0ea411040a67ed92bad5e0fd90dc2535.ppt
- Количество слайдов: 125

Patient Safety Monitoring in International Laboratories (SMILE) Quality Management in Hematology Laboratory. By Heidi Hanes 1

Patient Safety Monitoring in International Laboratories (SMILE) Objectives • At the end of this presentation should be able to: ØRecognize the four components of Quality Assurance. ØRecognize how to troubleshoot problems. ØDevelop own policy for all aspect of quality assurance. ØUnderstand what is necessary to undertake a program of Quality Management in Hematology Laboratory. 3

Patient Safety Monitoring in International Laboratories (SMILE) Quality Management includes • • Standardization Pre Analytical Control Post Analytical Control ØInternal Quality Control ØExternal Quality Assessment 4

Patient Safety Monitoring in International Laboratories (SMILE) 5

Patient Safety Monitoring in International Laboratories (SMILE) Standardization • Collaboration between groups. • Standard Operating Procedures • Problem solving for unsatisfactory performance • Test selection 6

PRE-ANALYTICAL CONTROL 7

Patient Safety Monitoring in International Laboratories (SMILE) Understanding functionality of your instrument: • • principles of operation startup or daily checks shutdown procedure normal sights and sounds of the instrument • familiarize staff to troubleshooting manual 8
Patient Safety Monitoring in International Laboratories (SMILE) Hematology Subsystems • Consist of 3 subsystems. Ø Electronic Ø Fluidic § Pneumatics (pressure and vacuums) § Hydraulics (liquids) Ø Reagent 9

Patient Safety Monitoring in International Laboratories (SMILE) Proper Specimen Collection • Analyze only specimens that were properly collected and stored. ØCorrect Tube ØCorrect amount of specimen in tube ØProper mixing ØCleanliness of puncture area ØStorage 10

Patient Safety Monitoring in International Laboratories (SMILE) Exercise 1 11
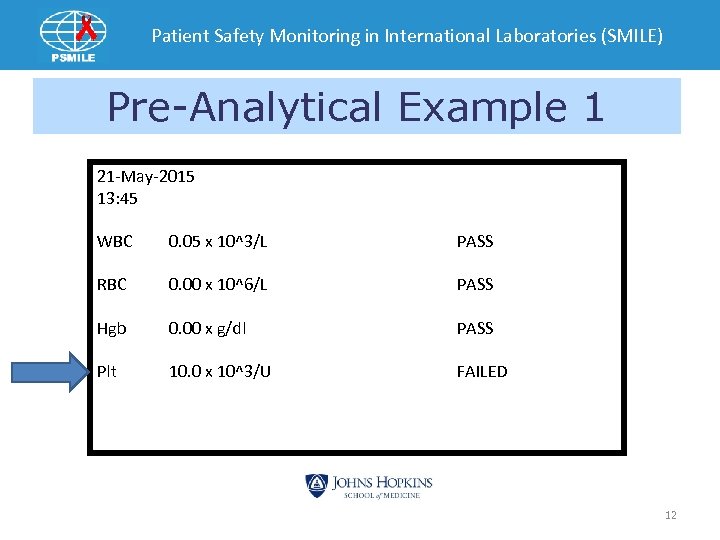
Patient Safety Monitoring in International Laboratories (SMILE) Pre-Analytical Example 1 21 -May-2015 13: 45 WBC 0. 05 x 10^3/L PASS RBC 0. 00 x 10^6/L PASS Hgb 0. 00 x g/dl PASS Plt 10. 0 x 10^3/U FAILED 12
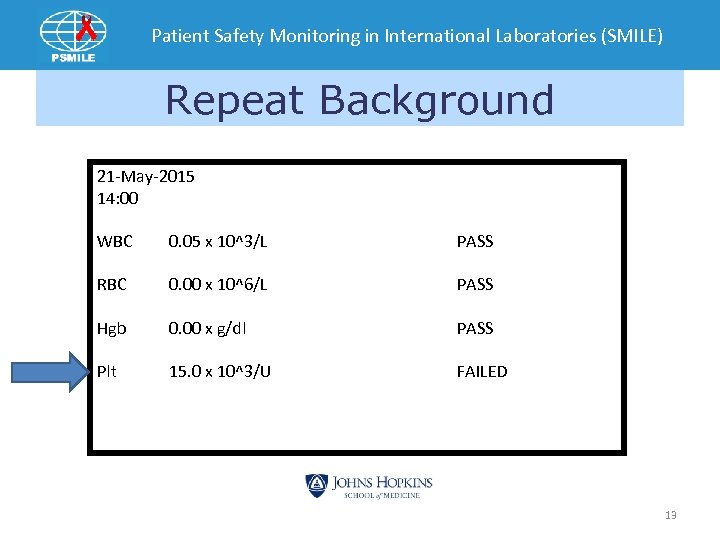
Patient Safety Monitoring in International Laboratories (SMILE) Repeat Background 21 -May-2015 14: 00 WBC 0. 05 x 10^3/L PASS RBC 0. 00 x 10^6/L PASS Hgb 0. 00 x g/dl PASS Plt 15. 0 x 10^3/U FAILED 13

Patient Safety Monitoring in International Laboratories (SMILE) Corrective Action • Check when reagent(s) last changed. • Change the most recently added reagent. • If none recently added change the diluent. • Rerun start-up 14
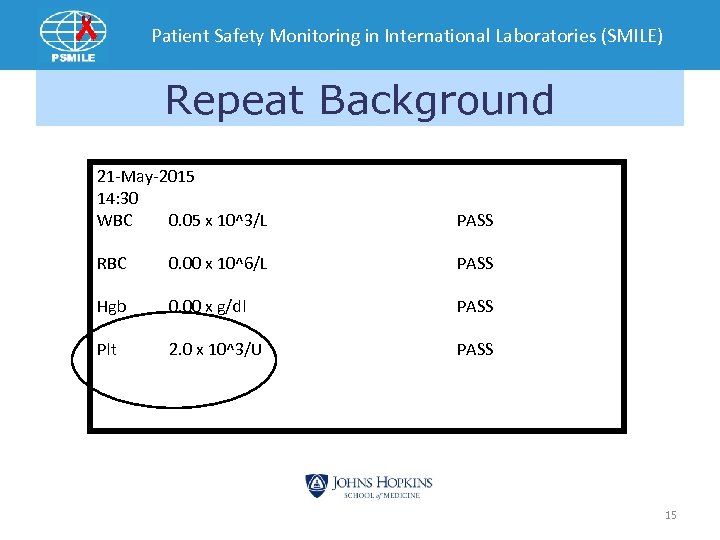
Patient Safety Monitoring in International Laboratories (SMILE) Repeat Background 21 -May-2015 14: 30 WBC 0. 05 x 10^3/L PASS RBC 0. 00 x 10^6/L PASS Hgb 0. 00 x g/dl PASS Plt 2. 0 x 10^3/U PASS 15

Patient Safety Monitoring in International Laboratories (SMILE) Causes for high background • The uptake tubing got contaminated. • Reagent agitated before connecting to instrument. • Dried reagent spills or leaks. 16
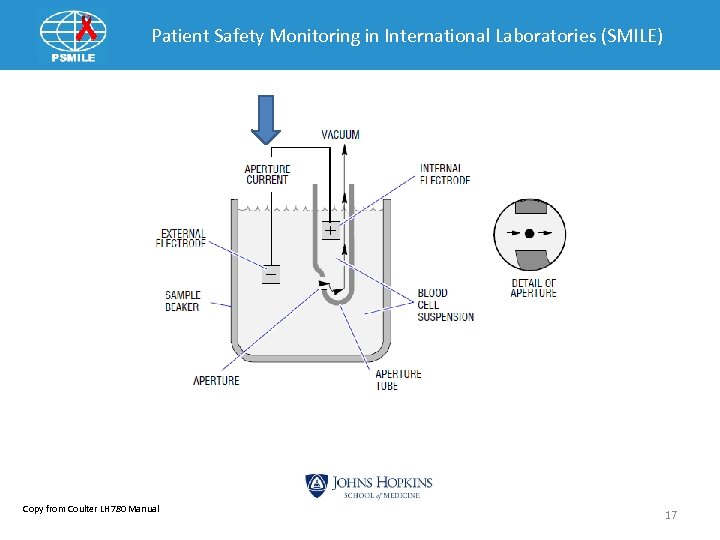
Patient Safety Monitoring in International Laboratories (SMILE) Copy from Coulter LH 780 Manual 17
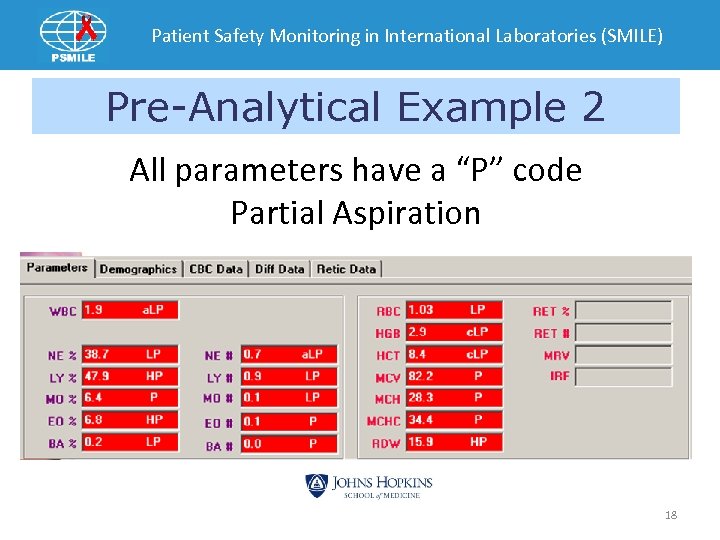
Patient Safety Monitoring in International Laboratories (SMILE) Pre-Analytical Example 2 All parameters have a “P” code Partial Aspiration 18

Patient Safety Monitoring in International Laboratories (SMILE) What can give this code? • • • Sample could be clotted. Hemoglobin is less than 4 g/dl Sample volume too low. The blood detector was turned off. Check your instrument manual. 19

Patient Safety Monitoring in International Laboratories (SMILE) Other Corrective Steps • Troubleshoot for leaks, kinks or plugs along the sample flow path. • Ensure the aspiration lines are clean. • Check for needle plugs. • Call Service Hot Line 20

Patient Safety Monitoring in International Laboratories (SMILE) Pre-Analytical Example 3 Get a 30 psi Pressure High Error 21

Patient Safety Monitoring in International Laboratories (SMILE) Solutions • Find the 30 psi regulator and adjust to 30 +/ -0. 2 psi. • Rerun several bloods and compare to see if any difference. • If unable to adjust or error occurs again call Technical Hot line. 22

Patient Safety Monitoring in International Laboratories (SMILE) 23

Post-Analytical Control Understanding your instrument results: 24

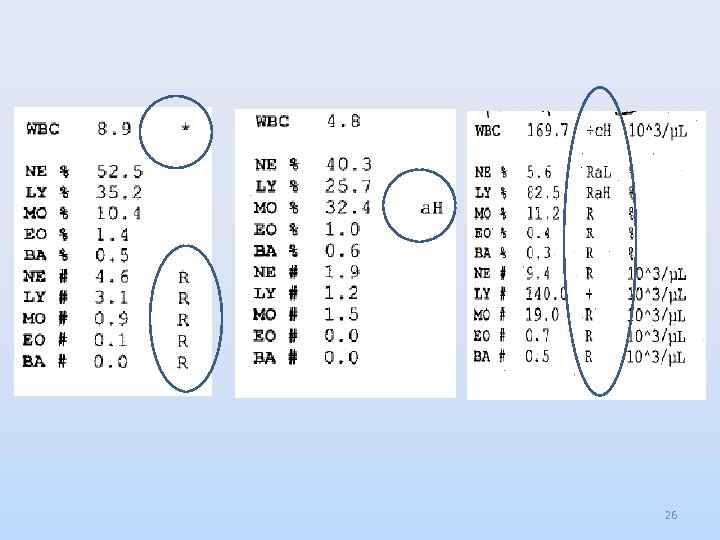
Flags • Can be a letter or symbol that appears to right of the result. ØManufacturer controlled ØAspiration, Linearity, interference ØLaboratory controlled ØCritical range ØDecision rules 25
26
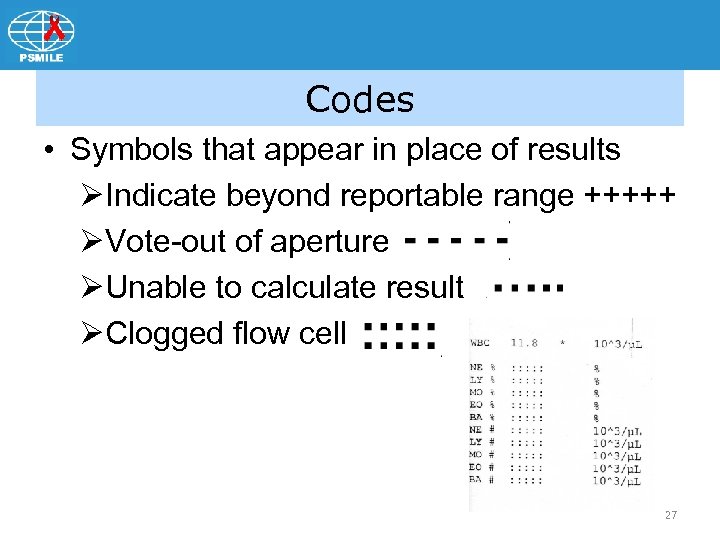
Codes • Symbols that appear in place of results ØIndicate beyond reportable range +++++ ØVote-out of aperture ØUnable to calculate result ØClogged flow cell 27

Patient Safety Monitoring in International Laboratories (SMILE) Messages • Usually manufacturer messages • Indicate possible abnormal cells, clumping, agglutination • Used to decide on reporting of instrument vs manual differential or verification of measured results. 28

Patient Safety Monitoring in International Laboratories (SMILE) I W 29

Patient Safety Monitoring in International Laboratories (SMILE) Patient Results • Are the results consistent with the patient’s condition? • Delta checks ØCan be set on instrument or LIS. ØChecks against previous result. ØValue set by laboratory. 30
H & H Check/Difference • Hgb x 3 = HCT +/- 3% • If > +/-3 can indicate a problem. ØInstrument ØSample 31

Patient Safety Monitoring in International Laboratories (SMILE) Exercise 2 32
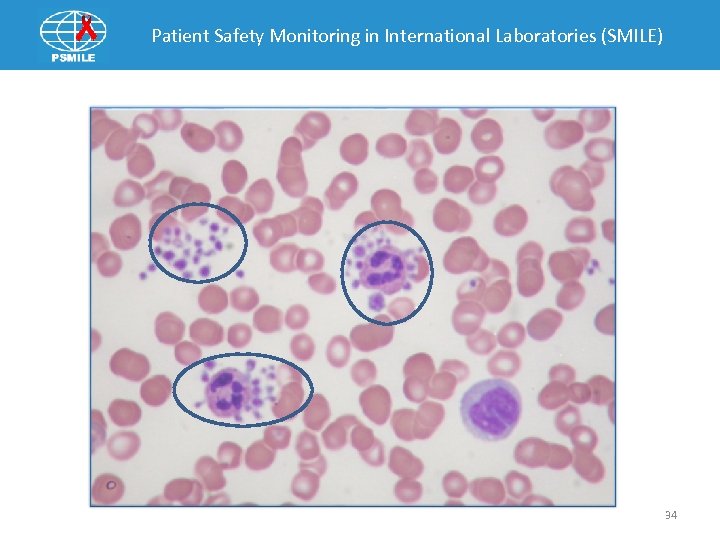
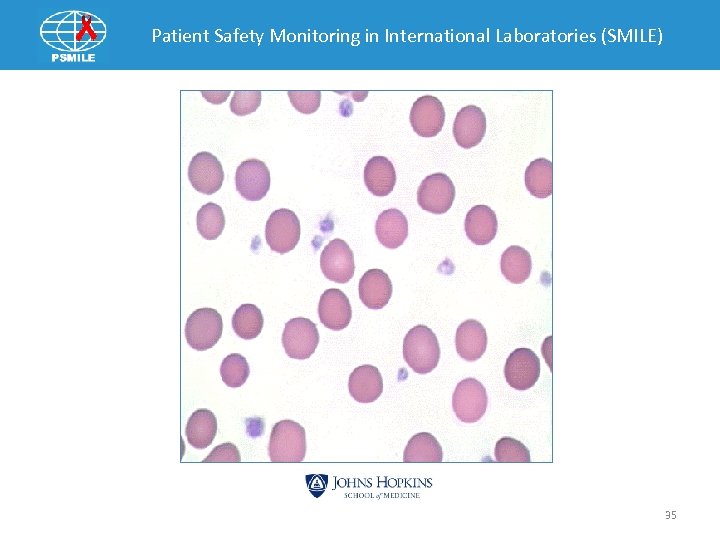
Patient Safety Monitoring in International Laboratories (SMILE) Post Analytical Example 1 Laboratory Results WBC - 8, 900/u. L RBC - 4, 460, 000/u. L Hgb -13. 4 g/dl HCT- 40. 7% Platelets - 56, 000/ul MCV - 91. 1. 1 fl MCH - 29. 9 pg MCHC - 32. 8 g/dl RDW - 23. 1% Instrument Differential Neutrophil % - 52. 5% Lymphocyte % - 35. 2% Monocyte % - 10. 4% Eosinophil % - 1. 4% Basophil % - 0. 5% Flags WBC interference (*) Micro/Fragmented RBC Giant Platelets R (Review)-code on Platelets Platelet Clumps 33
Patient Safety Monitoring in International Laboratories (SMILE) 34
Patient Safety Monitoring in International Laboratories (SMILE) 35
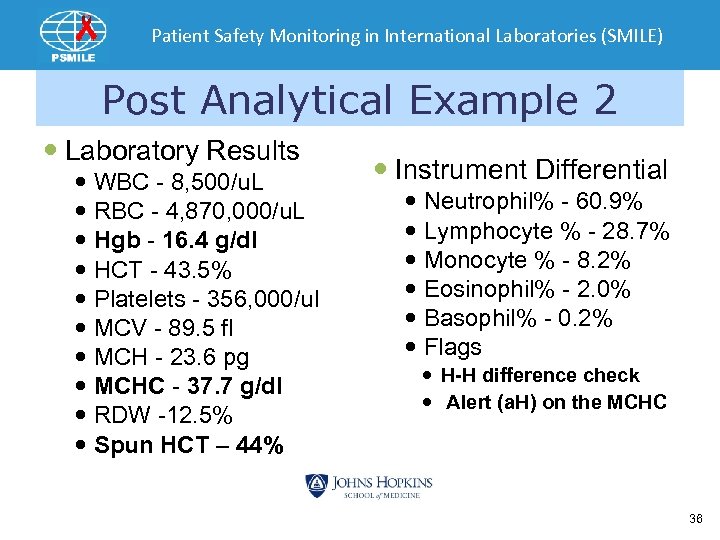
Patient Safety Monitoring in International Laboratories (SMILE) Post Analytical Example 2 Laboratory Results WBC - 8, 500/u. L RBC - 4, 870, 000/u. L Hgb - 16. 4 g/dl HCT - 43. 5% Platelets - 356, 000/ul MCV - 89. 5 fl MCH - 23. 6 pg MCHC - 37. 7 g/dl RDW -12. 5% Spun HCT – 44% Instrument Differential Neutrophil% - 60. 9% Lymphocyte % - 28. 7% Monocyte % - 8. 2% Eosinophil% - 2. 0% Basophil% - 0. 2% Flags H-H difference check Alert (a. H) on the MCHC 36

Patient Safety Monitoring in International Laboratories (SMILE) What is the Explanation ? Spun Plasma • The MCHC is > 36 • The spun hematocrit matches the instrument • It would appear that the Hgb is incorrect • Lipemia will falsely increase the hemoglobin result • Follow laboratory policy for lipemic samples 37
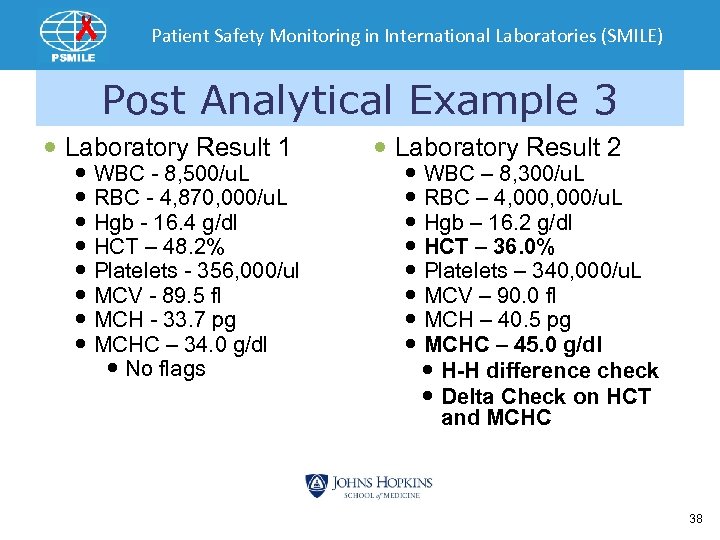
Patient Safety Monitoring in International Laboratories (SMILE) Post Analytical Example 3 Laboratory Result 1 WBC - 8, 500/u. L RBC - 4, 870, 000/u. L Hgb - 16. 4 g/dl HCT – 48. 2% Platelets - 356, 000/ul MCV - 89. 5 fl MCH - 33. 7 pg MCHC – 34. 0 g/dl No flags Laboratory Result 2 WBC – 8, 300/u. L RBC – 4, 000/u. L Hgb – 16. 2 g/dl HCT – 36. 0% Platelets – 340, 000/u. L MCV – 90. 0 fl MCH – 40. 5 pg MCHC – 45. 0 g/dl H-H difference check Delta Check on HCT and MCHC 38

Patient Safety Monitoring in International Laboratories (SMILE) 39
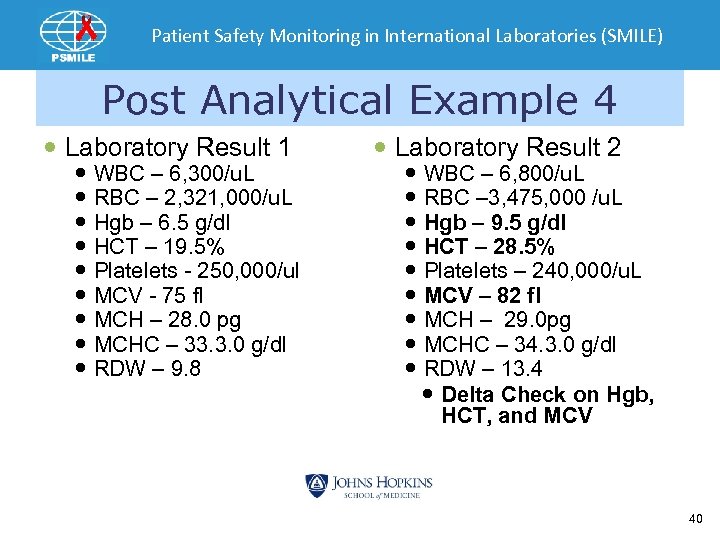
Patient Safety Monitoring in International Laboratories (SMILE) Post Analytical Example 4 Laboratory Result 1 WBC – 6, 300/u. L RBC – 2, 321, 000/u. L Hgb – 6. 5 g/dl HCT – 19. 5% Platelets - 250, 000/ul MCV - 75 fl MCH – 28. 0 pg MCHC – 33. 3. 0 g/dl RDW – 9. 8 Laboratory Result 2 WBC – 6, 800/u. L RBC – 3, 475, 000 /u. L Hgb – 9. 5 g/dl HCT – 28. 5% Platelets – 240, 000/u. L MCV – 82 fl MCH – 29. 0 pg MCHC – 34. 3. 0 g/dl RDW – 13. 4 Delta Check on Hgb, HCT, and MCV 40
Patient Safety Monitoring in International Laboratories (SMILE) 41
Patient Safety Monitoring in International Laboratories (SMILE) 42

Patient Safety Monitoring in International Laboratories (SMILE) 43

Patient Safety Monitoring in International Laboratories (SMILE) Analytical Control • Internal Quality Control • External Quality Assessment 44

Patient Safety Monitoring in International Laboratories (SMILE) INTERNAL QUALITY CONTROL 45

Patient Safety Monitoring in International Laboratories (SMILE) Internal Quality Control includes • Daily control specimen ØCommercial Control ØPatient Control • XB Analysis – Moving Average • Correlation/Comparison System • Policy- QC and Troubleshooting 46

Patient Safety Monitoring in International Laboratories (SMILE) Internal Quality Control Commercial Controls 47
Patient Safety Monitoring in International Laboratories (SMILE) Commercial Controls • Assayed vs Non-assayed • Introducing New QC Lot • Establishing QC Ranges 48

Patient Safety Monitoring in International Laboratories (SMILE) Lot–To-Lot Correlations 1. Set up new QC files for each control level of the new lot. 2. Verify the new lot by running each level of control three times in its respective file. 3. Ensure that the mean values of the control runs fall within the ranges published on the manufacturer assay. 4. Run each level twice a day for 3 -5 days to calculate new mean values for each analyte. 5. Compare the calculated mean values for each level to the range specified on the manufacturer assay sheet. 6. If the calculated mean is within range, enter it as the expected mean. 49

Patient Safety Monitoring in International Laboratories (SMILE) Establishing Means and Standard Deviations 1. Analyze the control(s) a minimum of 20 times across several days. 2. Take the average of these runs. 3. The average should be within the range state on the assay sheet. 4. Calculate a two Standard Deviation range from your results. 5. Incorporate this SD range around your new mean and monitor. 6. The mean and SD values should be periodically recalculated during the life of the new lot. 50
Patient Safety Monitoring in International Laboratories (SMILE) Factors to Consider for Means • Some hematology parameters, such as MCV, Platelets and RBC will start to change over time. • MCV will increase. • RBC values can decrease. • Platelets values will increase. • Mean, SD, and CV should be evaluated monthly. 51

Patient Safety Monitoring in International Laboratories (SMILE) Exercise 3 52
Patient Safety Monitoring in International Laboratories (SMILE) • • QC Example 1 Know variation of mean 2 fl Initial MCV mean – 84 fl Historical 2 SD – +/- 4 fl Use half known variation accommodate change Ø 1 fl • What should the mean be? Ø 85 • Established range – 81 -89 fl • Cumulative mean at end of product life should be 85. 53
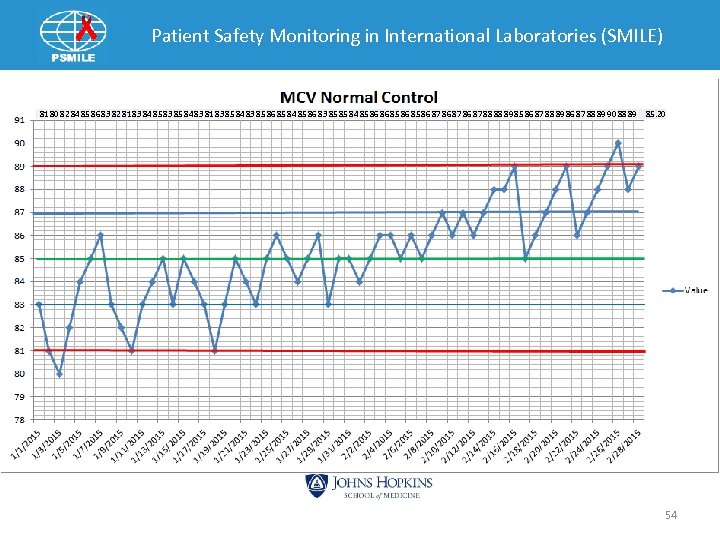
Patient Safety Monitoring in International Laboratories (SMILE) 81 80 82 84 85 86 83 82 81 83 84 85 83 85 84 83 81 83 85 84 83 85 86 85 84 85 86 83 85 85 84 85 86 86 85 86 87 88 88 89 85 86 87 88 89 90 88 89 85. 20 54
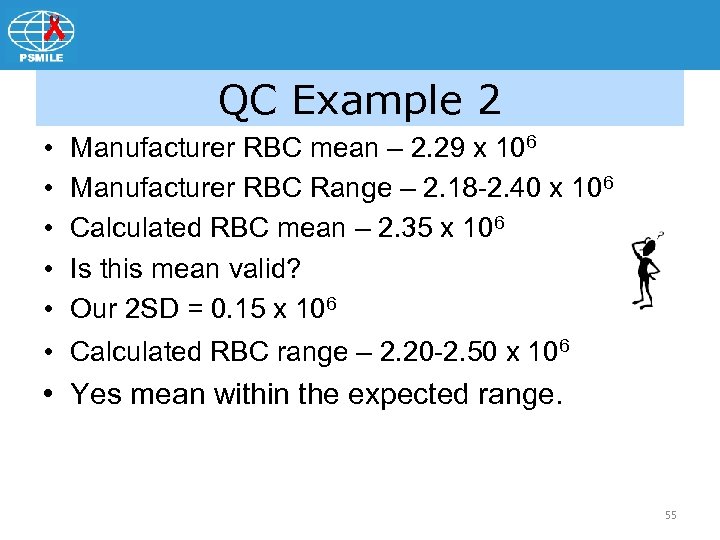
QC Example 2 • • • Manufacturer RBC mean – 2. 29 x 106 Manufacturer RBC Range – 2. 18 -2. 40 x 106 Calculated RBC mean – 2. 35 x 106 Is this mean valid? Our 2 SD = 0. 15 x 106 Calculated RBC range – 2. 20 -2. 50 x 106 • Yes mean within the expected range. 55
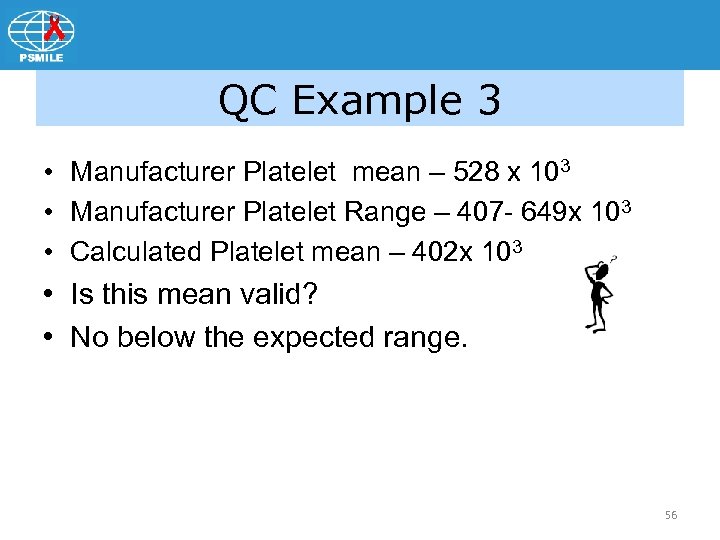
QC Example 3 • Manufacturer Platelet mean – 528 x 103 • Manufacturer Platelet Range – 407 - 649 x 103 • Calculated Platelet mean – 402 x 103 • Is this mean valid? • No below the expected range. 56

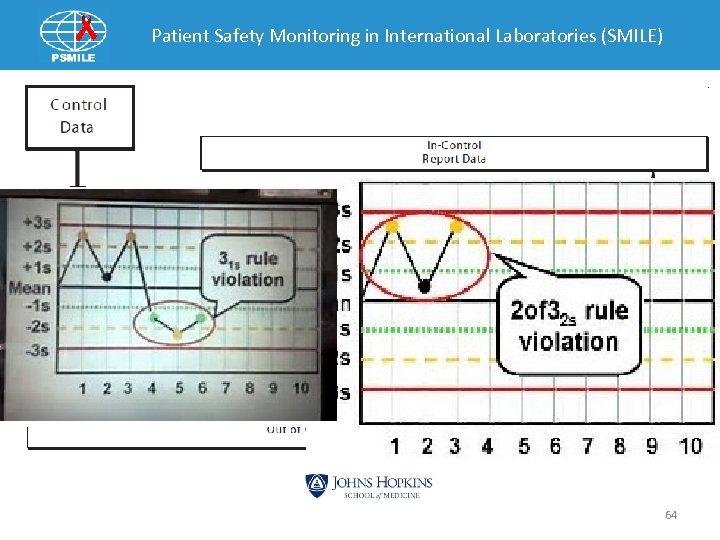
Commercial Control Monitoring Levey-Jennings Charts 57
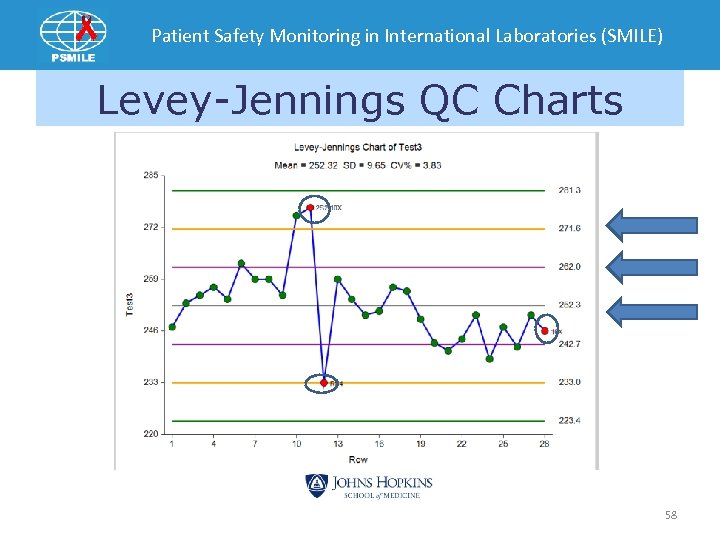
Patient Safety Monitoring in International Laboratories (SMILE) Levey-Jennings QC Charts 58
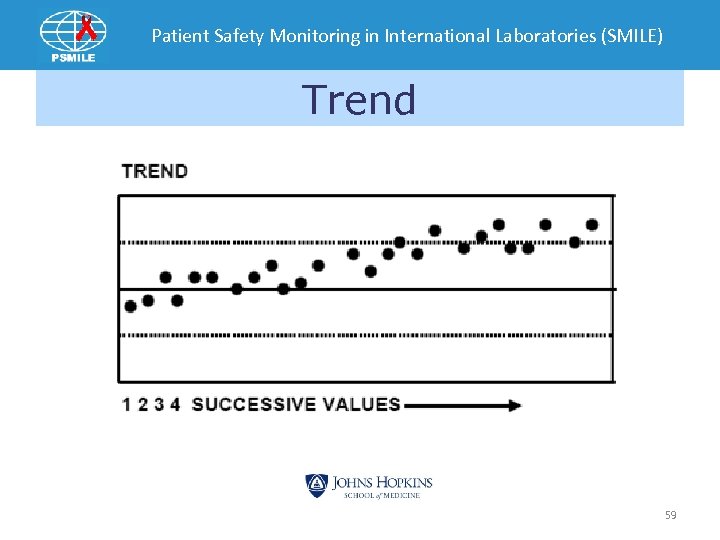
Patient Safety Monitoring in International Laboratories (SMILE) Trend 59
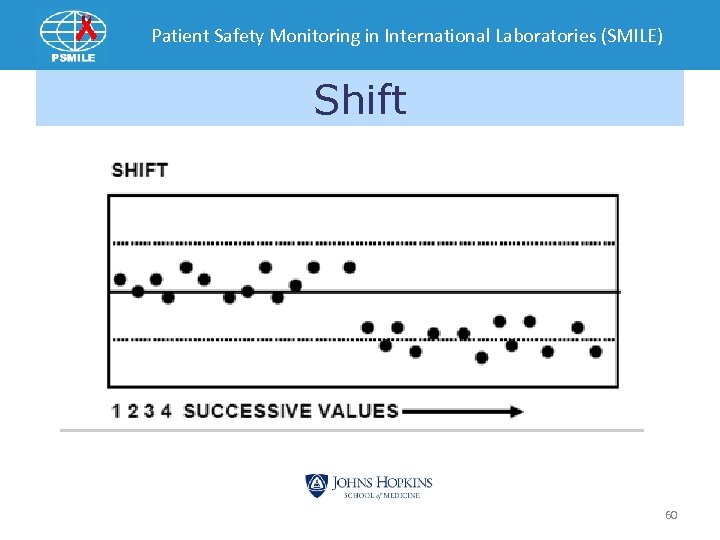
Patient Safety Monitoring in International Laboratories (SMILE) Shift 60
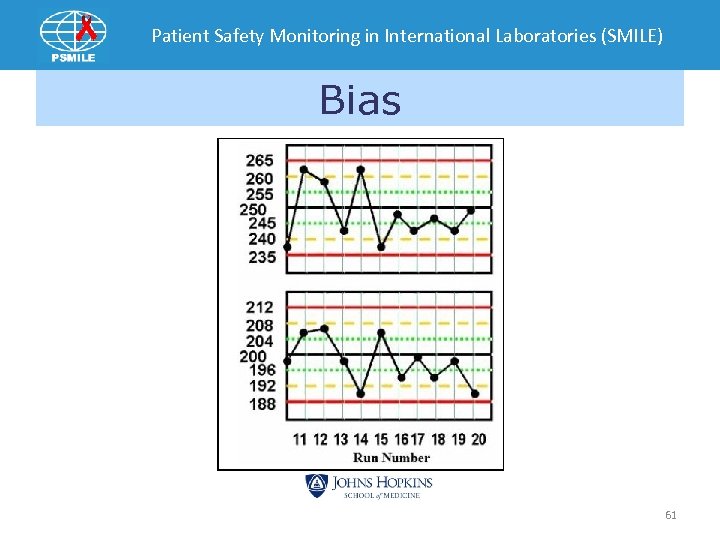
Patient Safety Monitoring in International Laboratories (SMILE) Bias 61
Patient Safety Monitoring in International Laboratories (SMILE) 62

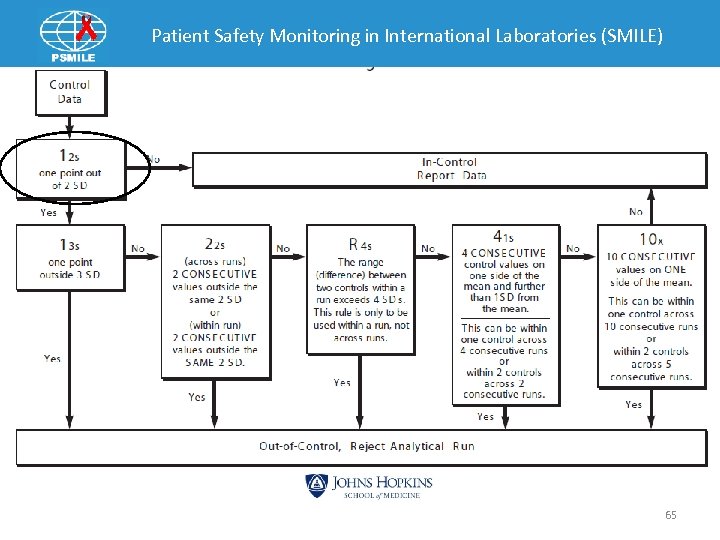
Patient Safety Monitoring in International Laboratories (SMILE) QC Policy • Each laboratory should adopt QC rules. • Establish policy/corrective action for controls that are “Out”. ØDocumentation is important. • Establish policy for Trends, bias and shifts. • Establish when calibration are required. 63
Patient Safety Monitoring in International Laboratories (SMILE) 64
Patient Safety Monitoring in International Laboratories (SMILE) 65

Patient Safety Monitoring in International Laboratories (SMILE) Policy Example • If control out +/-2 SD and a second Westgard rule is also seen Ø Rerun the control. Ø If another vial of control is available use it. Ø No more than 2 control reruns from same vial or new vial should be made. Ø If control still out begin troubleshooting do not report patient results. üCheck maintenance log üCheck reagent üCheck calibration date 66

Patient Safety Monitoring in International Laboratories (SMILE) Troubleshooting Guideline • Should have a policy on how to perform trouble shooting for out of range results. ØInstrument vs sample problem ØResolve problem before running patients • Can create a checklist. • Can also include a Corrective Action Flowchart. 67

Patient Safety Monitoring in International Laboratories (SMILE) Exercises 4 68
Patient Safety Monitoring in International Laboratories (SMILE) LJ Example 1 69
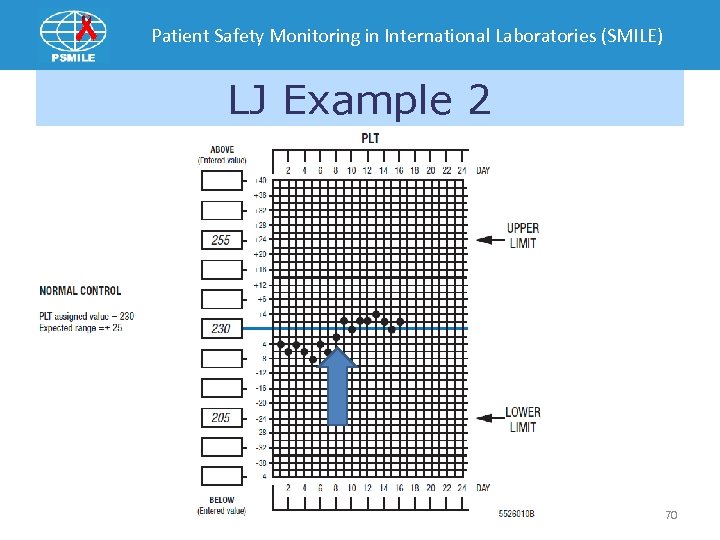
Patient Safety Monitoring in International Laboratories (SMILE) LJ Example 2 70
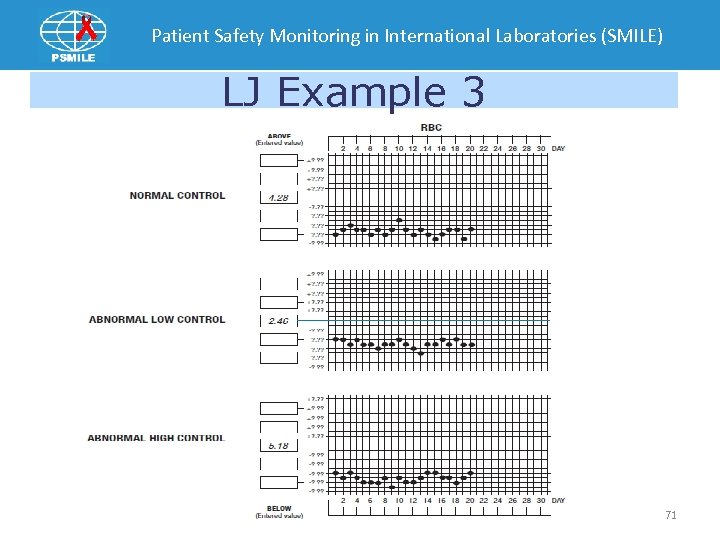
Patient Safety Monitoring in International Laboratories (SMILE) LJ Example 3 71
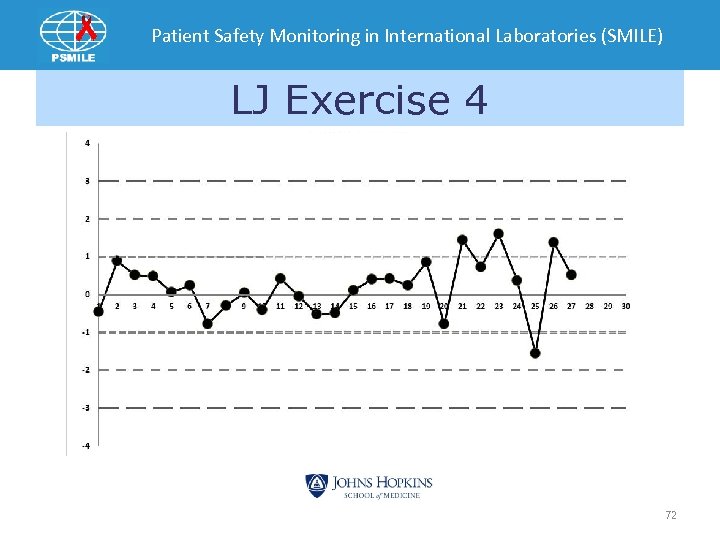
Patient Safety Monitoring in International Laboratories (SMILE) LJ Exercise 4 72
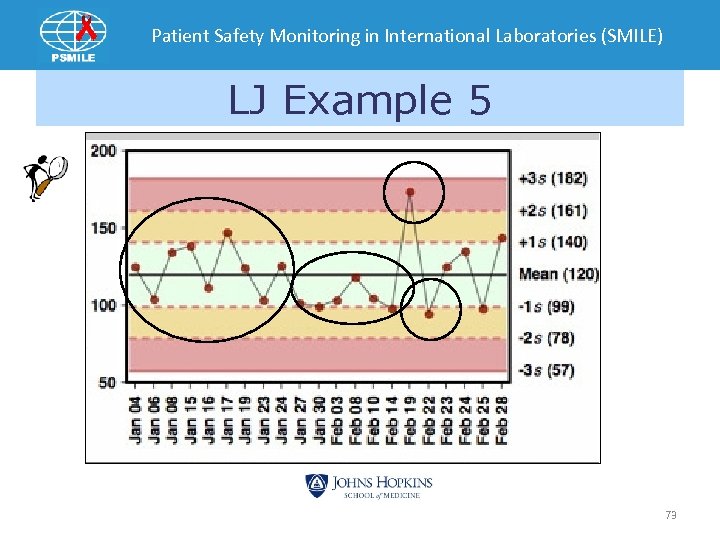
Patient Safety Monitoring in International Laboratories (SMILE) LJ Example 5 73

Patient Safety Monitoring in International Laboratories (SMILE) Internal Quality Control Retained Patient Controls 74
Patient Safety Monitoring in International Laboratories (SMILE) Retained Patient Control • Two-three patient samples with specific parameters. • Can be used over a 24 hour period. • Cost efficient. • Can be used to detect systematic error. • Transferable between instruments and modes. 75
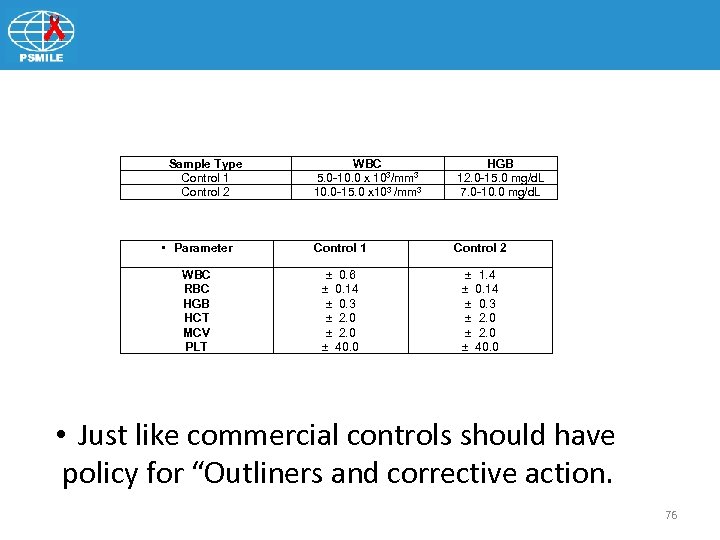
Sample Type Control 1 Control 2 WBC 5. 0 -10. 0 x 103/mm 3 10. 0 -15. 0 x 103 /mm 3 HGB 12. 0 -15. 0 mg/d. L 7. 0 -10. 0 mg/d. L • Parameter Control 1 Control 2 WBC RBC HGB HCT MCV PLT ± 0. 6 ± 0. 14 ± 0. 3 ± 2. 0 ± 40. 0 ± 1. 4 ± 0. 14 ± 0. 3 ± 2. 0 ± 40. 0 • Just like commercial controls should have policy for “Outliners and corrective action. 76
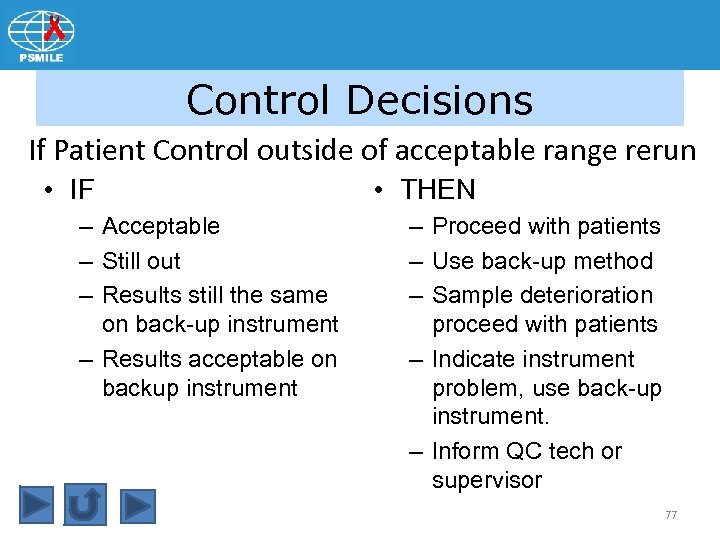
Control Decisions If Patient Control outside of acceptable range rerun • IF – Acceptable – Still out – Results still the same on back-up instrument – Results acceptable on backup instrument • THEN – Proceed with patients – Use back-up method – Sample deterioration proceed with patients – Indicate instrument problem, use back-up instrument. – Inform QC tech or supervisor 77

Patient Safety Monitoring in International Laboratories (SMILE) Exercises 5 78
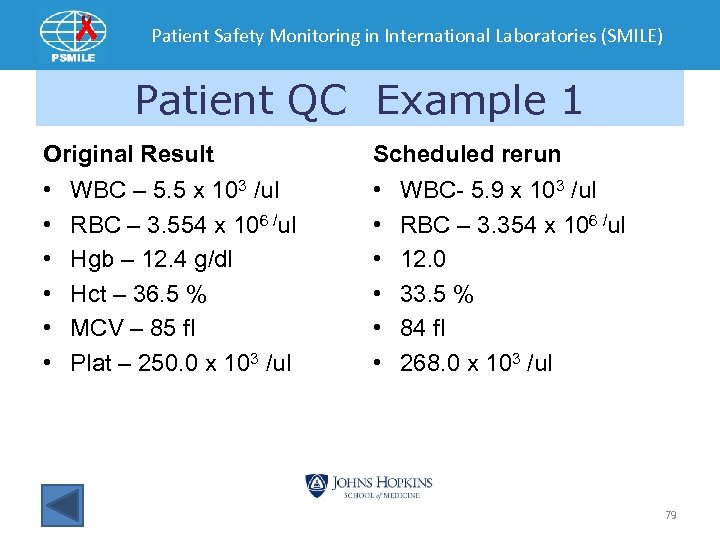
Patient Safety Monitoring in International Laboratories (SMILE) Patient QC Example 1 Original Result Scheduled rerun • • • WBC – 5. 5 x 103 /ul RBC – 3. 554 x 106 /ul Hgb – 12. 4 g/dl Hct – 36. 5 % MCV – 85 fl Plat – 250. 0 x 103 /ul WBC- 5. 9 x 103 /ul RBC – 3. 354 x 106 /ul 12. 0 33. 5 % 84 fl 268. 0 x 103 /ul 79
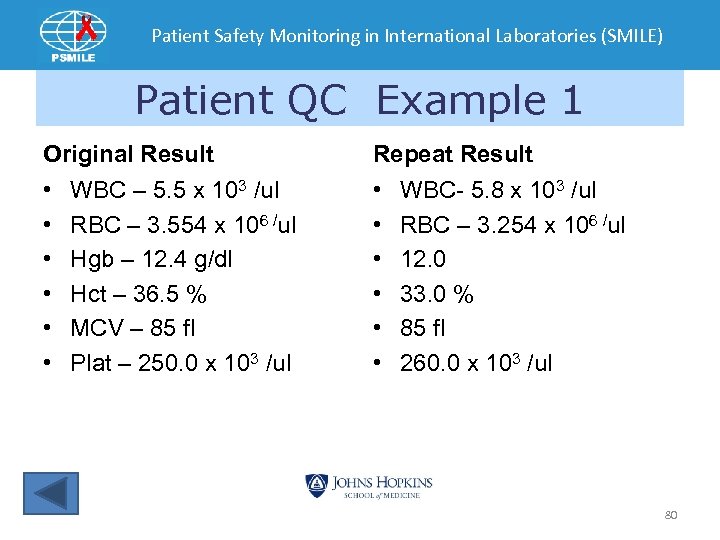
Patient Safety Monitoring in International Laboratories (SMILE) Patient QC Example 1 Original Result Repeat Result • • • WBC – 5. 5 x 103 /ul RBC – 3. 554 x 106 /ul Hgb – 12. 4 g/dl Hct – 36. 5 % MCV – 85 fl Plat – 250. 0 x 103 /ul WBC- 5. 8 x 103 /ul RBC – 3. 254 x 106 /ul 12. 0 33. 0 % 85 fl 260. 0 x 103 /ul 80
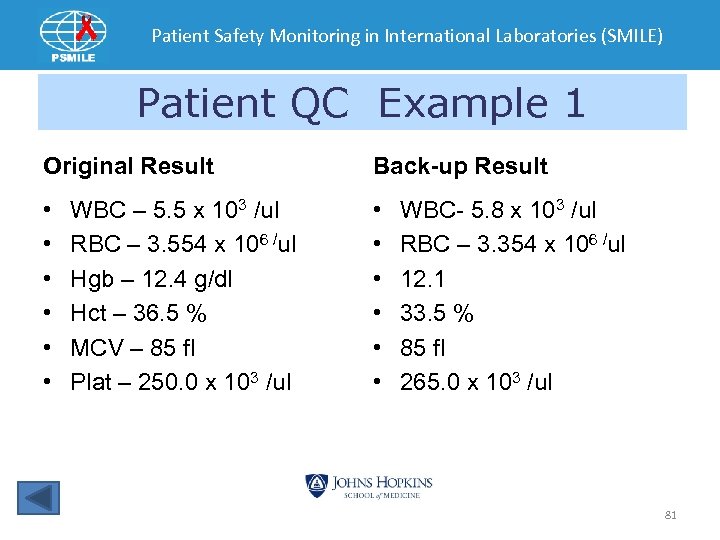
Patient Safety Monitoring in International Laboratories (SMILE) Patient QC Example 1 Original Result Back-up Result • • • WBC – 5. 5 x 103 /ul RBC – 3. 554 x 106 /ul Hgb – 12. 4 g/dl Hct – 36. 5 % MCV – 85 fl Plat – 250. 0 x 103 /ul WBC- 5. 8 x 103 /ul RBC – 3. 354 x 106 /ul 12. 1 33. 5 % 85 fl 265. 0 x 103 /ul 81

Patient Safety Monitoring in International Laboratories (SMILE) Internal Quality Control XB- Moving Average 82

Patient Safety Monitoring in International Laboratories (SMILE) XB – Moving Averages • Cost effective quality control method. • Allows for continuous monitoring of system performance. • Uses patient samples in conjunction with other controls. • Created by Brian S Bull. • Algorithm evaluates RBC indices. • Must run either 100/day or 400/week 83
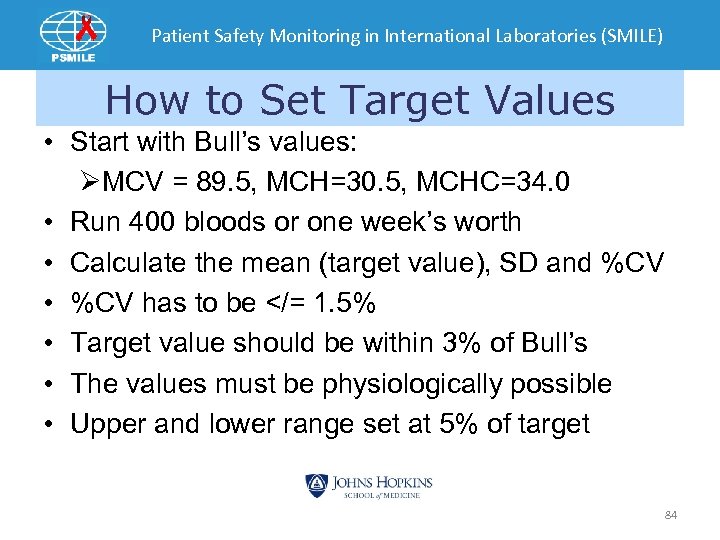
Patient Safety Monitoring in International Laboratories (SMILE) How to Set Target Values • Start with Bull’s values: ØMCV = 89. 5, MCH=30. 5, MCHC=34. 0 • Run 400 bloods or one week’s worth • Calculate the mean (target value), SD and %CV • %CV has to be </= 1. 5% • Target value should be within 3% of Bull’s • The values must be physiologically possible • Upper and lower range set at 5% of target 84

Patient Safety Monitoring in International Laboratories (SMILE) Reason Why Moving Average to be Out • Non-random population • Instrument problem • Calibration 85

Patient Safety Monitoring in International Laboratories (SMILE) Exercises 6 86
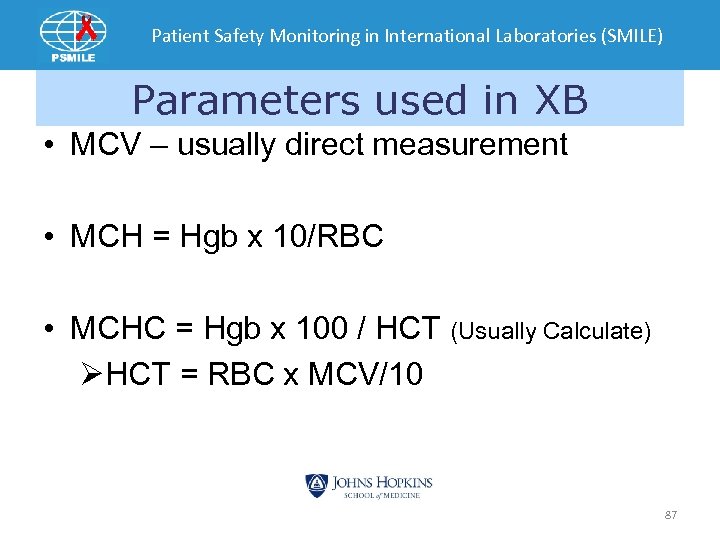
Patient Safety Monitoring in International Laboratories (SMILE) Parameters used in XB • MCV – usually direct measurement • MCH = Hgb x 10/RBC • MCHC = Hgb x 100 / HCT (Usually Calculate) ØHCT = RBC x MCV/10 87

Patient Safety Monitoring in International Laboratories (SMILE) How to troubleshoot with XB • MCV Ø Involves RBC aperture Ø Tell if problem with sizing or flow problem • MCH Ø Involves Hemoglobin sample which is part of WBC sample Ø Involves RBC count • MCHC Ø Involves all three parameters. 88
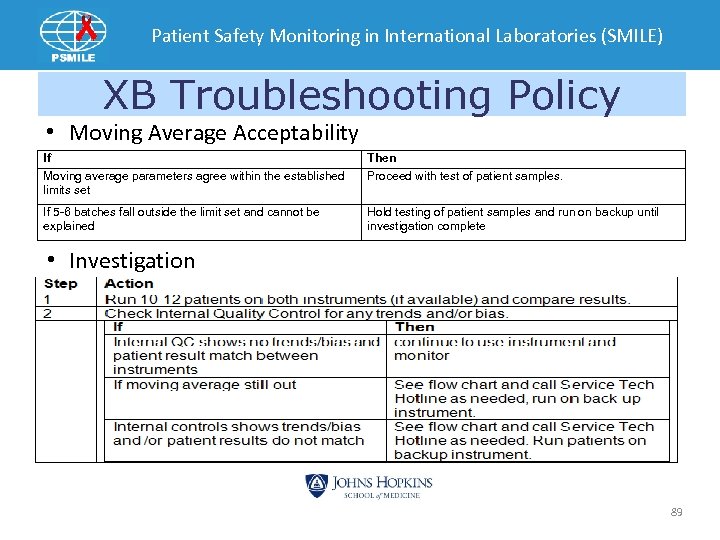
Patient Safety Monitoring in International Laboratories (SMILE) XB Troubleshooting Policy • Moving Average Acceptability If Moving average parameters agree within the established limits set Then Proceed with test of patient samples. If 5 -6 batches fall outside the limit set and cannot be explained Hold testing of patient samples and run on backup until investigation complete • Investigation 89

Patient Safety Monitoring in International Laboratories (SMILE) Example 1 90
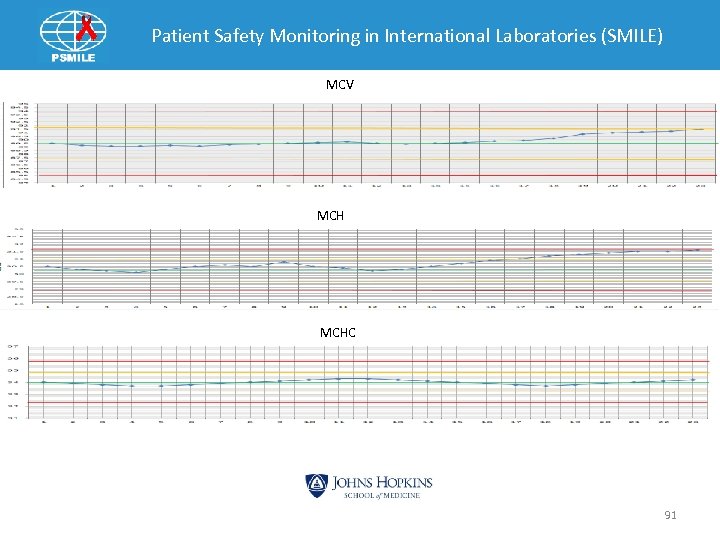
Patient Safety Monitoring in International Laboratories (SMILE) MCV MCHC 91
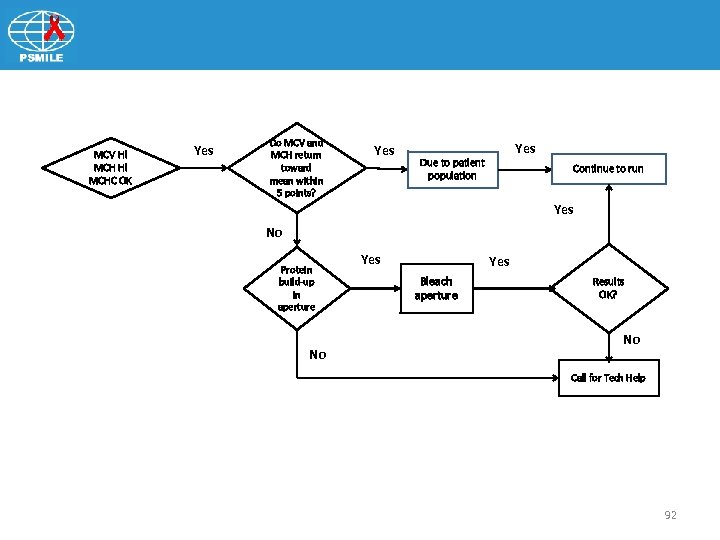
MCV Hi MCHC OK Yes Do MCV and MCH return toward mean within 5 points? Yes Due to patient population Continue to run Yes No Protein build-up in aperture No Yes Bleach aperture Results OK? No Call for Tech Help 92
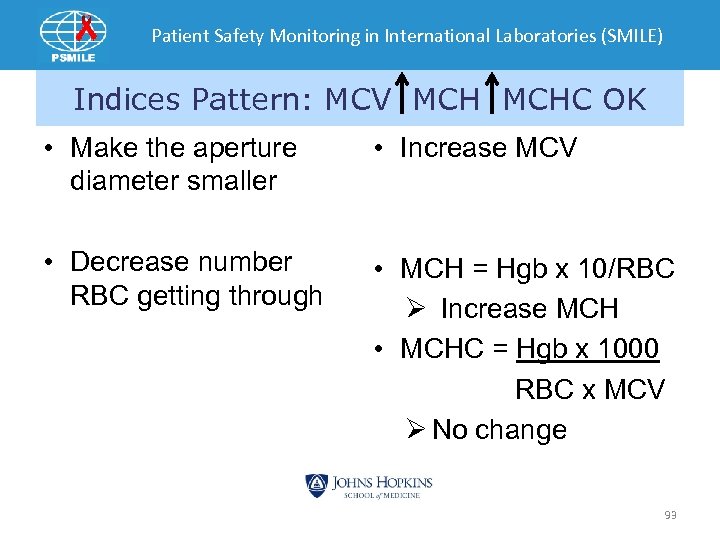
Patient Safety Monitoring in International Laboratories (SMILE) Indices Pattern: MCV MCHC OK • Make the aperture diameter smaller • Increase MCV • Decrease number RBC getting through • MCH = Hgb x 10/RBC Ø Increase MCH • MCHC = Hgb x 1000 RBC x MCV Ø No change 93

Patient Safety Monitoring in International Laboratories (SMILE) Example 2 94
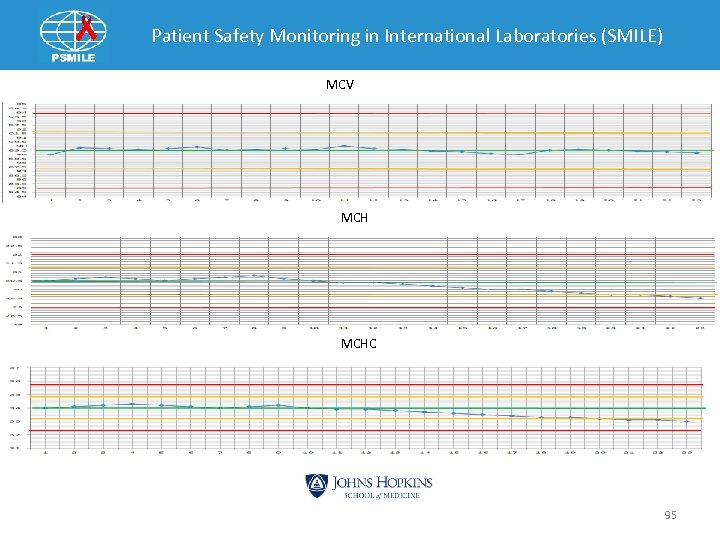
Patient Safety Monitoring in International Laboratories (SMILE) MCV MCHC 95
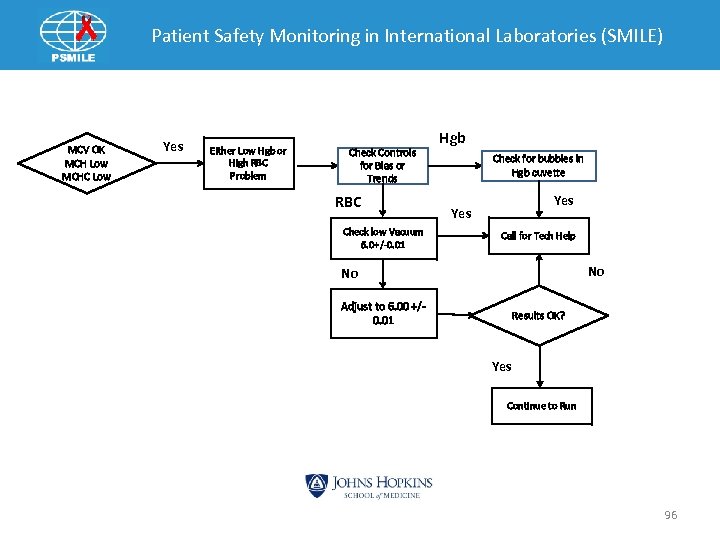
Patient Safety Monitoring in International Laboratories (SMILE) MCV OK MCH Low MCHC Low Yes Either Low Hgb or High RBC Problem Check Controls for Bias or Trends RBC Check low Vacuum 6. 0+/-0. 01 Hgb Check for bubbles in Hgb cuvette Yes Call for Tech Help No No Adjust to 6. 00 +/- 0. 01 Results OK? Yes Continue to Run 96
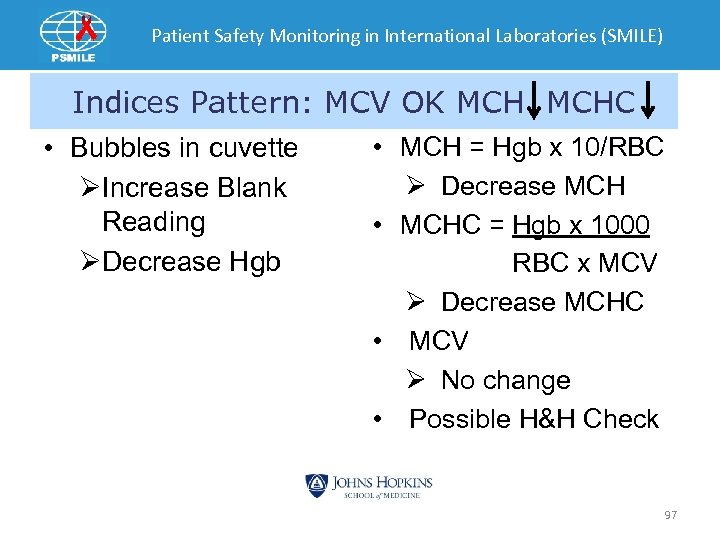
Patient Safety Monitoring in International Laboratories (SMILE) Indices Pattern: MCV OK MCHC • Bubbles in cuvette ØIncrease Blank Reading ØDecrease Hgb • MCH = Hgb x 10/RBC Ø Decrease MCH • MCHC = Hgb x 1000 RBC x MCV Ø Decrease MCHC • MCV Ø No change • Possible H&H Check 97
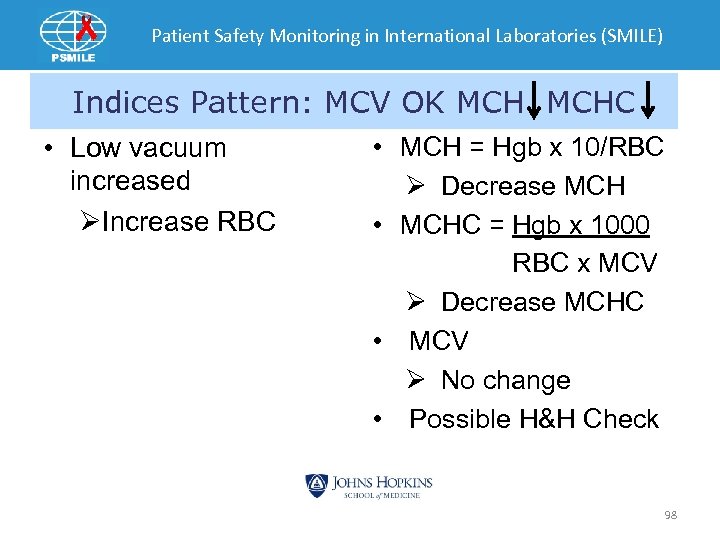
Patient Safety Monitoring in International Laboratories (SMILE) Indices Pattern: MCV OK MCHC • Low vacuum increased ØIncrease RBC • MCH = Hgb x 10/RBC Ø Decrease MCH • MCHC = Hgb x 1000 RBC x MCV Ø Decrease MCHC • MCV Ø No change • Possible H&H Check 98

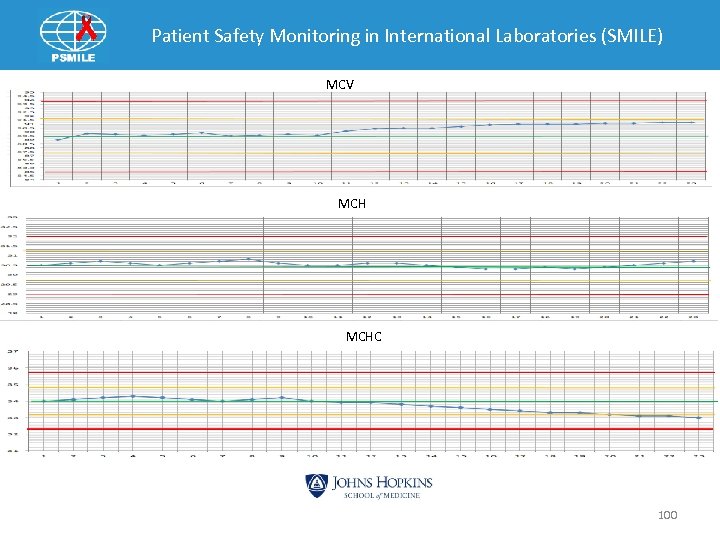
Patient Safety Monitoring in International Laboratories (SMILE) Example 3 99
Patient Safety Monitoring in International Laboratories (SMILE) MCV MCHC 100
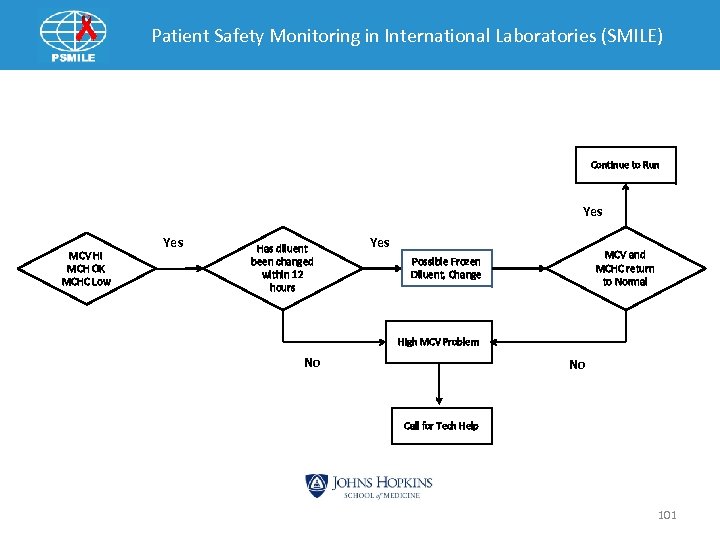
Patient Safety Monitoring in International Laboratories (SMILE) Continue to Run Yes MCV HI MCH OK MCHC Low Yes Has diluent been changed within 12 hours Yes MCV and MCHC return to Normal Possible Frozen Diluent, Change High MCV Problem No No Call for Tech Help 101
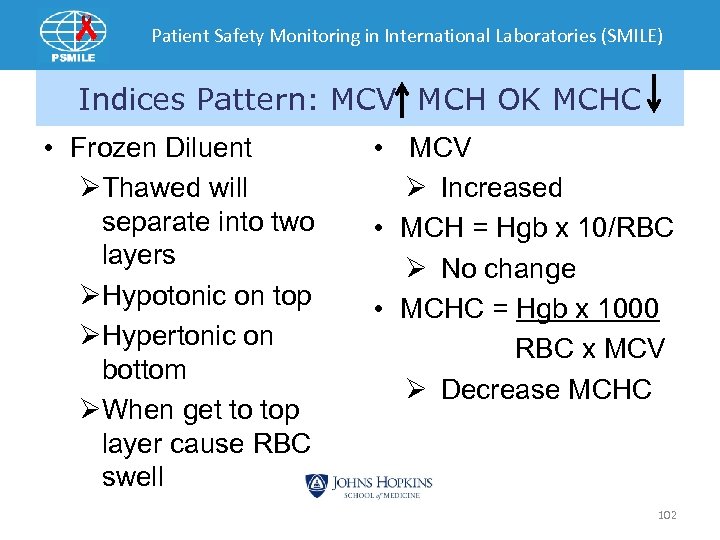
Patient Safety Monitoring in International Laboratories (SMILE) Indices Pattern: MCV MCH OK MCHC • Frozen Diluent ØThawed will separate into two layers ØHypotonic on top ØHypertonic on bottom ØWhen get to top layer cause RBC swell • MCV Ø Increased • MCH = Hgb x 10/RBC Ø No change • MCHC = Hgb x 1000 RBC x MCV Ø Decrease MCHC 102
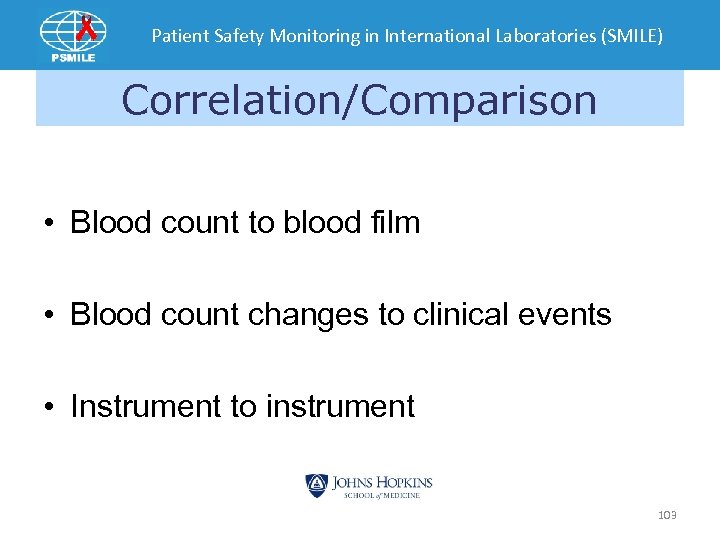
Patient Safety Monitoring in International Laboratories (SMILE) Correlation/Comparison • Blood count to blood film • Blood count changes to clinical events • Instrument to instrument 103

Patient Safety Monitoring in International Laboratories (SMILE) • • Factors to Consider with IQC Type of instrument – if fully automated. The size of the lab. The level of training of your staff. The number of specimens handled each day. • Dayshift vs 24 hour laboratory. • Country’s own regulations. • Accreditation requirements. 104

Patient Safety Monitoring in International Laboratories (SMILE) IQC Recommendations • A three level commercial control along with a retained patient control. • If patient numbers permits add XB to IQC • Commercial controls ØTwo levels at beginning, middle and end of shift • Retained Patient Controls ØAt equal times between commercial controls 105

Patient Safety Monitoring in International Laboratories (SMILE) QC Schedule Example • 8: 00 AM Commercial Controls level 1 and 2 Ø 10: 30 AM Retained Patient Controls • 1: 00 PM Commercial controls Level 2 and 3 Ø 3: 30 PM Retained Patient Controls • 6: 00 PM Commercial Controls Level 1 and 3 106

Patient Safety Monitoring in International Laboratories (SMILE) 107

Patient Safety Monitoring in International Laboratories (SMILE) EXTERNAL QUALITY CONTROL 108
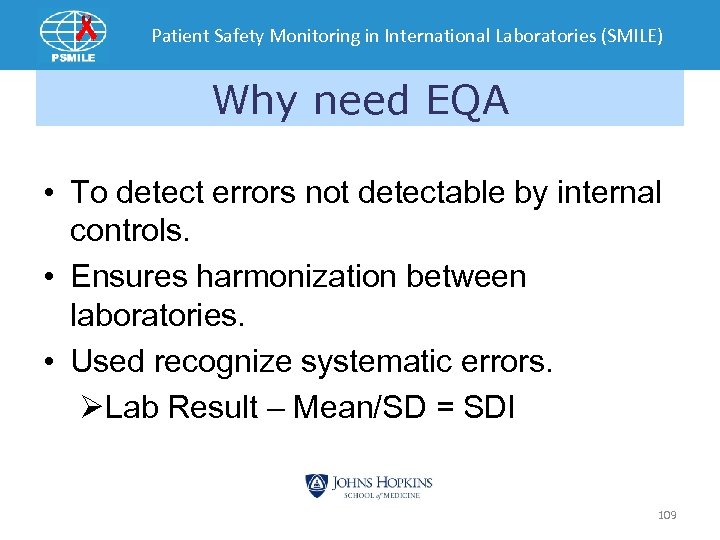
Patient Safety Monitoring in International Laboratories (SMILE) Why need EQA • To detect errors not detectable by internal controls. • Ensures harmonization between laboratories. • Used recognize systematic errors. ØLab Result – Mean/SD = SDI 109
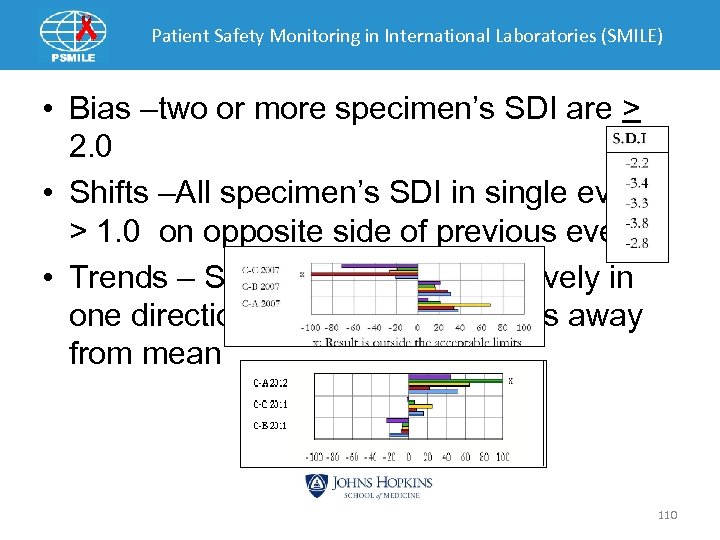
Patient Safety Monitoring in International Laboratories (SMILE) • Bias –two or more specimen’s SDI are > 2. 0 • Shifts –All specimen’s SDI in single event > 1. 0 on opposite side of previous event • Trends – SDIs increase progressively in one direction for three EQA events away from mean 110

Patient Safety Monitoring in International Laboratories (SMILE) SDI Interpretation • <1. 0 = satisfactory performance • 1. 0 -2. 0 = still acceptable but borderline • 2. 0 -3. 0 = requires review of techniques and check on calibration • >3. 0 = defect requiring urgent investigation 111

Patient Safety Monitoring in International Laboratories (SMILE) Exercises 7 112
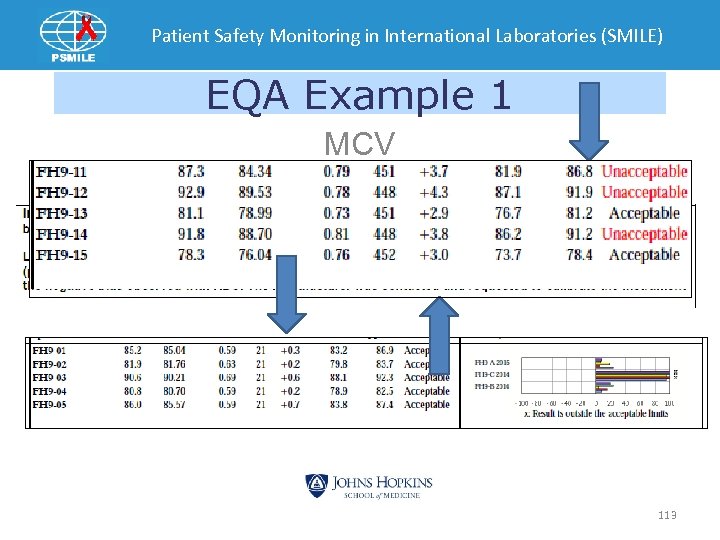
Patient Safety Monitoring in International Laboratories (SMILE) EQA Example 1 MCV 113
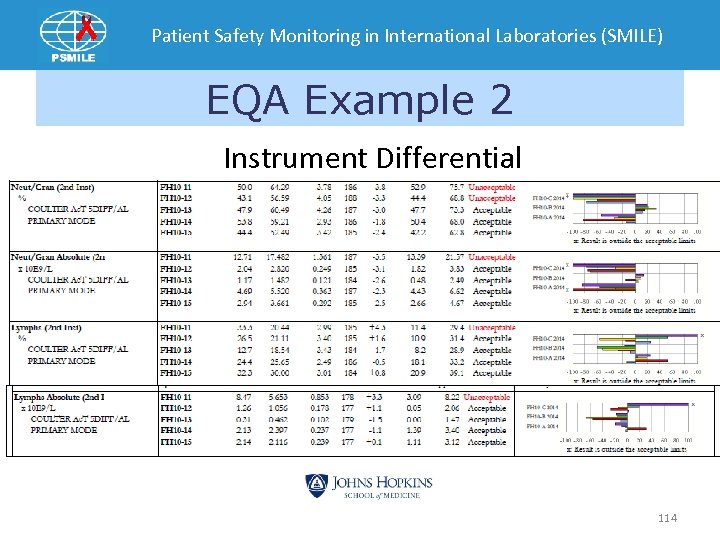
Patient Safety Monitoring in International Laboratories (SMILE) EQA Example 2 Instrument Differential 114
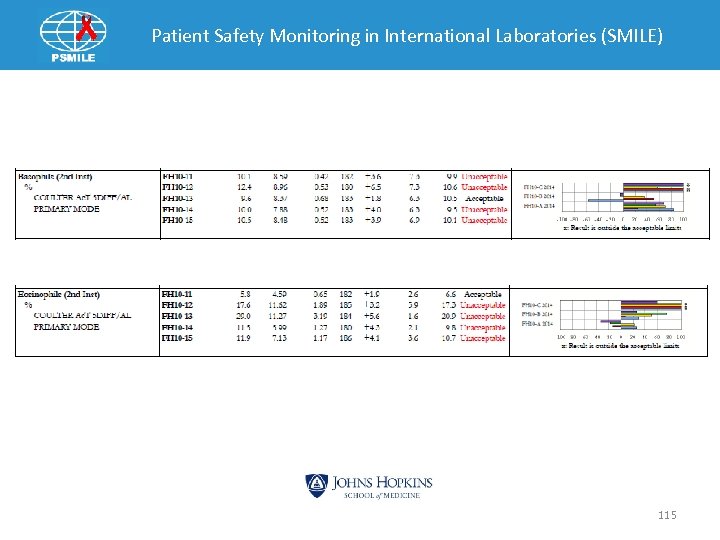
Patient Safety Monitoring in International Laboratories (SMILE) 115

Patient Safety Monitoring in International Laboratories (SMILE) INVESTIGATIVE ACTIONS AND ROOT CAUSE: Briefly discuss what actions were taken in this investigation and what you believe is the possible cause. 1. We checked to find out whethere were clerical errors and found out that there was none. 2. After replacing the flow cell, the EQA specimen which failed were re-run and all were within range. The cause of the EQA failure was a faulty flow cell in the CBC/Differential Analyzer. This was confirmed because after replacing the flow cell the EQA samples were re-run, all of the values that had previously failed now fell within acceptable limits of intended results. 116
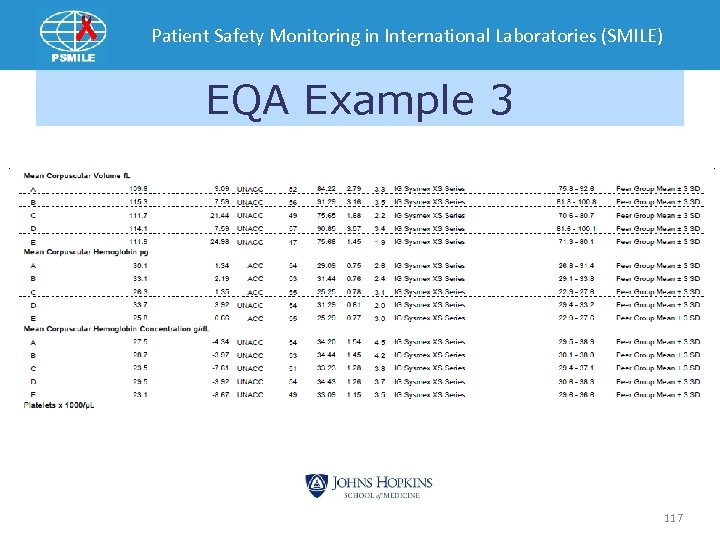
Patient Safety Monitoring in International Laboratories (SMILE) EQA Example 3 117
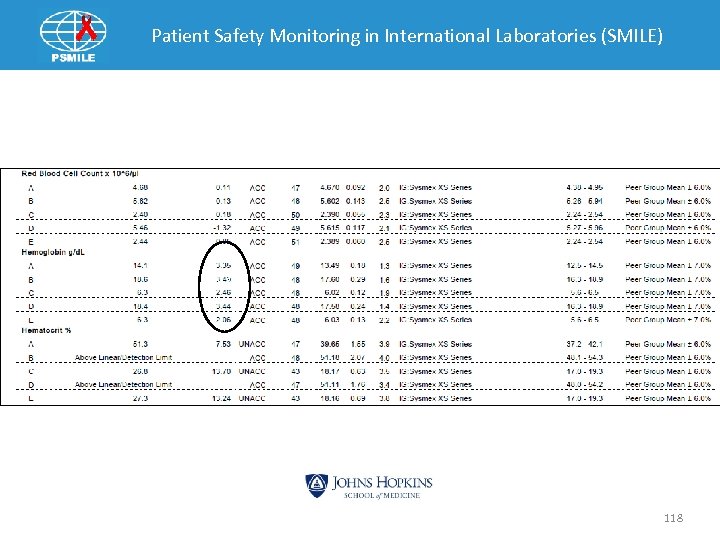
Patient Safety Monitoring in International Laboratories (SMILE) CV is 118
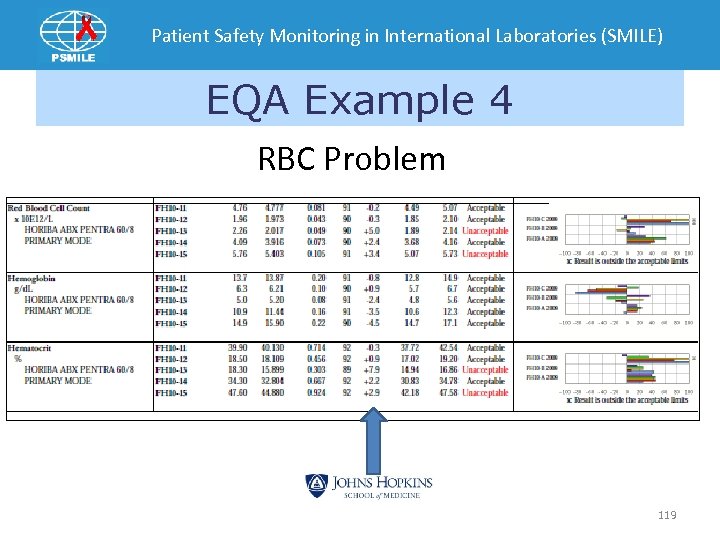
Patient Safety Monitoring in International Laboratories (SMILE) EQA Example 4 RBC Problem 119

Patient Safety Monitoring in International Laboratories (SMILE) 120
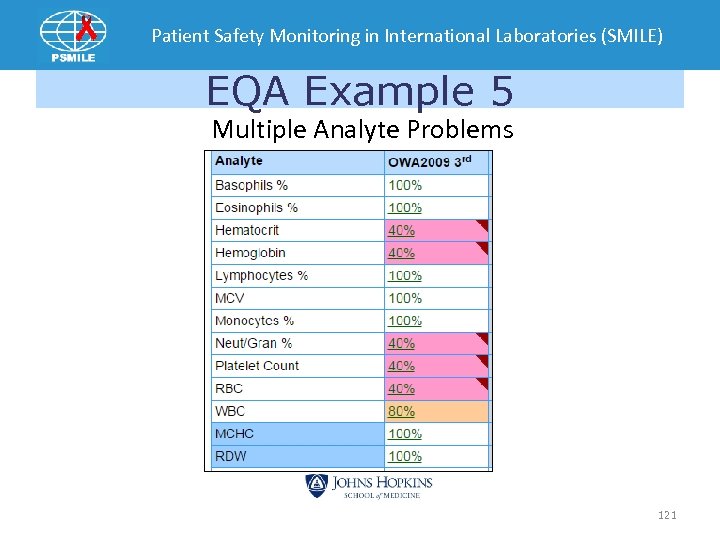
Patient Safety Monitoring in International Laboratories (SMILE) EQA Example 5 Multiple Analyte Problems 121

Patient Safety Monitoring in International Laboratories (SMILE) 122

Patient Safety Monitoring in International Laboratories (SMILE) 123

Patient Safety Monitoring in International Laboratories (SMILE) Summary and Conclusion • • Standardization Pre Analytic Control Post Analytic Control Analytical Control ØInternal Quality Control ØExternal Quality Assessment 124

Patient Safety Monitoring in International Laboratories (SMILE) Questions and Answers? 125

Patient Safety Monitoring in International Laboratories (SMILE) References • Quality Control (QC) Information and Troubleshooting Guide – Hematology – Beckman Coulter • The use of retained patient specimens for hematology quality control. Hackney JR, Cembrowski GS • An optimized quality control procedure for hematology analyzers with the use of retained patient specimens. Cembrowski GS, Lunetzky ES, Patrick CC, Wilson MK • Establishing Quality Control Means and Standard Deviation for Hematology Instrument, Streck • Quality Assurance in Hematology, WHO 126
0ea411040a67ed92bad5e0fd90dc2535.ppt