8330814c13703e030152a7a0e9d7d2e4.ppt
- Количество слайдов: 86

 PEDIATRIC ONCOLOGY: PAST, PRESENT AND FUTURE DIRECTIONS Stuart H. Gold The University of North Carolina at Chapel Hill
PEDIATRIC ONCOLOGY: PAST, PRESENT AND FUTURE DIRECTIONS Stuart H. Gold The University of North Carolina at Chapel Hill
 Conflicts I have no conflicts of interest I will discuss drugs used for non-FDA approved causes
Conflicts I have no conflicts of interest I will discuss drugs used for non-FDA approved causes
 Important principals to understand in Childhood Cancer Pediatric oncology cooperative groups Common childhood cancer types Childhood ALL as a paradigm Importance of role of cytogenetics in ALL advances Targeted/novel therapies Late effects What the present and future may hold
Important principals to understand in Childhood Cancer Pediatric oncology cooperative groups Common childhood cancer types Childhood ALL as a paradigm Importance of role of cytogenetics in ALL advances Targeted/novel therapies Late effects What the present and future may hold
 Case Presentation 1978 3 yo WM – Dx ALL – precursor B, WBC 9 k, induced with 3 drugs, progressive disease and CNS involvement, died without ever leaving the hospital with intractable seizures
Case Presentation 1978 3 yo WM – Dx ALL – precursor B, WBC 9 k, induced with 3 drugs, progressive disease and CNS involvement, died without ever leaving the hospital with intractable seizures
 Case Presentation 1982 5 year old WM- Dx ALL – precursor B – chromosomes revealed t(9; 22), Philadelphia chromosome, unclear of significance, treated with a 4 drug induction, early relapse, unable to get back into remission, and died 6 months after diagnosis
Case Presentation 1982 5 year old WM- Dx ALL – precursor B – chromosomes revealed t(9; 22), Philadelphia chromosome, unclear of significance, treated with a 4 drug induction, early relapse, unable to get back into remission, and died 6 months after diagnosis
 Case Presentation 1985 15 yo WM, WBC 35 k, precursor B ALL, cytogenetics revealed t(4; 11), bone marrow monthly for 2 years, no return of cytogenetic abnormality
Case Presentation 1985 15 yo WM, WBC 35 k, precursor B ALL, cytogenetics revealed t(4; 11), bone marrow monthly for 2 years, no return of cytogenetic abnormality
 Childhood Cancer 2% of all cancers occur in kids 10% of all childhood deaths Leading cause of death from disease Genetic lessons - heritable cancers Cooperative group trials – lessons Good cure rates
Childhood Cancer 2% of all cancers occur in kids 10% of all childhood deaths Leading cause of death from disease Genetic lessons - heritable cancers Cooperative group trials – lessons Good cure rates
 US Incidence of Childhood Cancer (S. E. E. R. Data) 0 -15 yo 9000 cases/yr 15 -19 yo 3700 cases/yr 19 -21 yo 1500 cases/yr ~2500 children and adolescents die yearly of cancer each year
US Incidence of Childhood Cancer (S. E. E. R. Data) 0 -15 yo 9000 cases/yr 15 -19 yo 3700 cases/yr 19 -21 yo 1500 cases/yr ~2500 children and adolescents die yearly of cancer each year
 Adult Cancer Adult cancer – 1. 5 million per year Prostate 219 k cases per year Lung 214 k cases per year Breast 180 k cases per year
Adult Cancer Adult cancer – 1. 5 million per year Prostate 219 k cases per year Lung 214 k cases per year Breast 180 k cases per year
 Childhood Cancer - Types AL - 23% (ALL: AML 4: 1) CNS - 21% Neuroblastoma - 7% Lymphoma - 6% Wilms’ Tumor - 6% Hodgkins - 5% Rhabdomyosarcoma - 3% Retinoblastoma - 3% Osteogenic Sarcoma - 2. 5% Ewing’s Sarcoma - 2%
Childhood Cancer - Types AL - 23% (ALL: AML 4: 1) CNS - 21% Neuroblastoma - 7% Lymphoma - 6% Wilms’ Tumor - 6% Hodgkins - 5% Rhabdomyosarcoma - 3% Retinoblastoma - 3% Osteogenic Sarcoma - 2. 5% Ewing’s Sarcoma - 2%
 Cooperative Groups Children’s Oncology Group A success story
Cooperative Groups Children’s Oncology Group A success story
 History 1955 First NCI sponsored cooperative group Acute Leukemia Chemotherapy Cooperative Study Group A 9 institutions First protocol: 6 MP vrs Azaserine for ALL N=125 Protocol was 9 pages Study lasted one year
History 1955 First NCI sponsored cooperative group Acute Leukemia Chemotherapy Cooperative Study Group A 9 institutions First protocol: 6 MP vrs Azaserine for ALL N=125 Protocol was 9 pages Study lasted one year
 History 1965 - Name change Children’s Cancer Study Group A 1969 - 1000 patients First phase I/II trials Surgical and Radiation Therapy Committees 1970 - Biology and Translational Research 1979 - Nursing, late effects, supportive care committees. Pathology ref ctr
History 1965 - Name change Children’s Cancer Study Group A 1969 - 1000 patients First phase I/II trials Surgical and Radiation Therapy Committees 1970 - Biology and Translational Research 1979 - Nursing, late effects, supportive care committees. Pathology ref ctr
 Tissue Banking - 2005 >15, 000 COG specimens provided to investigators >130 investigators received COG specimens 93 COG publications
Tissue Banking - 2005 >15, 000 COG specimens provided to investigators >130 investigators received COG specimens 93 COG publications
 History 2000 - the merger CCG + POG + NWTS + IRSG = Children’s Oncology Group 2013 - COG 8300 Members 222 Institutions Representing - US, Canada, Puerto Rico, Australia, Switzerland, Netherlands, New Zealand, Israel
History 2000 - the merger CCG + POG + NWTS + IRSG = Children’s Oncology Group 2013 - COG 8300 Members 222 Institutions Representing - US, Canada, Puerto Rico, Australia, Switzerland, Netherlands, New Zealand, Israel
 History <15 yo - >90% oncology pts on CCG/POG trials 15 -19 - <25% oncology pts enrolled Survival 10 yr post diagnosis 82%
History <15 yo - >90% oncology pts on CCG/POG trials 15 -19 - <25% oncology pts enrolled Survival 10 yr post diagnosis 82%
 History ~5000 patients enrolled per year on COG trials ~45, 000 cancer survivors in active follow-up
History ~5000 patients enrolled per year on COG trials ~45, 000 cancer survivors in active follow-up
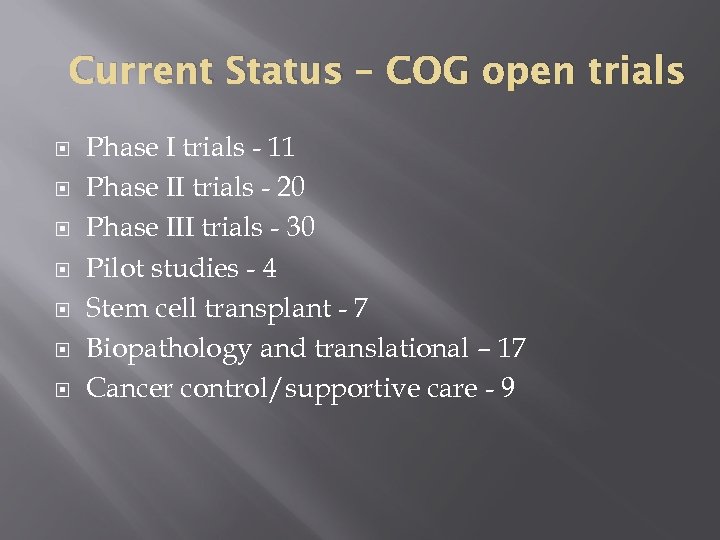 Current Status – COG open trials Phase I trials - 11 Phase II trials - 20 Phase III trials - 30 Pilot studies - 4 Stem cell transplant - 7 Biopathology and translational – 17 Cancer control/supportive care - 9
Current Status – COG open trials Phase I trials - 11 Phase II trials - 20 Phase III trials - 30 Pilot studies - 4 Stem cell transplant - 7 Biopathology and translational – 17 Cancer control/supportive care - 9
 UNC COG Open Trials Open to accrual 44 Closed to accrual but in f/u 40 Phase I – 1* Phase II – 8* Phase III – 19* Biology – 8 Other – 5* Three more studies to open soon 21 investigational drug studies
UNC COG Open Trials Open to accrual 44 Closed to accrual but in f/u 40 Phase I – 1* Phase II – 8* Phase III – 19* Biology – 8 Other – 5* Three more studies to open soon 21 investigational drug studies
 Protocol/Data Management Importance of data management COG with 1100 CRA UNC – 3 data managers Regulatory/supervisory/general General BMT and phase I/II
Protocol/Data Management Importance of data management COG with 1100 CRA UNC – 3 data managers Regulatory/supervisory/general General BMT and phase I/II
 Current complexities All these studies… PRC IRB Consents Annual Renewals Amendments Adverse events reporting Auditing HIPAA Finances CIRB 4 data deadlines per year – data score 99. 3%
Current complexities All these studies… PRC IRB Consents Annual Renewals Amendments Adverse events reporting Auditing HIPAA Finances CIRB 4 data deadlines per year – data score 99. 3%
 ALL Example Year Pt # Time Design #pages Consent 3 yr EFS 1955 125 1 yr to complete 2 arm study 9 0% 2005 ~4500 >5 yrs 5 arm 209 36 page >90%
ALL Example Year Pt # Time Design #pages Consent 3 yr EFS 1955 125 1 yr to complete 2 arm study 9 0% 2005 ~4500 >5 yrs 5 arm 209 36 page >90%
 ALL COG Trials - 2013 1 classification trial 5 trials for new dx 6 trials for recurrent disease 2 biology trials
ALL COG Trials - 2013 1 classification trial 5 trials for new dx 6 trials for recurrent disease 2 biology trials
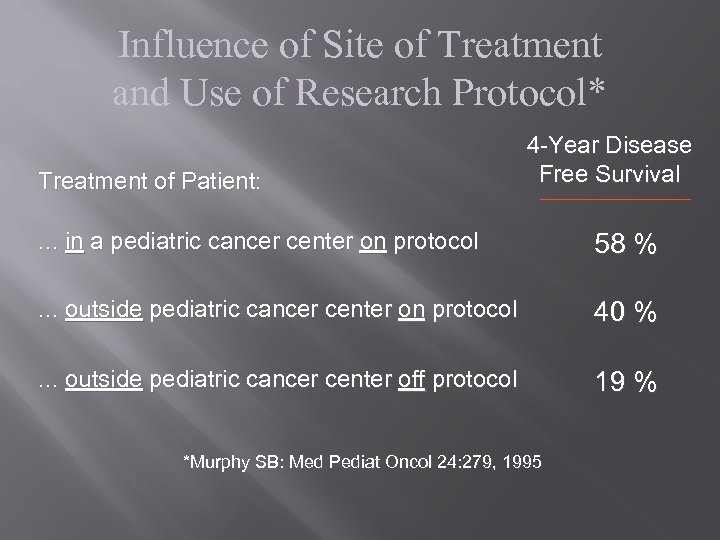 Influence of Site of Treatment and Use of Research Protocol* Treatment of Patient: 4 -Year Disease Free Survival . . . in a pediatric cancer center on protocol 58 % . . . outside pediatric cancer center on protocol 40 % . . . outside pediatric cancer center off protocol 19 % *Murphy SB: Med Pediat Oncol 24: 279, 1995
Influence of Site of Treatment and Use of Research Protocol* Treatment of Patient: 4 -Year Disease Free Survival . . . in a pediatric cancer center on protocol 58 % . . . outside pediatric cancer center on protocol 40 % . . . outside pediatric cancer center off protocol 19 % *Murphy SB: Med Pediat Oncol 24: 279, 1995
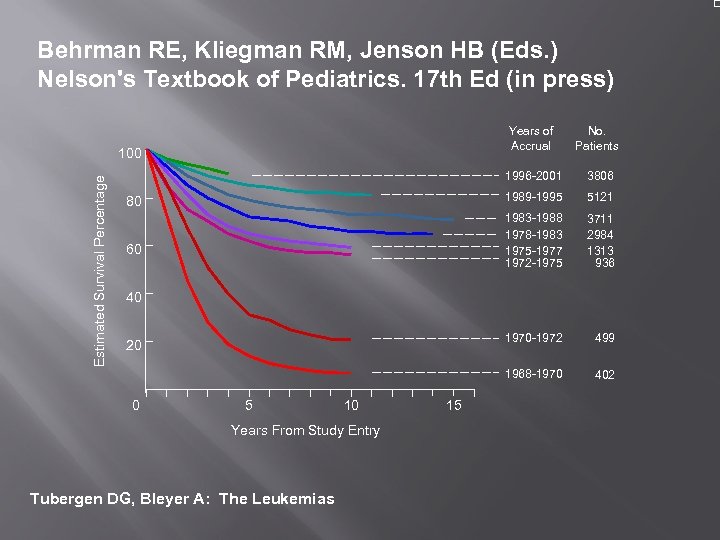 Behrman RE, Kliegman RM, Jenson HB (Eds. ) Nelson's Textbook of Pediatrics. 17 th Ed (in press) Years of Accrual 1996 -2001 499 1968 -1970 Estimated Survival Percentage 3711 2984 1313 936 1970 -1972 60 5121 1983 -1988 1978 -1983 1975 -1977 1972 -1975 80 3806 1989 -1995 100 No. Patients 402 40 20 0 5 10 Years From Study Entry Tubergen DG, Bleyer A: The Leukemias 15
Behrman RE, Kliegman RM, Jenson HB (Eds. ) Nelson's Textbook of Pediatrics. 17 th Ed (in press) Years of Accrual 1996 -2001 499 1968 -1970 Estimated Survival Percentage 3711 2984 1313 936 1970 -1972 60 5121 1983 -1988 1978 -1983 1975 -1977 1972 -1975 80 3806 1989 -1995 100 No. Patients 402 40 20 0 5 10 Years From Study Entry Tubergen DG, Bleyer A: The Leukemias 15
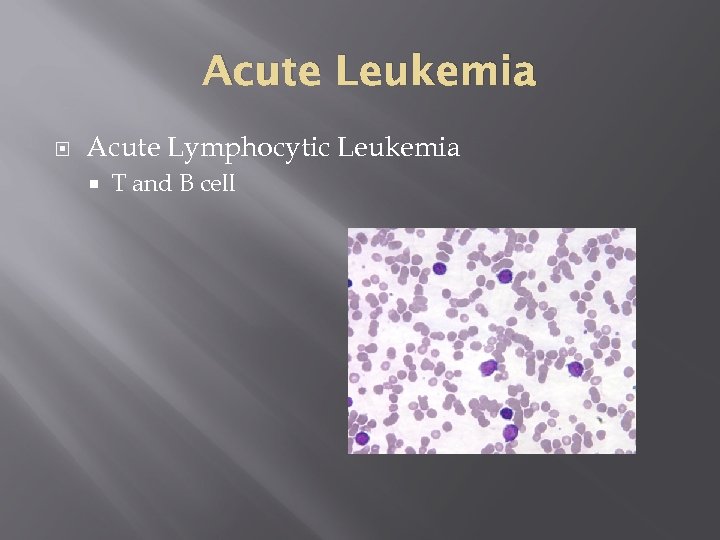 Acute Leukemia Acute Lymphocytic Leukemia T and B cell
Acute Leukemia Acute Lymphocytic Leukemia T and B cell
 ALL Most common childhood malignancy (25% of all pediatric cancers); 2500/yr in US Peak 2 -5 yr in industrialized countries More common in whites than AA, M>F (esp T cell)
ALL Most common childhood malignancy (25% of all pediatric cancers); 2500/yr in US Peak 2 -5 yr in industrialized countries More common in whites than AA, M>F (esp T cell)
 Presentation - AL Fever, purpura, fatigue Organomegaly, adenopathy Arthralgias, bone pain, arthritis Head ache, cranial nerve palsy Prolonged “viral illness” Skin rash Bleeding
Presentation - AL Fever, purpura, fatigue Organomegaly, adenopathy Arthralgias, bone pain, arthritis Head ache, cranial nerve palsy Prolonged “viral illness” Skin rash Bleeding
 ALL-Initial work-up Physical exam: testes, cranial nerves, papilledema, lymphadenopathy, organomegally Labs: cbc/diff; coags; chemistries, type and cross; cxr Blood smear review
ALL-Initial work-up Physical exam: testes, cranial nerves, papilledema, lymphadenopathy, organomegally Labs: cbc/diff; coags; chemistries, type and cross; cxr Blood smear review
 Oncologic Emergencies Tumor Lysis Leukocytosis Superior vena cava syndrome Fever, Infection and neutropenia
Oncologic Emergencies Tumor Lysis Leukocytosis Superior vena cava syndrome Fever, Infection and neutropenia
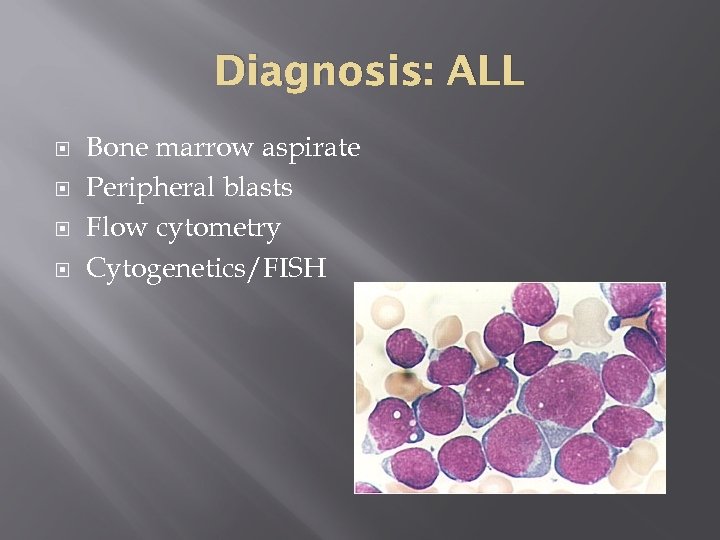 Diagnosis: ALL Bone marrow aspirate Peripheral blasts Flow cytometry Cytogenetics/FISH
Diagnosis: ALL Bone marrow aspirate Peripheral blasts Flow cytometry Cytogenetics/FISH
 Flow Cytometry at Diagnosis Each cell is counted and identified by specific cell surface markers Rapid test, blood or bone marrow Flow markers are patient specific Useful on future marrows to pick up minimal residual disease - MRD Helpful in defining a recurrence
Flow Cytometry at Diagnosis Each cell is counted and identified by specific cell surface markers Rapid test, blood or bone marrow Flow markers are patient specific Useful on future marrows to pick up minimal residual disease - MRD Helpful in defining a recurrence
 Cytogenetics at Diagnosis May take a while but some translocations assure a correct dx {Kathleen Rao} APML t(15; 17) CML t(9; 22) Burkitt’s Lymphoma t(8; 14) Solid tumors can mimic leukemia
Cytogenetics at Diagnosis May take a while but some translocations assure a correct dx {Kathleen Rao} APML t(15; 17) CML t(9; 22) Burkitt’s Lymphoma t(8; 14) Solid tumors can mimic leukemia
 Cytogenetics Numerical changes Most are diploid or hyper dip (>50 chrom); hyperdipoid is good; hypodiploid is bad Trisomy – 4, 10 – favorable prognosis Structural changes TEL-AML: t(12; 21)(p 12; q 22) - good prognosis t(9; 22) – Philadelphia chromosome - bad t(4; 11), MLL gene rearrangement – bad
Cytogenetics Numerical changes Most are diploid or hyper dip (>50 chrom); hyperdipoid is good; hypodiploid is bad Trisomy – 4, 10 – favorable prognosis Structural changes TEL-AML: t(12; 21)(p 12; q 22) - good prognosis t(9; 22) – Philadelphia chromosome - bad t(4; 11), MLL gene rearrangement – bad
 Prognostic features Age 1 -10 yo best White count <50 k best Immunophenotype – B cell best Ploidy – hypodiploid bad, hyperdiploid good Cytogenetics Rapidity of response to treatment Minimal residual disease
Prognostic features Age 1 -10 yo best White count <50 k best Immunophenotype – B cell best Ploidy – hypodiploid bad, hyperdiploid good Cytogenetics Rapidity of response to treatment Minimal residual disease
 Risk Groups: ALL Group wbc (/mm 3) standard <50, 000 Standard/low<50, 000 high >50, 000 infant T-cell Additional risk features: Minimal residual disease cytogenetics age(yr) 1 -9. 99 >10 (>13) <1 (<6 mo)
Risk Groups: ALL Group wbc (/mm 3) standard <50, 000 Standard/low<50, 000 high >50, 000 infant T-cell Additional risk features: Minimal residual disease cytogenetics age(yr) 1 -9. 99 >10 (>13) <1 (<6 mo)
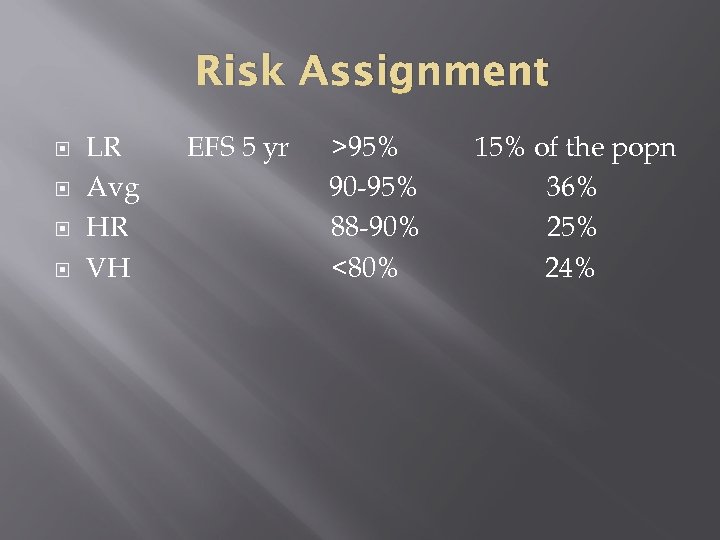 Risk Assignment LR Avg HR VH EFS 5 yr >95% 90 -95% 88 -90% <80% 15% of the popn 36% 25% 24%
Risk Assignment LR Avg HR VH EFS 5 yr >95% 90 -95% 88 -90% <80% 15% of the popn 36% 25% 24%
 Minimal Residual Disease - MRD Morphology – 2 -3% Routine flow cytometry - ~1% Flow cytometry MRD – 0. 01% PCR – 0. 001% Currently use 0. 01% at day 28 as positive
Minimal Residual Disease - MRD Morphology – 2 -3% Routine flow cytometry - ~1% Flow cytometry MRD – 0. 01% PCR – 0. 001% Currently use 0. 01% at day 28 as positive
 MRD – Bone Marrow d 29 4 year EFS <0. 01% 88% 0. 01 -0. 1 68% >0. 1 51%
MRD – Bone Marrow d 29 4 year EFS <0. 01% 88% 0. 01 -0. 1 68% >0. 1 51%
 Treatment Outline Induction, consolidation, interim maintenance, delayed intensification, maintenance Induction remission rates 95%+ Total treatment over 2 years for girls, 3 years for boys Intensive therapy for first ~8 months, weekly visits
Treatment Outline Induction, consolidation, interim maintenance, delayed intensification, maintenance Induction remission rates 95%+ Total treatment over 2 years for girls, 3 years for boys Intensive therapy for first ~8 months, weekly visits
 Major ALL Lessons Learned Delayed intensification, important Cranial Irradiation almost elliminated Rapidity of bone marrow response Dexamethasone improved outcome But not without toxicity Cytogenetics Minimal residual disease Prognostic features may change with therapy advances
Major ALL Lessons Learned Delayed intensification, important Cranial Irradiation almost elliminated Rapidity of bone marrow response Dexamethasone improved outcome But not without toxicity Cytogenetics Minimal residual disease Prognostic features may change with therapy advances
 Classification Study – ALL New things…. . Getting away from lots of bone marrows Risk stratifies Good cytogenetics – Trisomy 4, 10, t(12; 21) i. AMP 21 – new and bad
Classification Study – ALL New things…. . Getting away from lots of bone marrows Risk stratifies Good cytogenetics – Trisomy 4, 10, t(12; 21) i. AMP 21 – new and bad
 i. AMP 21 Intrachromosomal amplification of at least 4 copies of RUNX 1 on a single chromo Detected by FISH ~2% pre. B ALL Tend to be: older, lower WBC, 3 x risk of recurrence
i. AMP 21 Intrachromosomal amplification of at least 4 copies of RUNX 1 on a single chromo Detected by FISH ~2% pre. B ALL Tend to be: older, lower WBC, 3 x risk of recurrence
 i. AMP 21 - History Prior high risk trial Prior standard risk trial 70% vrs 88% EFS 81% vrs 94% EFS For both MRD positive or neg If i. AMP 21 positive on new avg risk trial Off protocol and to new HR protocol to VHR arm (<80% survival)
i. AMP 21 - History Prior high risk trial Prior standard risk trial 70% vrs 88% EFS 81% vrs 94% EFS For both MRD positive or neg If i. AMP 21 positive on new avg risk trial Off protocol and to new HR protocol to VHR arm (<80% survival)
 New lessons – Induction Dex vrs Pred Dexamethasone is better Dex – lower albumin, higher glu, more F/N, more infections, no diff in induction deaths Dex in induction predicts higher osteonecrosis >/= 10 yo Over 10 yo use pred, under dex Discontinuous dex in DI
New lessons – Induction Dex vrs Pred Dexamethasone is better Dex – lower albumin, higher glu, more F/N, more infections, no diff in induction deaths Dex in induction predicts higher osteonecrosis >/= 10 yo Over 10 yo use pred, under dex Discontinuous dex in DI
 New Lessons – Interim Maintenance High dose methotrexate better than escalating dose methotrexate for the high risk patient HD MTX no diff in toxicity No diff in neuro, sz, mucositis Decrease in BM and CNS recurrences Much higher cost and hospitalizations
New Lessons – Interim Maintenance High dose methotrexate better than escalating dose methotrexate for the high risk patient HD MTX no diff in toxicity No diff in neuro, sz, mucositis Decrease in BM and CNS recurrences Much higher cost and hospitalizations
 New Lessons - HR Methotrexate n=1209 Capizzi n=1217 BM relapse CNS DFS 82% vrs 75% 5. 5% 2. 6% HD MTX 3. 4% 1. 8%
New Lessons - HR Methotrexate n=1209 Capizzi n=1217 BM relapse CNS DFS 82% vrs 75% 5. 5% 2. 6% HD MTX 3. 4% 1. 8%
 Risk Adapted Strategies: Very High Risk Patients VHR >13 or CNS 3 or i. AMP 21 or MLL-R or hypodip MRD>0. 01% d 29 or Induction failures Trying new drug combinations Cyclophosphamid/Etoposide vrs Clofarabine/Cyclophosphamide/Etoposide Clofarabine arm too toxic
Risk Adapted Strategies: Very High Risk Patients VHR >13 or CNS 3 or i. AMP 21 or MLL-R or hypodip MRD>0. 01% d 29 or Induction failures Trying new drug combinations Cyclophosphamid/Etoposide vrs Clofarabine/Cyclophosphamide/Etoposide Clofarabine arm too toxic
 Lower Risk Patients See if can avoid anthracycline and alkylators …. . Reducing therapy and potential of long term side effect by adding moderate dose methotrexate Two imbedded QOL studies
Lower Risk Patients See if can avoid anthracycline and alkylators …. . Reducing therapy and potential of long term side effect by adding moderate dose methotrexate Two imbedded QOL studies
 Future - present Targeted Therapies Monoclonals Newer agents – Eg Tyrosine Kinase Inhibitors Refining chemotherapy Refining bone marrow transplant Improved supportive care Gene therapy Recognition and prevention and more research
Future - present Targeted Therapies Monoclonals Newer agents – Eg Tyrosine Kinase Inhibitors Refining chemotherapy Refining bone marrow transplant Improved supportive care Gene therapy Recognition and prevention and more research
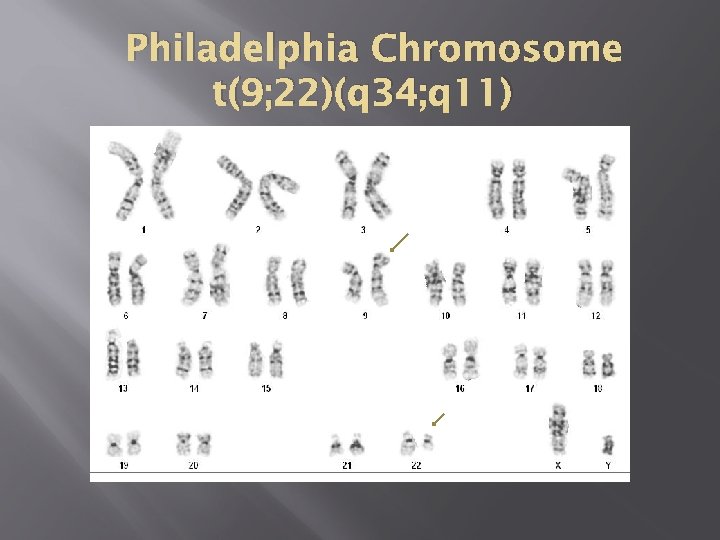 Philadelphia Chromosome t(9; 22)(q 34; q 11)
Philadelphia Chromosome t(9; 22)(q 34; q 11)
 Philadelphia Chromosome Breakpoint on chr 9 – c-abl Breakpoint on chr 22 – BCR Two new hybrid genes – bcr/abl on 22 is the major actor Protein product p 210 – augmented tyrosine kinase activity and autophosphorylates, translocated into the cytoplasm
Philadelphia Chromosome Breakpoint on chr 9 – c-abl Breakpoint on chr 22 – BCR Two new hybrid genes – bcr/abl on 22 is the major actor Protein product p 210 – augmented tyrosine kinase activity and autophosphorylates, translocated into the cytoplasm
 Imatinib First target therapy against tyrosine kinases Specifically blocks the activity of the bcr/abl protein Revolutionalized CML therapy… and now Ph+ ALL
Imatinib First target therapy against tyrosine kinases Specifically blocks the activity of the bcr/abl protein Revolutionalized CML therapy… and now Ph+ ALL
 Ph+ ALL Cytogenetics looks the same Different breakpoint Protein product p 190 Historically poor prognosis
Ph+ ALL Cytogenetics looks the same Different breakpoint Protein product p 190 Historically poor prognosis
 Ph+ ALL Chemotherapy 20 -30% cure Allo BMT 60% Chemo + Imatinib 88% 3 yr EFS Expert Rev Hematol. 2010 Dec; 3(6): 731 -42. doi: 10. 1586/ehm. 10. 60. Philadelphia chromosome-positive acute lymphoblastic leukemia in children: new and emerging treatment options. Schultz KR, Prestidge T, Camitta B. Source Division of Pedatric Hematology, Oncology, Blood and Marrow Transplantation, British Columbia Children's Hospital, University of British Columbia, Vancouver, BC, Canada. kschultz@interchange. ubc. ca
Ph+ ALL Chemotherapy 20 -30% cure Allo BMT 60% Chemo + Imatinib 88% 3 yr EFS Expert Rev Hematol. 2010 Dec; 3(6): 731 -42. doi: 10. 1586/ehm. 10. 60. Philadelphia chromosome-positive acute lymphoblastic leukemia in children: new and emerging treatment options. Schultz KR, Prestidge T, Camitta B. Source Division of Pedatric Hematology, Oncology, Blood and Marrow Transplantation, British Columbia Children's Hospital, University of British Columbia, Vancouver, BC, Canada. kschultz@interchange. ubc. ca
 Infant ALL – New Therapy Bad disease, esp under 6 mo with MLL-R FLT 3 kinase - highly expressed/activated in MLL-R ALL Lestaurtinib (CEP-701) selectively kills MLL-R cells (both in vivo and in vitro) No advantage to BMT over chemo
Infant ALL – New Therapy Bad disease, esp under 6 mo with MLL-R FLT 3 kinase - highly expressed/activated in MLL-R ALL Lestaurtinib (CEP-701) selectively kills MLL-R cells (both in vivo and in vitro) No advantage to BMT over chemo
 Other new ALL retrieval therapies -New vincristine strategies -Bortezomib – proteosome inhibitor -Blinatumomab – monoclonal antibody, bi-specific T-cell engager (Bi. TEs)– targets CD 19 -MLN 8237 – selective aurora kinase inhibitor -INCB 018424 - inhibits JAK family of kinases -Temsirolimus –m-Tor inhibitor
Other new ALL retrieval therapies -New vincristine strategies -Bortezomib – proteosome inhibitor -Blinatumomab – monoclonal antibody, bi-specific T-cell engager (Bi. TEs)– targets CD 19 -MLN 8237 – selective aurora kinase inhibitor -INCB 018424 - inhibits JAK family of kinases -Temsirolimus –m-Tor inhibitor
 Targeted and Novel Therapies NOT just for ALL For example: AML Lymphoma Neuroblastoma
Targeted and Novel Therapies NOT just for ALL For example: AML Lymphoma Neuroblastoma
 AML Gemtuzumab ozogamicin – anti CD 33 linked to cholicheamicin Bortezomib – proteosome inhibitor Sarafenib – multitargeted tyrosine kinase inhibitor
AML Gemtuzumab ozogamicin – anti CD 33 linked to cholicheamicin Bortezomib – proteosome inhibitor Sarafenib – multitargeted tyrosine kinase inhibitor
 Lymphoma Burkitt’s Lymphoma Rituximab – anti-CD 20 Combined with our standard chemotherapy backbone in high risk mature B cell lymphoma patients
Lymphoma Burkitt’s Lymphoma Rituximab – anti-CD 20 Combined with our standard chemotherapy backbone in high risk mature B cell lymphoma patients
 Neuroblastoma – a success story Anti-GD 2 monoclonal antibodies Uniformly expressed in neuroblastoma Normal: neurons, peripheral nerve fibers, skin melanocytes Targeted immunotherapy Passive immunotherapy – antibody dependent cellular toxicity
Neuroblastoma – a success story Anti-GD 2 monoclonal antibodies Uniformly expressed in neuroblastoma Normal: neurons, peripheral nerve fibers, skin melanocytes Targeted immunotherapy Passive immunotherapy – antibody dependent cellular toxicity
 Neuroblastoma Given with IL-2 and GM-CSF Pain, vascular leak, hypersensitivity 2 year EFS 66% vrs 46% (p=0. 01) Yu AL, Gilman AL, Ozkaynak MF, et al: Anti-GD 2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363: 1324 -34, 2010
Neuroblastoma Given with IL-2 and GM-CSF Pain, vascular leak, hypersensitivity 2 year EFS 66% vrs 46% (p=0. 01) Yu AL, Gilman AL, Ozkaynak MF, et al: Anti-GD 2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 363: 1324 -34, 2010
 Neuroblastoma - MIBG Norepinephrine analogue Meta-iodobenzylguanidine Concentrated in the sympathetic nervous system I-123 – used radiographically I-131 – used therapeutically
Neuroblastoma - MIBG Norepinephrine analogue Meta-iodobenzylguanidine Concentrated in the sympathetic nervous system I-123 – used radiographically I-131 – used therapeutically
 Neuroblastoma 2 cycles chemo stem cell harvest 2 cycles of chemo surgery 1 cycle of chemo MIBG therapy with SCR Autologous bone marrow transplant radiation therapy immunotherapy (5 cycles chimeric antibody, GMCSF, IL 2, isotretinoin)
Neuroblastoma 2 cycles chemo stem cell harvest 2 cycles of chemo surgery 1 cycle of chemo MIBG therapy with SCR Autologous bone marrow transplant radiation therapy immunotherapy (5 cycles chimeric antibody, GMCSF, IL 2, isotretinoin)
 After Therapy Long term side effects and issues….
After Therapy Long term side effects and issues….
 Cancer Survivors – S. E. E. R. United States 270, 000 childhood cancer survivors 1/640 (20 -39 yo) is a cancer survivor
Cancer Survivors – S. E. E. R. United States 270, 000 childhood cancer survivors 1/640 (20 -39 yo) is a cancer survivor
 Late Effects Dependent on age of child Dependent on type of tumor and location Dependent on treatment Chemotherapy Irradiation therapy Surgery
Late Effects Dependent on age of child Dependent on type of tumor and location Dependent on treatment Chemotherapy Irradiation therapy Surgery
 Late Effects Growth/development - radiation, IT Cognitive - radiation, intrathecals Endocrine - radiation Infertility - alkylators Secondary malignancies - VP 16, Alkylators Cardiac (anthracyclines) Hepatotoxicity - 6 MP, 6 TG, MTX Pulmonary – bleo Bone – AVN - steroids
Late Effects Growth/development - radiation, IT Cognitive - radiation, intrathecals Endocrine - radiation Infertility - alkylators Secondary malignancies - VP 16, Alkylators Cardiac (anthracyclines) Hepatotoxicity - 6 MP, 6 TG, MTX Pulmonary – bleo Bone – AVN - steroids
 Late Effects Emotional
Late Effects Emotional
 Other Issues Transitioning back to primary care giver Transitioning to adult oncologist
Other Issues Transitioning back to primary care giver Transitioning to adult oncologist
 Case Presentation 1978 3 yo WM – Dx ALL – precursor B, WBC 9 k, induced with 3 drugs, progressive disease and CNS involvement, died without ever leaving the hospital with intractable seizures 2013 >95% children ALL achieve a remission Induction Deaths ~0. 005%
Case Presentation 1978 3 yo WM – Dx ALL – precursor B, WBC 9 k, induced with 3 drugs, progressive disease and CNS involvement, died without ever leaving the hospital with intractable seizures 2013 >95% children ALL achieve a remission Induction Deaths ~0. 005%
 Case Presentation 1982 5 year old WM- Dx ALL – precursor B – chromosomes revealed t(9; 22), unclear of significance, treated with a 4 drug induction, early relapse, unable to get back into remission, and died 6 months after diagnosis
Case Presentation 1982 5 year old WM- Dx ALL – precursor B – chromosomes revealed t(9; 22), unclear of significance, treated with a 4 drug induction, early relapse, unable to get back into remission, and died 6 months after diagnosis
 Case Presentation May 2009 7 yo, 1 week history of cough, fever, bruising and rt knee pain. Found to have pancytopenia and hepatosplenomegaly. WBC 3, hgb 8, plt 8 – precursor B ALL Standard Risk until cytogenetics returned Ph+
Case Presentation May 2009 7 yo, 1 week history of cough, fever, bruising and rt knee pain. Found to have pancytopenia and hepatosplenomegaly. WBC 3, hgb 8, plt 8 – precursor B ALL Standard Risk until cytogenetics returned Ph+
 Case Presentation Treated on Ph+ clinical trial with intensive chemotherapy + dasatinib Treated from 5/09 – 3/12 Off therapy BM, cytogenetics and FISH nl PCR 27/100, 000 + Treated with continuous dasatinib PCR 11/100, 00 + March 2013
Case Presentation Treated on Ph+ clinical trial with intensive chemotherapy + dasatinib Treated from 5/09 – 3/12 Off therapy BM, cytogenetics and FISH nl PCR 27/100, 000 + Treated with continuous dasatinib PCR 11/100, 00 + March 2013
 Case Presentation 1985 15 yo WM, WBC 35 k, precursor B ALL, cytogenetics revealed t(4; 11), bone marrow monthly for 2 years, no return of cytogenetic abnormality –treated as HR 2013 – married with children, Heme/onc nurse
Case Presentation 1985 15 yo WM, WBC 35 k, precursor B ALL, cytogenetics revealed t(4; 11), bone marrow monthly for 2 years, no return of cytogenetic abnormality –treated as HR 2013 – married with children, Heme/onc nurse
 Case presentation November 2012 15 month old, fever, cytopenias, septic hip, WBC 6. 3, hgb 5. 5, plt 9 Started treatment on standard risk Cytogenetics t(9; 11)(p 22; q 23) Moved to very high risk protocl Doing well
Case presentation November 2012 15 month old, fever, cytopenias, septic hip, WBC 6. 3, hgb 5. 5, plt 9 Started treatment on standard risk Cytogenetics t(9; 11)(p 22; q 23) Moved to very high risk protocl Doing well
 UNC Ped Heme/Onc Around since about 1960 1990 ~35 -40 new oncology pt per year 149 new children with cancer 2012 Adding a new physician (total 11) Added 1 new nurse practitioner (total 7) Two managers of our data, adding one more
UNC Ped Heme/Onc Around since about 1960 1990 ~35 -40 new oncology pt per year 149 new children with cancer 2012 Adding a new physician (total 11) Added 1 new nurse practitioner (total 7) Two managers of our data, adding one more
 UNC Ped Heme/Onc Eleven Physicians 5 General Ped Heme/Onc clinical –Phase 1/2 1 Hemophilia/thrombosis/clinical and bench research 2 BMT 1 Sickle cell 1 combo general heme/onc & bench research 1 Cellular therapies doc
UNC Ped Heme/Onc Eleven Physicians 5 General Ped Heme/Onc clinical –Phase 1/2 1 Hemophilia/thrombosis/clinical and bench research 2 BMT 1 Sickle cell 1 combo general heme/onc & bench research 1 Cellular therapies doc
 UNC Ped Heme Onc Seven nurse practitioners One sickle cell One bmt One inpt genl heme/onc Four outpt genl heme/onc
UNC Ped Heme Onc Seven nurse practitioners One sickle cell One bmt One inpt genl heme/onc Four outpt genl heme/onc
 UNC Programs Pediatric Oncology Brain Tumor Survivors clinic Pediatric Hematology Sickle cell Hemophilia Thrombosis Vascular Malformations Pediatric BMT/cellular Therapies/ID
UNC Programs Pediatric Oncology Brain Tumor Survivors clinic Pediatric Hematology Sickle cell Hemophilia Thrombosis Vascular Malformations Pediatric BMT/cellular Therapies/ID
 UNC Contact Main office 919 -966 -1178 Page operator 919 -966 -4131
UNC Contact Main office 919 -966 -1178 Page operator 919 -966 -4131
 UNC Ped Heme/Onc Hospital School teachers Recreational therapists Psychologists Art therapist Nurses – clinic and the ward Pediatric Surgeons Radiation Oncology
UNC Ped Heme/Onc Hospital School teachers Recreational therapists Psychologists Art therapist Nurses – clinic and the ward Pediatric Surgeons Radiation Oncology
 Ped Hem/Onc Pediatric Radiologists Cytogenetics folks Special hematology Pathology And all of our Pediatric consultants True team effort, multidisciplinary approach
Ped Hem/Onc Pediatric Radiologists Cytogenetics folks Special hematology Pathology And all of our Pediatric consultants True team effort, multidisciplinary approach



