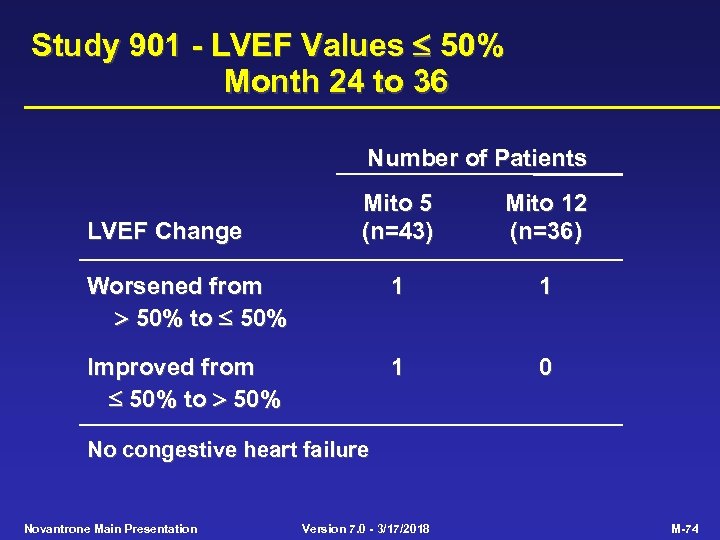 Study 901 - LVEF Values 50% Month 24 to 36 Number of Patients Mito 5 (n=43) Mito 12 (n=36) Worsened from 50% to 50% 1 1 Improved from 50% to 50% 1 0 LVEF Change No congestive heart failure Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-74
Study 901 - LVEF Values 50% Month 24 to 36 Number of Patients Mito 5 (n=43) Mito 12 (n=36) Worsened from 50% to 50% 1 1 Improved from 50% to 50% 1 0 LVEF Change No congestive heart failure Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-74
 Study 901 - Additional Safety Information • No deaths • Pregnancy: 2 reported – 1 placebo - terminated pregnancy – 1 mitoxantrone 12 mg/m 2 - normal child Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-75
Study 901 - Additional Safety Information • No deaths • Pregnancy: 2 reported – 1 placebo - terminated pregnancy – 1 mitoxantrone 12 mg/m 2 - normal child Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-75
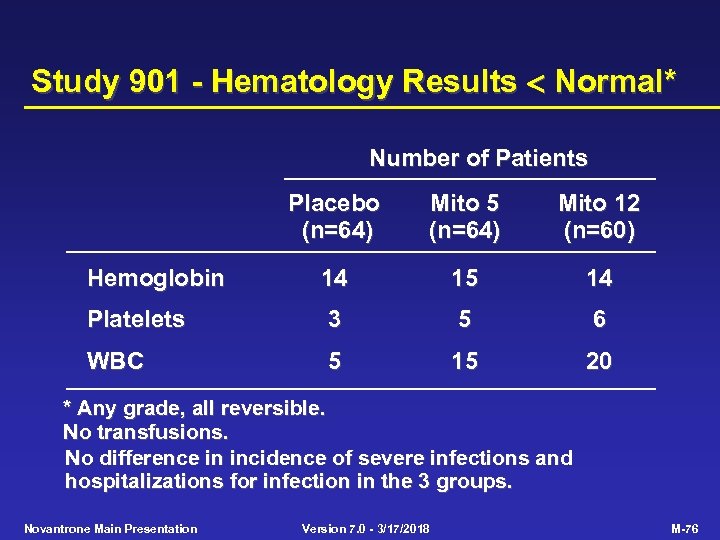 Study 901 - Hematology Results Normal* Number of Patients Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Hemoglobin 14 15 14 Platelets 3 5 6 WBC 5 15 20 * Any grade, all reversible. No transfusions. No difference in incidence of severe infections and hospitalizations for infection in the 3 groups. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-76
Study 901 - Hematology Results Normal* Number of Patients Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Hemoglobin 14 15 14 Platelets 3 5 6 WBC 5 15 20 * Any grade, all reversible. No transfusions. No difference in incidence of severe infections and hospitalizations for infection in the 3 groups. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-76
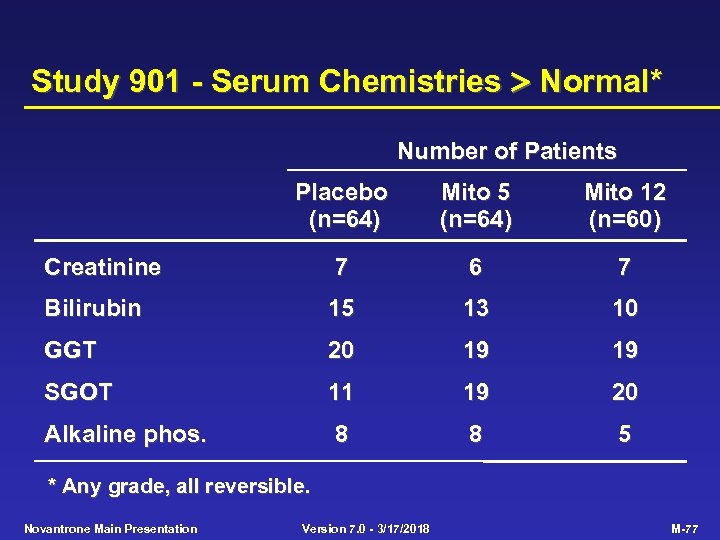 Study 901 - Serum Chemistries Normal* Number of Patients Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Creatinine 7 6 7 Bilirubin 15 13 10 GGT 20 19 19 SGOT 11 19 20 Alkaline phos. 8 8 5 * Any grade, all reversible. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-77
Study 901 - Serum Chemistries Normal* Number of Patients Placebo (n=64) Mito 5 (n=64) Mito 12 (n=60) Creatinine 7 6 7 Bilirubin 15 13 10 GGT 20 19 19 SGOT 11 19 20 Alkaline phos. 8 8 5 * Any grade, all reversible. Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-77
 Study 902 - Adverse Events • Profile similar to Study 901 • 3 adverse events considered severe (amenorrhea, depression/anorexia, contact lens intolerance) • No deaths on study • No cardiac toxicity (echocardiogram at baseline and M 6) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-78
Study 902 - Adverse Events • Profile similar to Study 901 • 3 adverse events considered severe (amenorrhea, depression/anorexia, contact lens intolerance) • No deaths on study • No cardiac toxicity (echocardiogram at baseline and M 6) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-78
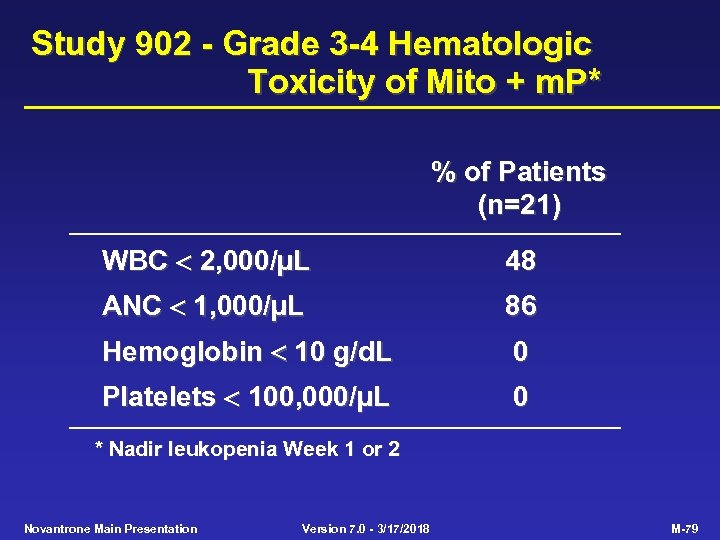 Study 902 - Grade 3 -4 Hematologic Toxicity of Mito + m. P* % of Patients (n=21) WBC 2, 000/µL 48 ANC 1, 000/µL 86 Hemoglobin 10 g/d. L 0 Platelets 100, 000/µL 0 * Nadir leukopenia Week 1 or 2 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-79
Study 902 - Grade 3 -4 Hematologic Toxicity of Mito + m. P* % of Patients (n=21) WBC 2, 000/µL 48 ANC 1, 000/µL 86 Hemoglobin 10 g/d. L 0 Platelets 100, 000/µL 0 * Nadir leukopenia Week 1 or 2 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-79
 Study 903 - Long Term Safety Database • • • Academic Clinic of Ulm University, Germany Professor Erich Mauch Retrospective analysis Mitoxantrone given between 11/88 and 9/98 454 patients All patients included ECTRIM - 1999 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-80
Study 903 - Long Term Safety Database • • • Academic Clinic of Ulm University, Germany Professor Erich Mauch Retrospective analysis Mitoxantrone given between 11/88 and 9/98 454 patients All patients included ECTRIM - 1999 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-80
 Study 903 - Data Collection and Quality Assurance • Staff hired exclusively for data collection • Collection of specified safety and efficacy data • Attempt to obtain most recent follow-up • Information on treatment off site not collected • All CRFs reviewed by Dr. Mauch for accuracy Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-81
Study 903 - Data Collection and Quality Assurance • Staff hired exclusively for data collection • Collection of specified safety and efficacy data • Attempt to obtain most recent follow-up • Information on treatment off site not collected • All CRFs reviewed by Dr. Mauch for accuracy Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-81
 Study 903 - Treatment Guidelines • Mitoxantrone 12 mg/m 2 every 3 months • Dose adjustment or interval change as needed • Treatment discontinued if: – Not tolerated – Good disease response – Attempt to become pregnant Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-82
Study 903 - Treatment Guidelines • Mitoxantrone 12 mg/m 2 every 3 months • Dose adjustment or interval change as needed • Treatment discontinued if: – Not tolerated – Good disease response – Attempt to become pregnant Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-82
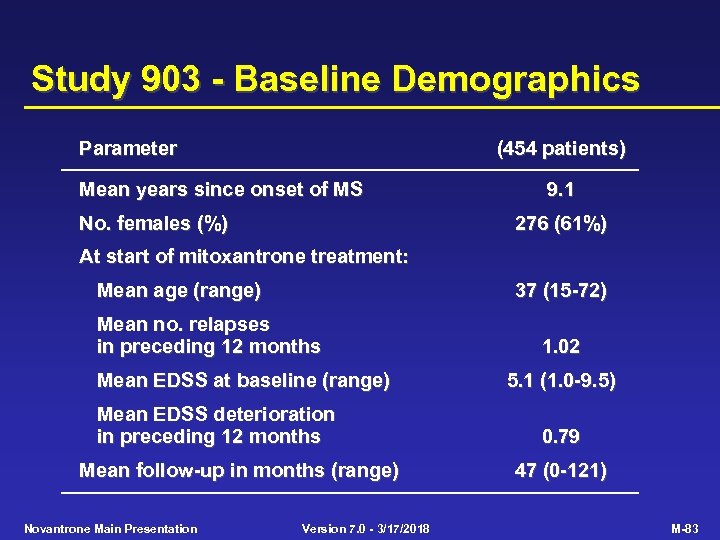 Study 903 - Baseline Demographics Parameter (454 patients) Mean years since onset of MS No. females (%) 9. 1 276 (61%) At start of mitoxantrone treatment: Mean age (range) 37 (15 -72) Mean no. relapses in preceding 12 months Mean EDSS at baseline (range) Mean EDSS deterioration in preceding 12 months Mean follow-up in months (range) Novantrone Main Presentation Version 7. 0 - 3/17/2018 1. 02 5. 1 (1. 0 -9. 5) 0. 79 47 (0 -121) M-83
Study 903 - Baseline Demographics Parameter (454 patients) Mean years since onset of MS No. females (%) 9. 1 276 (61%) At start of mitoxantrone treatment: Mean age (range) 37 (15 -72) Mean no. relapses in preceding 12 months Mean EDSS at baseline (range) Mean EDSS deterioration in preceding 12 months Mean follow-up in months (range) Novantrone Main Presentation Version 7. 0 - 3/17/2018 1. 02 5. 1 (1. 0 -9. 5) 0. 79 47 (0 -121) M-83
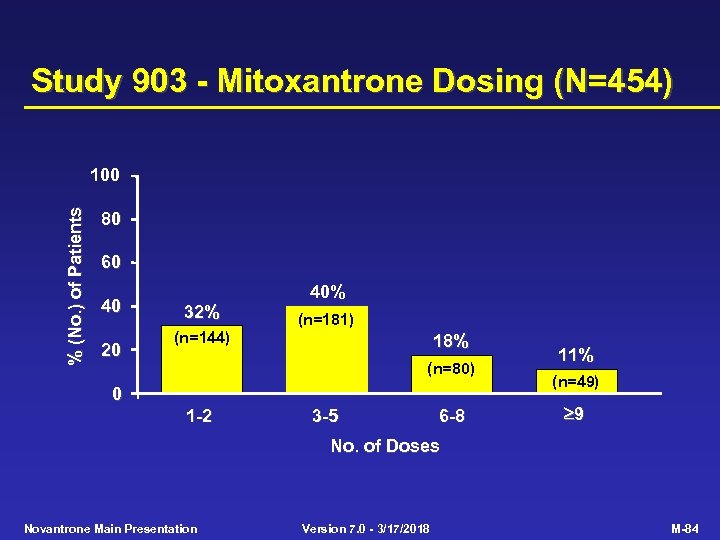 Study 903 - Mitoxantrone Dosing (N=454) % (No. ) of Patients 100 80 60 40 20 32% 40% (n=181) (n=144) 18% (n=80) 0 1 -2 3 -5 6 -8 11% (n=49) 9 No. of Doses Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-84
Study 903 - Mitoxantrone Dosing (N=454) % (No. ) of Patients 100 80 60 40 20 32% 40% (n=181) (n=144) 18% (n=80) 0 1 -2 3 -5 6 -8 11% (n=49) 9 No. of Doses Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-84
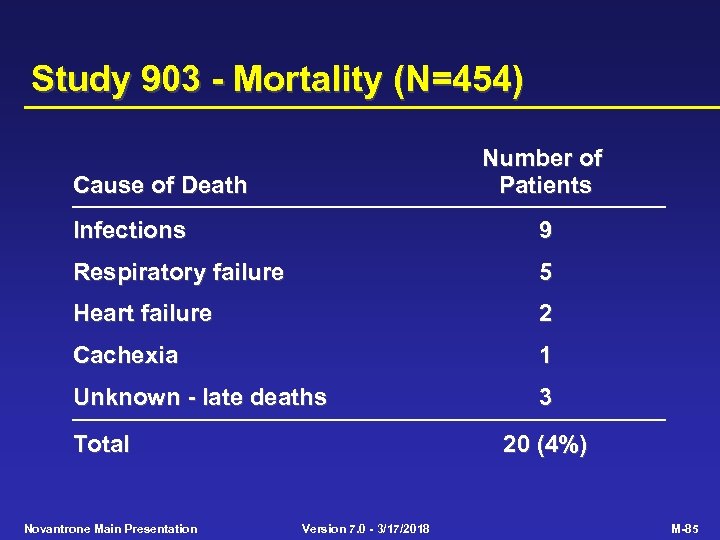 Study 903 - Mortality (N=454) Number of Patients Cause of Death Infections 9 Respiratory failure 5 Heart failure 2 Cachexia 1 Unknown - late deaths 3 Total Novantrone Main Presentation 20 (4%) Version 7. 0 - 3/17/2018 M-85
Study 903 - Mortality (N=454) Number of Patients Cause of Death Infections 9 Respiratory failure 5 Heart failure 2 Cachexia 1 Unknown - late deaths 3 Total Novantrone Main Presentation 20 (4%) Version 7. 0 - 3/17/2018 M-85
 Study 903 - Pregnancies • Pregnancies – 4 during mitoxantrone treatment – 5 after discontinuing treatment • Births – 6 normal – 1 pregnant at data collection – 2 outcomes not available Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-86
Study 903 - Pregnancies • Pregnancies – 4 during mitoxantrone treatment – 5 after discontinuing treatment • Births – 6 normal – 1 pregnant at data collection – 2 outcomes not available Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-86
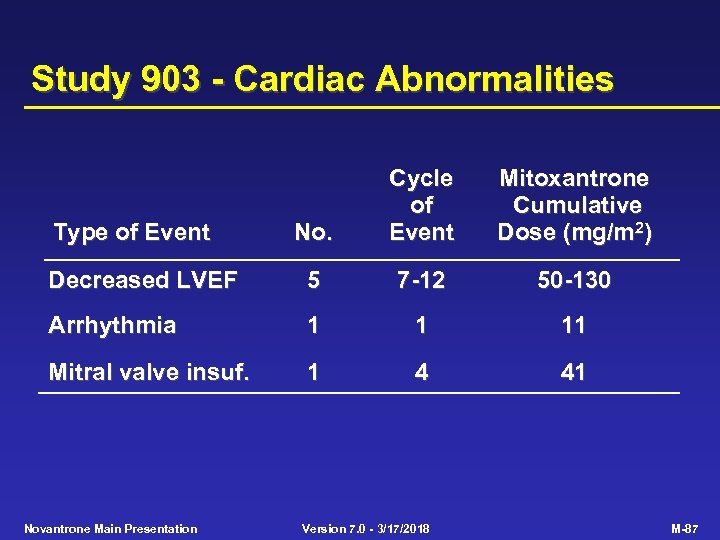 Study 903 - Cardiac Abnormalities No. Cycle of Event Mitoxantrone Cumulative Dose (mg/m 2) Decreased LVEF 5 7 -12 50 -130 Arrhythmia 1 1 11 Mitral valve insuf. 1 4 41 Type of Event Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-87
Study 903 - Cardiac Abnormalities No. Cycle of Event Mitoxantrone Cumulative Dose (mg/m 2) Decreased LVEF 5 7 -12 50 -130 Arrhythmia 1 1 11 Mitral valve insuf. 1 4 41 Type of Event Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-87
 Mitoxantrone Cardiac Effects in Cancer Patients* • Mitoxantrone given every 3 -4 weeks • Often with other chemotherapy/chest radiation • At cumulative dose of 140 mg/m 2 – – Congestive heart failure = 2. 6% Moderate to severe LVEF = 13% • Occur during or within a year of completing mitoxantrone therapy * Published literature and Immunex databases Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-88
Mitoxantrone Cardiac Effects in Cancer Patients* • Mitoxantrone given every 3 -4 weeks • Often with other chemotherapy/chest radiation • At cumulative dose of 140 mg/m 2 – – Congestive heart failure = 2. 6% Moderate to severe LVEF = 13% • Occur during or within a year of completing mitoxantrone therapy * Published literature and Immunex databases Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-88
 Mitoxantrone Safety Conclusions • Adverse events mild or moderate • Manageable and reversible • Dose up to 100 mg/m 2 in MS trials not associated with congestive heart failure • Myelosuppression – Reversible within 1 - 3 weeks – Not associated with severe infection • No secondary leukemia or myelodysplasia Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-89
Mitoxantrone Safety Conclusions • Adverse events mild or moderate • Manageable and reversible • Dose up to 100 mg/m 2 in MS trials not associated with congestive heart failure • Myelosuppression – Reversible within 1 - 3 weeks – Not associated with severe infection • No secondary leukemia or myelodysplasia Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-89
 Risk & Benefit Assessment • Well characterized adverse events – Monitoring – Management • Clear benefit in progressive MS • Benefits outweigh risks – Disease stage without satisfactory therapy Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-90
Risk & Benefit Assessment • Well characterized adverse events – Monitoring – Management • Clear benefit in progressive MS • Benefits outweigh risks – Disease stage without satisfactory therapy Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-90
 Acute Adverse Events • • Mild to moderate intensity Nausea and emesis: transient, manageable Alopecia mild, reversible Leukopenia – Grade 3 -4 in 50% of patients – Between day 7 -14 – Reversible by day 21 – Risk of neutropenic fever low Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-91
Acute Adverse Events • • Mild to moderate intensity Nausea and emesis: transient, manageable Alopecia mild, reversible Leukopenia – Grade 3 -4 in 50% of patients – Between day 7 -14 – Reversible by day 21 – Risk of neutropenic fever low Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-91
 Laboratory Monitoring • Serum chemistries (including LFT) – Before each course • Hemogram – Before each course – If fever develops at expected leukocyte nadir Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-92
Laboratory Monitoring • Serum chemistries (including LFT) – Before each course • Hemogram – Before each course – If fever develops at expected leukocyte nadir Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-92
 Cardiac Monitoring • LVEF assessment • • – At baseline – At cumulative dose of 100 mg/m 2 Assess risk/benefit and monitor LVEF before each course if: – Cumulative dose 100 mg/m 2 – LVEF decreases by 15% from baseline Discontinue therapy if: – LVEF 50% – Cumulative dose 140 mg/m 2 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-93
Cardiac Monitoring • LVEF assessment • • – At baseline – At cumulative dose of 100 mg/m 2 Assess risk/benefit and monitor LVEF before each course if: – Cumulative dose 100 mg/m 2 – LVEF decreases by 15% from baseline Discontinue therapy if: – LVEF 50% – Cumulative dose 140 mg/m 2 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-93
 Pregnancy • Mitoxantrone should not be used in pregnant women or in women attempting to become pregnant (already in label) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-94
Pregnancy • Mitoxantrone should not be used in pregnant women or in women attempting to become pregnant (already in label) Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-94
 Benefit of Mitoxantrone - Study 901 • Slows progression of neurologic impairment – Decreases 1. 0 EDSS progression by 64% • Reduces relapses – Decreases treated relapses by 69% • Lessens CNS lesions – Substantial decrease in number of patients with Gd-enhanced lesions – Slows progression of T 2 w lesions Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-95
Benefit of Mitoxantrone - Study 901 • Slows progression of neurologic impairment – Decreases 1. 0 EDSS progression by 64% • Reduces relapses – Decreases treated relapses by 69% • Lessens CNS lesions – Substantial decrease in number of patients with Gd-enhanced lesions – Slows progression of T 2 w lesions Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-95
 Benefit of Mitoxantrone Supportive Studies • Study 902 (m. P vs Mitoxantrone + m. P) – Slows progression of neurologic impairment (EDSS - 83%) – Decreases relapse rate (77%) – Decreases number of patients with new Gd+ lesions (86%) • Study 903 – Therapy feasible in practice setting Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-96
Benefit of Mitoxantrone Supportive Studies • Study 902 (m. P vs Mitoxantrone + m. P) – Slows progression of neurologic impairment (EDSS - 83%) – Decreases relapse rate (77%) – Decreases number of patients with new Gd+ lesions (86%) • Study 903 – Therapy feasible in practice setting Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-96
 Discussion Novantrone Main Presentation Version 7. 0 - M-
Discussion Novantrone Main Presentation Version 7. 0 - M-
 Adequacy of Controlled Study 901 • Well designed, randomized, placebo • • • controlled Prospectively defined entry criteria, endpoints, number of patients, and statistical analyses Effect on disability and relapse using established scales MRI prospectively limited to subset Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-98
Adequacy of Controlled Study 901 • Well designed, randomized, placebo • • • controlled Prospectively defined entry criteria, endpoints, number of patients, and statistical analyses Effect on disability and relapse using established scales MRI prospectively limited to subset Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-98
 Adequacy of Controlled Study 902 • Well designed, randomized, controlled • Prospectively defined entry criteria, endpoints • Design typical of MRI-based trial • Effect on disability and relapse using established scales Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-99
Adequacy of Controlled Study 902 • Well designed, randomized, controlled • Prospectively defined entry criteria, endpoints • Design typical of MRI-based trial • Effect on disability and relapse using established scales Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-99
 Mitoxantrone Slows Progression of Neurologic Disability - Study 901 • • Used 3 complementary disability scales All 3 primary disability endpoints met Secondary disability endpoints met No rebound one year after stopping therapy Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-100
Mitoxantrone Slows Progression of Neurologic Disability - Study 901 • • Used 3 complementary disability scales All 3 primary disability endpoints met Secondary disability endpoints met No rebound one year after stopping therapy Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-100
 Mitoxantrone Slows Progression of Neurologic Disability - Study 902 • EDSS assessment unmasked • Results robust – Significant difference between mitoxantrone and control – Consistency of EDSS results in the 2 randomized studies Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-101
Mitoxantrone Slows Progression of Neurologic Disability - Study 902 • EDSS assessment unmasked • Results robust – Significant difference between mitoxantrone and control – Consistency of EDSS results in the 2 randomized studies Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-101
 Mitoxantrone Decreases Relapse • Relapse definition/severity – prospectively defined in both studies • Consistency of results in the 2 studies • Highly significant vs control group • Consistency in all relapse evaluations Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-102
Mitoxantrone Decreases Relapse • Relapse definition/severity – prospectively defined in both studies • Consistency of results in the 2 studies • Highly significant vs control group • Consistency in all relapse evaluations Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-102
 MRI Results - Study 901 • MRI evaluations – In subset of patients – No stratification by baseline MRI – Study not sized for MRI endpoints • Decreased Gd+ lesions – Consistent with clinical results – Suggests biologic effect Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-103
MRI Results - Study 901 • MRI evaluations – In subset of patients – No stratification by baseline MRI – Study not sized for MRI endpoints • Decreased Gd+ lesions – Consistent with clinical results – Suggests biologic effect Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-103
 MRI Results - Study 902 • • • Design typical of MRI-based trial Nine monthly scans per patient MRI findings – Highly significant – Consistent with clinical results – Indicate mitoxantrone biologic effect • Consistent MRI results in both studies Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-104
MRI Results - Study 902 • • • Design typical of MRI-based trial Nine monthly scans per patient MRI findings – Highly significant – Consistent with clinical results – Indicate mitoxantrone biologic effect • Consistent MRI results in both studies Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-104
 Dose - 12 mg/m 2 Every 3 Months • Investigational dose in Study 901 • Significantly better than placebo for all primary and most secondary variables • Dose of 20 mg (Study 902) 12 mg/m 2 • Substantial safety information in Study 901, 902, 903, and oncology safety databases Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-105
Dose - 12 mg/m 2 Every 3 Months • Investigational dose in Study 901 • Significantly better than placebo for all primary and most secondary variables • Dose of 20 mg (Study 902) 12 mg/m 2 • Substantial safety information in Study 901, 902, 903, and oncology safety databases Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-105
 Target Population • Patients with progressive forms of MS excluding primary progressive MS Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-106
Target Population • Patients with progressive forms of MS excluding primary progressive MS Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-106
 Mitoxantrone in Multiple Sclerosis: A Clinician’s Perspective Fred D. Lublin, M. D. Professor of Neurology MCP Hahnemann University Novantrone Main Presentation Version 7. 0 - M-
Mitoxantrone in Multiple Sclerosis: A Clinician’s Perspective Fred D. Lublin, M. D. Professor of Neurology MCP Hahnemann University Novantrone Main Presentation Version 7. 0 - M-
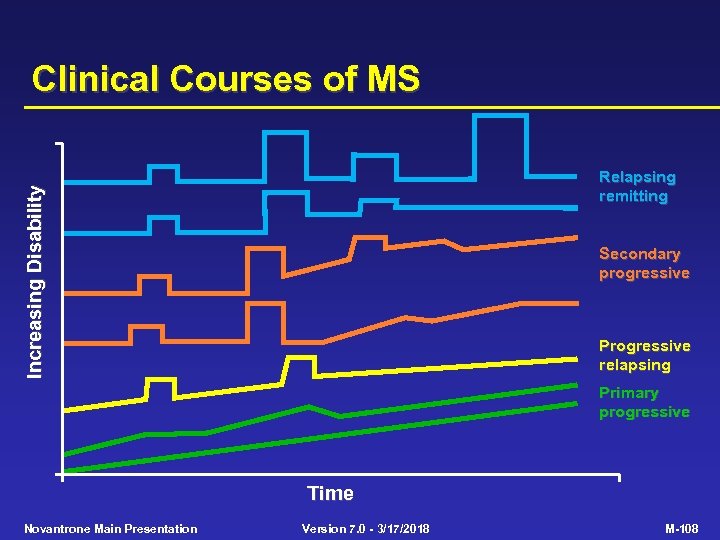 Clinical Courses of MS Increasing Disability Relapsing remitting Secondary progressive Progressive relapsing Primary progressive Time Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-108
Clinical Courses of MS Increasing Disability Relapsing remitting Secondary progressive Progressive relapsing Primary progressive Time Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-108
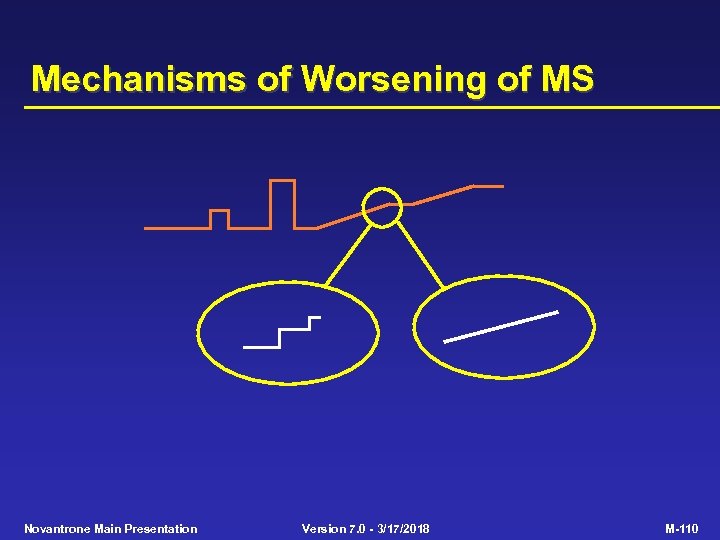
 Mechanisms of Worsening of MS • Relapsing-remitting disease with incomplete recovery (step-wise worsening) • Gradual, progressive worsening independent of relapses Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-109
Mechanisms of Worsening of MS • Relapsing-remitting disease with incomplete recovery (step-wise worsening) • Gradual, progressive worsening independent of relapses Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-109
 Mechanisms of Worsening of MS Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-110
Mechanisms of Worsening of MS Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-110
 Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-111
Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-111
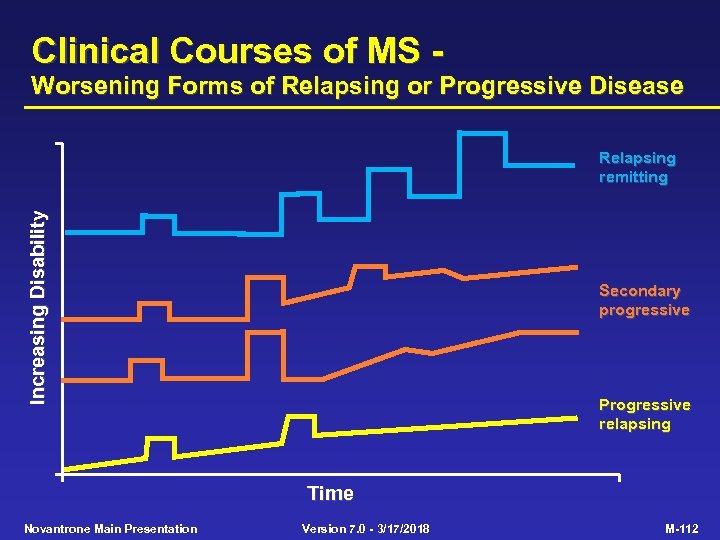 Clinical Courses of MS - Worsening Forms of Relapsing or Progressive Disease Increasing Disability Relapsing remitting Secondary progressive Progressive relapsing Time Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-112
Clinical Courses of MS - Worsening Forms of Relapsing or Progressive Disease Increasing Disability Relapsing remitting Secondary progressive Progressive relapsing Time Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-112
 Conclusions Novantrone Main Presentation Version 7. 0 - M-
Conclusions Novantrone Main Presentation Version 7. 0 - M-
 Effectiveness of Mitoxantrone in MS • Study 901 – Primary endpoint - clinical – MRI data consistent with clinical findings • Study 902 – Primary endpoint - MRI – Clinical data consistent with MRI findings Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-114
Effectiveness of Mitoxantrone in MS • Study 901 – Primary endpoint - clinical – MRI data consistent with clinical findings • Study 902 – Primary endpoint - MRI – Clinical data consistent with MRI findings Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-114
 Proposed Use of Mitoxantrone in MS • To slow progression of neurologic disability and reduce relapse rate • Patients with progressive forms of MS excluding primary progressive MS • Dose 12 mg/m 2 every 3 months • Disease control for 2 -3 years beneficial • Patients with limited therapeutic options Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-115
Proposed Use of Mitoxantrone in MS • To slow progression of neurologic disability and reduce relapse rate • Patients with progressive forms of MS excluding primary progressive MS • Dose 12 mg/m 2 every 3 months • Disease control for 2 -3 years beneficial • Patients with limited therapeutic options Novantrone Main Presentation Version 7. 0 - 3/17/2018 M-115


