ab1ce6292d560cf28d3ef3975bc25361.ppt
- Количество слайдов: 18
 Patient Safety Special Interest Group (PSSIG) and Government Projects Special Interest Group Meeting at HEALTH LEVEL 7 Ali Rashidee, MD. MS. Agency for Healthcare Research and Quality
Patient Safety Special Interest Group (PSSIG) and Government Projects Special Interest Group Meeting at HEALTH LEVEL 7 Ali Rashidee, MD. MS. Agency for Healthcare Research and Quality
 PATIENT SAFETY: Estimates of medical errors The Harvard Medical Practice Study (Brennan TA et al. and Leape LL et al. Results of the Harvard Medical Practice Study. New England Journal of Medicine 324(6): 370 -376, and 377 -384 respectively, 1991. – adverse events in 3. 7% of hospitalization, and about 28% of these attributable to negligence. Although about 71% of these caused disabling injuries that lasted less than six months, 2. 6% caused permanent disability and 13. 6 percent lead to death. The Colorado and Utah Hospital Discharge Study (Thomas EJ et al. Incidence and Types of Adverse Events and Negligent Care in Utah and Colorado. Medical Care, Spring 2000) – adverse events in 2. 9% of hospitalizations, and 6. 6 % of these lead to death, and over half assessed to be preventable. When extrapolated to 33. 6 million admissions to US hospitals in 1997, the results of a study in Colorado and Utah conducted by Thomas E J et al. imply that at least 44, 000 Americans dies each year as a result of medical errors. Another study by Leape L L et al. . at Harvard Medical Practice Study, 1991 suggests 98, 000 deaths due to medical errors- AHA Hospital Statistics.
PATIENT SAFETY: Estimates of medical errors The Harvard Medical Practice Study (Brennan TA et al. and Leape LL et al. Results of the Harvard Medical Practice Study. New England Journal of Medicine 324(6): 370 -376, and 377 -384 respectively, 1991. – adverse events in 3. 7% of hospitalization, and about 28% of these attributable to negligence. Although about 71% of these caused disabling injuries that lasted less than six months, 2. 6% caused permanent disability and 13. 6 percent lead to death. The Colorado and Utah Hospital Discharge Study (Thomas EJ et al. Incidence and Types of Adverse Events and Negligent Care in Utah and Colorado. Medical Care, Spring 2000) – adverse events in 2. 9% of hospitalizations, and 6. 6 % of these lead to death, and over half assessed to be preventable. When extrapolated to 33. 6 million admissions to US hospitals in 1997, the results of a study in Colorado and Utah conducted by Thomas E J et al. imply that at least 44, 000 Americans dies each year as a result of medical errors. Another study by Leape L L et al. . at Harvard Medical Practice Study, 1991 suggests 98, 000 deaths due to medical errors- AHA Hospital Statistics.
 Burden of medical errors • Even when using the lower estimate, deaths due to medical errors exceed the number attributable to the 8 th leading cause of death. Death: Final data for 1997. CDC-National Vital Statistics Reports. 47(19): 27, 1999. • More people die in a given year as a result of medical errors than from motor vehicle accidents (~44, 000), breast cancer (~43, 000) or AIDS(~16, 500). Births and Deaths: Preliminary data for 1998. CDC, National Vital Statistics Reports. 47(25): 6, 1999. • Medication error along, occurring either in or out of hospitals, are estimated to account for 7000 deaths annually. Phillips DP et al. Increase in US medication error deaths between 1983 and 1993. The Lancet, 351: 643 -44, 1998. • Total national cost of preventable adverse events are estimated between 17 billion of which health care costs represent one half. Thomas EJ et al. Cost of Medical Injuries in Utah and Colorado. Inquiry 36: 225 -264, 1999 and Johnson WJ et al. The economic consequences of medical injuries, JAMA. 267: 2487 -2492, 1992. • The Quality in Australian Health Care Study (Wilson RM et al. The Quality in Australian Health Care Study. The Medical Journal of Australia. 163(9): 458 -71, 1995 – 16. 6 percent of hospital admissions involved adverse events, half of those considered preventable. About 14 percent of adverse events were found to have resulted in permanent disability, with 4. 9 percent resulting in death.
Burden of medical errors • Even when using the lower estimate, deaths due to medical errors exceed the number attributable to the 8 th leading cause of death. Death: Final data for 1997. CDC-National Vital Statistics Reports. 47(19): 27, 1999. • More people die in a given year as a result of medical errors than from motor vehicle accidents (~44, 000), breast cancer (~43, 000) or AIDS(~16, 500). Births and Deaths: Preliminary data for 1998. CDC, National Vital Statistics Reports. 47(25): 6, 1999. • Medication error along, occurring either in or out of hospitals, are estimated to account for 7000 deaths annually. Phillips DP et al. Increase in US medication error deaths between 1983 and 1993. The Lancet, 351: 643 -44, 1998. • Total national cost of preventable adverse events are estimated between 17 billion of which health care costs represent one half. Thomas EJ et al. Cost of Medical Injuries in Utah and Colorado. Inquiry 36: 225 -264, 1999 and Johnson WJ et al. The economic consequences of medical injuries, JAMA. 267: 2487 -2492, 1992. • The Quality in Australian Health Care Study (Wilson RM et al. The Quality in Australian Health Care Study. The Medical Journal of Australia. 163(9): 458 -71, 1995 – 16. 6 percent of hospital admissions involved adverse events, half of those considered preventable. About 14 percent of adverse events were found to have resulted in permanent disability, with 4. 9 percent resulting in death.
 IOM Reports IOM reports have consistently emphasized the need for enhancing patient safety and healthcare quality: § Patient Safety: A New Standard for Care ( 2003) § Priority Areas for National Action: Transforming Healthcare quality (2003) § Crossing the Quality Chasm (2001) § To Err is Human (2000) § Computer-based Patient Record (1991, 1997)
IOM Reports IOM reports have consistently emphasized the need for enhancing patient safety and healthcare quality: § Patient Safety: A New Standard for Care ( 2003) § Priority Areas for National Action: Transforming Healthcare quality (2003) § Crossing the Quality Chasm (2001) § To Err is Human (2000) § Computer-based Patient Record (1991, 1997)
 IOM Recommendations The future health care models needs to actively pursue many key elements: • Consider care to be a continuous process • Knowledge is shared & information flows freely • Decision making is evidence-based, with protocol/process support • Safety is a system property • Transparency is a necessity • Care delivery is Team-based • Cooperation among the clinicians is a priority Patient Safety will be of paramount importance as healthcare becomes exponentially complex, and the discipline needs to encompass entire spectrum of healthcare.
IOM Recommendations The future health care models needs to actively pursue many key elements: • Consider care to be a continuous process • Knowledge is shared & information flows freely • Decision making is evidence-based, with protocol/process support • Safety is a system property • Transparency is a necessity • Care delivery is Team-based • Cooperation among the clinicians is a priority Patient Safety will be of paramount importance as healthcare becomes exponentially complex, and the discipline needs to encompass entire spectrum of healthcare.
 Definitions § Original IOM Errors report: “An adverse event is defined as an injury caused by medical management [commission] rather than by the underlying disease or condition of the patient. ” § Patient Safety definition: “An adverse event resulting in unintended harm to the patient by an act of commission or omission rather than by the underlying disease or condition of the patient. ”
Definitions § Original IOM Errors report: “An adverse event is defined as an injury caused by medical management [commission] rather than by the underlying disease or condition of the patient. ” § Patient Safety definition: “An adverse event resulting in unintended harm to the patient by an act of commission or omission rather than by the underlying disease or condition of the patient. ”
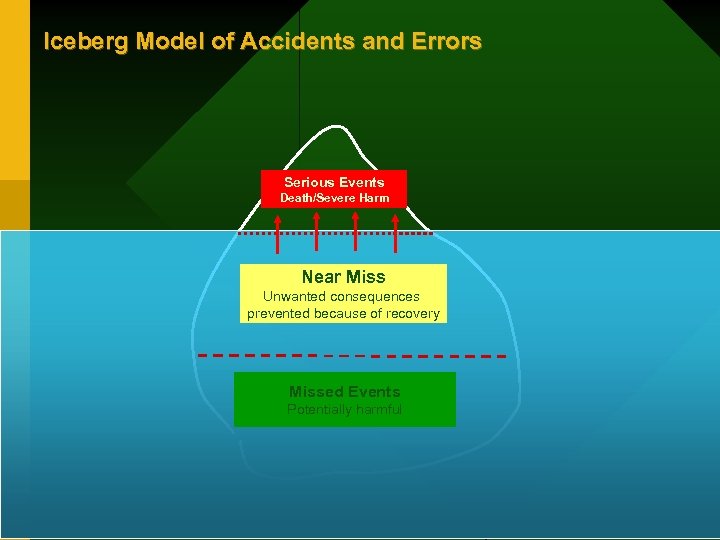 Iceberg Model of Accidents and Errors Serious Events Death/Severe Harm Near Miss Unwanted consequences prevented because of recovery Missed Events Potentially harmful
Iceberg Model of Accidents and Errors Serious Events Death/Severe Harm Near Miss Unwanted consequences prevented because of recovery Missed Events Potentially harmful
 Mission of the PSSIG Support the HL 7 mission to create and promote its standards by defining the information system interoperability components required for interchange of patient safety information and the reporting of adverse events.
Mission of the PSSIG Support the HL 7 mission to create and promote its standards by defining the information system interoperability components required for interchange of patient safety information and the reporting of adverse events.
 Contributions to HL 7 Standardization The PSSIG works on contributing to the HL 7 processes and products to: § Create scenarios to develop the requirements for identifying and sharing medical errors, adverse events, contributory factor analysis, and other patient safety related information. § Identify clinical decision support needs to facilitate patient safety § Further develop Domain Information Models (DIMs), Refined Message Information Models (RMIMs), and messages which can be communicated between systems that serve ‘Patient Safety’ needs § Identify the set of trigger events to initiate the transmission of such data § Create clinical content and document architecture for collecting and sharing medical errors/adverse event data. § Identify, develop and promote the use of standard terminology to support the collection and sharing of medical errors/adverse events § Enable and promote the use of these standards and make the standards as widely available as possible.
Contributions to HL 7 Standardization The PSSIG works on contributing to the HL 7 processes and products to: § Create scenarios to develop the requirements for identifying and sharing medical errors, adverse events, contributory factor analysis, and other patient safety related information. § Identify clinical decision support needs to facilitate patient safety § Further develop Domain Information Models (DIMs), Refined Message Information Models (RMIMs), and messages which can be communicated between systems that serve ‘Patient Safety’ needs § Identify the set of trigger events to initiate the transmission of such data § Create clinical content and document architecture for collecting and sharing medical errors/adverse event data. § Identify, develop and promote the use of standard terminology to support the collection and sharing of medical errors/adverse events § Enable and promote the use of these standards and make the standards as widely available as possible.
 Formal Relationships with other HL 7 Groups The PSSIG is being sponsored by the Regulated Clinical Research Information Management (RCRIM) Technical Committee. The PSSIG will work closely with the: § Patient Care Technical Committee to define settings and processes and information needs in medical care that effect patient safety § Electronic Health Record Special Interest Group to identify and help develop patient safety related functionalities within electronic medical record environment § Medication Special Interest Group to ensure proper medication usage (drug-drug interaction, contraindications, drug concentration and dosing, dispensing, etc. )
Formal Relationships with other HL 7 Groups The PSSIG is being sponsored by the Regulated Clinical Research Information Management (RCRIM) Technical Committee. The PSSIG will work closely with the: § Patient Care Technical Committee to define settings and processes and information needs in medical care that effect patient safety § Electronic Health Record Special Interest Group to identify and help develop patient safety related functionalities within electronic medical record environment § Medication Special Interest Group to ensure proper medication usage (drug-drug interaction, contraindications, drug concentration and dosing, dispensing, etc. )
 Formal Relationships with other HL 7 Groups § Structured Document Technical Committee to accommodate the patient safety information needs within structured technical documents § Clinical Decision Support Technical Committee to address patient safety needs at the point-of-care from a evidence-based healthcare delivery perspective; and § Vocabulary Technical Committee to improve patient safety related vocabularies (coding for diseases, adverse events/medical errors, drugs, vaccines, devices, procedures, clinical terminology, regulatory terminology, etc. )
Formal Relationships with other HL 7 Groups § Structured Document Technical Committee to accommodate the patient safety information needs within structured technical documents § Clinical Decision Support Technical Committee to address patient safety needs at the point-of-care from a evidence-based healthcare delivery perspective; and § Vocabulary Technical Committee to improve patient safety related vocabularies (coding for diseases, adverse events/medical errors, drugs, vaccines, devices, procedures, clinical terminology, regulatory terminology, etc. )
 Relationships with Other Partners This SIG will work with the: § IOM Board on Healthcare Services: Data Standards for Promoting Patient Safety § Joint Commission on Accreditation of Healthcare Organizations § Center for Medicare and Medicaid Services, Food and Drug Administration, Centers for Disease control and Prevention, Agency for Healthcare Research and Quality § Consolidated Health Informatics Initiative, and § Other national/international government and non-government stakeholders interested in addressing adverse events/medical error related challenges and promoting patient safety.
Relationships with Other Partners This SIG will work with the: § IOM Board on Healthcare Services: Data Standards for Promoting Patient Safety § Joint Commission on Accreditation of Healthcare Organizations § Center for Medicare and Medicaid Services, Food and Drug Administration, Centers for Disease control and Prevention, Agency for Healthcare Research and Quality § Consolidated Health Informatics Initiative, and § Other national/international government and non-government stakeholders interested in addressing adverse events/medical error related challenges and promoting patient safety.
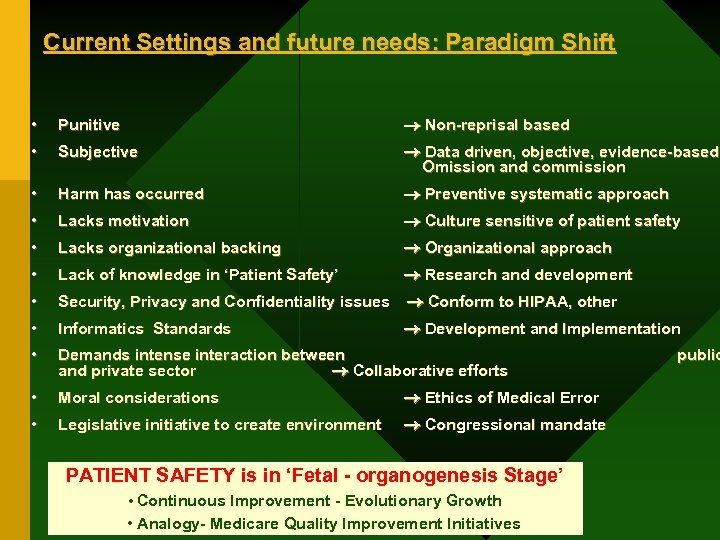 Current Settings and future needs: Paradigm Shift • Punitive Non-reprisal based • Subjective Data driven, objective, evidence-based, Omission and commission • Harm has occurred Preventive systematic approach • Lacks motivation Culture sensitive of patient safety • Lacks organizational backing Organizational approach • Lack of knowledge in ‘Patient Safety’ Research and development • Security, Privacy and Confidentiality issues Conform to HIPAA, other • Informatics Standards Development and Implementation • Demands intense interaction between and private sector Collaborative efforts • Moral considerations Ethics of Medical Error • Legislative initiative to create environment Congressional mandate PATIENT SAFETY is in ‘Fetal - organogenesis Stage’ • Continuous Improvement - Evolutionary Growth • Analogy- Medicare Quality Improvement Initiatives public
Current Settings and future needs: Paradigm Shift • Punitive Non-reprisal based • Subjective Data driven, objective, evidence-based, Omission and commission • Harm has occurred Preventive systematic approach • Lacks motivation Culture sensitive of patient safety • Lacks organizational backing Organizational approach • Lack of knowledge in ‘Patient Safety’ Research and development • Security, Privacy and Confidentiality issues Conform to HIPAA, other • Informatics Standards Development and Implementation • Demands intense interaction between and private sector Collaborative efforts • Moral considerations Ethics of Medical Error • Legislative initiative to create environment Congressional mandate PATIENT SAFETY is in ‘Fetal - organogenesis Stage’ • Continuous Improvement - Evolutionary Growth • Analogy- Medicare Quality Improvement Initiatives public
 Work in Progress § Individual Case Safety Reporting – Purpose: Enable individual case safety reporting from providers, and between information systems – Progress: • FDA has been working on this. Drug adverse event messaging being balloted at the RCRIM TC level • Work on Device, Blood Product, Vaccine case safety reporting going on § Notifiable diseases - case notification related messaging § Nosocomial Infection related messaging
Work in Progress § Individual Case Safety Reporting – Purpose: Enable individual case safety reporting from providers, and between information systems – Progress: • FDA has been working on this. Drug adverse event messaging being balloted at the RCRIM TC level • Work on Device, Blood Product, Vaccine case safety reporting going on § Notifiable diseases - case notification related messaging § Nosocomial Infection related messaging
 Patient Safety Task Force: AHRQ, CDC, CMS, FDA. NATIONAL PATIENT SAFETY NETWORK (NPSN) § Adverse Event Reporting System - FDA § Vaccine Adverse Event Reporting System, co-admin by CDC and FDA § Biological Product Deviation Reporting System - FDA § Fatalities - FDA, a sub-system of BPDR § Manufacturer and User Data Experience -Medical Device - FDA, and § National Healthcare Safety Network and its subcomponents – CDC § Collection of Contextual Information § Contributory Factor Analysis - Eindhoven Model § Safety related Terminology § Development of Knowledgebase and Decision Support
Patient Safety Task Force: AHRQ, CDC, CMS, FDA. NATIONAL PATIENT SAFETY NETWORK (NPSN) § Adverse Event Reporting System - FDA § Vaccine Adverse Event Reporting System, co-admin by CDC and FDA § Biological Product Deviation Reporting System - FDA § Fatalities - FDA, a sub-system of BPDR § Manufacturer and User Data Experience -Medical Device - FDA, and § National Healthcare Safety Network and its subcomponents – CDC § Collection of Contextual Information § Contributory Factor Analysis - Eindhoven Model § Safety related Terminology § Development of Knowledgebase and Decision Support
 How will NPSN bring ‘VALUE’ to the care givers? • Monitoring – Number, type of adverse events (serious/non-serious, sentinel, preventable/non-preventable etc. ) – Identify ‘Safety’ related week points in a health care delivery system – Contributory Factor analysis to guide corrective action – Assessment of outcomes of corrective actions • Modeling – unidentified/new unique events that may reflect preventable threat • Mindfulness – Heightened awareness of adverse events - reduce complacency – Active engagement, ownership – Feedback After T van der Schaaf. Disillusionment and Empowerment!
How will NPSN bring ‘VALUE’ to the care givers? • Monitoring – Number, type of adverse events (serious/non-serious, sentinel, preventable/non-preventable etc. ) – Identify ‘Safety’ related week points in a health care delivery system – Contributory Factor analysis to guide corrective action – Assessment of outcomes of corrective actions • Modeling – unidentified/new unique events that may reflect preventable threat • Mindfulness – Heightened awareness of adverse events - reduce complacency – Active engagement, ownership – Feedback After T van der Schaaf. Disillusionment and Empowerment!
 What other Government Projects are working on the same/similar theme? A. B. C.
What other Government Projects are working on the same/similar theme? A. B. C.
 What are the common goals? A. B. C. What are the best ways to work together?
What are the common goals? A. B. C. What are the best ways to work together?


