6533bb442e9c2c48cf4473456a5eb279.ppt
- Количество слайдов: 81
 Pediatric Hearing Loss
Pediatric Hearing Loss
 Introduction • Hearing loss is common • 1 in 1000 babies have significant hearing loss at birth • 10 in 1000 children have significant hearing loss at school (inc. mild and moderate) • 80% of all children will have at least one middle ear infection
Introduction • Hearing loss is common • 1 in 1000 babies have significant hearing loss at birth • 10 in 1000 children have significant hearing loss at school (inc. mild and moderate) • 80% of all children will have at least one middle ear infection
 And Mild Unilateral Hearing Loss has an Impact. . . • Significant reduction in language acquisition and speech with prolonged mild unilateral hearing loss. http: //www. cdc. gov/ncbddd/ehdi/documents/unilateralh l/Mild_Uni_2005%20 Workshop_Proceedings. pdf
And Mild Unilateral Hearing Loss has an Impact. . . • Significant reduction in language acquisition and speech with prolonged mild unilateral hearing loss. http: //www. cdc. gov/ncbddd/ehdi/documents/unilateralh l/Mild_Uni_2005%20 Workshop_Proceedings. pdf
 Paediatric Sensorineural Loss • Sensorineural Hearing Loss (Nerve deafness) – Genetic and Idiopathic (70%) – Acquired (30%) • Low birth weight/ NICU residents • Hyperbilirubinaemia • Ear and Head trauma • Anoxia and Hypoxia • Ototoxic Drugs and Chemicals • Infectious Diseases • Recurrent otitis media
Paediatric Sensorineural Loss • Sensorineural Hearing Loss (Nerve deafness) – Genetic and Idiopathic (70%) – Acquired (30%) • Low birth weight/ NICU residents • Hyperbilirubinaemia • Ear and Head trauma • Anoxia and Hypoxia • Ototoxic Drugs and Chemicals • Infectious Diseases • Recurrent otitis media
 Diagnosis • SWISH (Statewide Infant Hearing Screening Programme) aims to test every child born in NSW and identify significant hearing loss by the age of 3 months • Parental (other carers) clues • Family physician
Diagnosis • SWISH (Statewide Infant Hearing Screening Programme) aims to test every child born in NSW and identify significant hearing loss by the age of 3 months • Parental (other carers) clues • Family physician
 Diagnosis • History ✦ Risk factors for SNHL ✦ Auditory milestones: – 3 months: Startled by loud sounds and calmed by familiar sounds – 6 months: ability to localise – 9 months: respond to name & mimic environmental sounds – 12 months: First meaningful words – 18 months: Vocabulary of 20 words or more – 24 months: Small sentences
Diagnosis • History ✦ Risk factors for SNHL ✦ Auditory milestones: – 3 months: Startled by loud sounds and calmed by familiar sounds – 6 months: ability to localise – 9 months: respond to name & mimic environmental sounds – 12 months: First meaningful words – 18 months: Vocabulary of 20 words or more – 24 months: Small sentences
 Diagnosis • Examination – Local • • • Auricle Canal Tympanic membrane (pneumatic otoscopy) – Regional • Syndromic features – Tuning Forks • Difficult under 6 yrs
Diagnosis • Examination – Local • • • Auricle Canal Tympanic membrane (pneumatic otoscopy) – Regional • Syndromic features – Tuning Forks • Difficult under 6 yrs
 Treatment for Paediatric Sensorineural Hearing Loss • Hearing assistive techniques – Preferential seating – Techniques to use at home • Rehabilitation – Hearing Aids – FM systems • Cochlear Implantation
Treatment for Paediatric Sensorineural Hearing Loss • Hearing assistive techniques – Preferential seating – Techniques to use at home • Rehabilitation – Hearing Aids – FM systems • Cochlear Implantation
 Etiology § Congenital HL § 50% Genetic § 50% Acquired § Childhood Onset HL § 50% Genetic § 25% Acquired § 25% Unknown
Etiology § Congenital HL § 50% Genetic § 50% Acquired § Childhood Onset HL § 50% Genetic § 25% Acquired § 25% Unknown
 Genetic HL § 75% non-syndromal § 25% syndromal § § 75% autosomal recessive (AR) 25% autosomal dominant (AD) 1 -2% X-linked Rare mitochondrial
Genetic HL § 75% non-syndromal § 25% syndromal § § 75% autosomal recessive (AR) 25% autosomal dominant (AD) 1 -2% X-linked Rare mitochondrial
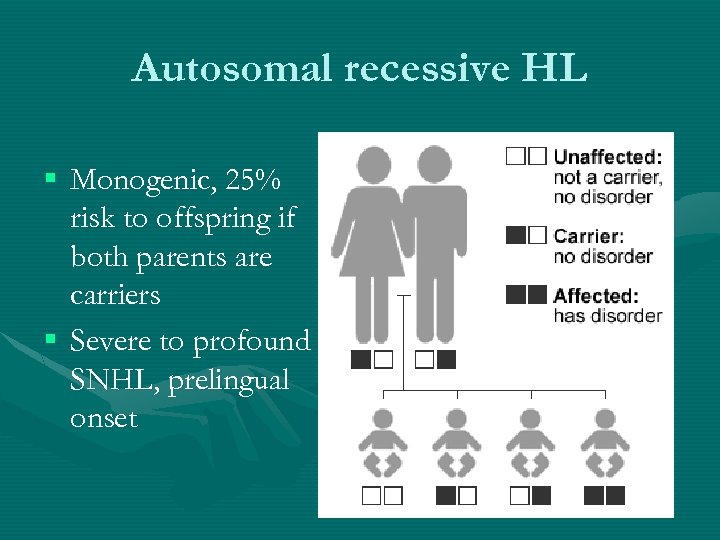 Autosomal recessive HL § Monogenic, 25% risk to offspring if both parents are carriers § Severe to profound SNHL, prelingual onset
Autosomal recessive HL § Monogenic, 25% risk to offspring if both parents are carriers § Severe to profound SNHL, prelingual onset
 Autosomal recessive syndromal HL § § Usher syndrome Pendred Jervel and Lange Nielsen Goldenhar (Oculoauriculoverterbral spectrum)
Autosomal recessive syndromal HL § § Usher syndrome Pendred Jervel and Lange Nielsen Goldenhar (Oculoauriculoverterbral spectrum)
 Epidemiology • congenital SNHL 1 -3 per 1000 per live births • 10 x greater for infants with 1 or more risk factor than those with no risk factors, ie 2% to 5%. • late-onset and acquired hearing loss in childhood 6 x higher than the incidence of hearing loss in the neonatal period • 1% all children have HL
Epidemiology • congenital SNHL 1 -3 per 1000 per live births • 10 x greater for infants with 1 or more risk factor than those with no risk factors, ie 2% to 5%. • late-onset and acquired hearing loss in childhood 6 x higher than the incidence of hearing loss in the neonatal period • 1% all children have HL
 Evaluation • History: – intrauterine infections (most commmon prenatal cause) – perinatal infection, maternal drug abuse, low Apgar score (most common perinatal causes) – Prematurity, NICU stay, bilirubinemia, family history. – Meningitis (most commmon postnatal cause) • Physical: microscopic exam; auricle, periauricular pits, craniofacial abnormalities, • +/- ocular, thyroid, skin, limb exams look for syndromic cause
Evaluation • History: – intrauterine infections (most commmon prenatal cause) – perinatal infection, maternal drug abuse, low Apgar score (most common perinatal causes) – Prematurity, NICU stay, bilirubinemia, family history. – Meningitis (most commmon postnatal cause) • Physical: microscopic exam; auricle, periauricular pits, craniofacial abnormalities, • +/- ocular, thyroid, skin, limb exams look for syndromic cause
 Evaluation • OAE • ABR – TORCH, meningitis, family hx, craniofacial abnormalities, birth weight <1. 5 kg, neonatal hyperbilirubinemia, Apgar <4 at 1 minutes, <6 at 5 minutes, prolonged NICU stay or ECMO or mechanical vent, exposure to ototoxic meds. • Behavior observation audiometry (birth to 6 mos) • Visual Reinforcement Audiometry (6 mos-3 yrs) • Conventional play audiometry (3 -6 yrs) • Standard Audiometry (6 yrs+)
Evaluation • OAE • ABR – TORCH, meningitis, family hx, craniofacial abnormalities, birth weight <1. 5 kg, neonatal hyperbilirubinemia, Apgar <4 at 1 minutes, <6 at 5 minutes, prolonged NICU stay or ECMO or mechanical vent, exposure to ototoxic meds. • Behavior observation audiometry (birth to 6 mos) • Visual Reinforcement Audiometry (6 mos-3 yrs) • Conventional play audiometry (3 -6 yrs) • Standard Audiometry (6 yrs+)
 Ancillary Tests Imaging: CT temporal bone: inner ear disorders, cholesteatoma, & osteodysplasias. CBC, lipid profile, Ig. M assay for TORCH (Toxoplasmosis, Other[syphilis], Rubella, Cytomegalovirus, Herpes simplex) Connexin-26 test Other tests as indicated by ddx.
Ancillary Tests Imaging: CT temporal bone: inner ear disorders, cholesteatoma, & osteodysplasias. CBC, lipid profile, Ig. M assay for TORCH (Toxoplasmosis, Other[syphilis], Rubella, Cytomegalovirus, Herpes simplex) Connexin-26 test Other tests as indicated by ddx.
 Causes of HL • • • 5 -10% prenatal causes (TORCH, teratogens) 5 -15% perinatal causes (hypoxemia etc) 10 -20% postnatal causes (meningitis etc) 20 -30% UNKNOWN 30 -50% genetic
Causes of HL • • • 5 -10% prenatal causes (TORCH, teratogens) 5 -15% perinatal causes (hypoxemia etc) 10 -20% postnatal causes (meningitis etc) 20 -30% UNKNOWN 30 -50% genetic
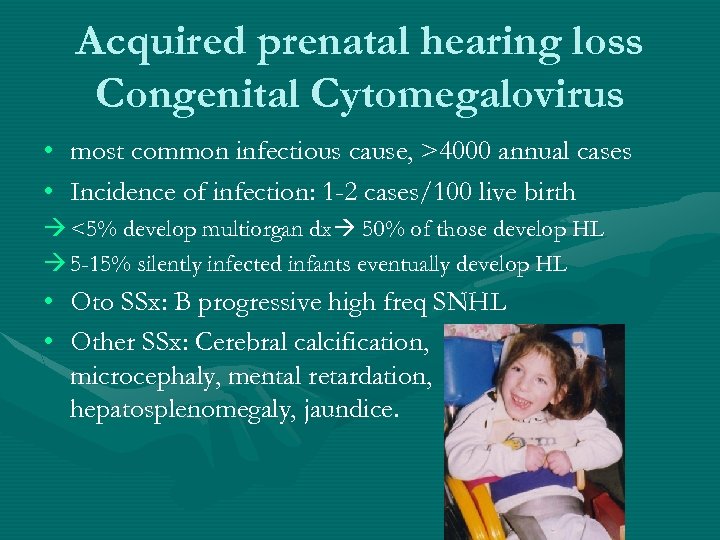 Acquired prenatal hearing loss Congenital Cytomegalovirus • most common infectious cause, >4000 annual cases • Incidence of infection: 1 -2 cases/100 live birth <5% develop multiorgan dx 50% of those develop HL 5 -15% silently infected infants eventually develop HL • Oto SSx: B progressive high freq SNHL • Other SSx: Cerebral calcification, microcephaly, mental retardation, hepatosplenomegaly, jaundice.
Acquired prenatal hearing loss Congenital Cytomegalovirus • most common infectious cause, >4000 annual cases • Incidence of infection: 1 -2 cases/100 live birth <5% develop multiorgan dx 50% of those develop HL 5 -15% silently infected infants eventually develop HL • Oto SSx: B progressive high freq SNHL • Other SSx: Cerebral calcification, microcephaly, mental retardation, hepatosplenomegaly, jaundice.
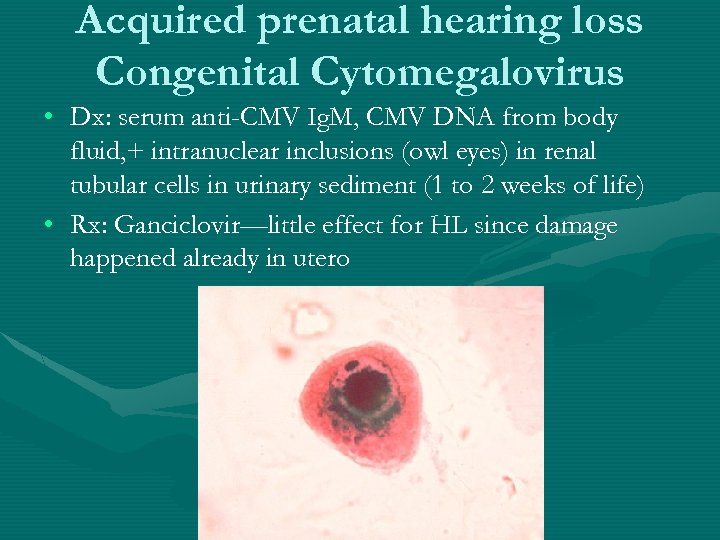 Acquired prenatal hearing loss Congenital Cytomegalovirus • Dx: serum anti-CMV Ig. M, CMV DNA from body fluid, + intranuclear inclusions (owl eyes) in renal tubular cells in urinary sediment (1 to 2 weeks of life) • Rx: Ganciclovir—little effect for HL since damage happened already in utero
Acquired prenatal hearing loss Congenital Cytomegalovirus • Dx: serum anti-CMV Ig. M, CMV DNA from body fluid, + intranuclear inclusions (owl eyes) in renal tubular cells in urinary sediment (1 to 2 weeks of life) • Rx: Ganciclovir—little effect for HL since damage happened already in utero
 Acquired prenatal hearing loss Congenital Syphilis • • • Pathophysio: transplacental transmission, 100% inoculation rate 40% perinatal death Oto SSx Hennebert sign (aka +fistula sign) – Early deafness birth to 3 yo – delayed 8 -20 yo. • Other SSx: Hutchinson triad: abnormal central incisors Hutchinson teeth), interstitial keratitis of the eye, • Dx: RPR, VDRL(sensitive); FTA-ABS(specific) • Tx: PCN (aka
Acquired prenatal hearing loss Congenital Syphilis • • • Pathophysio: transplacental transmission, 100% inoculation rate 40% perinatal death Oto SSx Hennebert sign (aka +fistula sign) – Early deafness birth to 3 yo – delayed 8 -20 yo. • Other SSx: Hutchinson triad: abnormal central incisors Hutchinson teeth), interstitial keratitis of the eye, • Dx: RPR, VDRL(sensitive); FTA-ABS(specific) • Tx: PCN (aka
 • • • Acquired prenatal hearing loss Congenital Rubella Rare since vaccination (0 -3 per year now in USA) Pathophysio: vasculitis resulting in tissue necrosis Oto SSx: B often asymmetric severe to profound SNHL • Other SSx: growth delay, learning disability, congenital heart disease, and ocular, endocrinologic, and neurologic abnormalities. • Dx: urine/throat/amniotic fluid clx, antirubella Ig. M
• • • Acquired prenatal hearing loss Congenital Rubella Rare since vaccination (0 -3 per year now in USA) Pathophysio: vasculitis resulting in tissue necrosis Oto SSx: B often asymmetric severe to profound SNHL • Other SSx: growth delay, learning disability, congenital heart disease, and ocular, endocrinologic, and neurologic abnormalities. • Dx: urine/throat/amniotic fluid clx, antirubella Ig. M
 Inner Ear Dysmorphologies • Time frame: membranous labyrinth is interrupted during 1 st trimester • Etiologies: Genetic or teratogenic exposure • Classifications – membranous labyrinth ONLY (seen at autopsy) – Osseous & membranous labyrinth ( seen in CT)
Inner Ear Dysmorphologies • Time frame: membranous labyrinth is interrupted during 1 st trimester • Etiologies: Genetic or teratogenic exposure • Classifications – membranous labyrinth ONLY (seen at autopsy) – Osseous & membranous labyrinth ( seen in CT)
 Inner Ear Dysmorphologies • Incidence: 20% congenital SNHL will show abnormal inner ear on CT temporal bone – Bony: Dilated Vestibular aqueduct >cochlea>SCC (as reflected by modern imaging technology)
Inner Ear Dysmorphologies • Incidence: 20% congenital SNHL will show abnormal inner ear on CT temporal bone – Bony: Dilated Vestibular aqueduct >cochlea>SCC (as reflected by modern imaging technology)
 Inner Ear Dysmorphologies membranous labyrinth ONLY • Complete membranous labyrinthine dysplasia (Siebenmann-Bing) • Limited membranous labyrinthine dysplasia – Scheibe dysplasia (cochleosaccular dysplasia) MOST common membranous labyrinthine dysplasia – Cochlear basal turn dysplasia
Inner Ear Dysmorphologies membranous labyrinth ONLY • Complete membranous labyrinthine dysplasia (Siebenmann-Bing) • Limited membranous labyrinthine dysplasia – Scheibe dysplasia (cochleosaccular dysplasia) MOST common membranous labyrinthine dysplasia – Cochlear basal turn dysplasia
 Bing-Siebenmann • Extremely rare • Associated with Jervell and Lange-Nielsen syndrome and Usher syndrome.
Bing-Siebenmann • Extremely rare • Associated with Jervell and Lange-Nielsen syndrome and Usher syndrome.
 Scheibe dysplasia cochleosaccular dysplasia • Pathophysio: incomplete development of the pars inferior – Cochlea dysplasia: severa in the basal turn, lessen toward apex, or severe throughout – Saccule: collapsed – Organ of Corti: partial or completely missing – SCCs & utricle: NORMAL • Oto. SSx: SNHL • Associated w/ Usher syndrome & Waardenburg syndrome
Scheibe dysplasia cochleosaccular dysplasia • Pathophysio: incomplete development of the pars inferior – Cochlea dysplasia: severa in the basal turn, lessen toward apex, or severe throughout – Saccule: collapsed – Organ of Corti: partial or completely missing – SCCs & utricle: NORMAL • Oto. SSx: SNHL • Associated w/ Usher syndrome & Waardenburg syndrome
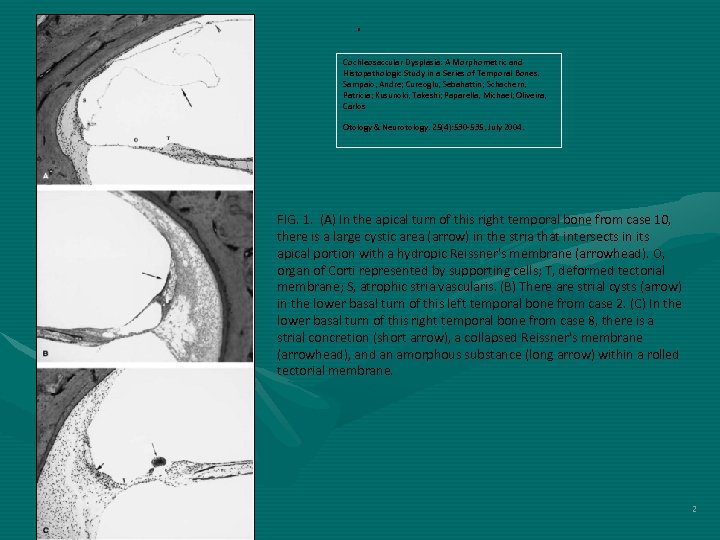 . Cochleosaccular Dysplasia: A Morphometric and Histopathologic Study in a Series of Temporal Bones. Sampaio, Andre; Cureoglu, Sebahattin; Schachern, Patricia; Kusunoki, Takeshi; Paparella, Michael; Oliveira, Carlos Otology & Neurotology. 25(4): 530 -535, July 2004. FIG. 1. (A) In the apical turn of this right temporal bone from case 10, there is a large cystic area (arrow) in the stria that intersects in its apical portion with a hydropic Reissner's membrane (arrowhead). O, organ of Corti represented by supporting cells; T, deformed tectorial membrane; S, atrophic stria vascularis. (B) There are strial cysts (arrow) in the lower basal turn of this left temporal bone from case 2. (C) In the lower basal turn of this right temporal bone from case 8, there is a strial concretion (short arrow), a collapsed Reissner's membrane (arrowhead), and an amorphous substance (long arrow) within a rolled tectorial membrane. 2
. Cochleosaccular Dysplasia: A Morphometric and Histopathologic Study in a Series of Temporal Bones. Sampaio, Andre; Cureoglu, Sebahattin; Schachern, Patricia; Kusunoki, Takeshi; Paparella, Michael; Oliveira, Carlos Otology & Neurotology. 25(4): 530 -535, July 2004. FIG. 1. (A) In the apical turn of this right temporal bone from case 10, there is a large cystic area (arrow) in the stria that intersects in its apical portion with a hydropic Reissner's membrane (arrowhead). O, organ of Corti represented by supporting cells; T, deformed tectorial membrane; S, atrophic stria vascularis. (B) There are strial cysts (arrow) in the lower basal turn of this left temporal bone from case 2. (C) In the lower basal turn of this right temporal bone from case 8, there is a strial concretion (short arrow), a collapsed Reissner's membrane (arrowhead), and an amorphous substance (long arrow) within a rolled tectorial membrane. 2
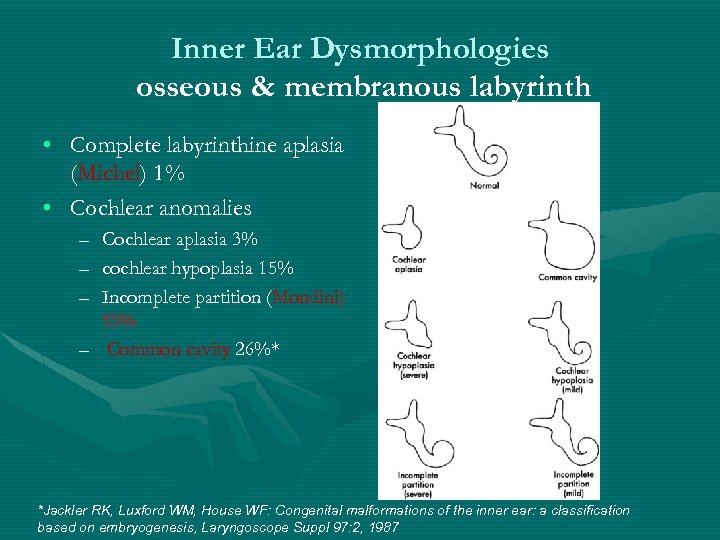 Inner Ear Dysmorphologies osseous & membranous labyrinth • Complete labyrinthine aplasia (Michel) 1% • Cochlear anomalies – Cochlear aplasia 3% – cochlear hypoplasia 15% – Incomplete partition (Mondini) 55% – Common cavity 26%* *Jackler RK, Luxford WM, House WF: Congenital malformations of the inner ear: a classification based on embryogenesis, Laryngoscope Suppl 97: 2, 1987
Inner Ear Dysmorphologies osseous & membranous labyrinth • Complete labyrinthine aplasia (Michel) 1% • Cochlear anomalies – Cochlear aplasia 3% – cochlear hypoplasia 15% – Incomplete partition (Mondini) 55% – Common cavity 26%* *Jackler RK, Luxford WM, House WF: Congenital malformations of the inner ear: a classification based on embryogenesis, Laryngoscope Suppl 97: 2, 1987
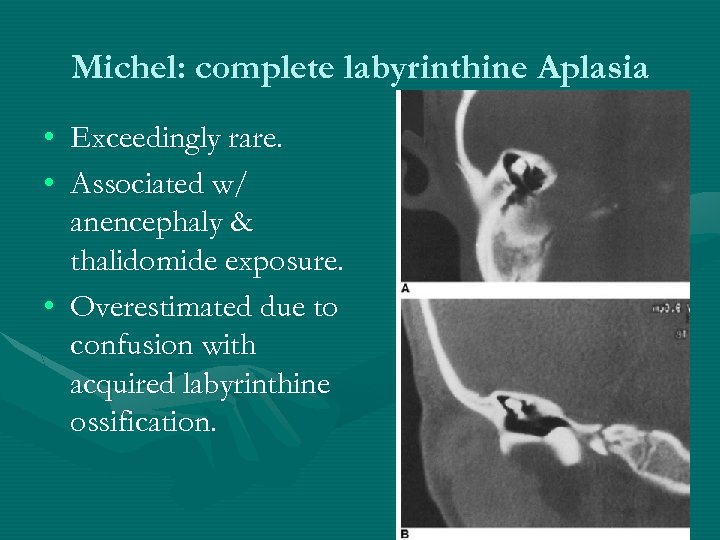 Michel: complete labyrinthine Aplasia • Exceedingly rare. • Associated w/ anencephaly & thalidomide exposure. • Overestimated due to confusion with acquired labyrinthine ossification.
Michel: complete labyrinthine Aplasia • Exceedingly rare. • Associated w/ anencephaly & thalidomide exposure. • Overestimated due to confusion with acquired labyrinthine ossification.
 Mondini: incomplete partition • Pathphysio: arrest at 7 th week gestation 1. 5 turn cochlea • Oto SSx: normal to profound SNHL • Other SSx: – 20% SCC deformities; – dilated cochlear aquaduct: perilymphatic gushers & meningitis
Mondini: incomplete partition • Pathphysio: arrest at 7 th week gestation 1. 5 turn cochlea • Oto SSx: normal to profound SNHL • Other SSx: – 20% SCC deformities; – dilated cochlear aquaduct: perilymphatic gushers & meningitis
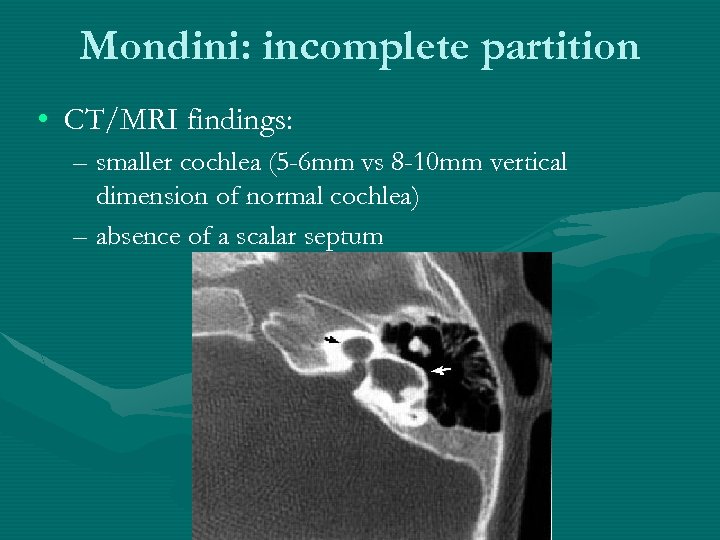 Mondini: incomplete partition • CT/MRI findings: – smaller cochlea (5 -6 mm vs 8 -10 mm vertical dimension of normal cochlea) – absence of a scalar septum
Mondini: incomplete partition • CT/MRI findings: – smaller cochlea (5 -6 mm vs 8 -10 mm vertical dimension of normal cochlea) – absence of a scalar septum
 Common Cavity • Pathphysio: arrest at 4 th week otocyst stage or later • CT/MRI findings: – Empty ovoid space (average 7 mm vertically, 10 mm horizontally) – Common cavity cochlear ANTERIOR to the IAC on axial CT • Oto SSx: variable SNHL, usually poor
Common Cavity • Pathphysio: arrest at 4 th week otocyst stage or later • CT/MRI findings: – Empty ovoid space (average 7 mm vertically, 10 mm horizontally) – Common cavity cochlear ANTERIOR to the IAC on axial CT • Oto SSx: variable SNHL, usually poor
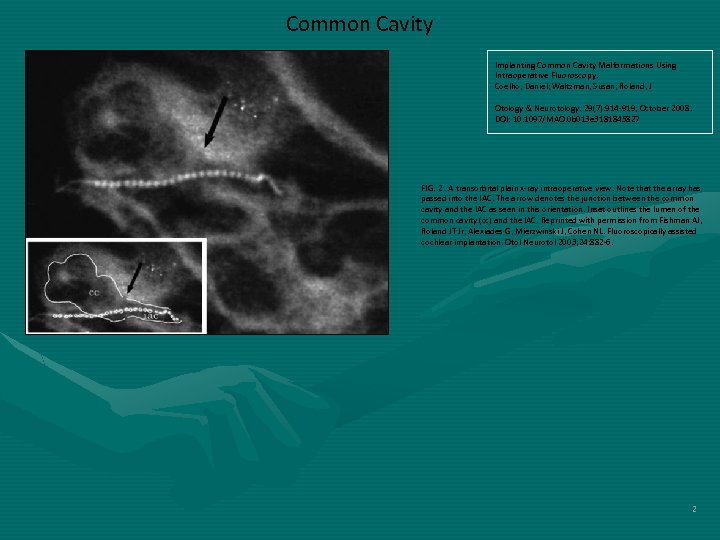 Common Cavity Implanting Common Cavity Malformations Using Intraoperative Fluoroscopy. Coelho, Daniel; Waltzman, Susan; Roland, J Otology & Neurotology. 29(7): 914 -919, October 2008. DOI: 10. 1097/MAO. 0 b 013 e 3181845827 FIG. 2. A transorbital plain x-ray intraoperative view. Note that the array has passed into the IAC. The arrow denotes the junction between the common cavity and the IAC as seen in this orientation. Inset outlines the lumen of the common cavity (cc) and the IAC. Reprinted with permission from Fishman AJ, Roland JT Jr, Alexiades G, Mierzwinski J, Cohen NL. Fluoroscopically assisted cochlear implantation. Otol Neurotol 2003; 24: 882 -6. 2
Common Cavity Implanting Common Cavity Malformations Using Intraoperative Fluoroscopy. Coelho, Daniel; Waltzman, Susan; Roland, J Otology & Neurotology. 29(7): 914 -919, October 2008. DOI: 10. 1097/MAO. 0 b 013 e 3181845827 FIG. 2. A transorbital plain x-ray intraoperative view. Note that the array has passed into the IAC. The arrow denotes the junction between the common cavity and the IAC as seen in this orientation. Inset outlines the lumen of the common cavity (cc) and the IAC. Reprinted with permission from Fishman AJ, Roland JT Jr, Alexiades G, Mierzwinski J, Cohen NL. Fluoroscopically assisted cochlear implantation. Otol Neurotol 2003; 24: 882 -6. 2
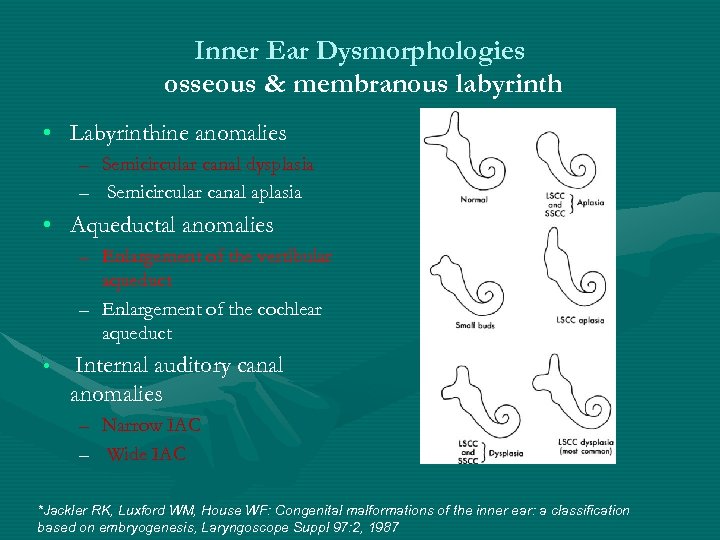 Inner Ear Dysmorphologies osseous & membranous labyrinth • Labyrinthine anomalies – Semicircular canal dysplasia – Semicircular canal aplasia • Aqueductal anomalies – Enlargement of the vestibular aqueduct – Enlargement of the cochlear aqueduct • Internal auditory canal anomalies – Narrow IAC – Wide IAC *Jackler RK, Luxford WM, House WF: Congenital malformations of the inner ear: a classification based on embryogenesis, Laryngoscope Suppl 97: 2, 1987
Inner Ear Dysmorphologies osseous & membranous labyrinth • Labyrinthine anomalies – Semicircular canal dysplasia – Semicircular canal aplasia • Aqueductal anomalies – Enlargement of the vestibular aqueduct – Enlargement of the cochlear aqueduct • Internal auditory canal anomalies – Narrow IAC – Wide IAC *Jackler RK, Luxford WM, House WF: Congenital malformations of the inner ear: a classification based on embryogenesis, Laryngoscope Suppl 97: 2, 1987
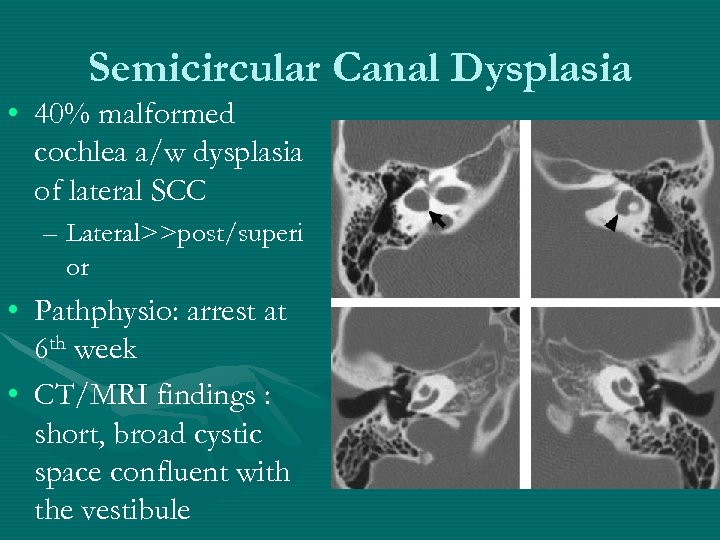 Semicircular Canal Dysplasia • 40% malformed cochlea a/w dysplasia of lateral SCC – Lateral>>post/superi or • Pathphysio: arrest at 6 th week • CT/MRI findings : short, broad cystic space confluent with the vestibule
Semicircular Canal Dysplasia • 40% malformed cochlea a/w dysplasia of lateral SCC – Lateral>>post/superi or • Pathphysio: arrest at 6 th week • CT/MRI findings : short, broad cystic space confluent with the vestibule
 Enlargement of the Vestibular Aqueduct • Epid: most common radiographically detectable malformation of the inner ear • Pathphysio: Acquired abnormal communication between the subarachnoid space and the fluid chambers of the inner ear • Oto SSx: – born w/ normal or mildly impaired hearing that gradually worsens; – hearing variable, 40% profound SNHL – CHL possible: AVOID STAPEDECTOMY! (a/w perilymphatic gusher)
Enlargement of the Vestibular Aqueduct • Epid: most common radiographically detectable malformation of the inner ear • Pathphysio: Acquired abnormal communication between the subarachnoid space and the fluid chambers of the inner ear • Oto SSx: – born w/ normal or mildly impaired hearing that gradually worsens; – hearing variable, 40% profound SNHL – CHL possible: AVOID STAPEDECTOMY! (a/w perilymphatic gusher)
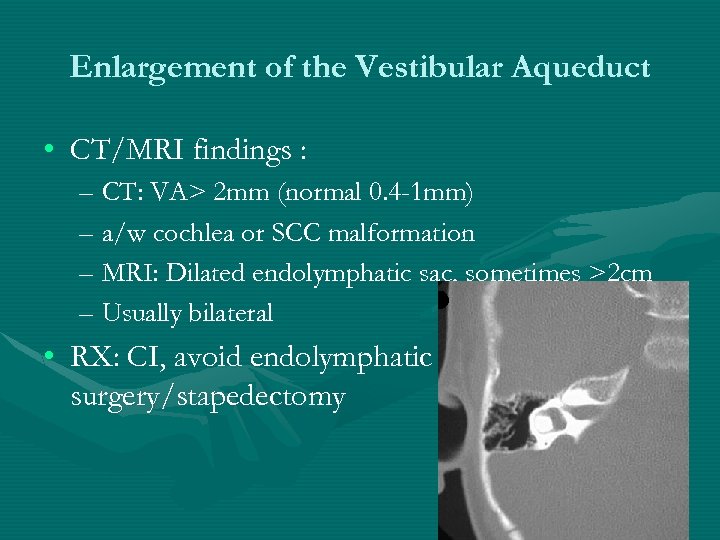 Enlargement of the Vestibular Aqueduct • CT/MRI findings : – CT: VA> 2 mm (normal 0. 4 -1 mm) – a/w cochlea or SCC malformation – MRI: Dilated endolymphatic sac, sometimes >2 cm – Usually bilateral • RX: CI, avoid endolymphatic surgery/stapedectomy
Enlargement of the Vestibular Aqueduct • CT/MRI findings : – CT: VA> 2 mm (normal 0. 4 -1 mm) – a/w cochlea or SCC malformation – MRI: Dilated endolymphatic sac, sometimes >2 cm – Usually bilateral • RX: CI, avoid endolymphatic surgery/stapedectomy
 Wide Internal Auditory Canal • Usually incidental finding in normal hearing subjects • CT/MRI findings : IAC>10 mm • a/w spontaenous CSF otorrhea & gusher during stapes surgery obtain CT for congenital CHL!
Wide Internal Auditory Canal • Usually incidental finding in normal hearing subjects • CT/MRI findings : IAC>10 mm • a/w spontaenous CSF otorrhea & gusher during stapes surgery obtain CT for congenital CHL!
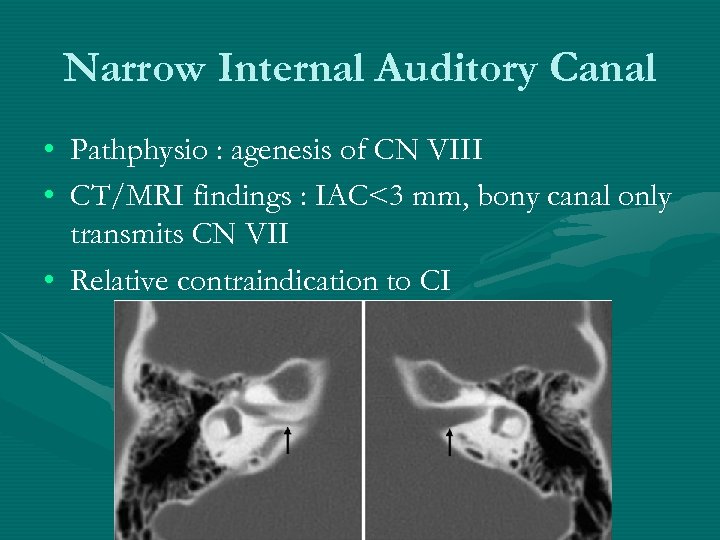 Narrow Internal Auditory Canal • Pathphysio : agenesis of CN VIII • CT/MRI findings : IAC<3 mm, bony canal only transmits CN VII • Relative contraindication to CI
Narrow Internal Auditory Canal • Pathphysio : agenesis of CN VIII • CT/MRI findings : IAC<3 mm, bony canal only transmits CN VII • Relative contraindication to CI
 GENETIC HL • >50% non-syndromic – 75% to 80% autosomal recessive – 15% to 20% autosomal dominant – 1% to 2% is X-linked. – <<1% mitochondrial inheritance
GENETIC HL • >50% non-syndromic – 75% to 80% autosomal recessive – 15% to 20% autosomal dominant – 1% to 2% is X-linked. – <<1% mitochondrial inheritance
 Autosomal Recessive Disorders Usher syndrome • • • Most common cause of congenital deafness 50% deaf-blind in USA Pathophy: unknown, could also be autosomal dominant, X-linked • SSx: Variable SNHL, progressive retinitis pigmentosa • Dx: Electroretinography
Autosomal Recessive Disorders Usher syndrome • • • Most common cause of congenital deafness 50% deaf-blind in USA Pathophy: unknown, could also be autosomal dominant, X-linked • SSx: Variable SNHL, progressive retinitis pigmentosa • Dx: Electroretinography
 Usher syndrome subtypes • I: profound congenital SNHL, No vestibular response Blind by childhood, most common • II: moderate to severe SNHL, normal vestibular response, blind by early adulthood • III: progressive SNHL, progressive vestibular dysfunction, varied progression in blindness
Usher syndrome subtypes • I: profound congenital SNHL, No vestibular response Blind by childhood, most common • II: moderate to severe SNHL, normal vestibular response, blind by early adulthood • III: progressive SNHL, progressive vestibular dysfunction, varied progression in blindness
 Autosomal Recessive Disorders Pendred syndrome • Pathophy: Defect in tyrosine iodination from pendrin (chloride/iodide transporter) • Oto. SSx: severe to profound SNHL, a/w Mondini deformity, dilated vestibular aqueducts. • Other SSx: multinodular goiter in 8 -14 yo • Dx: + perchlorate test • Rx: Thyroid supplement
Autosomal Recessive Disorders Pendred syndrome • Pathophy: Defect in tyrosine iodination from pendrin (chloride/iodide transporter) • Oto. SSx: severe to profound SNHL, a/w Mondini deformity, dilated vestibular aqueducts. • Other SSx: multinodular goiter in 8 -14 yo • Dx: + perchlorate test • Rx: Thyroid supplement
 Autosomal Recessive Disorders Jervell and Lange-Nielsen Syndrome • • • Pathophy: mutation in potassium channel Oto. SSx: B severe to profound SNHL Other SSx: cardiac abnormalities, recurrent syncope, sudden death • Dx: EKG ( prolonged QT, large T-wave) • Rx: beta-blocker, HA
Autosomal Recessive Disorders Jervell and Lange-Nielsen Syndrome • • • Pathophy: mutation in potassium channel Oto. SSx: B severe to profound SNHL Other SSx: cardiac abnormalities, recurrent syncope, sudden death • Dx: EKG ( prolonged QT, large T-wave) • Rx: beta-blocker, HA
 Autosomal Recessive Disorders Goldenhar Syndrome • aka Hemifacial Microsomia/ Oculoauriculovertebral spectrum • Pathophy: uncertain, malformation of 1 st and 2 nd arch derivatives • Oto. SSx: – microtia/EAC atresia, ossicular malformation CHL – abnormal CN VII, SCC, oval window SNHL
Autosomal Recessive Disorders Goldenhar Syndrome • aka Hemifacial Microsomia/ Oculoauriculovertebral spectrum • Pathophy: uncertain, malformation of 1 st and 2 nd arch derivatives • Oto. SSx: – microtia/EAC atresia, ossicular malformation CHL – abnormal CN VII, SCC, oval window SNHL
 Autosomal Recessive Disorders Goldenhar Syndrome • Other SSx: – – Ocular: epibulbar dermoids, colobomas of upper eyelids Vertebral: fusion or absence of cervical vertebrae Facial asymmetry Mild mental retardation • Dx: PE
Autosomal Recessive Disorders Goldenhar Syndrome • Other SSx: – – Ocular: epibulbar dermoids, colobomas of upper eyelids Vertebral: fusion or absence of cervical vertebrae Facial asymmetry Mild mental retardation • Dx: PE
 Autosomal Dominant Disorders Waardenberg Syndrome • • • Pathophy: abnormal tyrosine metabolism Oto. SSx: U/B SNHL, +/- vestibular dysfunction Other SSx: – Pigmentary abnormalities iriditis, forelock, skin depigmentation – Dystopia canthorum – Synophrys – Flat nasal root, – Hypoplastic alae (heterchromic white patch
Autosomal Dominant Disorders Waardenberg Syndrome • • • Pathophy: abnormal tyrosine metabolism Oto. SSx: U/B SNHL, +/- vestibular dysfunction Other SSx: – Pigmentary abnormalities iriditis, forelock, skin depigmentation – Dystopia canthorum – Synophrys – Flat nasal root, – Hypoplastic alae (heterchromic white patch
 Autosomal Dominant Disorders Waardenberg Syndrome • • Subtypes I: + telecanthus, 36 -66. 7% SNHL II: -telecanthus, 57 -85% SNHL III: type 1 + hypoplasia or contracture of the upper limbs. (=Klein-Waardenburg syndrome) • IV: WS + Hirschsprung disease (Waardenburg-Shah syndrome) autosomal recessive • Dx: clinical H&P, family Hx
Autosomal Dominant Disorders Waardenberg Syndrome • • Subtypes I: + telecanthus, 36 -66. 7% SNHL II: -telecanthus, 57 -85% SNHL III: type 1 + hypoplasia or contracture of the upper limbs. (=Klein-Waardenburg syndrome) • IV: WS + Hirschsprung disease (Waardenburg-Shah syndrome) autosomal recessive • Dx: clinical H&P, family Hx
 Autosomal Dominant Disorders Stickler Syndrome • =Progressive Arthro-Ophthalmpathy • Pathophy: mutation in type II and type XI collagen, variable phenotype; 1: 10, 000 • Oto. SSx: progressive SNHL, MHL ( from ETD of clefting) • Other SSx: – – myopia, retinal detachment Marfanoid habitus joint hypermobilities Midline clefting • Dx: clinical H&P, family Hx
Autosomal Dominant Disorders Stickler Syndrome • =Progressive Arthro-Ophthalmpathy • Pathophy: mutation in type II and type XI collagen, variable phenotype; 1: 10, 000 • Oto. SSx: progressive SNHL, MHL ( from ETD of clefting) • Other SSx: – – myopia, retinal detachment Marfanoid habitus joint hypermobilities Midline clefting • Dx: clinical H&P, family Hx
 Autosomal Dominant Disorders Branchio-Oto-Renal Syndrome • • • =Melnick Fraser Syndrome, 1 in 40, 000 newborns Pathophy: branchial arches, otic & renal abnormal development Oto. SSx: – preauricular ear pits/tags, microtia, EAC stenosis; middle/inner ear anomalites – 50% MHL, 30% CHL, 20% SNHL • Other SSx: varied renal abnormalities (agenesis to mild dysplasia) • Dx: Renal US or pyelography; renal abnormalities frequently asymptomatic
Autosomal Dominant Disorders Branchio-Oto-Renal Syndrome • • • =Melnick Fraser Syndrome, 1 in 40, 000 newborns Pathophy: branchial arches, otic & renal abnormal development Oto. SSx: – preauricular ear pits/tags, microtia, EAC stenosis; middle/inner ear anomalites – 50% MHL, 30% CHL, 20% SNHL • Other SSx: varied renal abnormalities (agenesis to mild dysplasia) • Dx: Renal US or pyelography; renal abnormalities frequently asymptomatic
 Autosomal Dominant Disorders Treacher Collins Syndrome • =Mandibulofacial dysostosis • Pathophy: uncertain. • Oto. SSx: microtia/EAC atresia, preauricular fistulas, malformed ossicle CHL, widened aqueduct, aberrant CN VII • Other SSx: mandibular hypoplasia-fishmouth; downward slanting palpebral fissures, coloboma of lower eyelids, palate defects. Choanal atresia • Dx: clinical H&P, family Hx • Rx: BAHA, possible atresia repair
Autosomal Dominant Disorders Treacher Collins Syndrome • =Mandibulofacial dysostosis • Pathophy: uncertain. • Oto. SSx: microtia/EAC atresia, preauricular fistulas, malformed ossicle CHL, widened aqueduct, aberrant CN VII • Other SSx: mandibular hypoplasia-fishmouth; downward slanting palpebral fissures, coloboma of lower eyelids, palate defects. Choanal atresia • Dx: clinical H&P, family Hx • Rx: BAHA, possible atresia repair
 Autosomal Dominant Disorders Neurofibromatosis I • • =Von Recklinghausen disease Pathophy: NF 1 in chromosome 17 Oto. SSx: retrocochlear HL NF 1 (2/7 characters) – – – – >6 café-au-lait spots 2 or more neurofibromas or 1 plexiform neurofibroma Axillary or groin freckling Optic nerve glioma Lisch nodules (eye hamartomas) Bony lesions +family Hx • 5% risk of U vestibular schwannoma
Autosomal Dominant Disorders Neurofibromatosis I • • =Von Recklinghausen disease Pathophy: NF 1 in chromosome 17 Oto. SSx: retrocochlear HL NF 1 (2/7 characters) – – – – >6 café-au-lait spots 2 or more neurofibromas or 1 plexiform neurofibroma Axillary or groin freckling Optic nerve glioma Lisch nodules (eye hamartomas) Bony lesions +family Hx • 5% risk of U vestibular schwannoma
 Autosomal Dominant Disorders Neurofibromatosis 2 • • • Pathophy: mutation in Merlin ( tumor suppressor gene) in chromosome 22 Oto. SSx: retrocochlear HL NF 2 – B vestibular schwannoma by 2 nd decade of life – Family h/o NFII in a 1 st degree relative PLUS – A) unilateral vestibular schwannoma at <30 yo – B) 2 neurofibroma + other intracranial & spinal cord tumors (gliomas/schwannomas/meningiomas)
Autosomal Dominant Disorders Neurofibromatosis 2 • • • Pathophy: mutation in Merlin ( tumor suppressor gene) in chromosome 22 Oto. SSx: retrocochlear HL NF 2 – B vestibular schwannoma by 2 nd decade of life – Family h/o NFII in a 1 st degree relative PLUS – A) unilateral vestibular schwannoma at <30 yo – B) 2 neurofibroma + other intracranial & spinal cord tumors (gliomas/schwannomas/meningiomas)
 Autosomal Dominant Disorders Apert Syndrome • • • =Acrocephalosyndactyly Pathophy: autosomal dominant or sporadic Oto. SSx: Stapes fixation CHL, patent cochlear aqueduct, large subarcuate fossa • Other SSx: – lobster claw hands – midface abnormalites (hypertelorism, proptosis, saddle nose, high-arched palate) – craniofacial dysostosis – trapezoid mouth
Autosomal Dominant Disorders Apert Syndrome • • • =Acrocephalosyndactyly Pathophy: autosomal dominant or sporadic Oto. SSx: Stapes fixation CHL, patent cochlear aqueduct, large subarcuate fossa • Other SSx: – lobster claw hands – midface abnormalites (hypertelorism, proptosis, saddle nose, high-arched palate) – craniofacial dysostosis – trapezoid mouth
 Autosomal Dominant Disorders Crouzon Syndrome • • • = Craniofacial dysostosis , Pathophy: unknown Oto. SSx: microtia/EAC atresia, malformed ossicle CHL, Other SSx: midface abnormalites (hypertelorism, small maxilla, exophthalmos, parrot nose, short upper lip, craniofacial dysostosis, mandibular prognathism
Autosomal Dominant Disorders Crouzon Syndrome • • • = Craniofacial dysostosis , Pathophy: unknown Oto. SSx: microtia/EAC atresia, malformed ossicle CHL, Other SSx: midface abnormalites (hypertelorism, small maxilla, exophthalmos, parrot nose, short upper lip, craniofacial dysostosis, mandibular prognathism
 Sex-linked Disorders Alport Disease • Pathophy: 80% X-linked or autosomal dominant/recessive. Abnormal Type IV collagen formation in glomerular basement renal failure • Oto. SSx: B degeneration of organ of Corti and stria slowly progressive SNHL • Other SSx: hematuria, progressive nephritis, macular/corneal lesions • Dx: skin or renal bx w/ electron microscopy, UA • Rx: HD, renal transplant.
Sex-linked Disorders Alport Disease • Pathophy: 80% X-linked or autosomal dominant/recessive. Abnormal Type IV collagen formation in glomerular basement renal failure • Oto. SSx: B degeneration of organ of Corti and stria slowly progressive SNHL • Other SSx: hematuria, progressive nephritis, macular/corneal lesions • Dx: skin or renal bx w/ electron microscopy, UA • Rx: HD, renal transplant.
 What Causes Hearing Loss? Non-Genetic • Infections • Drug-Related • Traumas/ Exposures • Structural Genetic • Unknown
What Causes Hearing Loss? Non-Genetic • Infections • Drug-Related • Traumas/ Exposures • Structural Genetic • Unknown
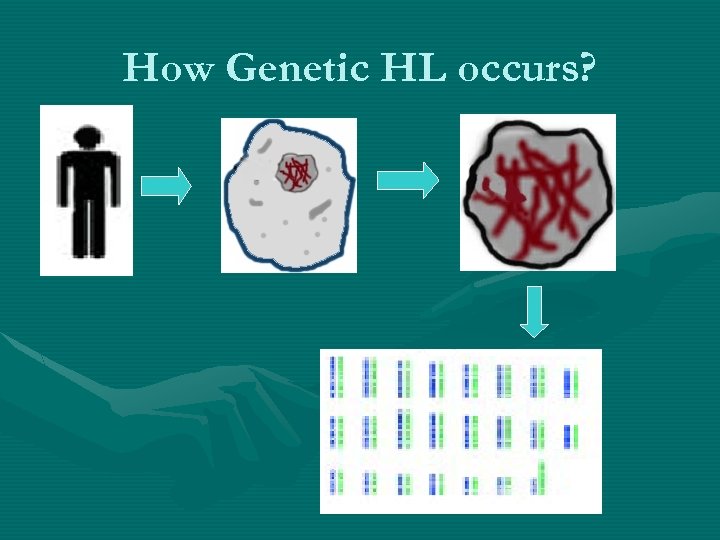 How Genetic HL occurs?
How Genetic HL occurs?
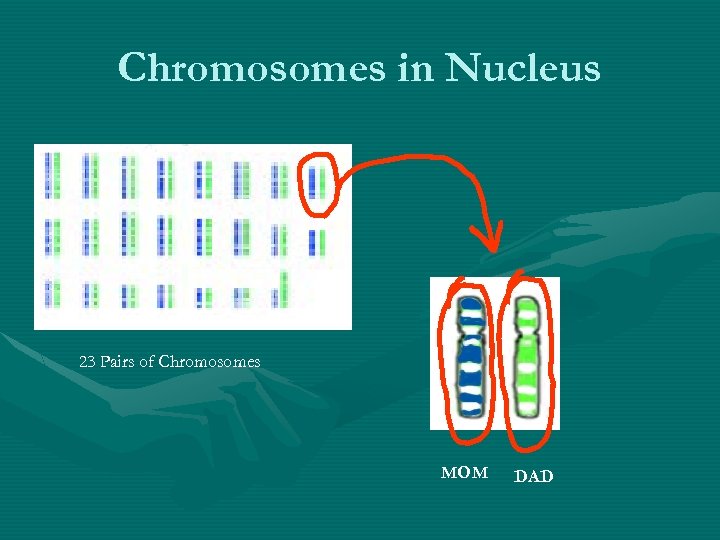 Chromosomes in Nucleus 23 Pairs of Chromosomes MOM DAD
Chromosomes in Nucleus 23 Pairs of Chromosomes MOM DAD
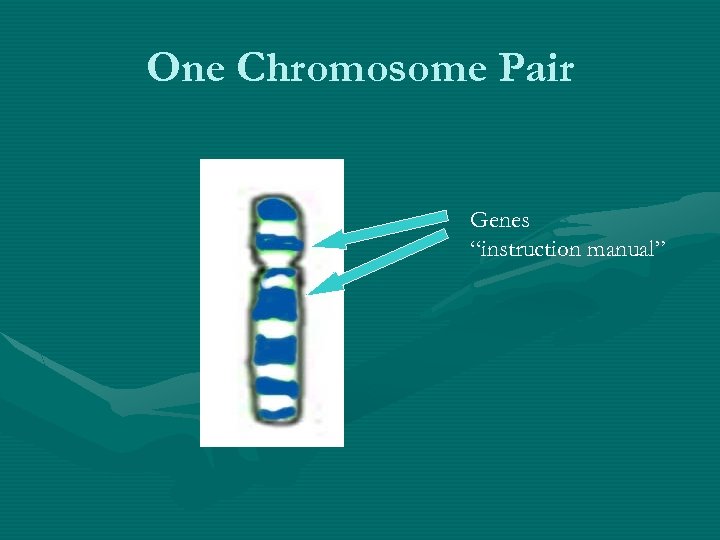 One Chromosome Pair Genes “instruction manual”
One Chromosome Pair Genes “instruction manual”
 Genes
Genes
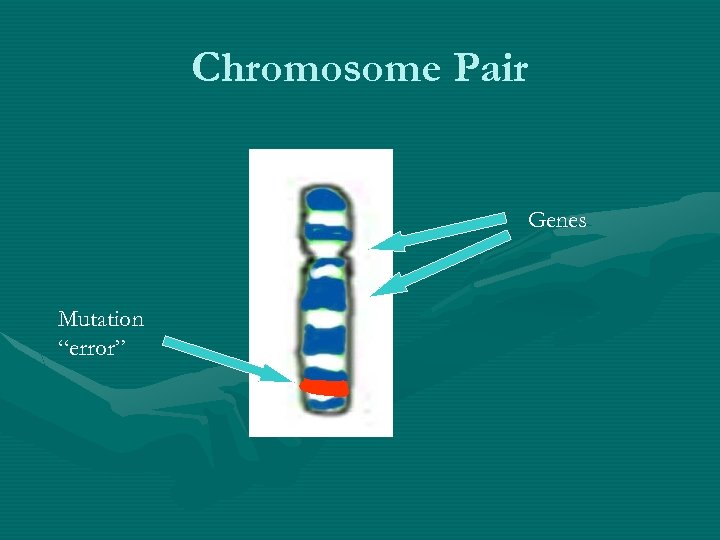 Chromosome Pair Genes Mutation “error”
Chromosome Pair Genes Mutation “error”
 How Is Mutation Inherited? • • Dominant ~15% Recessive ~80% X-Linked ~2% Mitochondrial >2%
How Is Mutation Inherited? • • Dominant ~15% Recessive ~80% X-Linked ~2% Mitochondrial >2%
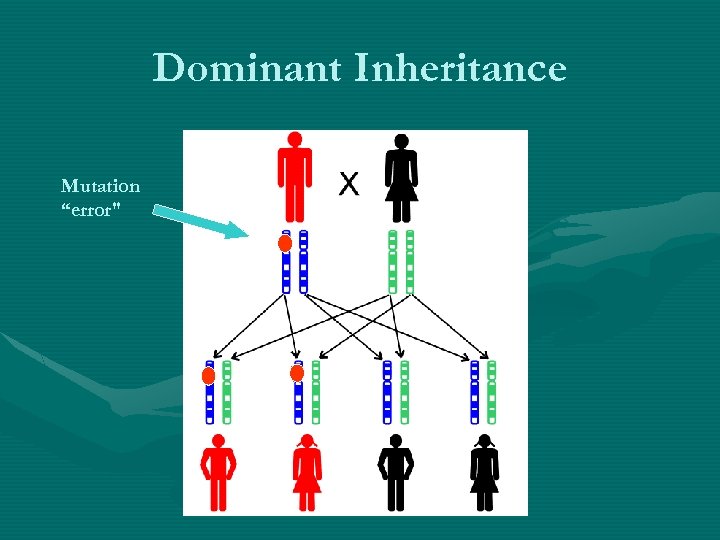 Dominant Inheritance Mutation “error"
Dominant Inheritance Mutation “error"
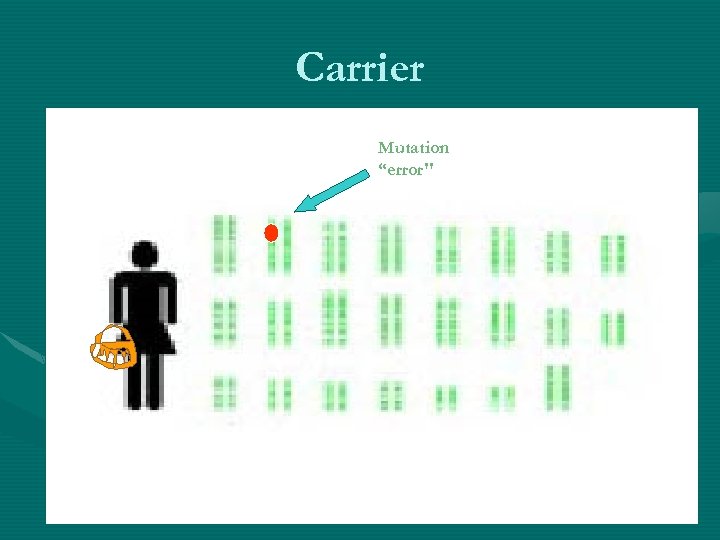 Carrier Mutation “error"
Carrier Mutation “error"
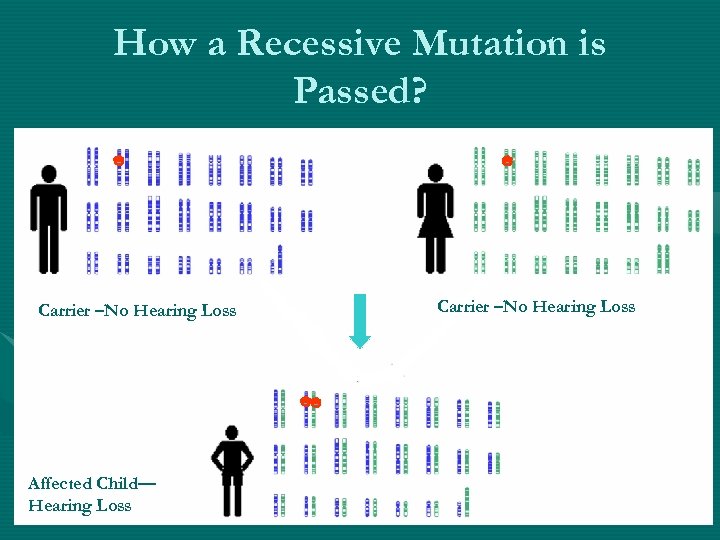 How a Recessive Mutation is Passed? Carrier –No Hearing Loss Affected Child— Hearing Loss Carrier –No Hearing Loss
How a Recessive Mutation is Passed? Carrier –No Hearing Loss Affected Child— Hearing Loss Carrier –No Hearing Loss
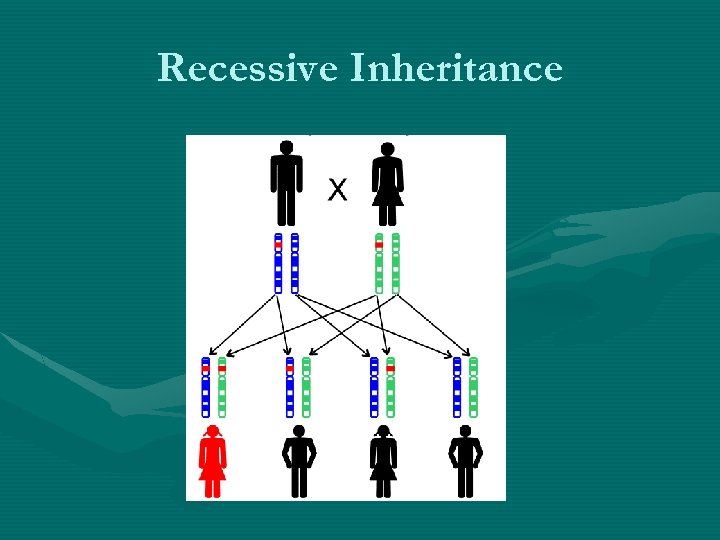 Recessive Inheritance
Recessive Inheritance
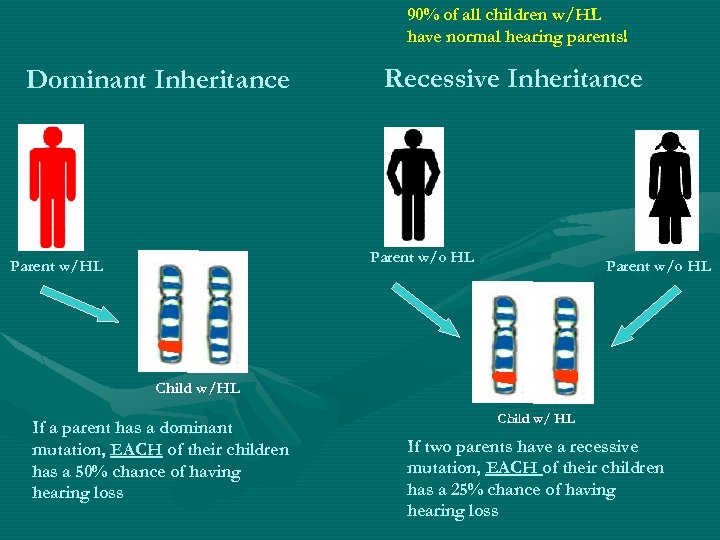 90% of all children w/HL have normal hearing parents! Dominant Inheritance Recessive Inheritance Parent w/o HL Child w/HL If a parent has a dominant mutation, EACH of their children has a 50% chance of having hearing loss Child w/ HL If two parents have a recessive mutation, EACH of their children has a 25% chance of having hearing loss
90% of all children w/HL have normal hearing parents! Dominant Inheritance Recessive Inheritance Parent w/o HL Child w/HL If a parent has a dominant mutation, EACH of their children has a 50% chance of having hearing loss Child w/ HL If two parents have a recessive mutation, EACH of their children has a 25% chance of having hearing loss
 HOW? WHO? WHY?
HOW? WHO? WHY?
 HOW Do We Know If HL is Genetic?
HOW Do We Know If HL is Genetic?
 WHO Should Have a Genetic Test? • Everybody with Sensorineural HL Also 2 Mutations in Cx 26!!
WHO Should Have a Genetic Test? • Everybody with Sensorineural HL Also 2 Mutations in Cx 26!!
 WHY Should We Have a Genetic Test? ? Benefits for Genetic Testing • a definite cause • family members realize that they are carriers & determine risk factors for future children • helps to find appropriate treatment/ management
WHY Should We Have a Genetic Test? ? Benefits for Genetic Testing • a definite cause • family members realize that they are carriers & determine risk factors for future children • helps to find appropriate treatment/ management
 Limitations for Genetic Testing • • • does not necessarily find the answer severity of HL may not be predicted a person may have mutations, but not have HL
Limitations for Genetic Testing • • • does not necessarily find the answer severity of HL may not be predicted a person may have mutations, but not have HL
 Things to Consider 1. Talk to knowledgeable professional § Primary Care/ Pediatrician § Clinical Geneticist § ENT § Genetic Counselor § Audiologist § Clinical Molecular Geneticist
Things to Consider 1. Talk to knowledgeable professional § Primary Care/ Pediatrician § Clinical Geneticist § ENT § Genetic Counselor § Audiologist § Clinical Molecular Geneticist
 Things to Consider 2. What tests are done? • • 3. Cost Cx 26 Cx 30 Mitochondrial Tests Pendred
Things to Consider 2. What tests are done? • • 3. Cost Cx 26 Cx 30 Mitochondrial Tests Pendred
 UNDERSTANDING TEST RESULTS (example Cx 26)
UNDERSTANDING TEST RESULTS (example Cx 26)
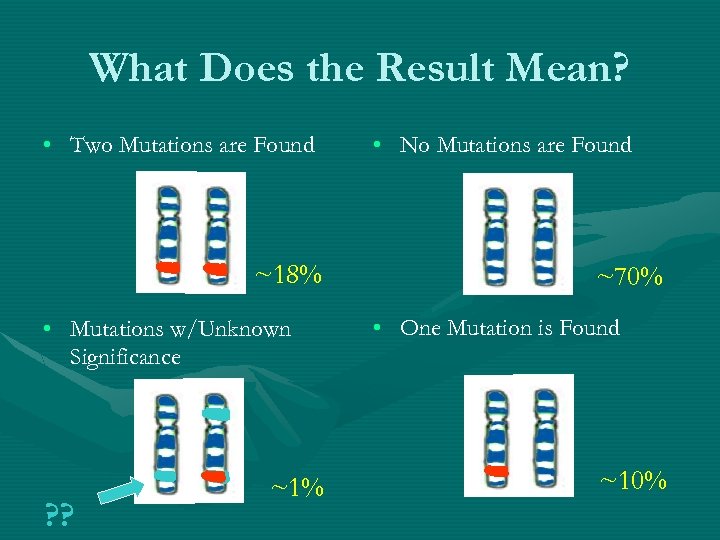 What Does the Result Mean? • Two Mutations are Found ~18% • Mutations w/Unknown Significance ? ? ~1% • No Mutations are Found ~70% • One Mutation is Found ~10%
What Does the Result Mean? • Two Mutations are Found ~18% • Mutations w/Unknown Significance ? ? ~1% • No Mutations are Found ~70% • One Mutation is Found ~10%
 One Mutation Found • • Mutation unrelated to deafness Test did not find 2 nd mutation Dominant mutation There may be a mutation in another gene
One Mutation Found • • Mutation unrelated to deafness Test did not find 2 nd mutation Dominant mutation There may be a mutation in another gene
 Future in Genetics and HL • More Genetic Tests Gene. Chip Technology
Future in Genetics and HL • More Genetic Tests Gene. Chip Technology
 Research Studies • Connexin 26 Study- individuals with Cx 26 mutations • Genetic Testing and Counseling Study - If you or your child has had genetic testing for hearing loss and you are willing to fill out a questionnaire • Gene. Chip Study - individuals with hearing loss who and parents with normal hearing • Novel Gene Discovery Study - five or more family members with hearing loss
Research Studies • Connexin 26 Study- individuals with Cx 26 mutations • Genetic Testing and Counseling Study - If you or your child has had genetic testing for hearing loss and you are willing to fill out a questionnaire • Gene. Chip Study - individuals with hearing loss who and parents with normal hearing • Novel Gene Discovery Study - five or more family members with hearing loss


