b677ae127d113d121681e97c347a4377.ppt
- Количество слайдов: 23

PCI Unmet Clinical Needs For SVG Distal embolisation No reflow High rate of restenosis For AMI / other thrombus loaded lesions Distal embolisation No reflow Patients with DES Late ST due to incomplete endothelialization Bleeding complications due to long term Plavix use
The Evolution of Stents Bare Metal 1 st Drug Eluting 2 nd MGuard Generation 3 rd Generation While addressing the restenosis problem, today's stent technology overlooks the adverse effects of acute embolization

About MGuard’s Technology • • • Micron Circular Knitting Technology Net Material: Poly Ethylene Terephthalate (PET) Fiber diameter: 20 μm Aperture size at expanded state: ~180μm X 200μm System profile - 1. 3 mm Net secured to the distal and proximal end of the stent
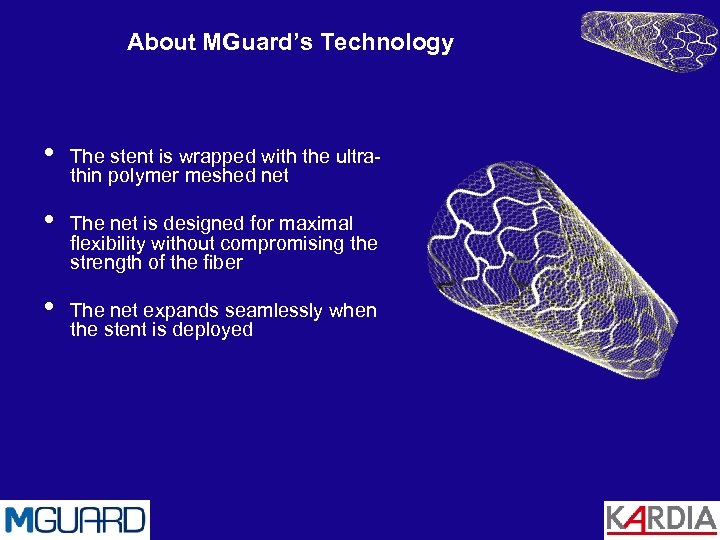
About MGuard’s Technology • • • The stent is wrapped with the ultrathin polymer meshed net The net is designed for maximal flexibility without compromising the strength of the fiber The net expands seamlessly when the stent is deployed

About MGuard’s Clinical Benefits MGuard is designed to 1. Trap thrombotic material to protect bloodstream from embolic debris 2. Reduce vessel injury and restenosis rate 3. Maintain standard procedure Injury lowered by MGuard

About MGuard’s Clinical Benefits MGuard Lifelong Embolic Protection • MGuard blocks embolic showers and plaque detachment from the arterial wall, blocking debris at the source during and post procedure. • MGuard addresses the risk of suboptimal perfusion and no-reflow in occluded lesions.
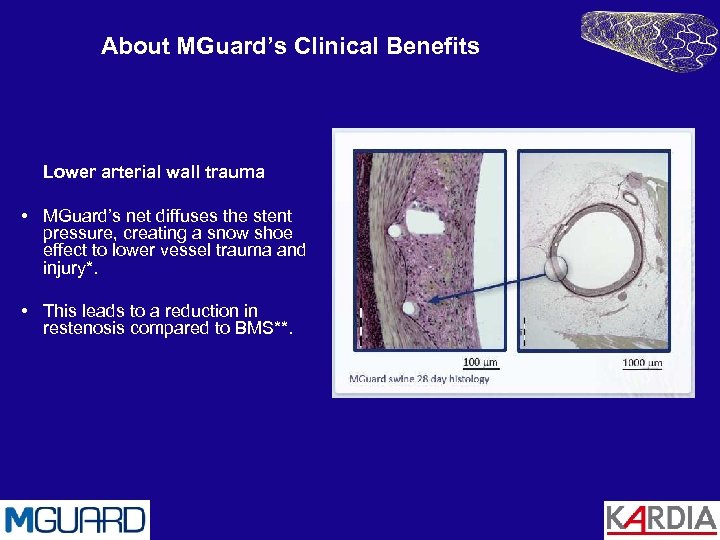
About MGuard’s Clinical Benefits Lower arterial wall trauma • MGuard’s net diffuses the stent pressure, creating a snow shoe effect to lower vessel trauma and injury*. • This leads to a reduction in restenosis compared to BMS**.
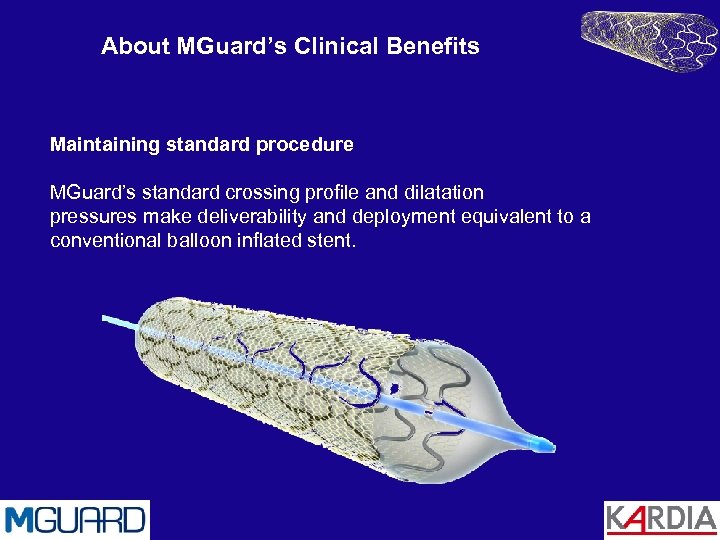
About MGuard’s Clinical Benefits Maintaining standard procedure MGuard’s standard crossing profile and dilatation pressures make deliverability and deployment equivalent to a conventional balloon inflated stent.

MGuard Trials • Preclinical - Coronary Porcine Study • MGuard FIM Trial • The GUARD Trial - Brazil Multicenter Study • The MAGICAL Study MGuard in Acute MI Trial • i. MOS – MGuard International Registry

Animal Trials Porcine studies by CBSET Inc. Cambridge, MA A comparison of MGuard with standard BMS Methods 9 Swine with a total of 21 stents: 5 MGuard, 6 BMS (control) 6 Month FU Trial Results No animal morbidity No device thrombosis Low inflammatory response (0. 8 ± 0. 3 on a scale of 0 -3) Low Schwartz injury score (0. 15 ± 0. 1) Exceptionally good endothelization (4 ± 0)
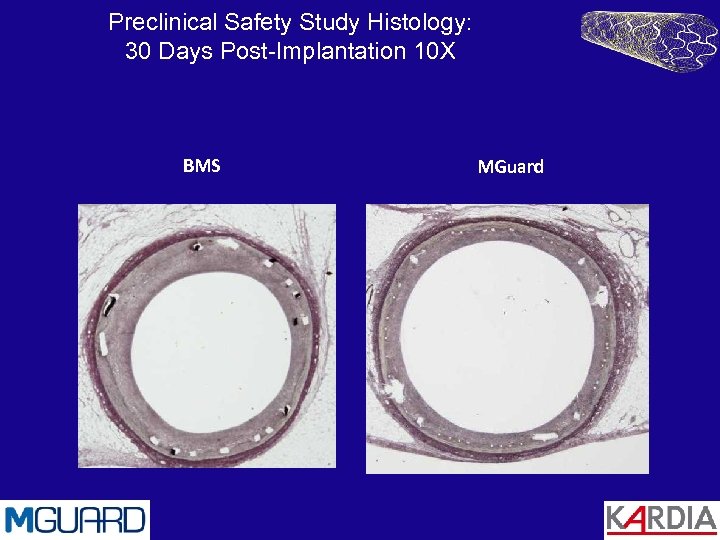
Preclinical Safety Study Histology: 30 Days Post-Implantation 10 X BMS MGuard
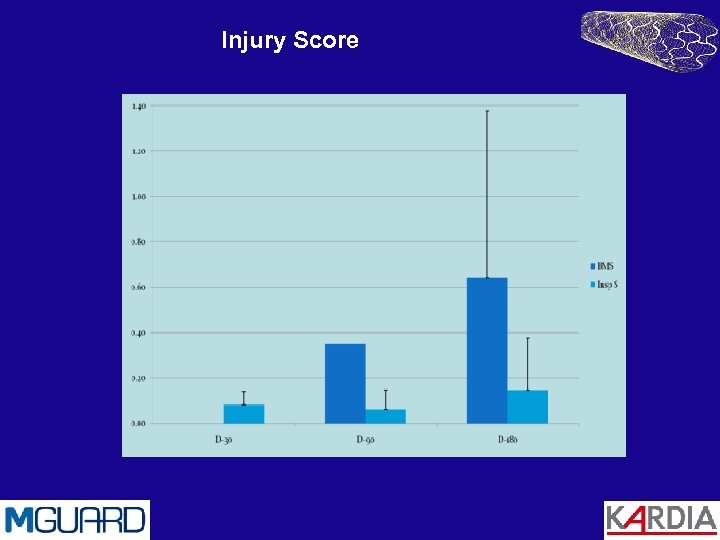
Injury Score
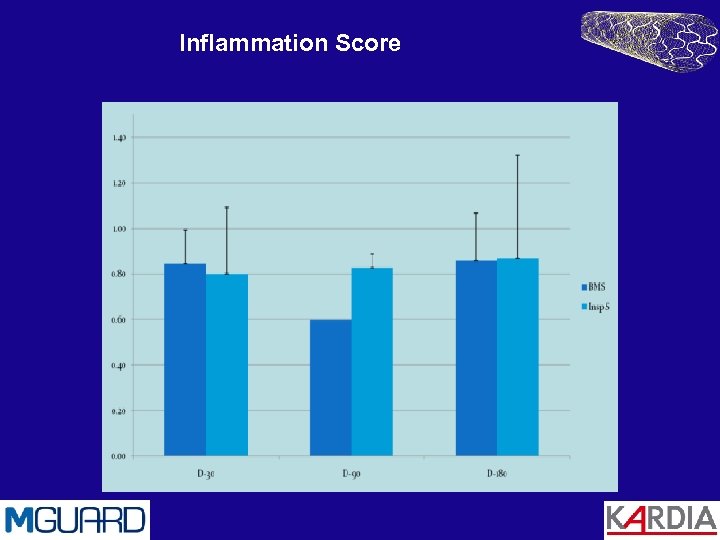
Inflammation Score
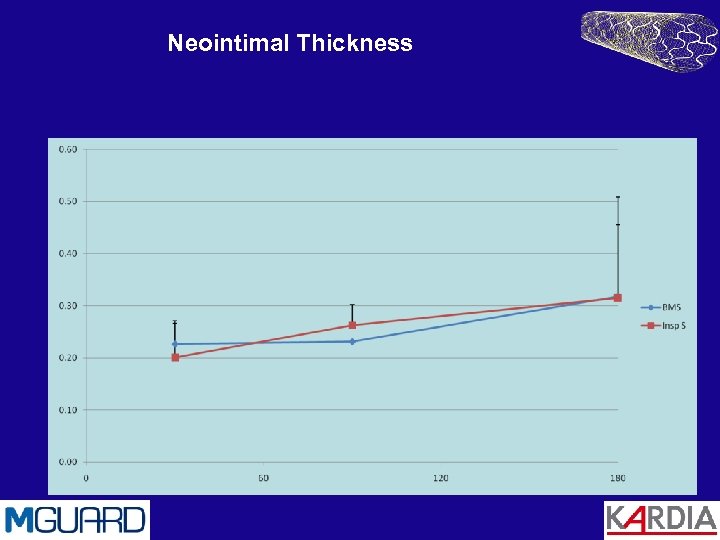
Neointimal Thickness

MGuard First in Man Study Primary endpoint: 30 days MACE Secondary endpoints: Device success Procedural success TIMI flow post procedure 6 Months MACE 6 Months Late Lumen Loss Participating centers: Eberhard Grube ; Helios Heart Center, Siegburg, Germany Karl Eugen Hauptmann ; Trier, Germany
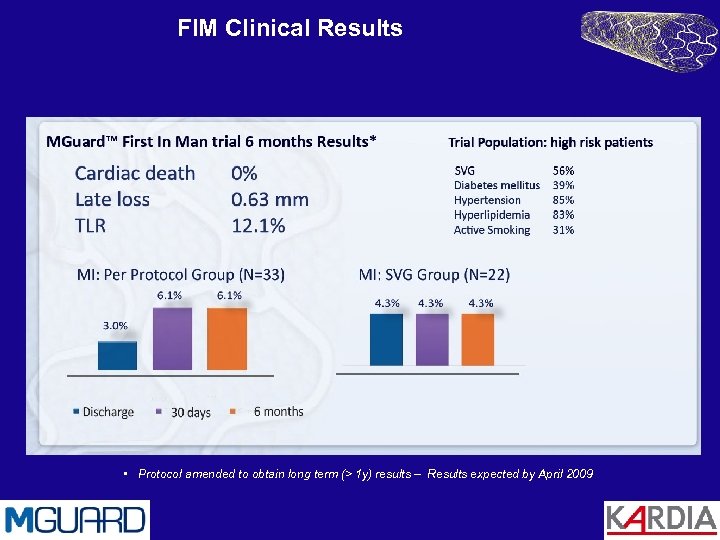
FIM Clinical Results • Protocol amended to obtain long term (> 1 y) results – Results expected by April 2009

Case Report #1 - MGuard in SVG • • • 78 year old female with history of hypercholesterolemia, hypertension, smoking and diabetes. Coronary artery bypass surgery in 1993 and presented with progressive angina (CCS-III). Coronary angiography revealed subtotal occluded (99%) vein graft to the Right Coronary Artery. 2 MGuard stents were implanted Procedural success with: • No clinical adverse events • No CPK rise • No no-reflow post PCI
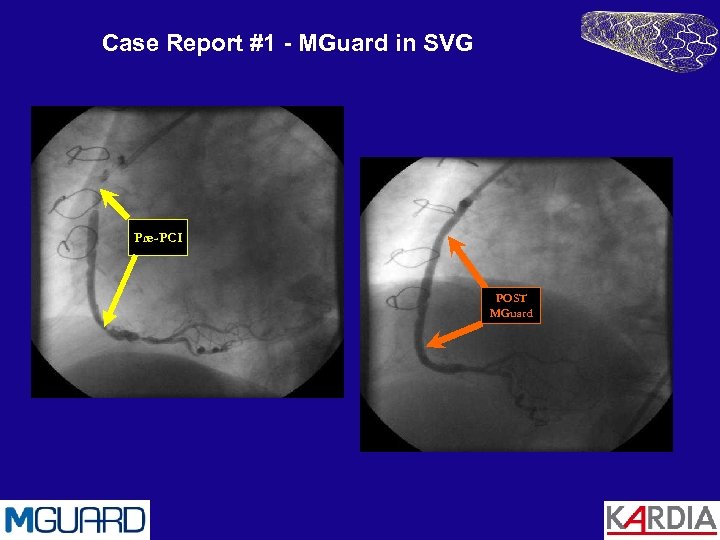
Case Report #1 - MGuard in SVG Pre-PCI POST MGuard
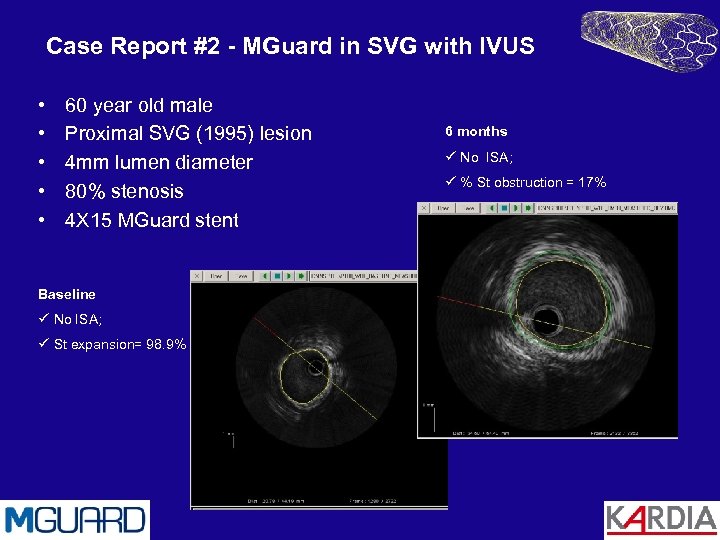
Case Report #2 - MGuard in SVG with IVUS • • • 60 year old male Proximal SVG (1995) lesion 4 mm lumen diameter 80% stenosis 4 X 15 MGuard stent Baseline ü No ISA; ü St expansion= 98. 9% 6 months ü No ISA; ü % St obstruction = 17%
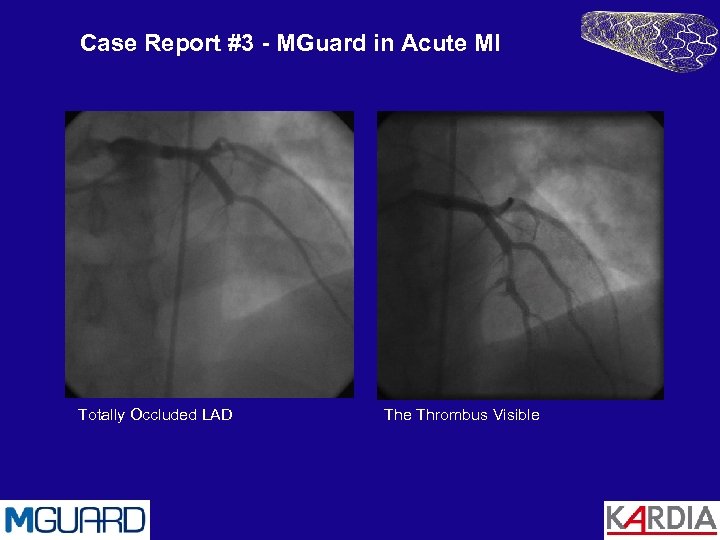
Case Report #3 - MGuard in Acute MI Totally Occluded LAD The Thrombus Visible
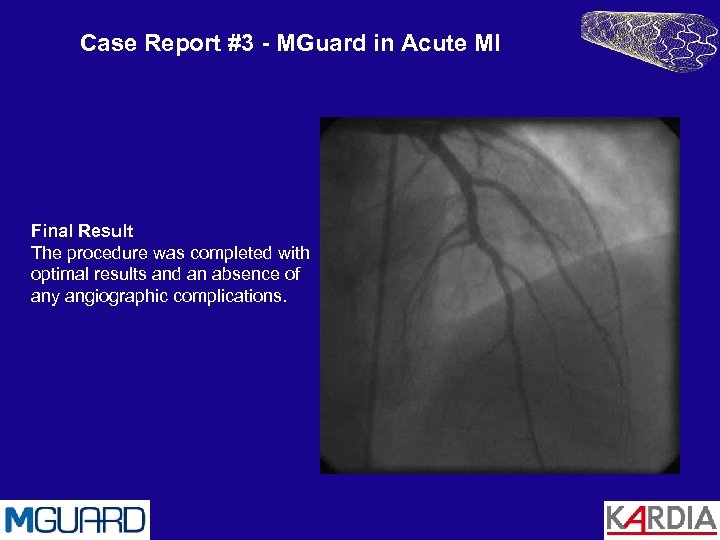
Case Report #3 - MGuard in Acute MI Final Result The procedure was completed with optimal results and an absence of any angiographic complications.
MGuard Technical Data Stent : Stent material: Stainless steel 316 Stent design: Low profile Strut thickness: 100μm, low profile stent design. Catheter: Rapid exchange delivery system 0. 014” guide wire compatible Guiding catheter: 6 F Nominal Pressure: 6 atm. Rated Burst Pressure: ≤ 3. 0 mm: 16 atm. ≥ 3. 5 mm: 14 atm. Radio-opaque markers: Proximal and Distal Balloon Characteristic: Semi - compliant Usable Catheter Length: 1420 mm ± 20 mm
b677ae127d113d121681e97c347a4377.ppt