e42817a31835184d82fd488ccad1b1d2.ppt
- Количество слайдов: 12

Performance Improvement in Microbiology Laboratory MLAB 2434 – Microbiology Keri Brophy-Martinez

Terminology ¢ ¢ “Quality Control” has always been a part of the clinical laboratory, but had been used exclusively for analytical activities. QC is designated to ensure the medical reliability of lab data. We now know that we must analyze pre -analytical, and postanalytical activities and the terminology is NOW called “Performance Improvement” (PI)

Indicators/Monitors of Performance Improvement ¢ ¢ Process Monitors l Results are complied and evaluated routinely, allows for identification of disruptions Outcome Monitors l Measurements as a result of processes Focus Monitors l Initiated in response to a suspected problem Benchmarking l Seeking an industry’s or profession’s best practices l Helps provide a starting point for improvement
Terminology ¢ Quality Assurance (QA) is defined as taking measures to ensure high quality patient care. Involves monitoring all parts of a system and implementing changes when suboptimal performance is identified
Terminology (cont’d) ¢ Preanalytical Stage Test ordering l Order transcription l Patient preparation l Specimen collection l Specimen identification l Specimen transport l
Terminology (cont’d) ¢ Analytical Stage l ¢ Sample testing Postanalytical Stage Result transcription l Result delivery l Result review l Action taken on basis of result l

Quality Control Procedures ¢ Daily temperature checks l l ¢ NIST (National Institute of Standards & Technology) Thermometer calibration (once when received from manufacturer) Equipment preventive maintenance program – includes routine maintenance such as oiling, cleaning, replacing filters, etc.

Quality Control Procedures (cont’d) ¢ All prepared media must be quality controlled prior to use l l ¢ ¢ If media is purchased, can rely on manufacturer’s QC, but lab must obtain a statement of quality control from manufacturer All shipments should be inspected as it comes to lab HOWEVER, chocolate and selective agars for Neisseria and Campylobacter MUST be rechecked by lab Test media using ATCC strains Records maintained for 2 years Reagents should be QC’ed on each day of use, new lot numbers, and/or with each test run
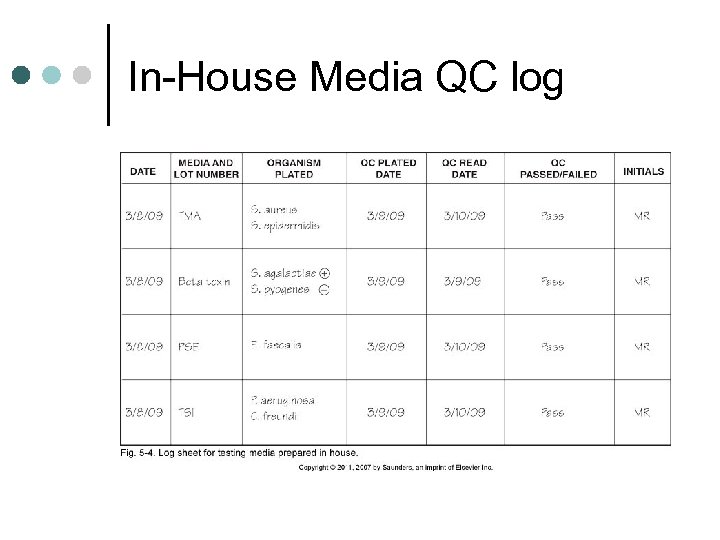
In-House Media QC log
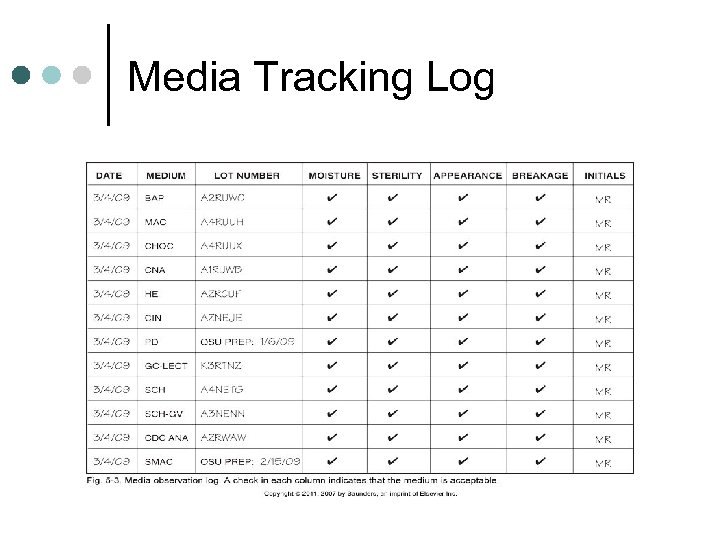
Media Tracking Log

Quality Control Procedures (cont’d) Antimicrobial Susceptibility QC ¢ Personnel Competency – proficiency testing is one way this can be assured (CLIA requires this) ¢ Continuing Education ¢ QC Manual – must be reviewed and signed at least annually ¢

References ¢ Mahon, C. R. , Lehman, D. C. , & Manuselis, G. (2011). Textbook of Diagnostic Microbiology (4 th ed. ). Maryland Heights, MO: Saunders.
e42817a31835184d82fd488ccad1b1d2.ppt