d9c2f427e1bb1bb52d2fea0be911551e.ppt
- Количество слайдов: 67

PENN CENTER FOR EVIDENCE-BASED PRACTICE Hospital-based Comparative Effectiveness Centers: Improving the Quality, Safety and Cost-Effectiveness of Patient Care Thru Evidence-based Practice at the Systems Level Craig A Umscheid, MD, MSCE, FACP Assistant Professor of Medicine and Epidemiology Director, Center for Evidence-based Practice Medical Director, Clinical Decision Support Senior Associate Director, ECRI-Penn AHRQ EPC TEACH Plenary August 8 th, 2013

Outline w Case w Defining CER and HTA w Practicing EBM at a “systems” level thru hospital-based HTA • Synthesizing evidence for decision-making • Clinical decision support • Education in EBM w Conclusions 2
Case: Chlorhexidine to Reduce Surgical Site Infections Chlorhexidine: $13 per patient Betadine: 60 cents per patient 3
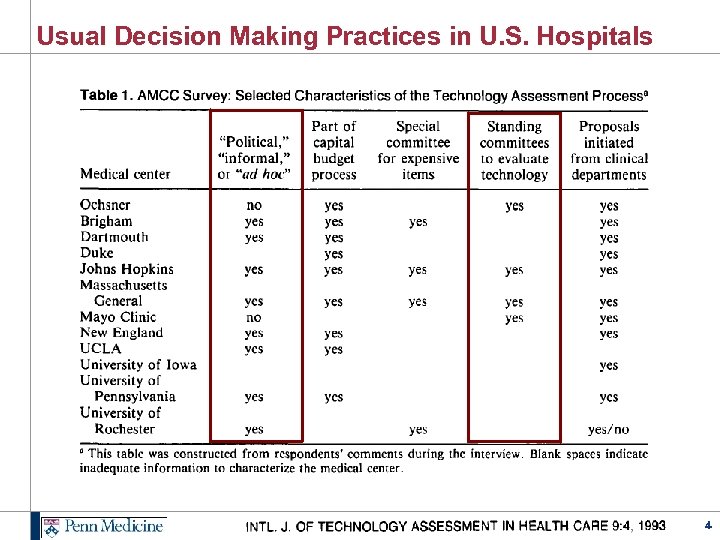
Usual Decision Making Practices in U. S. Hospitals 4
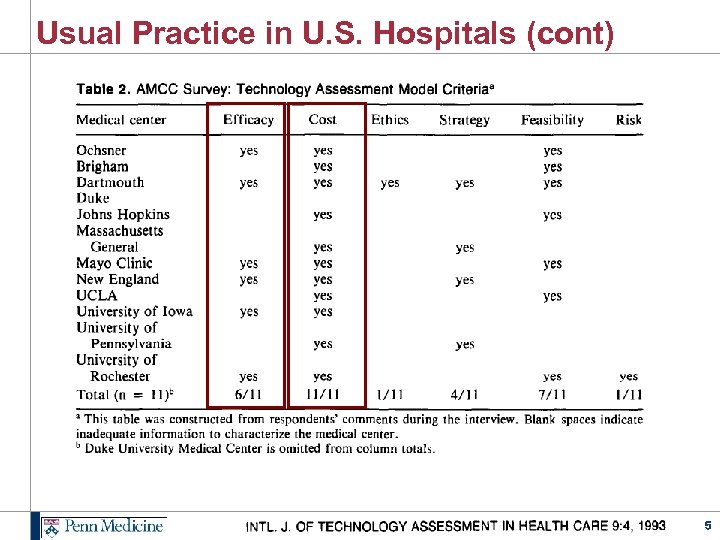
Usual Practice in U. S. Hospitals (cont) 5

Is there a better way? How about CER and HTA? 6
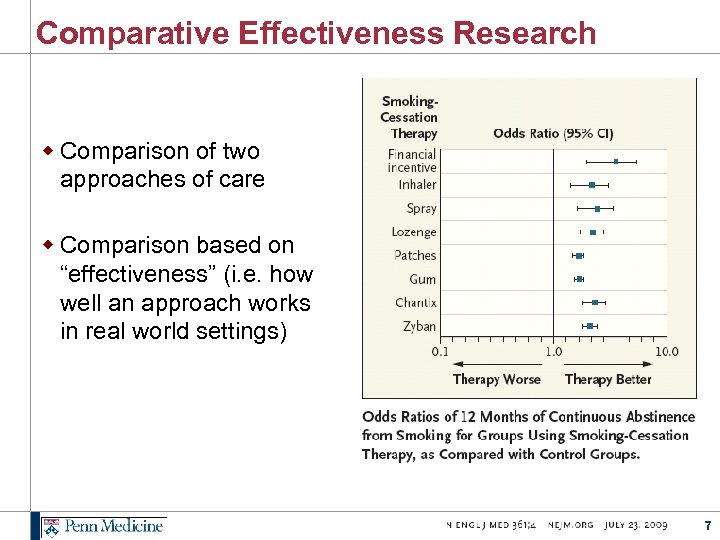
Comparative Effectiveness Research w Comparison of two approaches of care w Comparison based on “effectiveness” (i. e. how well an approach works in real world settings) 7

Health Technology Assessment w Also referred to as Healthcare Technology Assessment or Medical Technology Assessment w Form of policy research that systematically examines short and long term consequences of a health technology w Technologies are defined broadly as drugs, devices, procedures, and processes of care w Outcomes can include efficacy, effectiveness, safety, cost, ethical or social consequences w Goal is to inform decision making in policy and practice (as opposed to the goal of research, which is often to contribute to generalizable knowledge) IJTAHC. 25: Supplement 1 (2009) 8
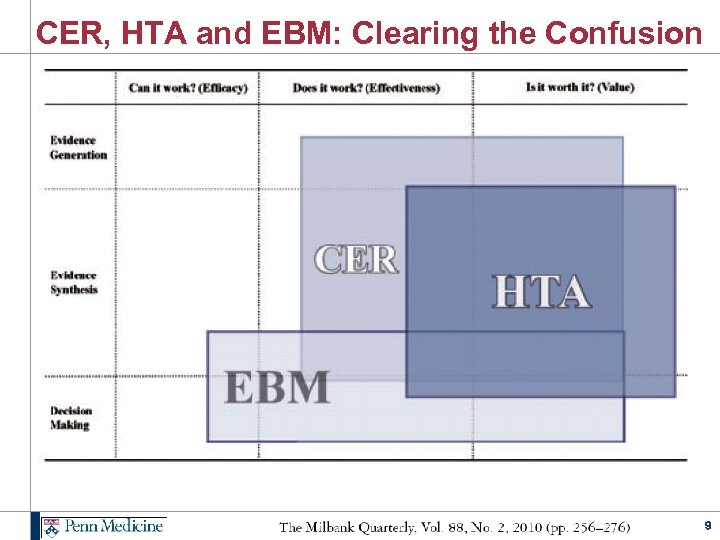
CER, HTA and EBM: Clearing the Confusion 9

What is Hospital-based HTA / CE ? 10

Hospital-based Health Tech Assessment J Gen Intern Med. 2010; 25(12): 1352– 5. 11
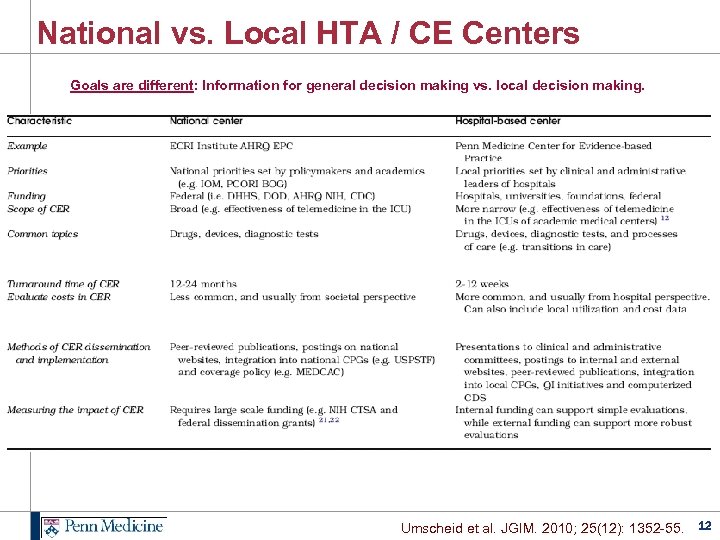
National vs. Local HTA / CE Centers Goals are different: Information for general decision making vs. local decision making. Umscheid et al. JGIM. 2010; 25(12): 1352 -55. 12
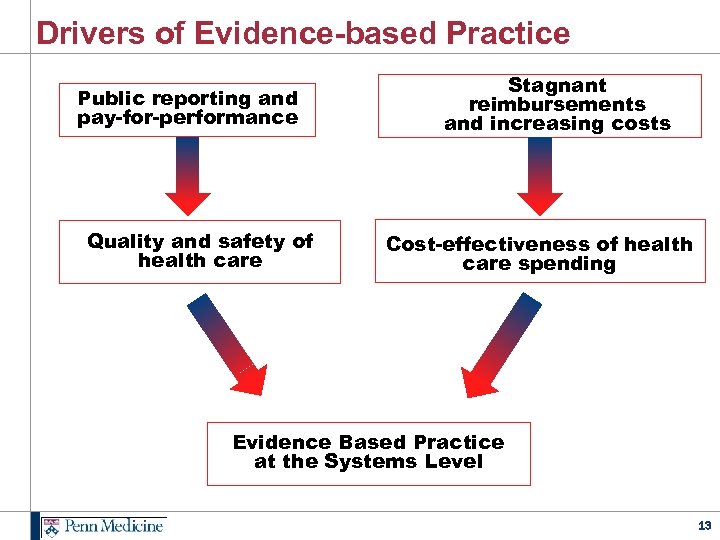
Drivers of Evidence-based Practice Public reporting and pay-for-performance Quality and safety of health care Stagnant reimbursements and increasing costs Cost-effectiveness of health care spending Evidence Based Practice at the Systems Level 13
Drivers of Evidence-based Practice Public reporting and pay-for-performance Quality and safety of health care Stagnant reimbursements and increasing costs Cost-effectiveness of health care spending Evidence Based Practice at the Systems Level 14

International Models for HB-HTA Slides courtesy of Marco Marchetti, Director, HTA Unit, A. Gemelli University Hospital, Rome, Italy 15

16
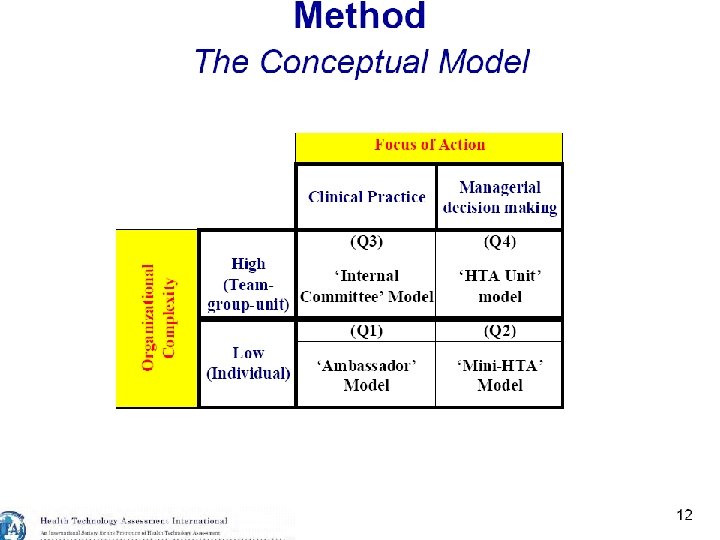
17
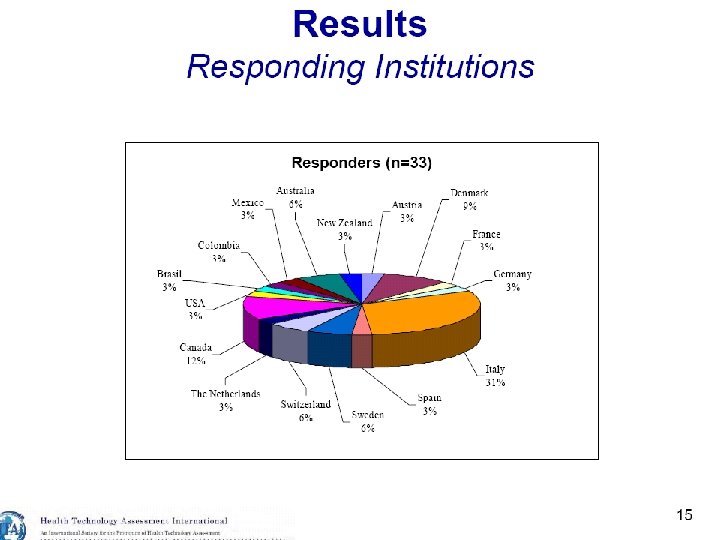
18
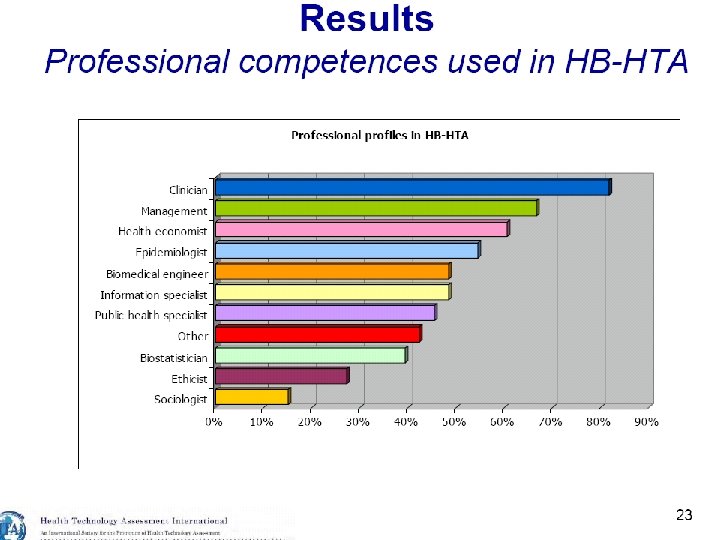
19
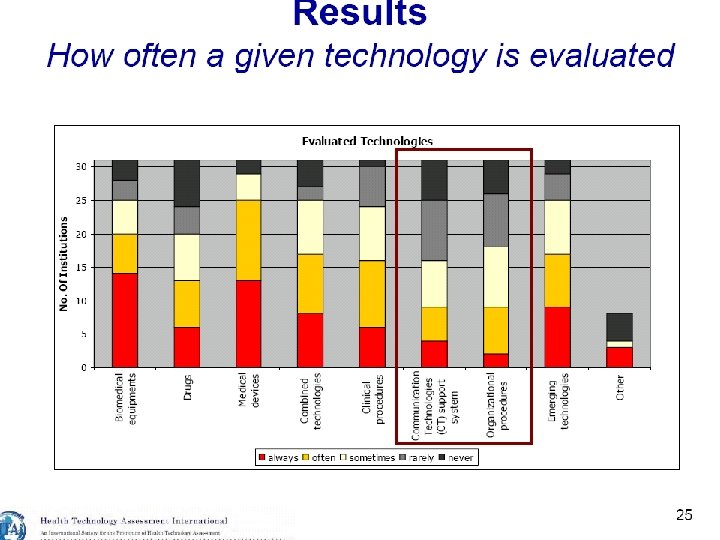
20
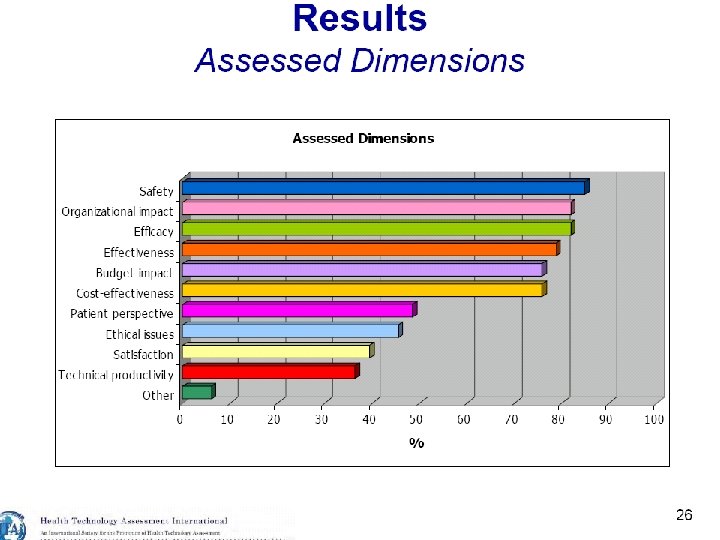
21
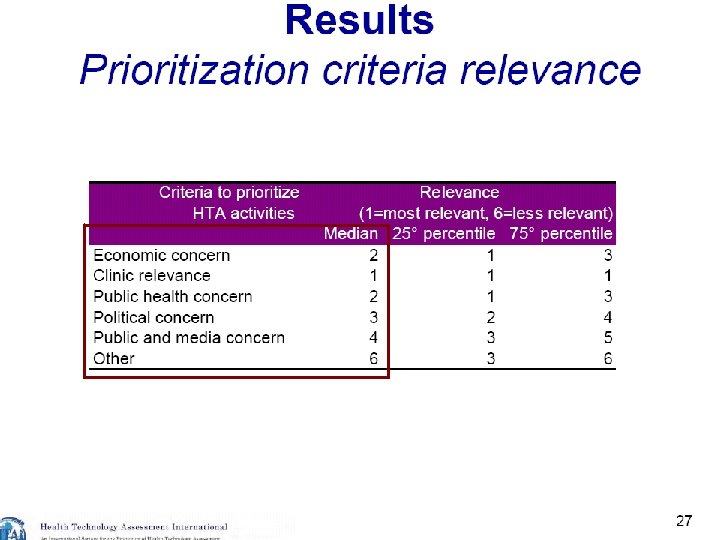
22
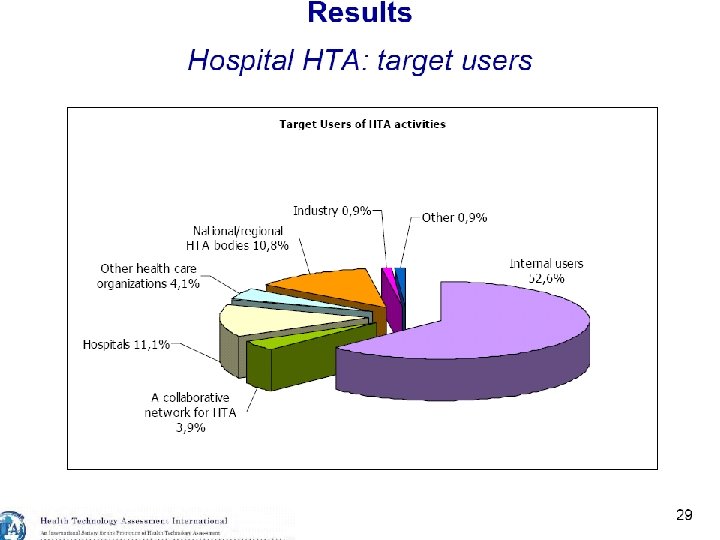
23
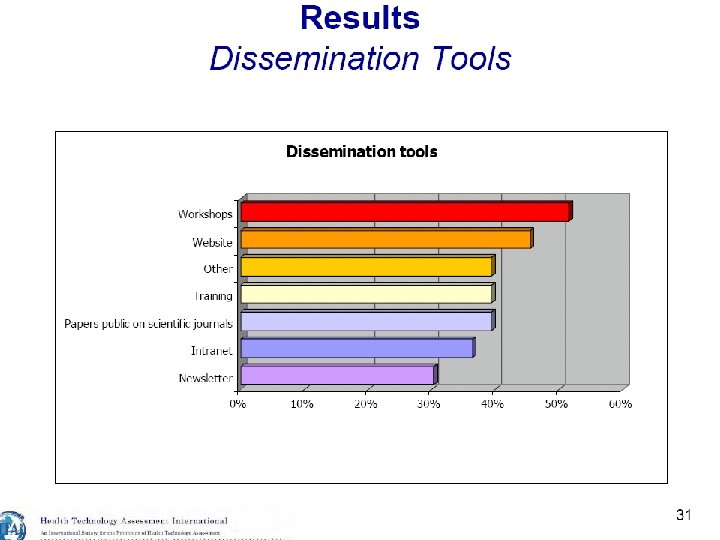
24

Models of HB-HTA in the US 25
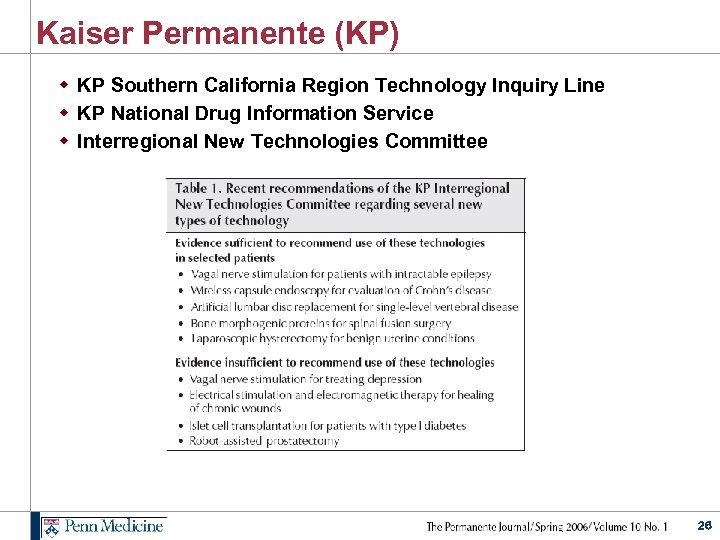
Kaiser Permanente (KP) w KP Southern California Region Technology Inquiry Line w KP National Drug Information Service w Interregional New Technologies Committee 26
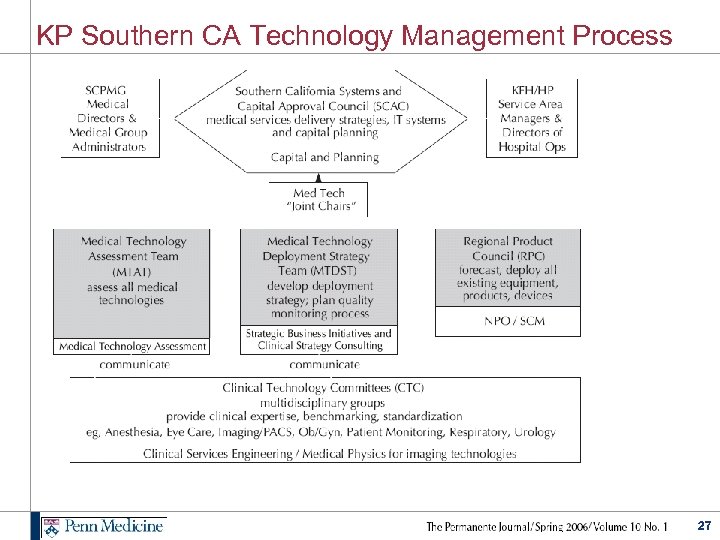
KP Southern CA Technology Management Process 27

Penn Medicine Center for Evidence-based Practice (CEP) 28
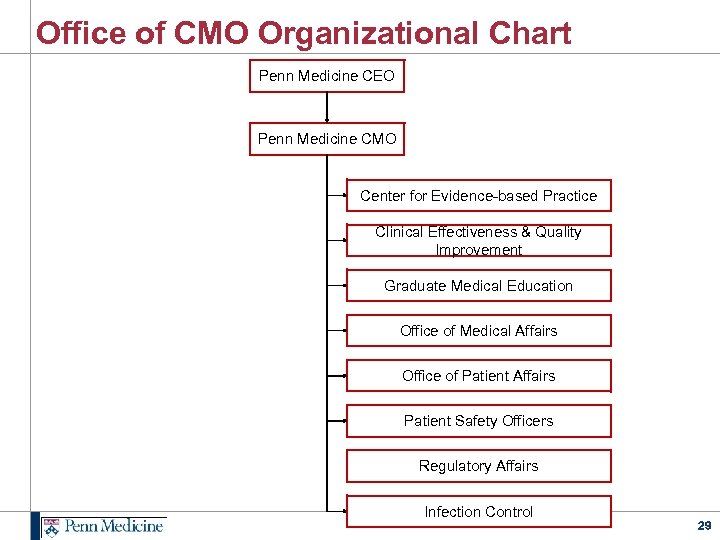
Office of CMO Organizational Chart Penn Medicine CEO Penn Medicine CMO Center for Evidence-based Practice Clinical Effectiveness & Quality Improvement Graduate Medical Education Office of Medical Affairs Office of Patient Affairs Patient Safety Officers Regulatory Affairs Infection Control 29

Center for Evidence-based Practice: Mission and Approach “To support the quality, safety and value of patient care at Penn through evidence-based practice. ” • Perform reviews of the medical literature to inform clinical practice, policy, purchasing and formulary decisions in and outside of Penn • Help translate evidence into practice at Penn through computerized clinical decision support (CDS) • Offer education in evidence-based decision making to trainees, staff and faculty in and outside of Penn Umscheid et al. JGIM. 2010; 25(12): 1352 -55. 30

Framework for Evidence-based Guidance 1. Define the clinical issue of concern 2. Perform systematic search for existing evidence 3. Identify or develop best practices 4. Implement best practices 5. Monitor the impact 31
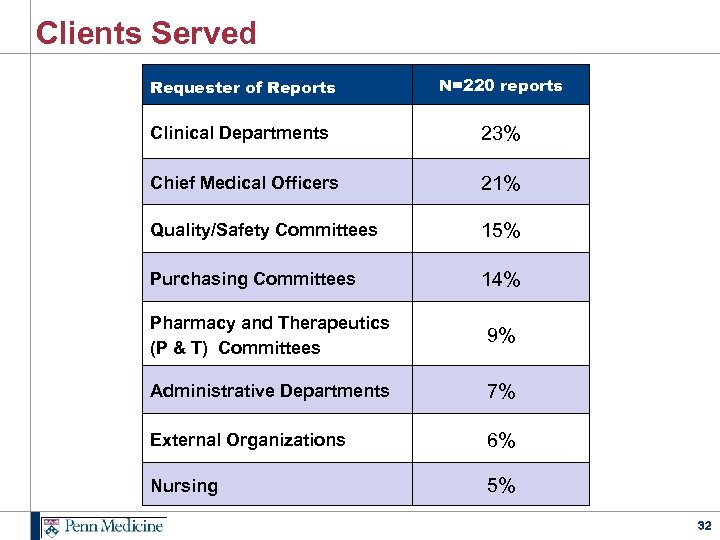
Clients Served Requester of Reports N=220 reports Clinical Departments 23% Chief Medical Officers 21% Quality/Safety Committees 15% Purchasing Committees 14% Pharmacy and Therapeutics (P & T) Committees 9% Administrative Departments 7% External Organizations 6% Nursing 5% 32
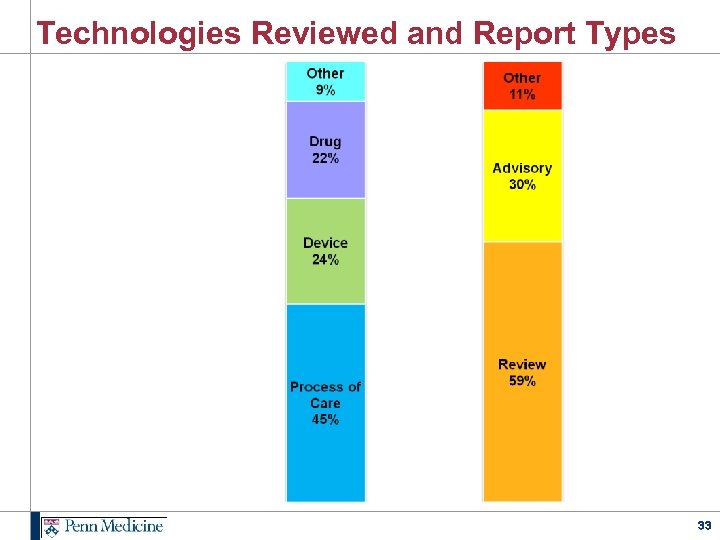
Technologies Reviewed and Report Types 33
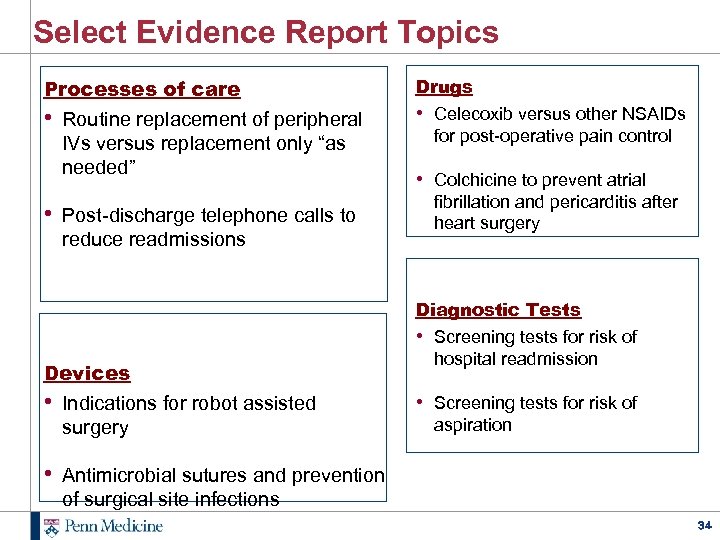
Select Evidence Report Topics Processes of care • Routine replacement of peripheral IVs versus replacement only “as needed” Drugs • Celecoxib versus other NSAIDs for post-operative pain control • Post-discharge telephone calls to fibrillation and pericarditis after heart surgery reduce readmissions Devices • Indications for robot assisted surgery • Colchicine to prevent atrial Diagnostic Tests • Screening tests for risk of hospital readmission • Screening tests for risk of aspiration • Antimicrobial sutures and prevention of surgical site infections 34
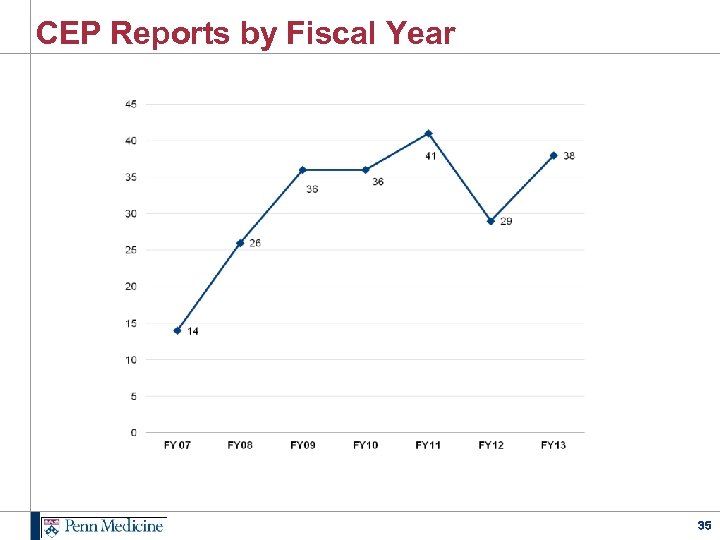
CEP Reports by Fiscal Year 35
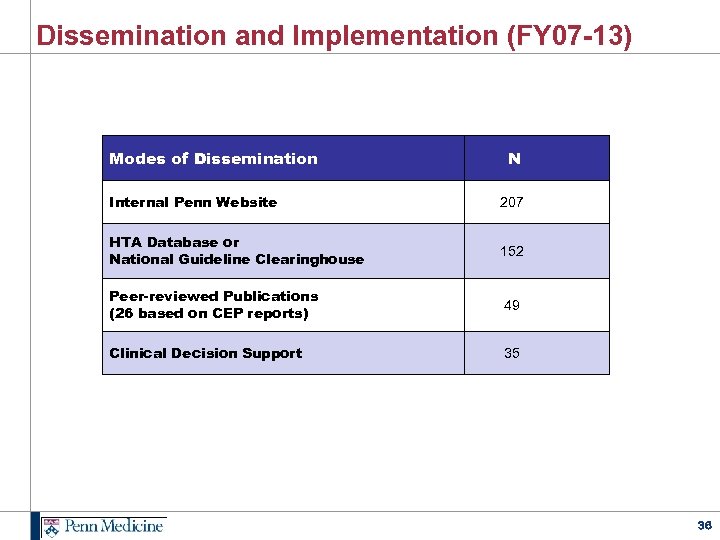
Dissemination and Implementation (FY 07 -13) Modes of Dissemination N Internal Penn Website 207 HTA Database or National Guideline Clearinghouse 152 Peer-reviewed Publications (26 based on CEP reports) 49 Clinical Decision Support 35 36

External Collaborations: CDC and AHRQ w Centers for Disease Control and Prevention (CDC) • Infection control guidelines w Agency for Healthcare Research and Quality (AHRQ) • One of 11 centers nationally awarded an “AHRQ Evidence-based Practice Center” contract • Perform evidence reviews to inform clinical practice guidelines and other forms of national healthcare policy 37
Computerized Clinical Decision Support (CDS) “Provides clinicians or patients with knowledge and information, intelligently filtered or presented, to enhance patient care. ” • Alerts (e. g. , drug allergies or interactions) • Reminders (e. g. , about best practices) • Order sets www. himss. org/cdsguide 38
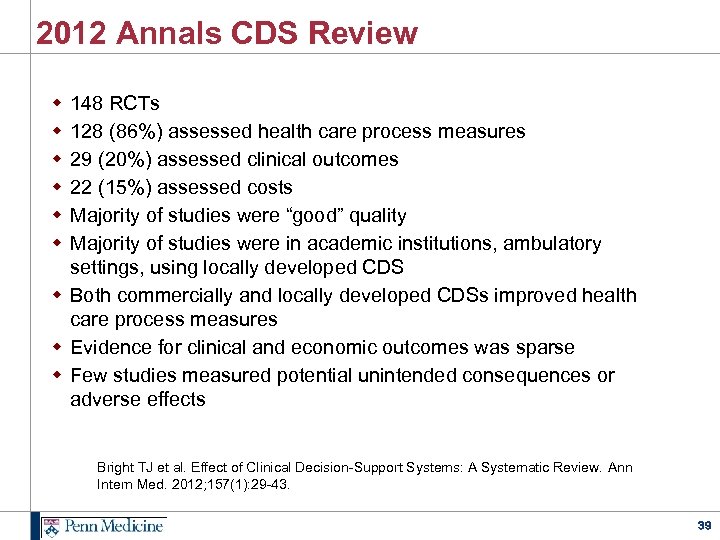
2012 Annals CDS Review w w w 148 RCTs 128 (86%) assessed health care process measures 29 (20%) assessed clinical outcomes 22 (15%) assessed costs Majority of studies were “good” quality Majority of studies were in academic institutions, ambulatory settings, using locally developed CDS w Both commercially and locally developed CDSs improved health care process measures w Evidence for clinical and economic outcomes was sparse w Few studies measured potential unintended consequences or adverse effects Bright TJ et al. Effect of Clinical Decision-Support Systems: A Systematic Review. Ann Intern Med. 2012; 157(1): 29 -43. 39

Predictors of Improved Practice with CDS w Meta-regression identified success features, including: • • • integration with charting or order entry system local user involvement in development automatic provision of decision support as part of clinician workflow provision of decision support at time and location of decision-making provision of a recommendation, not just an assessment Lobach D et al. Evidence Report No. 203. (Prepared by the Duke Evidence-based Practice Center under Contract No. 290 -2007 -10066 -I. ) AHRQ Publication No. 12 -E 001 -EF. Rockville, MD: Agency for Healthcare Research and Quality. April 2012. 40

CDS Five Rights Model To improve care outcomes with CDS one must provide: the Right Information… Evidence-based, useful for guiding action …to the Right Stakeholder… Both clinicians and patients …in the Right Format… Alerts, Order Sets, etc. …through the Right Channel… Internet, mobile devices, electronic health records …at the Right Point in the Workflow. To influence key decisions/actions www. himss. org/cdsguide 41

42 42

CDS Mission at Penn To continuously improve the safety, quality, and efficiency of patient care by ensuring that providers have the information needed to drive decisions that lead to optimal outcomes. 43

Primary CDS Activities at Penn 1. Evaluating and prioritizing new CDS proposals 2. Developing and deploying CDS interventions 3. Cataloguing and evaluating implemented interventions 44
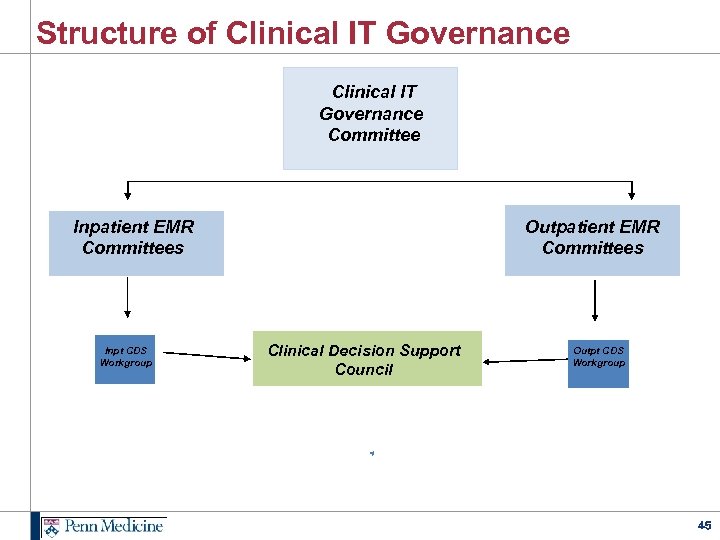
Structure of Clinical IT Governance Committee Inpatient EMR Committees Inpt CDS Workgroup Outpatient EMR Committees Clinical Decision Support Council Outpt CDS Workgroup 45
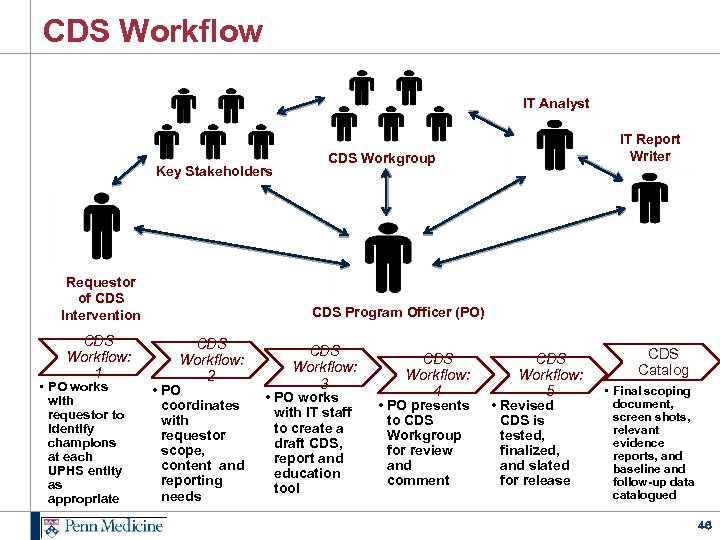
CDS Workflow IT Analyst Key Stakeholders Requestor of CDS Intervention CDS Workflow: 1 • PO works with requestor to identify champions at each UPHS entity as appropriate IT Report Writer CDS Workgroup CDS Program Officer (PO) CDS Workflow: 2 • PO coordinates with requestor scope, content and reporting needs CDS Workflow: 3 • PO works with IT staff to create a draft CDS, report and education tool CDS Workflow: 4 • PO presents to CDS Workgroup for review and comment CDS Workflow: 5 • Revised CDS is tested, finalized, and slated for release CDS Catalog • Final scoping document, screen shots, relevant evidence reports, and baseline and follow-up data catalogued 46

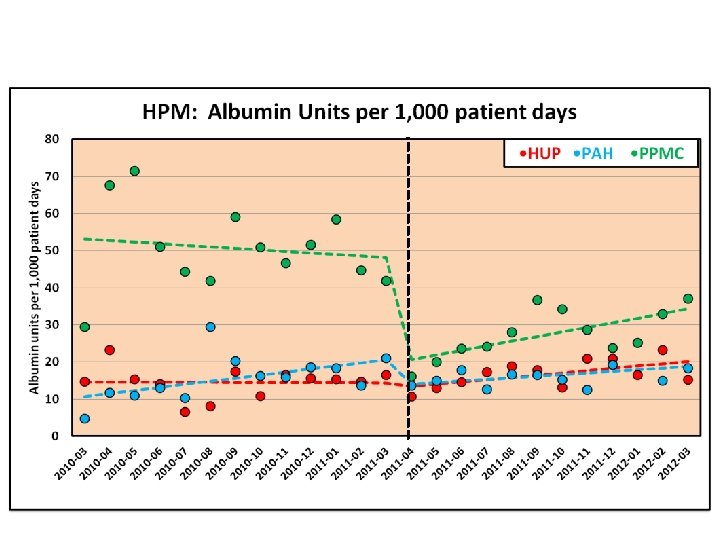
CEP CDS Interventions w 35 CEP reports have informed decision support interventions embedded in Penn’s electronic health record, including: • • Venous thromboembolism prophylaxis Readmission risk flag Foley catheter removal alert Albumin order set Red blood cell transfusion order set Nurse-driven protocol for vaccine assessment and administration Early warning system for sepsis Delirium management order set 47

223 pages! 48 48
CDS to Increase Use of Clot Prevention Meds 49

Clot Prevention CDS (continued) 50
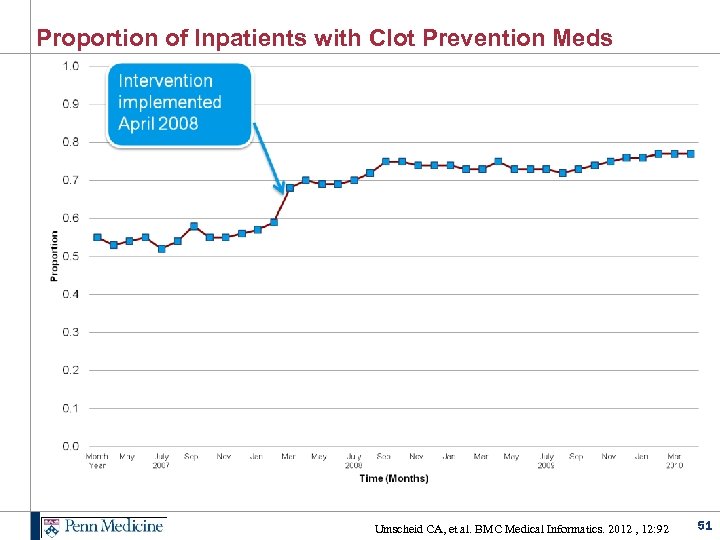
Proportion of Inpatients with Clot Prevention Meds Umscheid CA, et al. BMC Medical Informatics. 2012 , 12: 92 51
52 52
Risk Factors for 30 Day Readmission 53
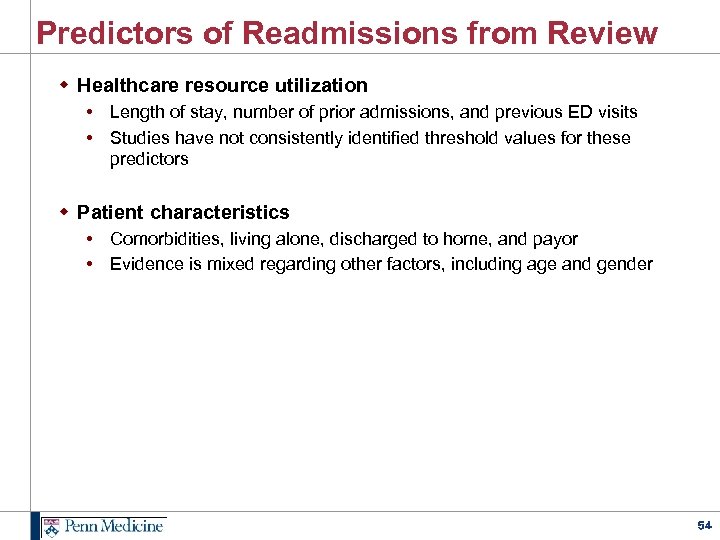
Predictors of Readmissions from Review w Healthcare resource utilization • Length of stay, number of prior admissions, and previous ED visits • Studies have not consistently identified threshold values for these predictors w Patient characteristics • Comorbidities, living alone, discharged to home, and payor • Evidence is mixed regarding other factors, including age and gender 54
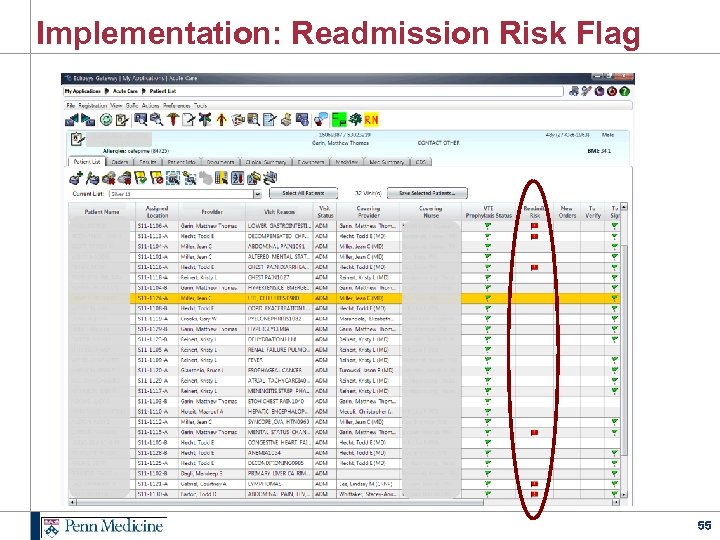
Implementation: Readmission Risk Flag 55
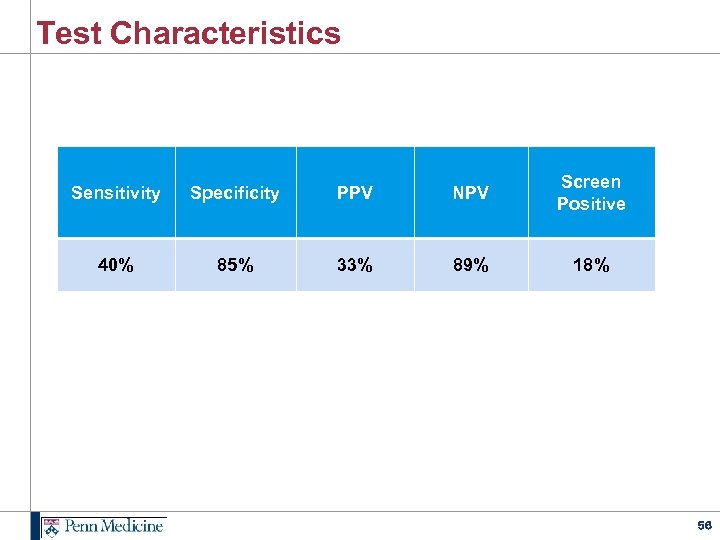
Test Characteristics Sensitivity Specificity PPV NPV Screen Positive 40% 85% 33% 89% 18% 56

CDC Guideline on Preventing Urinary Catheter Infections Full guideline at http: //www. cdc. gov/hicpac/index. html 57
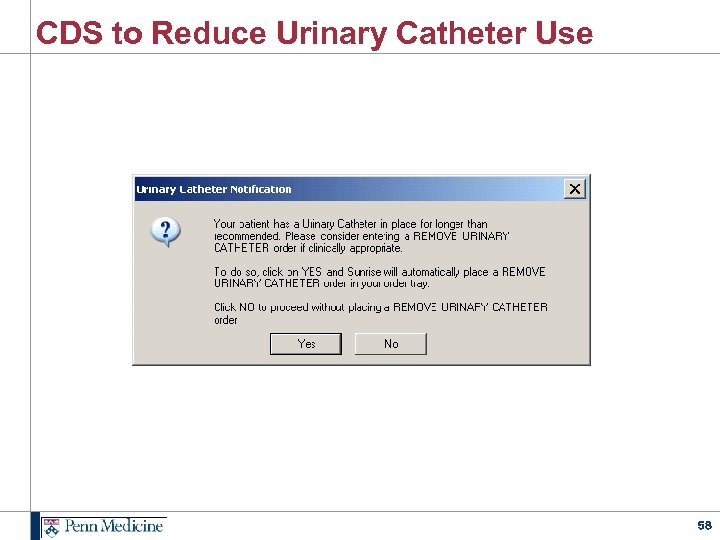
CDS to Reduce Urinary Catheter Use 58
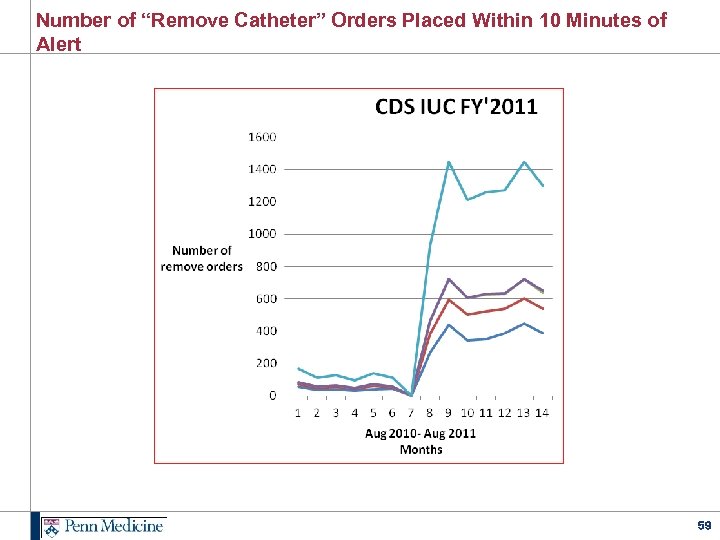
Number of “Remove Catheter” Orders Placed Within 10 Minutes of Alert 59
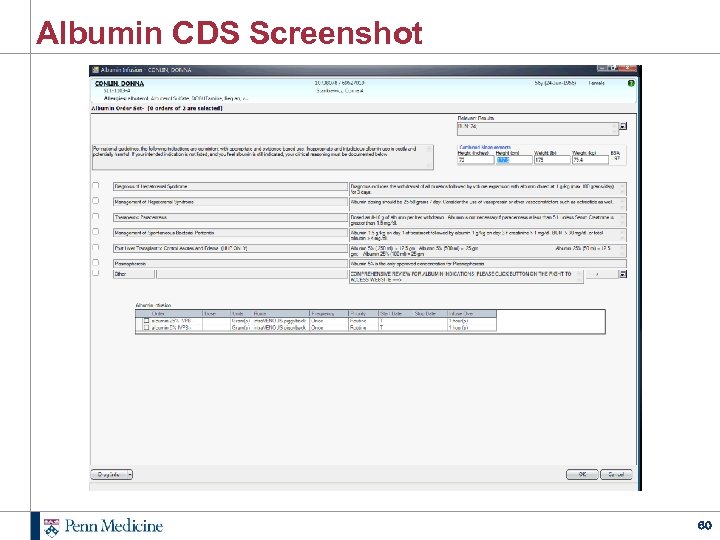
Albumin CDS Screenshot 60
CEP Educational Efforts Education Resident Education • • High Value Care Curriculum • Healthcare Systems Leadership and Quality Improvement Track • Clinical Investigator Toolbox “Academic detailing” thru distribution of Info. POEMs and PROVE • Critical Appraisal certificate course • Local and national conferences and workshops Fellow Education • Edu cati on Medical Student Education • Direct medical student EBM curriculum • Small group instructors in Epidemiology and Health Policy courses a c u Education d n E Systematic Review and Meta-analysis course Education Faculty and Staff Education 62
Back to Our Case: Chlorhexidine: $13 per patient Betadine: 60 cents per patient 63
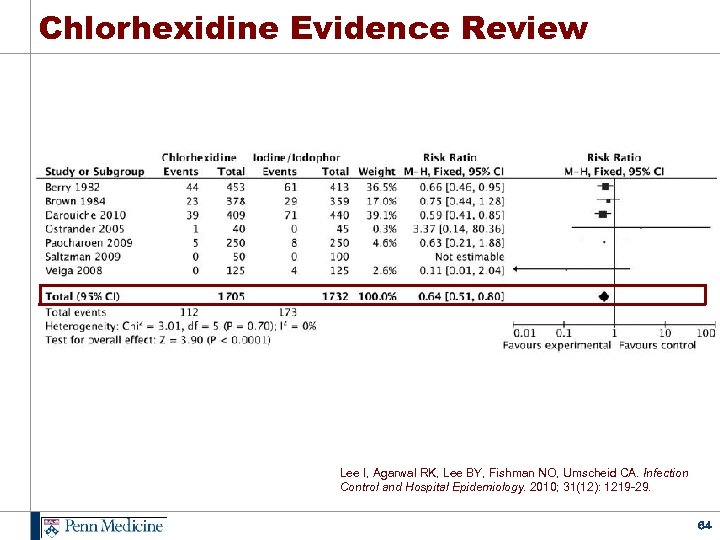
Chlorhexidine Evidence Review Lee I, Agarwal RK, Lee BY, Fishman NO, Umscheid CA. Infection Control and Hospital Epidemiology. 2010; 31(12): 1219 -29. 64
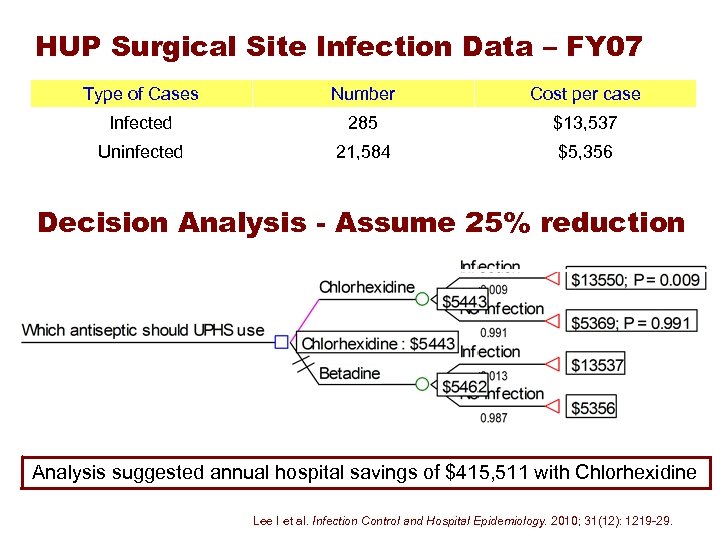
HUP Surgical Site Infection Data – FY 07 Type of Cases Number Cost per case Infected 285 $13, 537 Uninfected 21, 584 $5, 356 Decision Analysis - Assume 25% reduction Analysis suggested annual hospital savings of $415, 511 with Chlorhexidine Lee I et al. Infection Control and Hospital Epidemiology. 2010; 31(12): 1219 -29.

Conclusions w Evidence-based decision making impacts quality, safety and cost-effectiveness of care delivered to patients. w Despite this, infrastructures or centers to support such decision making in U. S. hospital and health care systems are not common. w Penn Medicine’s Center for Evidence Based Practice (CEP) is one of only a few academically based centers in the US with internal and external funding to support such work. w Penn’s CEP is enthusiastic about collaborating in the domains of operations, research and education to improve the quality, safety and value of care thru a systems approach to evidencebased practice. 66
Discussion craig. umscheid@uphs. upenn. edu http: //www. uphs. upenn. edu/cep/ 67
d9c2f427e1bb1bb52d2fea0be911551e.ppt