d324be376542b5fbe875022e50a1d6d7.ppt
- Количество слайдов: 40
 • Performance of the analytical techniques employed in characterization and certification of stainless steel materials by Ingemar Gustavsson Analytical Chemistry Group Corrosion and Metals Research Institute Drottning Kristinas väg 48 SE 114 28 Stockholm, Sweden E-mail: ingemar. gustavsson@simr. se
• Performance of the analytical techniques employed in characterization and certification of stainless steel materials by Ingemar Gustavsson Analytical Chemistry Group Corrosion and Metals Research Institute Drottning Kristinas väg 48 SE 114 28 Stockholm, Sweden E-mail: ingemar. gustavsson@simr. se
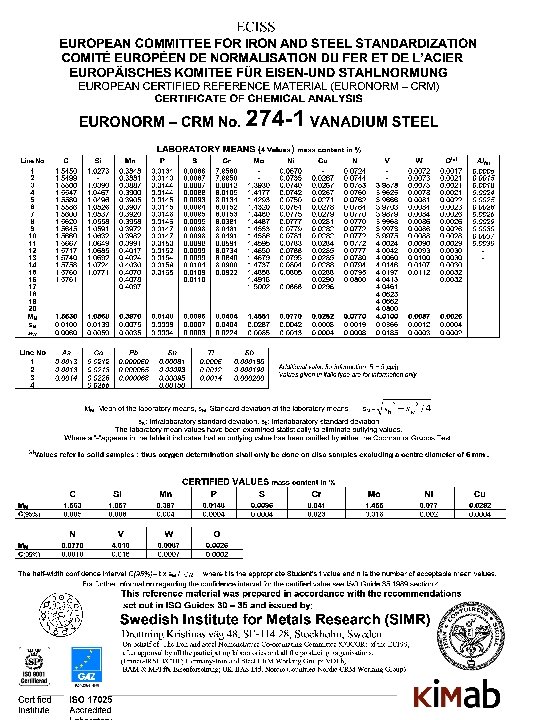
 Definitions of RMs and CRMs according to ISO-Guides 30 -35 ______________ • Reference Material (RM) • A material, sufficiently homogeneous and stable with respect to one or more properties, which have been established to be fit for its intended use in a measurement process • Certified Reference Material (CRM) • An RM characterized by a metrologically valid procedure, accompanied by a certificate that states the value of the specified property, its associated uncertainty, and a statement of metrological traceability
Definitions of RMs and CRMs according to ISO-Guides 30 -35 ______________ • Reference Material (RM) • A material, sufficiently homogeneous and stable with respect to one or more properties, which have been established to be fit for its intended use in a measurement process • Certified Reference Material (CRM) • An RM characterized by a metrologically valid procedure, accompanied by a certificate that states the value of the specified property, its associated uncertainty, and a statement of metrological traceability
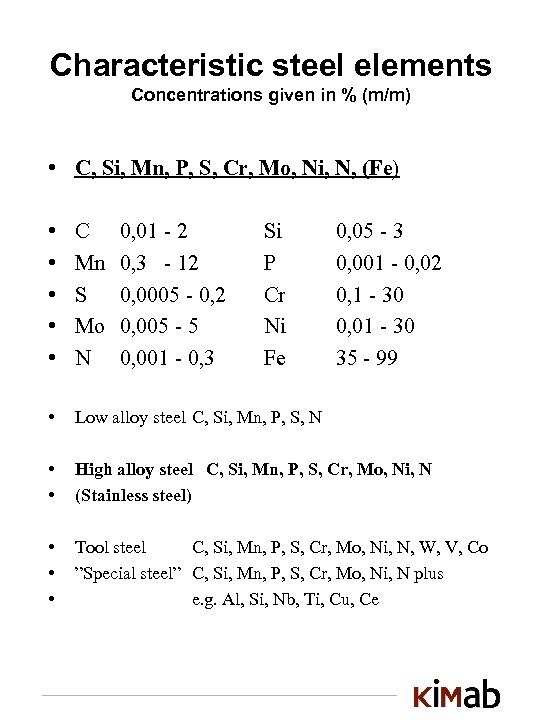 Characteristic steel elements Concentrations given in % (m/m) • C, Si, Mn, P, S, Cr, Mo, Ni, N, (Fe) • • • C Mn S Mo N • Low alloy steel C, Si, Mn, P, S, N • • High alloy steel C, Si, Mn, P, S, Cr, Mo, Ni, N (Stainless steel) • • • Tool steel C, Si, Mn, P, S, Cr, Mo, Ni, N, W, V, Co ”Special steel” C, Si, Mn, P, S, Cr, Mo, Ni, N plus e. g. Al, Si, Nb, Ti, Cu, Ce 0, 01 - 2 0, 3 - 12 0, 0005 - 0, 2 0, 005 - 5 0, 001 - 0, 3 Si P Cr Ni Fe 0, 05 - 3 0, 001 - 0, 02 0, 1 - 30 0, 01 - 30 35 - 99
Characteristic steel elements Concentrations given in % (m/m) • C, Si, Mn, P, S, Cr, Mo, Ni, N, (Fe) • • • C Mn S Mo N • Low alloy steel C, Si, Mn, P, S, N • • High alloy steel C, Si, Mn, P, S, Cr, Mo, Ni, N (Stainless steel) • • • Tool steel C, Si, Mn, P, S, Cr, Mo, Ni, N, W, V, Co ”Special steel” C, Si, Mn, P, S, Cr, Mo, Ni, N plus e. g. Al, Si, Nb, Ti, Cu, Ce 0, 01 - 2 0, 3 - 12 0, 0005 - 0, 2 0, 005 - 5 0, 001 - 0, 3 Si P Cr Ni Fe 0, 05 - 3 0, 001 - 0, 02 0, 1 - 30 0, 01 - 30 35 - 99
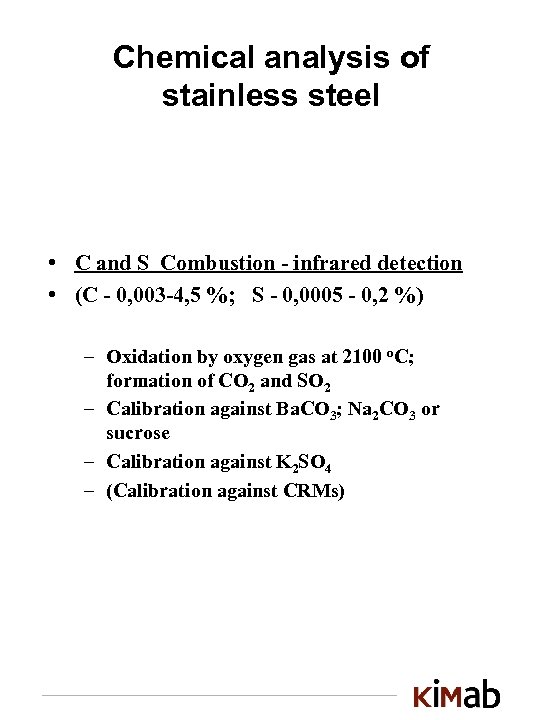 Chemical analysis of stainless steel • C and S Combustion - infrared detection • (C - 0, 003 -4, 5 %; S - 0, 0005 - 0, 2 %) – Oxidation by oxygen gas at 2100 o. C; formation of CO 2 and SO 2 – Calibration against Ba. CO 3; Na 2 CO 3 or sucrose – Calibration against K 2 SO 4 – (Calibration against CRMs)
Chemical analysis of stainless steel • C and S Combustion - infrared detection • (C - 0, 003 -4, 5 %; S - 0, 0005 - 0, 2 %) – Oxidation by oxygen gas at 2100 o. C; formation of CO 2 and SO 2 – Calibration against Ba. CO 3; Na 2 CO 3 or sucrose – Calibration against K 2 SO 4 – (Calibration against CRMs)
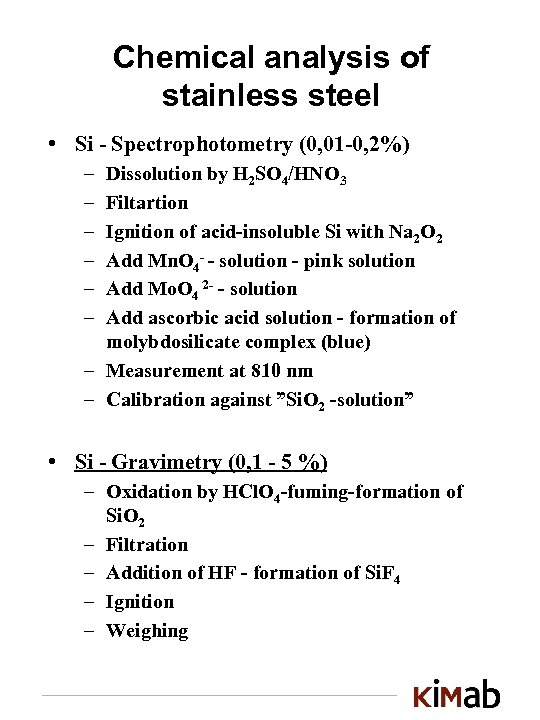 Chemical analysis of stainless steel • Si - Spectrophotometry (0, 01 -0, 2%) – – – Dissolution by H 2 SO 4/HNO 3 Filtartion Ignition of acid-insoluble Si with Na 2 O 2 Add Mn. O 4 - - solution - pink solution Add Mo. O 4 2 - - solution Add ascorbic acid solution - formation of molybdosilicate complex (blue) – Measurement at 810 nm – Calibration against ”Si. O 2 -solution” • Si - Gravimetry (0, 1 - 5 %) – Oxidation by HCl. O 4 -fuming-formation of Si. O 2 – Filtration – Addition of HF - formation of Si. F 4 – Ignition – Weighing
Chemical analysis of stainless steel • Si - Spectrophotometry (0, 01 -0, 2%) – – – Dissolution by H 2 SO 4/HNO 3 Filtartion Ignition of acid-insoluble Si with Na 2 O 2 Add Mn. O 4 - - solution - pink solution Add Mo. O 4 2 - - solution Add ascorbic acid solution - formation of molybdosilicate complex (blue) – Measurement at 810 nm – Calibration against ”Si. O 2 -solution” • Si - Gravimetry (0, 1 - 5 %) – Oxidation by HCl. O 4 -fuming-formation of Si. O 2 – Filtration – Addition of HF - formation of Si. F 4 – Ignition – Weighing
 Chemical analysis of stainless steel • Mn - AAS - flame (0, 002 - 2 %) – – – Dissolution by HCl/HNO 3 (HF) Add HCl. O 4 - fuming Filtration Measurement at 279, 5 nm Calibration against matrix-matched Mn solution
Chemical analysis of stainless steel • Mn - AAS - flame (0, 002 - 2 %) – – – Dissolution by HCl/HNO 3 (HF) Add HCl. O 4 - fuming Filtration Measurement at 279, 5 nm Calibration against matrix-matched Mn solution
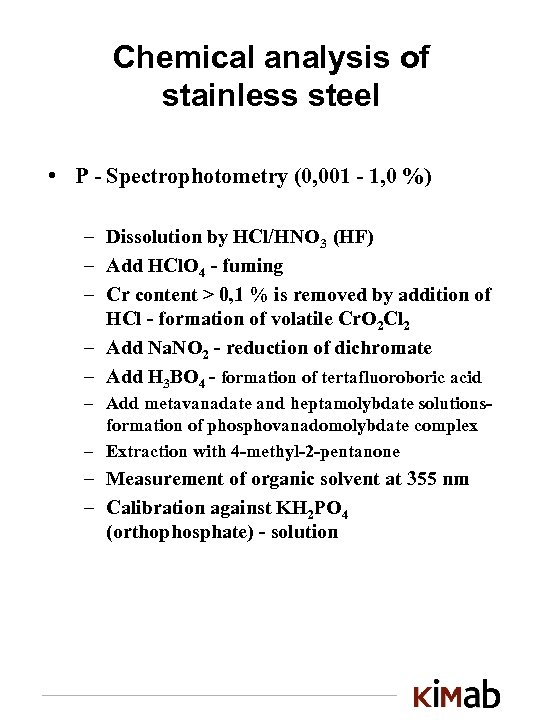 Chemical analysis of stainless steel • P - Spectrophotometry (0, 001 - 1, 0 %) – Dissolution by HCl/HNO 3 (HF) – Add HCl. O 4 - fuming – Cr content > 0, 1 % is removed by addition of HCl - formation of volatile Cr. O 2 Cl 2 – Add Na. NO 2 - reduction of dichromate – Add H 3 BO 4 - formation of tertafluoroboric acid – Add metavanadate and heptamolybdate solutionsformation of phosphovanadomolybdate complex – Extraction with 4 -methyl-2 -pentanone – Measurement of organic solvent at 355 nm – Calibration against KH 2 PO 4 (orthophosphate) - solution
Chemical analysis of stainless steel • P - Spectrophotometry (0, 001 - 1, 0 %) – Dissolution by HCl/HNO 3 (HF) – Add HCl. O 4 - fuming – Cr content > 0, 1 % is removed by addition of HCl - formation of volatile Cr. O 2 Cl 2 – Add Na. NO 2 - reduction of dichromate – Add H 3 BO 4 - formation of tertafluoroboric acid – Add metavanadate and heptamolybdate solutionsformation of phosphovanadomolybdate complex – Extraction with 4 -methyl-2 -pentanone – Measurement of organic solvent at 355 nm – Calibration against KH 2 PO 4 (orthophosphate) - solution
 Chemical analysis of stainless steel • Cr and V - Potentiometric titration Cr (1 - 30 %); V (0, 5 - 10 %) – Dissolution by HCl/HNO 3/HF in a. Teflon pressure vessel in micro-wave assisted system – Add H 3 PO 4/H 2 SO 4 - oxidation to Cr(VI) and to V(V), respectively – Titration with Fe(II) giving Cr(III) and V(IV) – Oxidation with Mn. O 4 - -solution giving V(V) and not Cr(VI) – Excess of Mn. O 4 - is reduced by NO 2– Excess of NO 2 - is eliminated by urea – Standardisation using pure K 2 Cr 2 O 7
Chemical analysis of stainless steel • Cr and V - Potentiometric titration Cr (1 - 30 %); V (0, 5 - 10 %) – Dissolution by HCl/HNO 3/HF in a. Teflon pressure vessel in micro-wave assisted system – Add H 3 PO 4/H 2 SO 4 - oxidation to Cr(VI) and to V(V), respectively – Titration with Fe(II) giving Cr(III) and V(IV) – Oxidation with Mn. O 4 - -solution giving V(V) and not Cr(VI) – Excess of Mn. O 4 - is reduced by NO 2– Excess of NO 2 - is eliminated by urea – Standardisation using pure K 2 Cr 2 O 7
 Chemical analysis of stainless steel • N - Melt extraction - Thermal Conductivity (0, 002 - 0, 6 %) – Melt extraction at 2700 o. C in graphite crucible and purging helium – Calibration against KNO 3 – (Calibration against CRMs)
Chemical analysis of stainless steel • N - Melt extraction - Thermal Conductivity (0, 002 - 0, 6 %) – Melt extraction at 2700 o. C in graphite crucible and purging helium – Calibration against KNO 3 – (Calibration against CRMs)
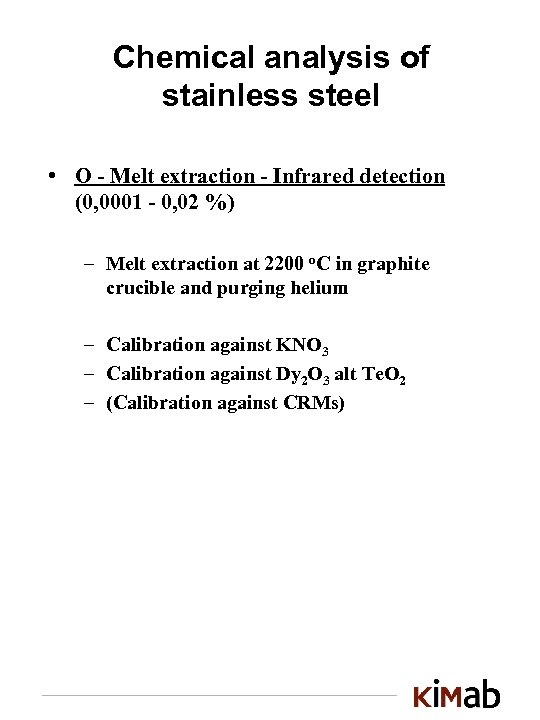 Chemical analysis of stainless steel • O - Melt extraction - Infrared detection (0, 0001 - 0, 02 %) – Melt extraction at 2200 o. C in graphite crucible and purging helium – Calibration against KNO 3 – Calibration against Dy 2 O 3 alt Te. O 2 – (Calibration against CRMs)
Chemical analysis of stainless steel • O - Melt extraction - Infrared detection (0, 0001 - 0, 02 %) – Melt extraction at 2200 o. C in graphite crucible and purging helium – Calibration against KNO 3 – Calibration against Dy 2 O 3 alt Te. O 2 – (Calibration against CRMs)
 Chemical analysis of stainless steel • Trace elements (< 0, 01 %) • Li, Be, B. . . Ti…Nb, Mo…Sn, Sb… Ce, La…W…Pb, Bi, Th, U – Dissolution in HCl/HNO 3/(HF) in a Teflon pressure vessel using a micro-wave assisted system – Dilution to suitable analytical volume – Addition of internal standard (usually 1 - 3) of Y, Rh, In, Re, Ir, Tl – Calibration against matrix-matched elemental solutions
Chemical analysis of stainless steel • Trace elements (< 0, 01 %) • Li, Be, B. . . Ti…Nb, Mo…Sn, Sb… Ce, La…W…Pb, Bi, Th, U – Dissolution in HCl/HNO 3/(HF) in a Teflon pressure vessel using a micro-wave assisted system – Dilution to suitable analytical volume – Addition of internal standard (usually 1 - 3) of Y, Rh, In, Re, Ir, Tl – Calibration against matrix-matched elemental solutions
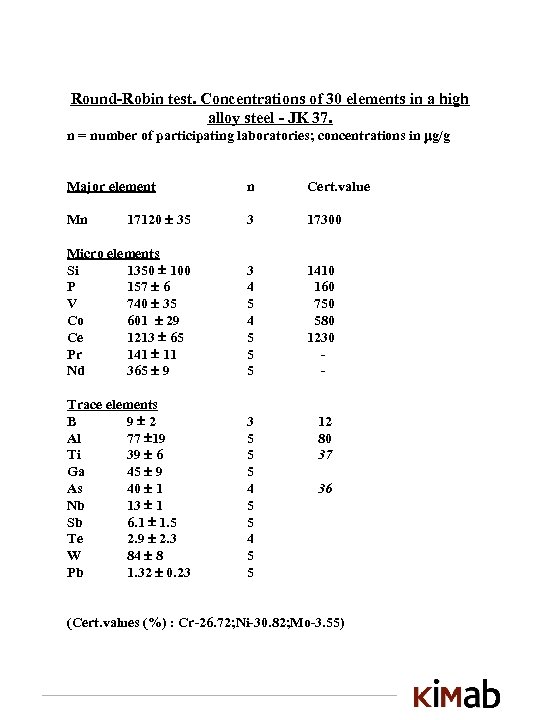 Round-Robin test. Concentrations of 30 elements in a high alloy steel - JK 37. n = number of participating laboratories; concentrations in g/g Major element n Cert. value 17120 35 3 17300 Micro elements Si 1350 100 P 157 6 V 740 35 Co 601 29 Ce 1213 65 Pr 141 11 Nd 365 9 3 4 5 5 5 1410 160 750 580 1230 - Trace elements B 9 2 Al 77 19 Ti 39 6 Ga 45 9 As 40 1 Nb 13 1 Sb 6. 1 1. 5 Te 2. 9 2. 3 W 84 8 Pb 1. 32 0. 23 3 5 5 5 4 5 5 12 80 37 Mn 36 (Cert. values (%) : Cr-26. 72; Ni-30. 82; Mo-3. 55)
Round-Robin test. Concentrations of 30 elements in a high alloy steel - JK 37. n = number of participating laboratories; concentrations in g/g Major element n Cert. value 17120 35 3 17300 Micro elements Si 1350 100 P 157 6 V 740 35 Co 601 29 Ce 1213 65 Pr 141 11 Nd 365 9 3 4 5 5 5 1410 160 750 580 1230 - Trace elements B 9 2 Al 77 19 Ti 39 6 Ga 45 9 As 40 1 Nb 13 1 Sb 6. 1 1. 5 Te 2. 9 2. 3 W 84 8 Pb 1. 32 0. 23 3 5 5 5 4 5 5 12 80 37 Mn 36 (Cert. values (%) : Cr-26. 72; Ni-30. 82; Mo-3. 55)
 Cont`d Round-Robin test. Concentrations of ultra-trace elements in a high alloy steel - JK 37. n = number of participating laboratories; concentration in g/g Ultra-trace elements Be Pd Ag Ba Ta Ir Pt Tl Bi <0. 1 <1 <0. 4 <1 <0. 20 0. 03 <0. 2 0. 33 0. 13 n 4 2 4 4 5 5 5 4 5 Trace or ultra-trace elements ? Ca Zn Se <12 <5 <10 4 2 2 Cert. value
Cont`d Round-Robin test. Concentrations of ultra-trace elements in a high alloy steel - JK 37. n = number of participating laboratories; concentration in g/g Ultra-trace elements Be Pd Ag Ba Ta Ir Pt Tl Bi <0. 1 <1 <0. 4 <1 <0. 20 0. 03 <0. 2 0. 33 0. 13 n 4 2 4 4 5 5 5 4 5 Trace or ultra-trace elements ? Ca Zn Se <12 <5 <10 4 2 2 Cert. value
 ICP-TOFMS - LECO Renaissance
ICP-TOFMS - LECO Renaissance
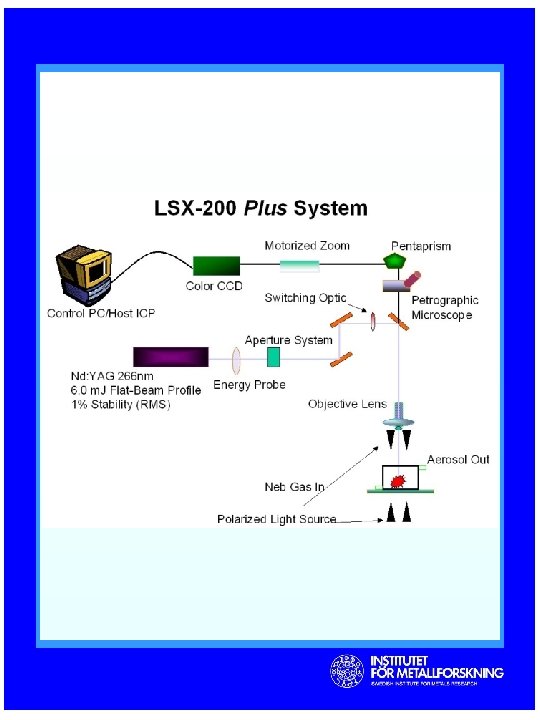
 Laser Ablation System CETAC LSX-200 plus
Laser Ablation System CETAC LSX-200 plus
 Instrument installation
Instrument installation
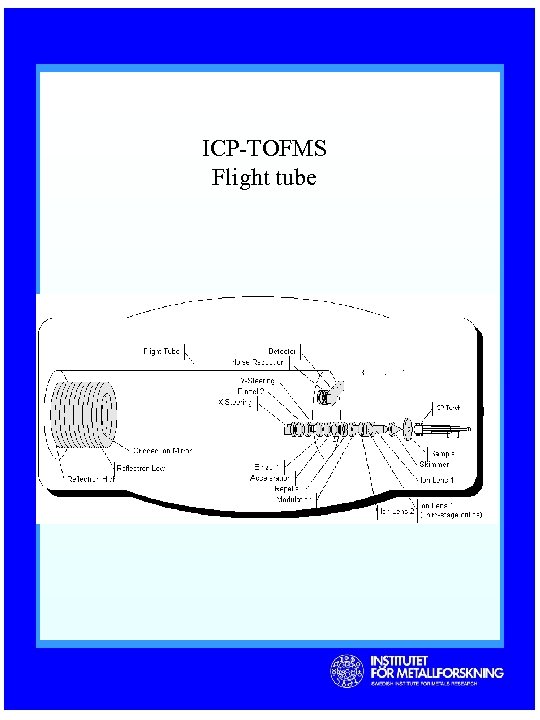 ICP-TOFMS Flight tube
ICP-TOFMS Flight tube
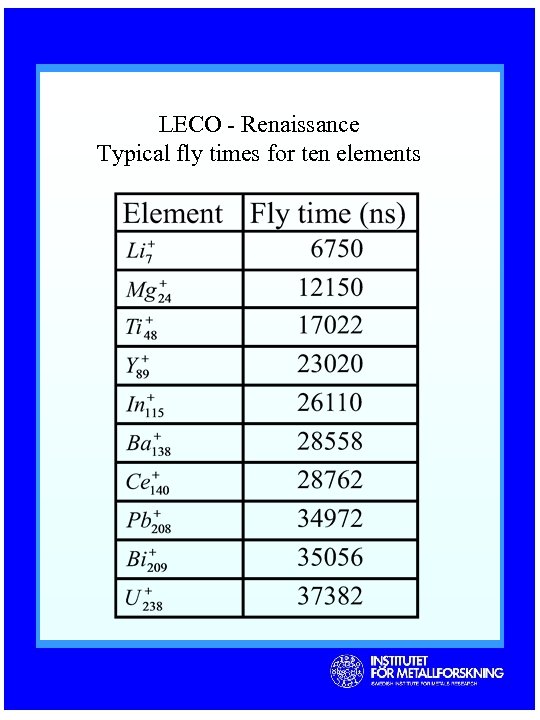 LECO - Renaissance Typical fly times for ten elements
LECO - Renaissance Typical fly times for ten elements
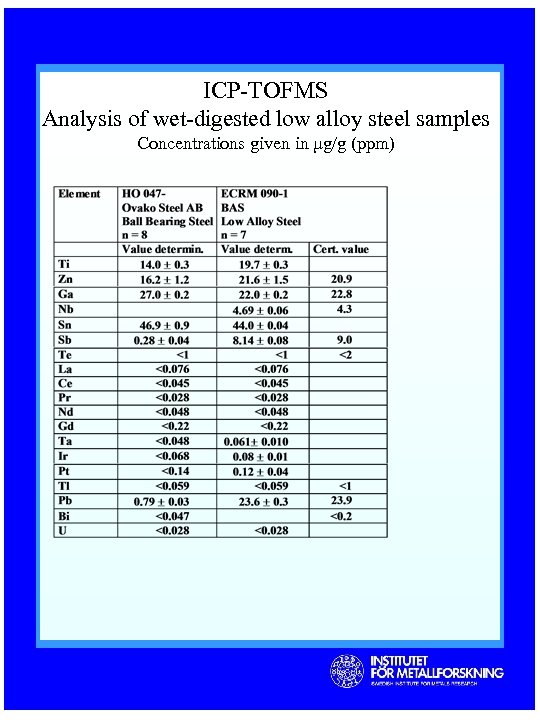 ICP-TOFMS Analysis of wet-digested low alloy steel samples Concentrations given in µg/g (ppm)
ICP-TOFMS Analysis of wet-digested low alloy steel samples Concentrations given in µg/g (ppm)
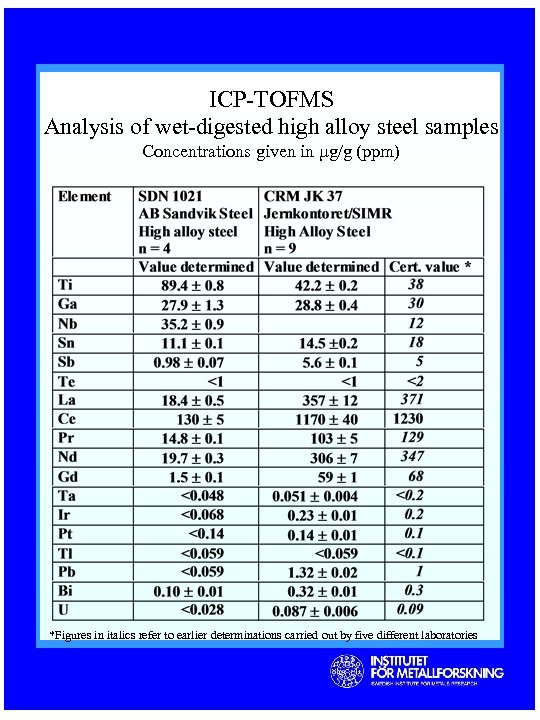 ICP-TOFMS Analysis of wet-digested high alloy steel samples Concentrations given in µg/g (ppm) *Figures in italics refer to earlier determinations carried out by five different laboratories
ICP-TOFMS Analysis of wet-digested high alloy steel samples Concentrations given in µg/g (ppm) *Figures in italics refer to earlier determinations carried out by five different laboratories
 Laser Ablation Copper scanning- spot size 200 µm
Laser Ablation Copper scanning- spot size 200 µm
 Laser Ablation Analysis of Copper samples: BAM 381 BAM 383 BAM 382 BAM 385 All BAM samples above have been determined in a traditional wet chemical way by ICP-TOFMS LASER measurements: Calibration with BAM 381, 384 and 385 Internal standard Sn 118 or Ag 107 Determination of BAM 383
Laser Ablation Analysis of Copper samples: BAM 381 BAM 383 BAM 382 BAM 385 All BAM samples above have been determined in a traditional wet chemical way by ICP-TOFMS LASER measurements: Calibration with BAM 381, 384 and 385 Internal standard Sn 118 or Ag 107 Determination of BAM 383
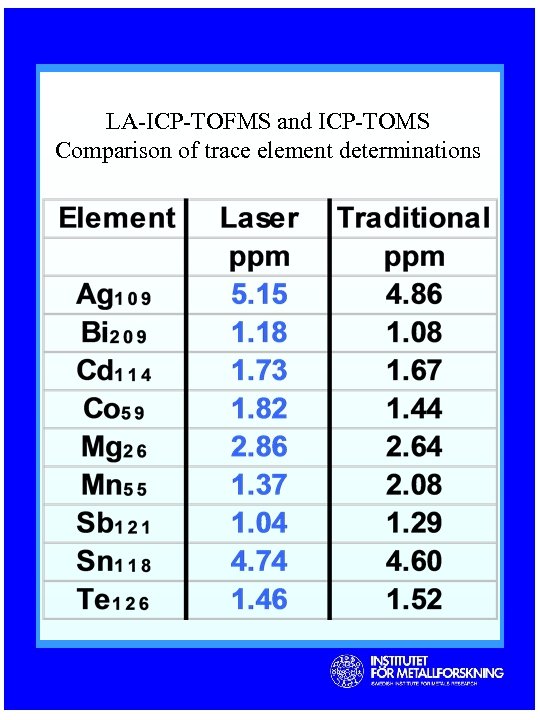 LA-ICP-TOFMS and ICP-TOMS Comparison of trace element determinations
LA-ICP-TOFMS and ICP-TOMS Comparison of trace element determinations
 Laser Ablation Analysis of Steel Samples: JK 1 C ECRM 090 -1 EZRM 179 -2 HOØ 47 (Ovako) Pure Iron Carbon Steel Tool Steel Ball Bearing Steel Calibration with: JK 1 C, ECRM 090 -1, EZRM 179 -2 Internal standards: Samples analysed: Ga and Ni HOØ 47 (Ovako)
Laser Ablation Analysis of Steel Samples: JK 1 C ECRM 090 -1 EZRM 179 -2 HOØ 47 (Ovako) Pure Iron Carbon Steel Tool Steel Ball Bearing Steel Calibration with: JK 1 C, ECRM 090 -1, EZRM 179 -2 Internal standards: Samples analysed: Ga and Ni HOØ 47 (Ovako)
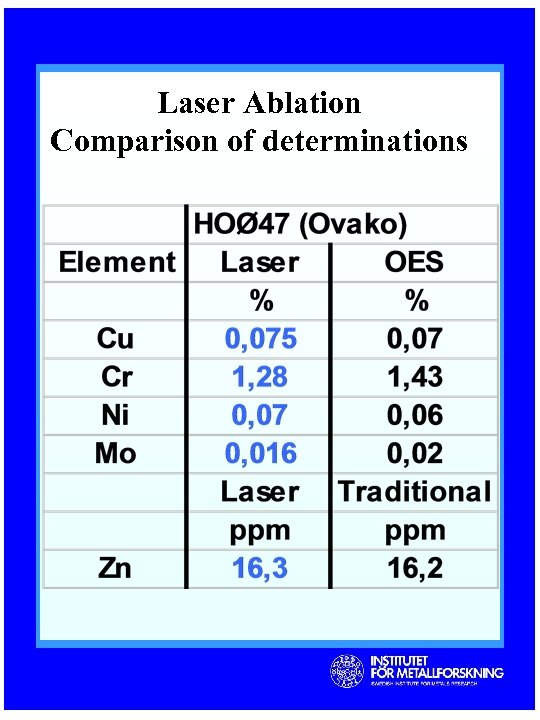 Laser Ablation Comparison of determinations
Laser Ablation Comparison of determinations
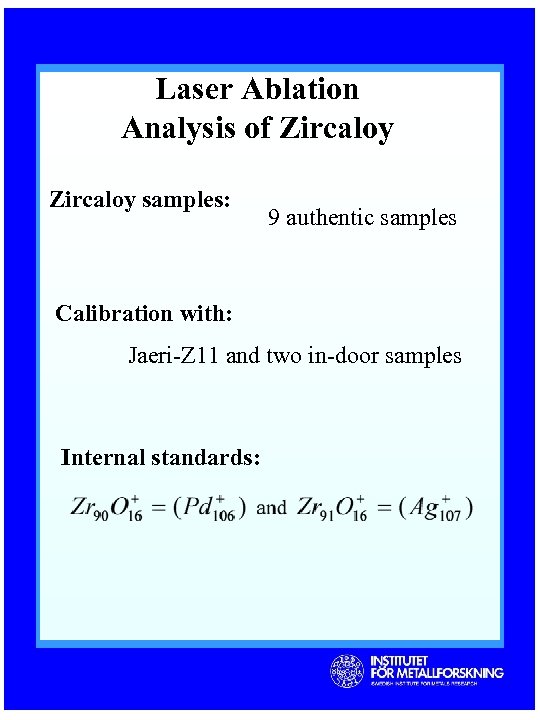 Laser Ablation Analysis of Zircaloy samples: 9 authentic samples Calibration with: Jaeri-Z 11 and two in-door samples Internal standards:
Laser Ablation Analysis of Zircaloy samples: 9 authentic samples Calibration with: Jaeri-Z 11 and two in-door samples Internal standards:
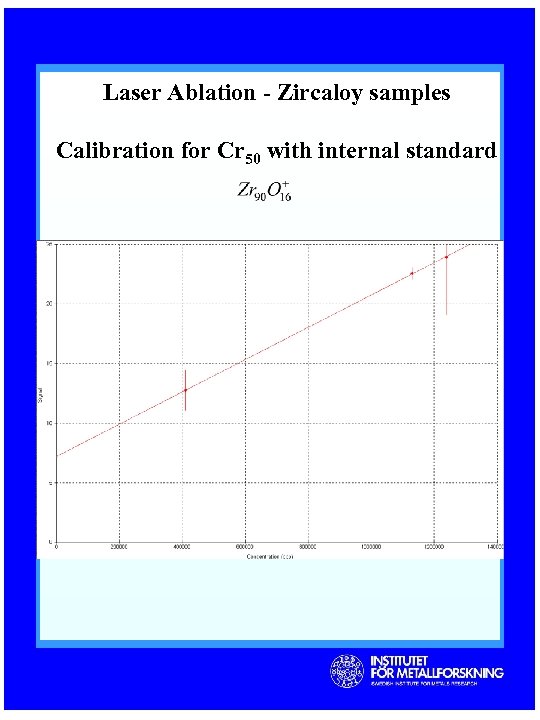 Laser Ablation - Zircaloy samples Calibration for Cr 50 with internal standard
Laser Ablation - Zircaloy samples Calibration for Cr 50 with internal standard
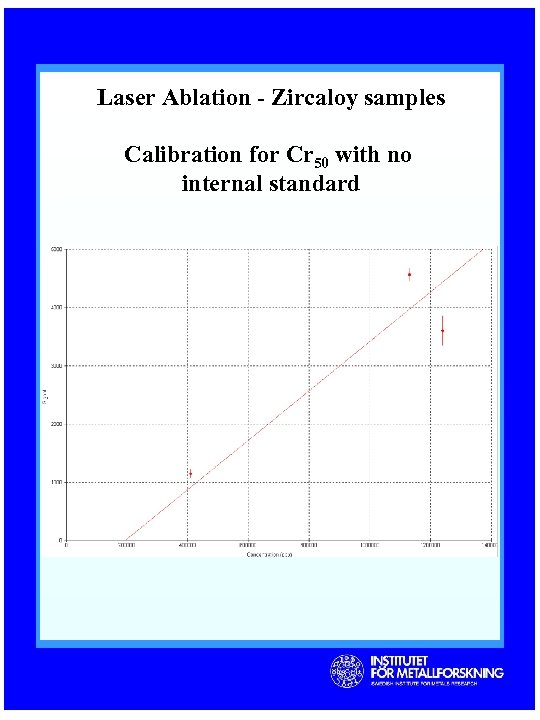 Laser Ablation - Zircaloy samples Calibration for Cr 50 with no internal standard
Laser Ablation - Zircaloy samples Calibration for Cr 50 with no internal standard
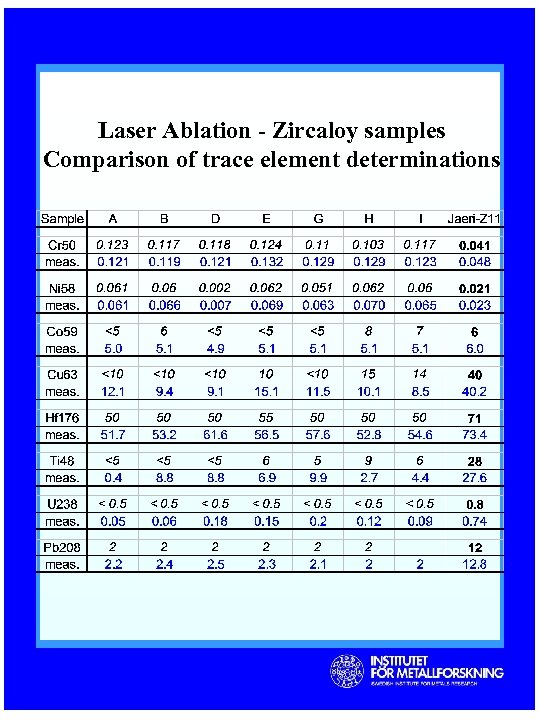 Laser Ablation - Zircaloy samples Comparison of trace element determinations
Laser Ablation - Zircaloy samples Comparison of trace element determinations
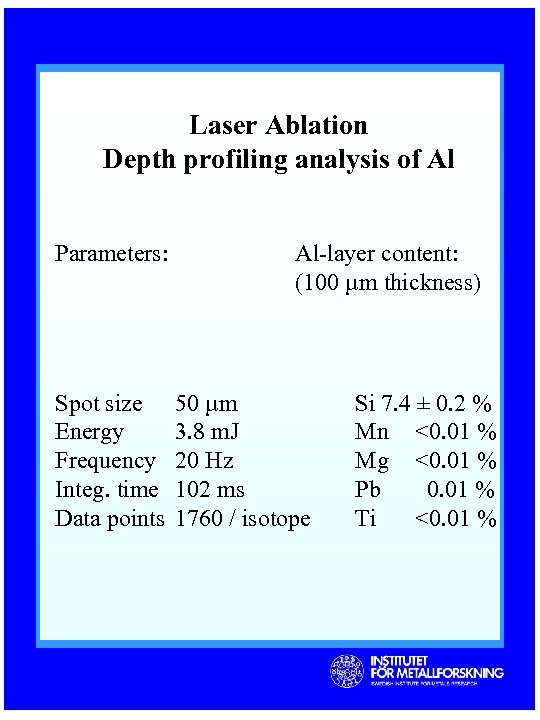 Laser Ablation Depth profiling analysis of Al Parameters: Spot size Energy Frequency Integ. time Data points Al-layer content: (100 µm thickness) 50 µm 3. 8 m. J 20 Hz 102 ms 1760 / isotope Si 7. 4 ± 0. 2 % Mn <0. 01 % Mg <0. 01 % Pb 0. 01 % Ti <0. 01 %
Laser Ablation Depth profiling analysis of Al Parameters: Spot size Energy Frequency Integ. time Data points Al-layer content: (100 µm thickness) 50 µm 3. 8 m. J 20 Hz 102 ms 1760 / isotope Si 7. 4 ± 0. 2 % Mn <0. 01 % Mg <0. 01 % Pb 0. 01 % Ti <0. 01 %
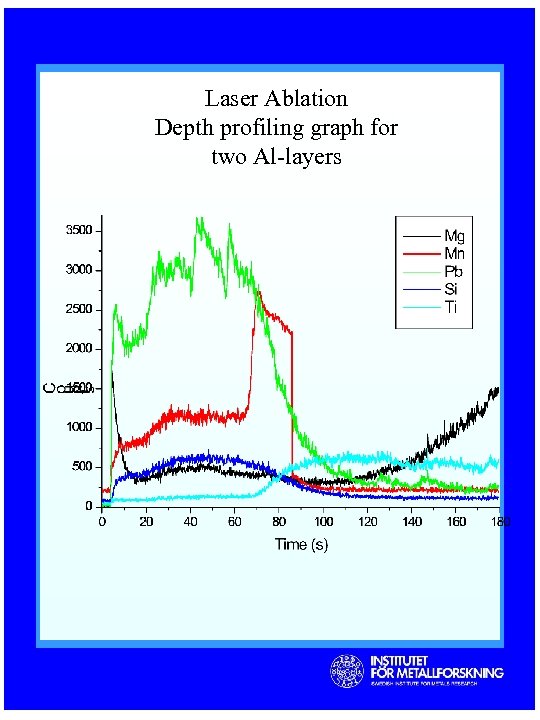 Laser Ablation Depth profiling graph for two Al-layers
Laser Ablation Depth profiling graph for two Al-layers
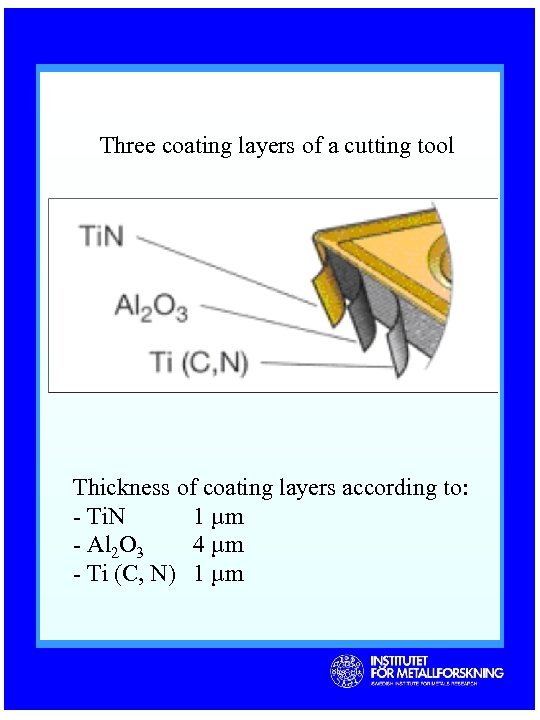 Three coating layers of a cutting tool Thickness of coating layers according to: - Ti. N 1 µm - Al 2 O 3 4 µm - Ti (C, N) 1 µm
Three coating layers of a cutting tool Thickness of coating layers according to: - Ti. N 1 µm - Al 2 O 3 4 µm - Ti (C, N) 1 µm
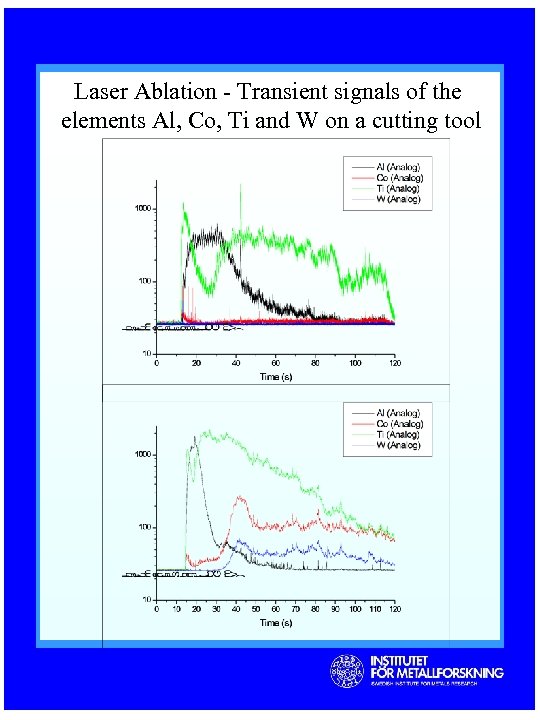 Laser Ablation - Transient signals of the elements Al, Co, Ti and W on a cutting tool
Laser Ablation - Transient signals of the elements Al, Co, Ti and W on a cutting tool
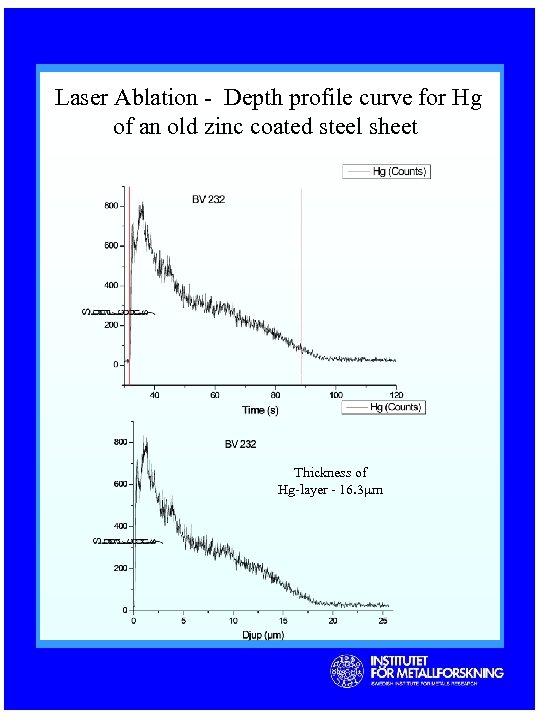 Laser Ablation - Depth profile curve for Hg of an old zinc coated steel sheet Thickness of Hg-layer - 16. 3µm
Laser Ablation - Depth profile curve for Hg of an old zinc coated steel sheet Thickness of Hg-layer - 16. 3µm
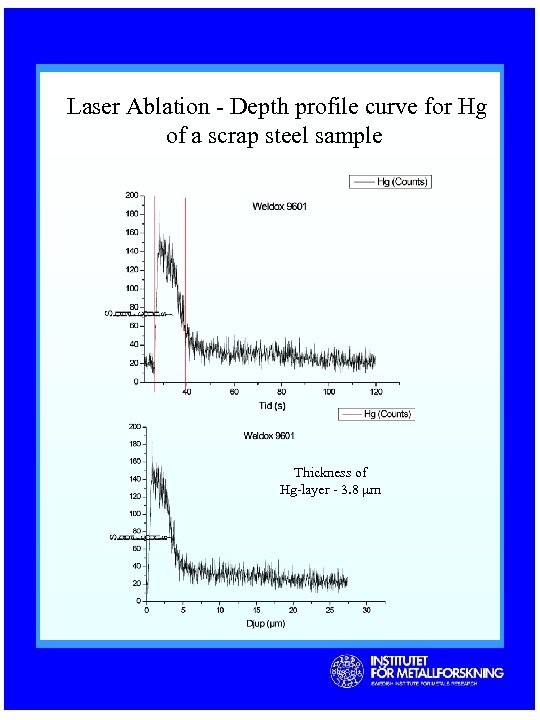 Laser Ablation - Depth profile curve for Hg of a scrap steel sample Thickness of Hg-layer - 3. 8 µm
Laser Ablation - Depth profile curve for Hg of a scrap steel sample Thickness of Hg-layer - 3. 8 µm
 Conclusions LA-ICP-TOFMS vs ICP-TOFMS: - a fast method compared with wet chemical analysis - a fast method for depth profile analysis - a tool for trace element determinations in steel and metals - a promising tool for determination of inhomogenieties (inclusions) in steel and metals
Conclusions LA-ICP-TOFMS vs ICP-TOFMS: - a fast method compared with wet chemical analysis - a fast method for depth profile analysis - a tool for trace element determinations in steel and metals - a promising tool for determination of inhomogenieties (inclusions) in steel and metals


