49d6bffde1e5a4a1baec99d18da3909a.ppt
- Количество слайдов: 80

Patient Care Devices Overview & Update HIMSS Webinar – 3 August Todd Cooper, Breakthrough Solutions Foundry Ken Fuchs, Mindray North America Steve Merritt, Baystate Health John Rhoads, Philips Healthcare 1

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 2

2009 – Obama vows to continue the HIT Plan begun by President Bush President Barack Obama announces an audacious plan: Computerize all health records within five years. - January 12, 2009 February 17, 2009 – the American Reinvestment and Recovery Act (ARRA) is signed into law • HITECH Act component of ARRA provides a $19 billion program to stimulate the adoption and use of HIT, with a focus on meaningful use of certified EHR-S 3

What about Device Connectivity? Download HITSP/TN 905 @ www. HITSP. org 4
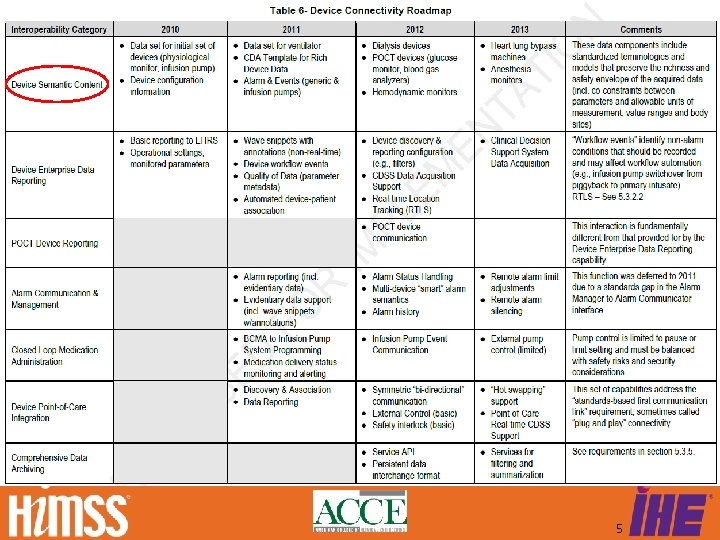
HITSP/TN 905 Roadmap 5

The Dream! Heterogeneity Enables… Multiple manufacturers + multiple device & application modalities coexisting & interoperating over a shared infrastructure “Best of Breed” Selection Ability to base product acquisition on most appropriate technology without requiring HIT re-engineering. Semantic Comparability Harmonized semantics (terminology & models) enable next generation applications, incl. real-time CDSS. Comprehensive, Real-time Availability Timely health care decisions based on richer, more complete information. Medical Device HIT: No longer a “nice to have” 6
The Benefits! Integration – Provider value propositions… Integrity of data – automatic population of all information systems – reducing medical errors Automated systems saves time for clinicians Improved agility of enterprises to meet varied patient loads Improved life-cycle cost of ownership Automated clinical data capture for EHR Access to patient data across devices and systems so custom communication interfaces can be eliminated. Allows for best of breed selection 7

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 8
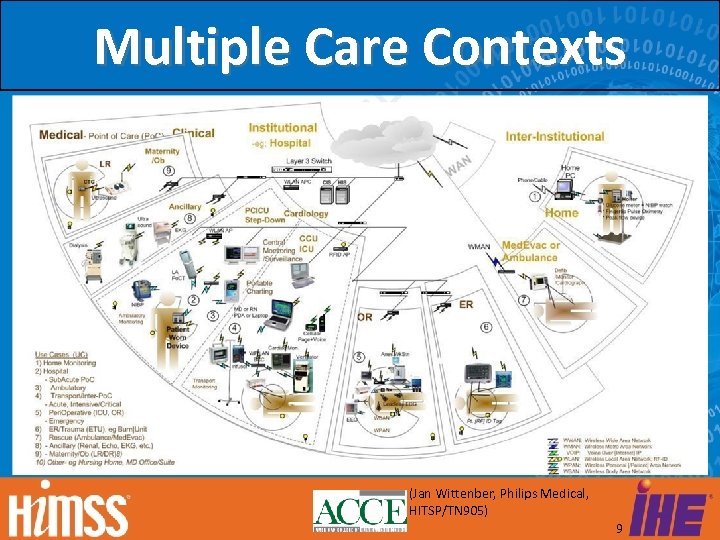
Multiple Care Contexts (Jan Wittenber, Philips Medical, HITSP/TN 905) 9
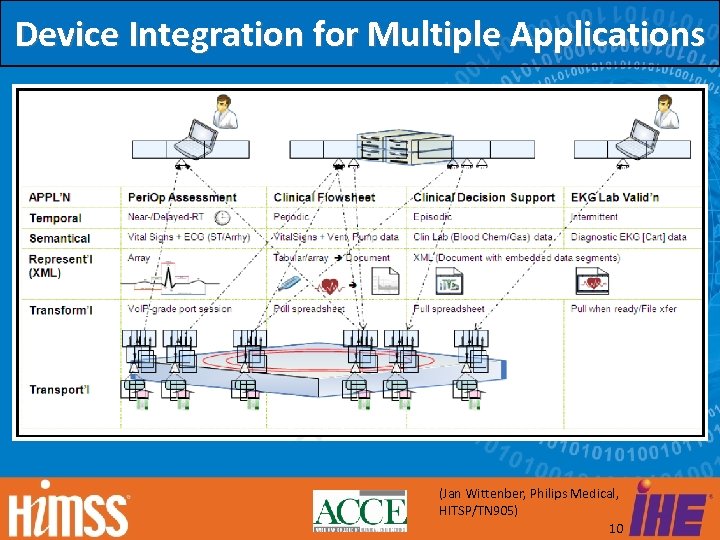
Device Integration for Multiple Applications (Jan Wittenber, Philips Medical, HITSP/TN 905) 10
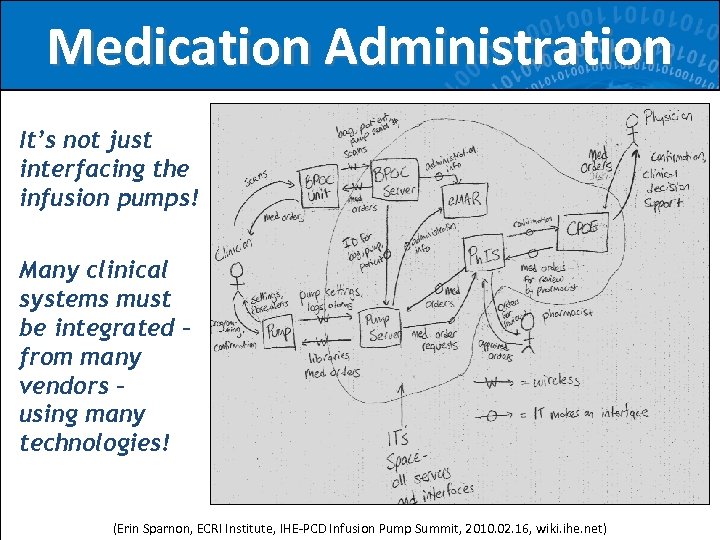
Medication Administration It’s not just interfacing the infusion pumps! Many clinical systems must be integrated – from many vendors – using many technologies! (Erin Sparnon, ECRI Institute, IHE-PCD Infusion Pump Summit, 2010. 02. 16, wiki. ihe. net) 11
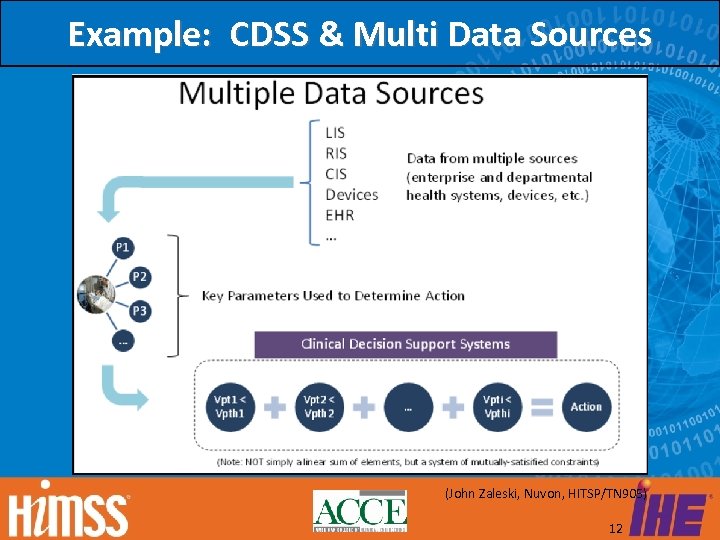
Example: CDSS & Multi Data Sources (John Zaleski, Nuvon, HITSP/TN 905) 12

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 13

IHE Patient Care Devices IHE-PCD Charter The Patient Care Device Domain is concerned with use cases in which at least one actor is a patient-centric point-of-care medical device. The PCD coordinates with other IHE clinical specialty based domains such as medical imaging and lab to ensure consistency of medical device integration solutions across all IHE technical frameworks. NOTE: Formed in 2005 & sponsored by HIMSS & ACCE 14
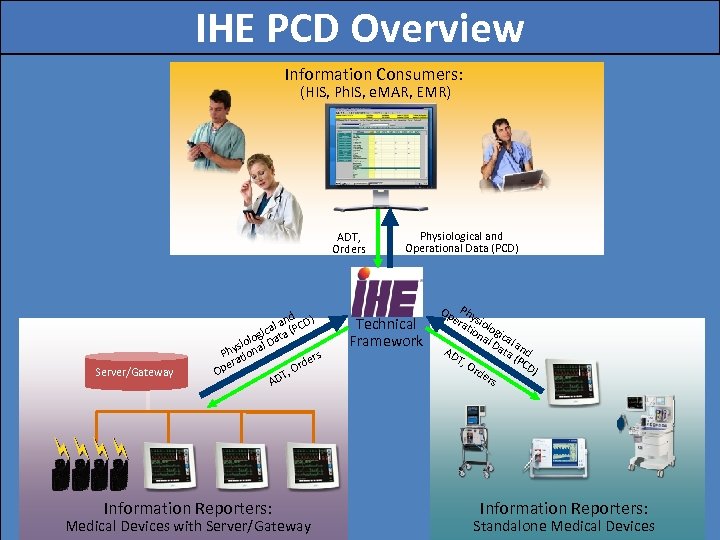
IHE PCD Overview Information Consumers: (HIS, Ph. IS, e. MAR, EMR) ADT, Orders Server/Gateway d ) l an (PCD a gic ta iolo al Da ys Ph tion rs rde era Op T, O AD Information Reporters: Medical Devices with Server/Gateway Physiological and Operational Data (PCD) Technical Framework Op Phy era sio tio log na ica l D l a ata nd AD (P T, CD Or ) de rs Information Reporters: Standalone Medical Devices 15
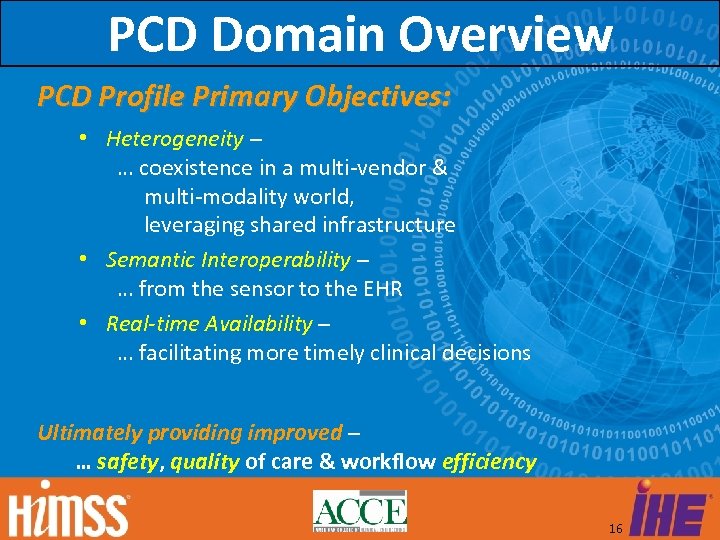
PCD Domain Overview PCD Profile Primary Objectives: • Heterogeneity – … coexistence in a multi-vendor & multi-modality world, leveraging shared infrastructure • Semantic Interoperability – … from the sensor to the EHR • Real-time Availability – … facilitating more timely clinical decisions Ultimately providing improved – … safety, quality of care & workflow efficiency 16

Profiles Simplify Development All implementation detail! 17
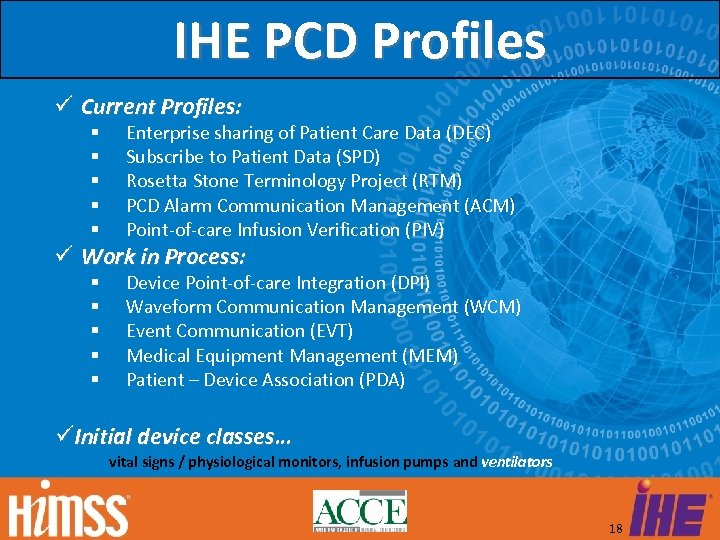
IHE PCD Profiles Current Profiles: Enterprise sharing of Patient Care Data (DEC) Subscribe to Patient Data (SPD) Rosetta Stone Terminology Project (RTM) PCD Alarm Communication Management (ACM) Point-of-care Infusion Verification (PIV) Work in Process: Device Point-of-care Integration (DPI) Waveform Communication Management (WCM) Event Communication (EVT) Medical Equipment Management (MEM) Patient – Device Association (PDA) Initial device classes… vital signs / physiological monitors, infusion pumps and ventilators 18
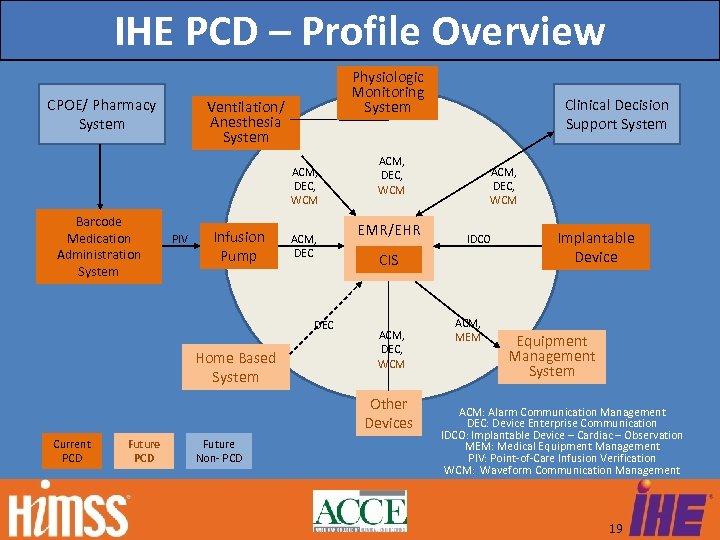
IHE PCD – Profile Overview CPOE/ Pharmacy System Physiologic Monitoring System Ventilation/ Anesthesia System ACM, DEC, WCM Barcode Medication Administration System PIV Infusion Pump ACM, DEC Home Based System ACM, DEC, WCM EMR/EHR Future PCD Future Non- PCD ACM, DEC, WCM IDCO CIS ACM, DEC, WCM Other Devices Current PCD Clinical Decision Support System ACM, MEM Implantable Device Equipment Management System ACM: Alarm Communication Management DEC: Device Enterprise Communication IDCO: Implantable Device – Cardiac – Observation MEM: Medical Equipment Management PIV: Point-of-Care Infusion Verification WCM: Waveform Communication Management 19

IHE Patient Care Devices 2009 White Paper Proposals Device Point-of-care Integration (DPI) Medical Equipment Management (MEM) – Complete! Medical Device Semantic Architecture Regulatory Considerations in Deploying Systems Incorporating IHE PCD Profiles IHE PCD Users Handbook What is & is not specified in PCD Profiles How to assess PCD profile support System verification & validation testing considerations 20

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 21

PCD Published Profiles DEC Profile Device to Enterprise Communication Trial Implementation 22

DEC Profiles • PCD DEC profile supports the communication of patient clinical data from devices. • This data can include: • • • Vital Signs (profile available) Alarm information (2008/2009 profile) Waveform information (emerging profile) Device configuration information (MEM) Etc. 23
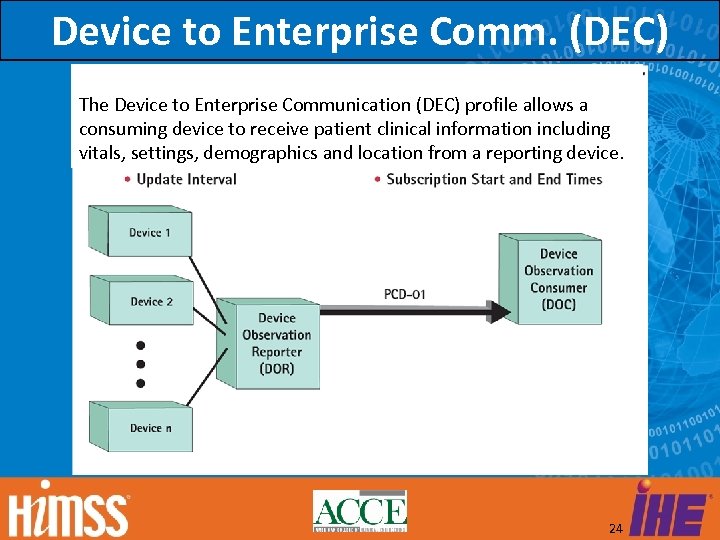
Device to Enterprise Comm. (DEC) The Device to Enterprise Communication (DEC) profile allows a consuming device to receive patient clinical information including vitals, settings, demographics and location from a reporting device. 24

PCD-01 Message Example MSH|^~&|INFO_SRC_PHILIPS^ACDE 48234567 ABCD^EUI-64||||20061215153500||ORU^R 01^ORU_R 01|PMS 116621490051| P|2. 5|||NE|AL||8859/1 PID|||AB 60001^^^Philips Medical^PI||Brooks^Albert^^^^^L||19610101|M PV 1||I|UNIT_1^^Bed 1 OBR|1|PMS 116621490051^INFO_SRC_PHILIPS^ACDE 48234567 ABCD^EUI-64| 69837^MDC_DEV_METER_PHYSIO_MULTI_PARAM_MDS^MDC|||20061215153500 OBX|1|ST|184326^MDC_ECG_STAT_ECT^MDC|1. 5130. 1. 184326|""||||||F OBX|2|ST|184327^MDC_ECG_STAT_RHY^MDC|1. 5130. 1. 184327|Sinus Rhythm||||||F OBX|3|NM|150456^MDC_PULS_OXIM_SAT_O 2^MDC|1. 5238. 1. 150456|99|262688^MDC_DIM_PERCENT^MDC|||||F OBX|4|NM|147842^MDC_ECG_HEART_RATE^MDC|1. 5130. 1. 147842|81|264864^MDC_DIM_BEAT_PER_MIN^MDC|||||F OBX|5|NM|150037^MDC_PRESS_BLD_ART_ABP_SYS^MDC|1. 5190. 1. 150036|126|266016^MDC_DIM_MMHG^MDC|||||F OBX|6|NM|150038^MDC_PRESS_BLD_ART_ABP_DIA^MDC|1. 5190. 1. 150036|76|266016^MDC_DIM_MMHG^MDC|||||F OBX|7|NM|150039^MDC_PRESS_BLD_ART_ABP_MEAN^MDC|1. 5190. 1. 150036|92|266016^MDC_DIM_MMHG^MDC|||||F OBX|8|NM|148065^MDC_ECG_V_P_C_CNT^MDC|1. 5130. 1. 148065|0|264864^MDC_DIM_BEAT_PER_MIN^MDC|||||F OBX|9|NM|150045^MDC_PRESS_BLD_ART_PULM_SYS^MDC|1. 5190. 1. 150044|26|266016^MDC_DIM_MMHG^MDC|||||F OBX|10|NM|150046^MDC_PRESS_BLD_ART_PULM_DIA^MDC|1. 5190. 1. 150044|9|266016^MDC_DIM_MMHG^MDC|||||F OBX|11|NM|150047^MDC_PRESS_BLD_ART_PULM_MEAN^MDC|1. 5190. 1. 150044|14|266016^MDC_DIM_MMHG^MDC|||||F OBX|12|NM|149538^MDC_PLETH_PULS_RATE^MDC|1. 5238. 1. 149538|55|264864^MDC_DIM_BEAT_PER_MIN^MDC|||||F OBX|13|NM|150067^MDC_PRESS_BLD_ATR_LEFT_MEAN^MDC|1. 5190. 1. 150064|4|266016^MDC_DIM_MMHG^MDC|||||F OBX|14|NM|150087^MDC_PRESS_BLD_VEN_CENT_MEAN^MDC|1. 5190. 1. 150084|12|266016^MDC_DIM_MMHG^MDC|||||F Note: Extensive message examples on the PCD FTP Site: ftp: //ftp. ihe. net/Patient_Care_Devices/Connectathons. Including. Process. And. Testing 25
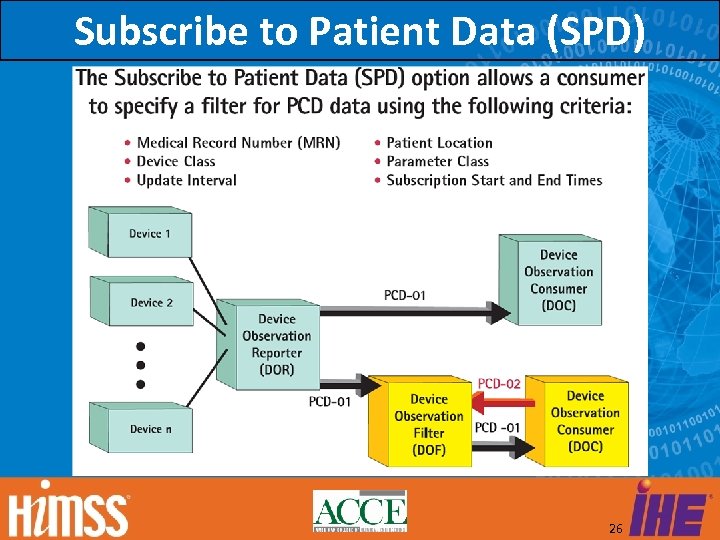
Subscribe to Patient Data (SPD) 26

PCD Published Profiles ACM Profile Alarm Communication Management Trial Implementation 27

Alarm Communication Management (ACM) Alarm Communication Management enables systems to deliver the right alarms, with the right priority, to the right individuals via devices with the right content, escalating to other individuals via devices (based on system configuration) 28
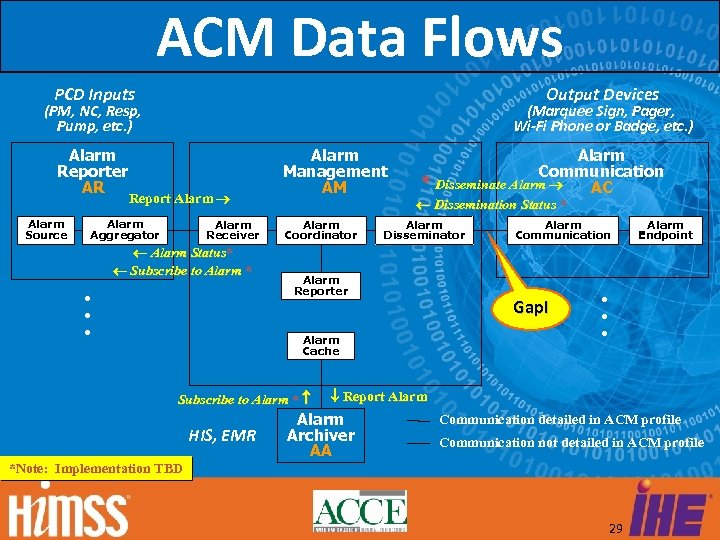
ACM Data Flows PCD Inputs Output Devices (PM, NC, Resp, Pump, etc. ) Alarm Reporter AR Alarm Source (Marquee Sign, Pager, Wi-Fi Phone or Badge, etc. ) Report Alarm Aggregator . . . Alarm Receiver Alarm Status* Subscribe to Alarm * Alarm Management AM Alarm Coordinator Alarm Communication * Disseminate Alarm AC Dissemination Status * Alarm Disseminator Alarm Reporter Alarm Cache Subscribe to Alarm * HIS, EMR Alarm Communication Gap! Alarm Endpoint . . . Report Alarm Archiver AA Communication detailed in ACM profile Communication not detailed in ACM profile *Note: Implementation TBD 29
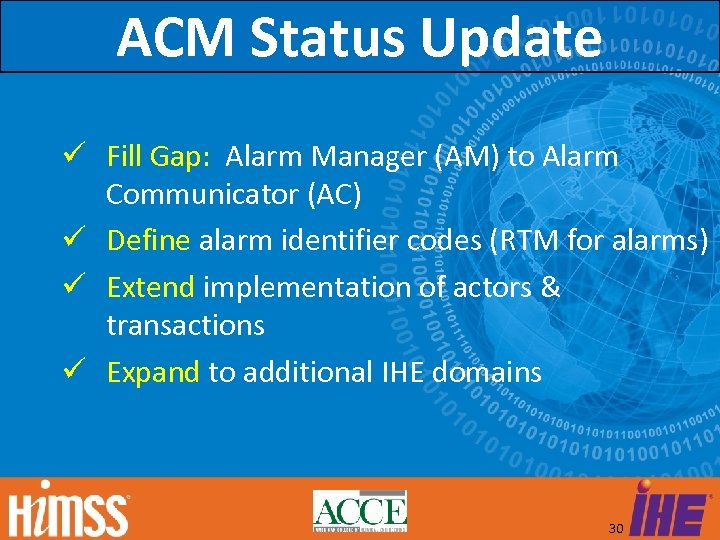
ACM Status Update Fill Gap: Alarm Manager (AM) to Alarm Communicator (AC) Define alarm identifier codes (RTM for alarms) Extend implementation of actors & transactions Expand to additional IHE domains 30

PCD Published Profiles PIV Profile Point-of-Care Infusion Verification Trial Implementation 31

PIV Objective Point-of-Care Infusion Verification supports the electronic transfer of infusion parameters from a Barcode Point of Care (BPOC) system, also known as a Bar Code Medication Administration (BCMA) system, to an infusion pump. 32
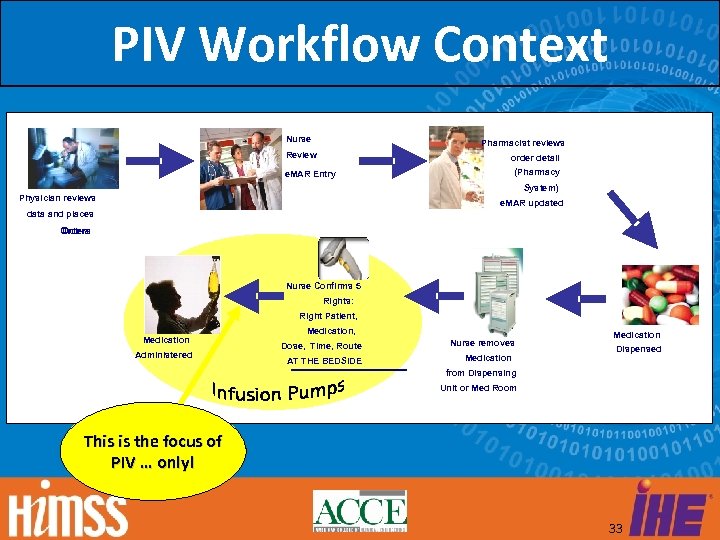
PIV Workflow Context Nurse Pharmacist reviews Review order detail (Pharmacy e. MAR Entry System) Physician reviews e. MAR updated data and places Orders Nurse Confirms 5 Rights: Right Patient, Medication Administered Medication, Dose, Time, Route AT THE BEDSIDE Nurse removes Medication Dispensed from Dispensing Unit or Med Room This is the focus of PIV … only! 33
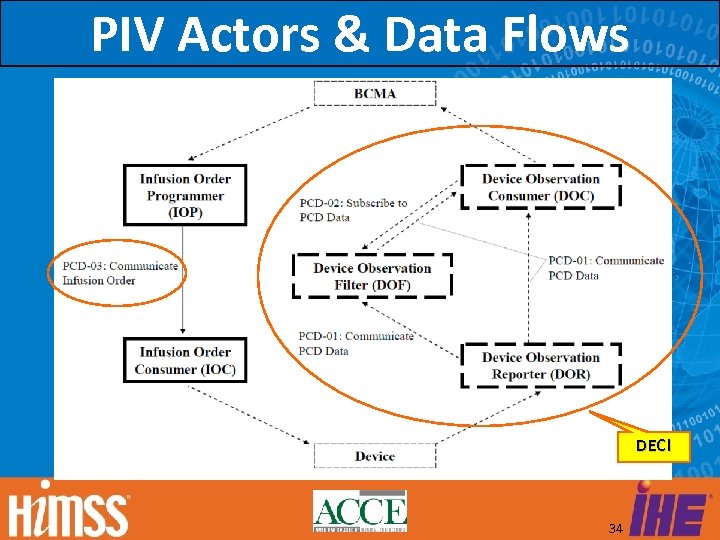
PIV Actors & Data Flows DEC! 34

PCD Published Profiles IDCO Profile Implantable Device – Cardiac – Observation Trial Implementation 35

IDCO Objective IDCO specifies the creation, transmission, and processing of discrete data elements and report attachments associated with implantable cardiac device interrogations (observations) or messages. 36
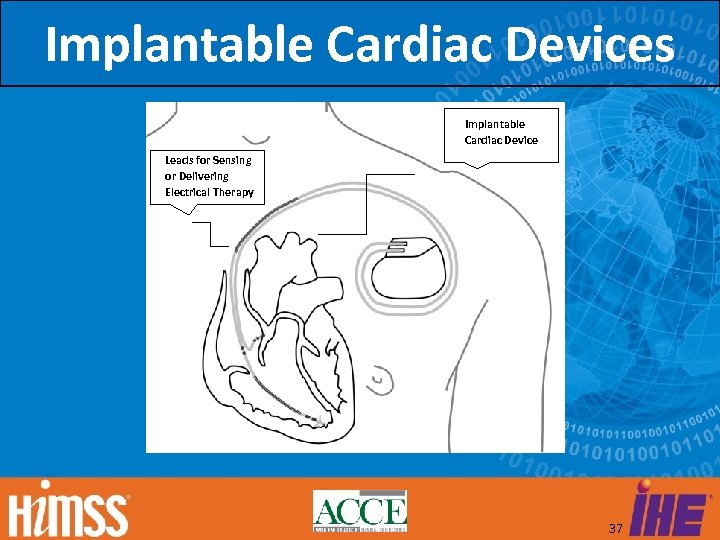
Implantable Cardiac Devices Implantable Cardiac Device Leads for Sensing or Delivering Electrical Therapy 37
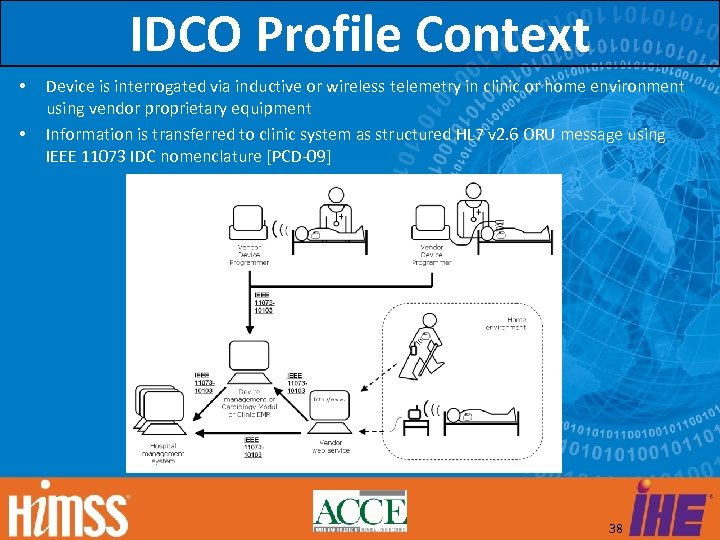
IDCO Profile Context • • Device is interrogated via inductive or wireless telemetry in clinic or home environment using vendor proprietary equipment Information is transferred to clinic system as structured HL 7 v 2. 6 ORU message using IEEE 11073 IDC nomenclature [PCD-09] 38

PCD Published Profiles RTM Profile Rosetta Terminology Mapping Trial Implementation 39
Rosetta for Semantic Interoperability 40
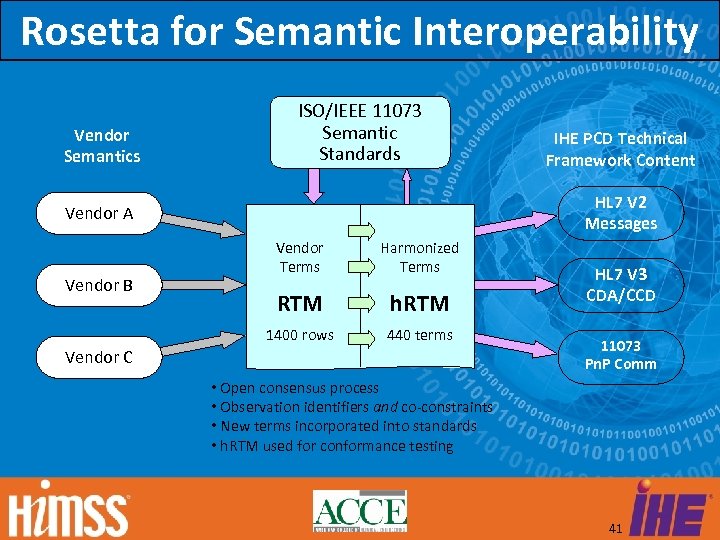
Rosetta for Semantic Interoperability Vendor Semantics ISO/IEEE 11073 Semantic Standards HL 7 V 2 Messages Vendor A Vendor Terms Harmonized Terms RTM h. RTM 1400 rows Vendor B IHE PCD Technical Framework Content 440 terms Vendor C HL 7 V 3 CDA/CCD 11073 Pn. P Comm • Open consensus process • Observation identifiers and co-constraints • New terms incorporated into standards • h. RTM used for conformance testing 41
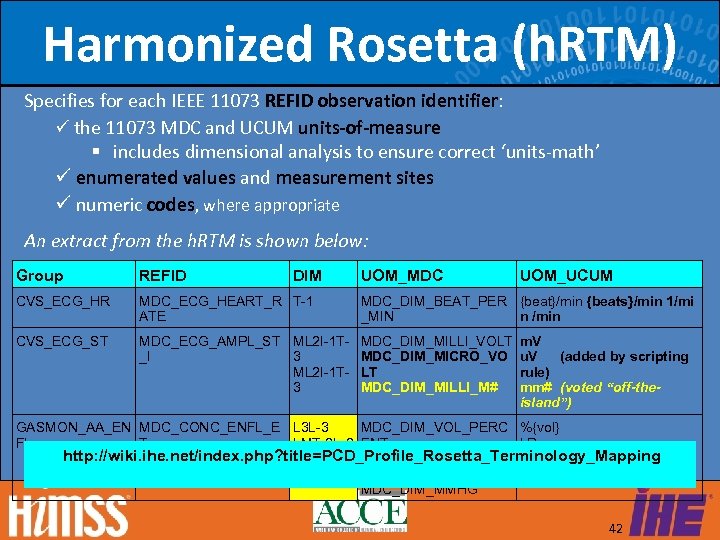
Harmonized Rosetta (h. RTM) Specifies for each IEEE 11073 REFID observation identifier: the 11073 MDC and UCUM units-of-measure includes dimensional analysis to ensure correct ‘units-math’ enumerated values and measurement sites numeric codes, where appropriate An extract from the h. RTM is shown below: Group REFID DIM UOM_MDC CVS_ECG_HR MDC_ECG_HEART_R T-1 ATE CVS_ECG_ST MDC_ECG_AMPL_ST ML 2 I-1 T- MDC_DIM_MILLI_VOLT _I 3 MDC_DIM_MICRO_VO ML 2 I-1 T- LT 3 MDC_DIM_MILLI_M# UOM_UCUM MDC_DIM_BEAT_PER {beat}/min {beats}/min 1/mi n /min _MIN m. V u. V (added by scripting rule) mm# (voted “off-theisland”) GASMON_AA_EN MDC_CONC_ENFL_E L 3 L-3 MDC_DIM_VOL_PERC %{vol} k. Pa FL T LMT-2 L-2 ENT http: //wiki. ihe. net/index. php? title=PCD_Profile_Rosetta_Terminology_Mapping LMT-2 L-2 MDC_DIM_KILO_PASC mm[Hg] AL MDC_DIM_MMHG 42

RTM & h. RTM Highlights Unified semantics and semantic model are essential prerequisites for safe and effective interoperability between devices and systems. The h. RTM rigorously defines what may be sent and informs recipients of what they may expect to receive. For each observation identifier, the h. RTM specifies the units-of-measure, enumerated values, measurement sites and other co-constraints. The h. RTM is based on the ISO/IEEE 11073 standards and leverages and extends that work by using an open consensus process. The h. RTM is publicly available for IHE PCD clinical devices and will be available shortly for IEEE 11073 personal health devices. The h. RTM supports message conformance testing frameworks that can be used for both clinical and personal health devices. 43
PCD Emerging Profiles Waveform Communication Management (WCM) Medical Equipment Management (MEM) Event Communication Device Point-of-Care Integration (DPI) Real Time Location Tracking Real-time data archiving and communication Mobile, enterprise-wide, reliable vital signs monitoring … many more! 44
![PCD Emerging Profiles Device Point-of-Care Integration [DPI] 45 PCD Emerging Profiles Device Point-of-Care Integration [DPI] 45](https://present5.com/presentation/49d6bffde1e5a4a1baec99d18da3909a/image-45.jpg)
PCD Emerging Profiles Device Point-of-Care Integration [DPI] 45

Device Point-of-Care Integration DPI: Scope – Device Point-of-care Integration (DPI) is concerned with use cases that include care contexts that fall within the stated charter of the IHE PCD, namely where "at least one actor is a regulated patient centric point-of-care medical device, " and that require device-to-device communication. 46
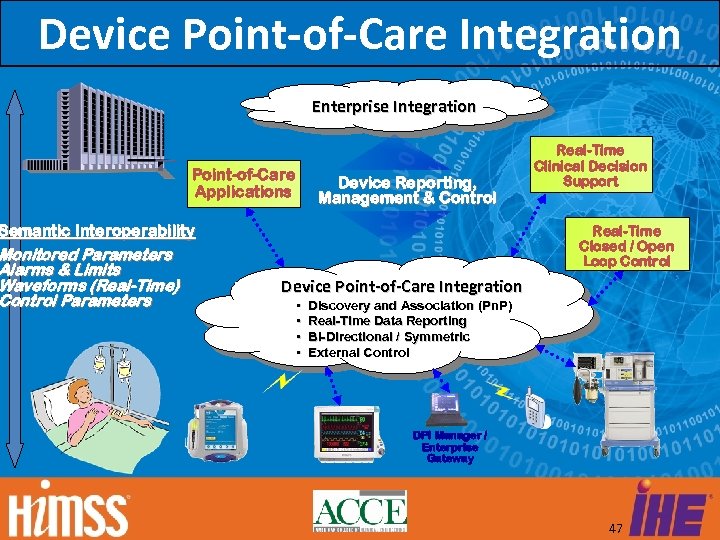
Device Point-of-Care Integration Enterprise Integration Point-of-Care Applications Device Reporting, Management & Control Semantic Interoperability Monitored Parameters Alarms & Limits Waveforms (Real-Time) Control Parameters Real-Time Clinical Decision Support Real-Time Closed / Open Loop Control Device Point-of-Care Integration • • Discovery and Association (Pn. P) Real-Time Data Reporting Bi-Directional / Symmetric External Control DPI Manager / Enterprise Gateway 47
![PCD Emerging Profiles Medical Equipment Management [MEM] Imagine … What if I. T. & PCD Emerging Profiles Medical Equipment Management [MEM] Imagine … What if I. T. &](https://present5.com/presentation/49d6bffde1e5a4a1baec99d18da3909a/image-48.jpg)
PCD Emerging Profiles Medical Equipment Management [MEM] Imagine … What if I. T. & device vendors gave me EVERYTHING I ever wanted? ! 48

MEM (Medical Equipment Management)
MEM Status 2009 – White Paper Ongoing work • Cycle 5 Brief Profile Proposal • Real Time Location Tracking • Location Boundary Alarms • Battery Management • Semantic Content Requirements 50
![PCD Emerging Profiles Waveform Communication Management [WCM] 51 PCD Emerging Profiles Waveform Communication Management [WCM] 51](https://present5.com/presentation/49d6bffde1e5a4a1baec99d18da3909a/image-51.jpg)
PCD Emerging Profiles Waveform Communication Management [WCM] 51
![WCM Objective Waveform Communication Management will extend the [DEC] profile to provide a method WCM Objective Waveform Communication Management will extend the [DEC] profile to provide a method](https://present5.com/presentation/49d6bffde1e5a4a1baec99d18da3909a/image-52.jpg)
WCM Objective Waveform Communication Management will extend the [DEC] profile to provide a method for passing near real-time waveform data using HL 7 v 2 observation messages between a Reporter and a Consumer. • A filter will be an optional element STATUS: • Supplement currently undergoing TC review 52
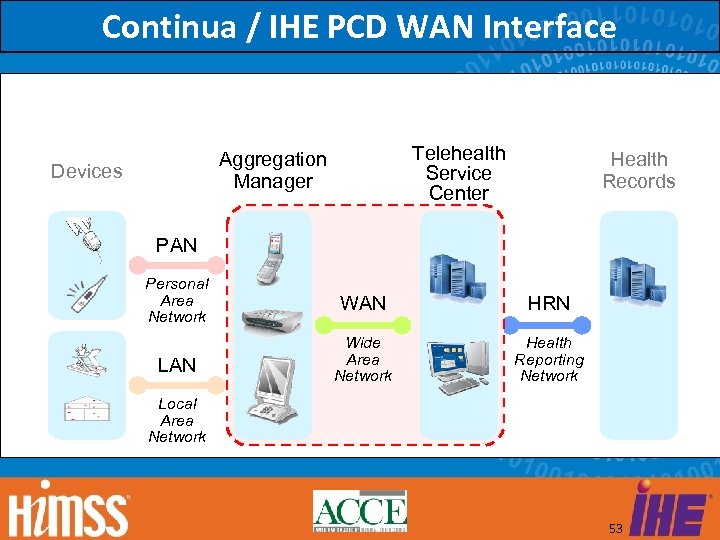
Continua / IHE PCD WAN Interface Telehealth Service Center Aggregation Manager Devices Health Records PAN Personal Area Network WAN HRN LAN Wide Area Network Health Reporting Network Local Area Network 53
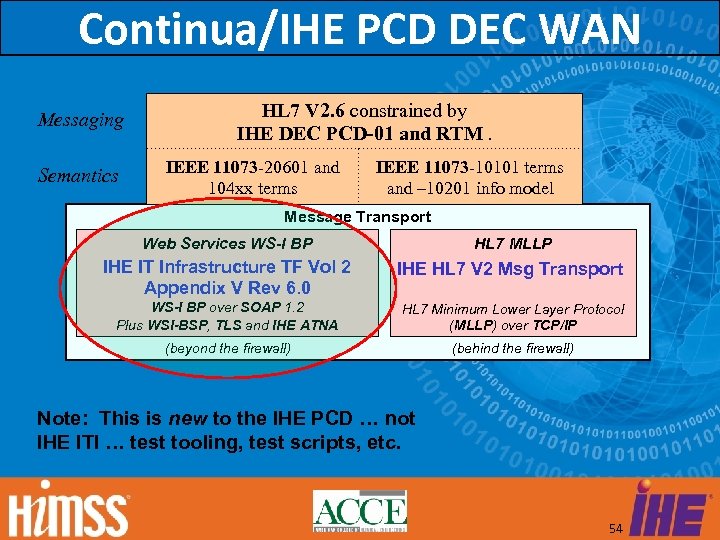
Continua/IHE PCD DEC WAN Messaging Semantics HL 7 V 2. 6 constrained by IHE DEC PCD-01 and RTM. IEEE 11073 -20601 and 104 xx terms IEEE 11073 -10101 terms and – 10201 info model Message Transport Web Services WS-I BP HL 7 MLLP IHE IT Infrastructure TF Vol 2 Appendix V Rev 6. 0 IHE HL 7 V 2 Msg Transport WS-I BP over SOAP 1. 2 Plus WSI-BSP, TLS and IHE ATNA HL 7 Minimum Lower Layer Protocol (MLLP) over TCP/IP (beyond the firewall) (behind the firewall) Note: This is new to the IHE PCD … not IHE ITI … test tooling, test scripts, etc. 54

PCD & ASTM “ICE”/CIMIT MDPn. P ICE-PCD Analysis Committee (ICE-PAC) – JWG underway to perform a map & gap analysis between “ICE” use case analyses, ISO/IEEE 11073 standards & the IHE DPI WG. (From ICE-PAC Overview Update 2009. 06. 22 to IHE PCD) 55

Design Engineers Clinical Engineers Clinicians RDC Project ~ Building on Clinical Requirements Clinical Scenario Gathering Requirements from Clinical System Providing Traceability of System Requirements Clinical Workflow Analysis Risk Analysis System Modeling Technical Solution and Clinical Implementation Provide Systems Level Risk Analysis Provides Traceability to Mitigating Risk Designing for the System, not modifying the system causing unintended consequences General Building Blocks and Interactions between the blocks are key to Safety If General Building Blocks make up Specific Systems then Systems are safe 56
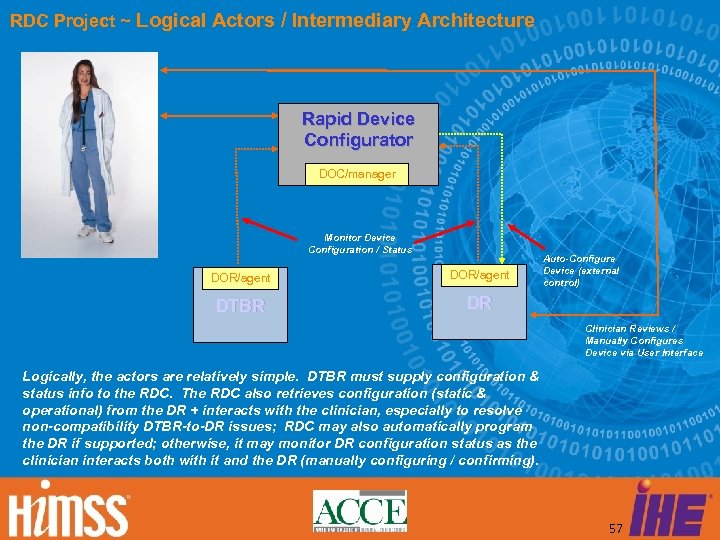
RDC Project ~ Logical Actors / Intermediary Architecture Rapid Device Configurator DOC/manager Monitor Device Configuration / Status DOR/agent DTBR Auto-Configure Device (external control) DR Clinician Reviews / Manually Configures Device via User Interface Logically, the actors are relatively simple. DTBR must supply configuration & status info to the RDC. The RDC also retrieves configuration (static & operational) from the DR + interacts with the clinician, especially to resolve non-compatibility DTBR-to-DR issues; RDC may also automatically program the DR if supported; otherwise, it may monitor DR configuration status as the clinician interacts both with it and the DR (manually configuring / confirming). 57

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 58

IHE Standards Adoption Process Develop technical specifications Testing at Connectathons IHE Demonstrations Products with IHE Identify available standards (e. g. HL 7, DICOM, IEEE, IETF) Document Use Case Requirements Improved safety, quality & efficiency! Easy to integrate products 59 59 59

Locked in the … dungeon? ! 60
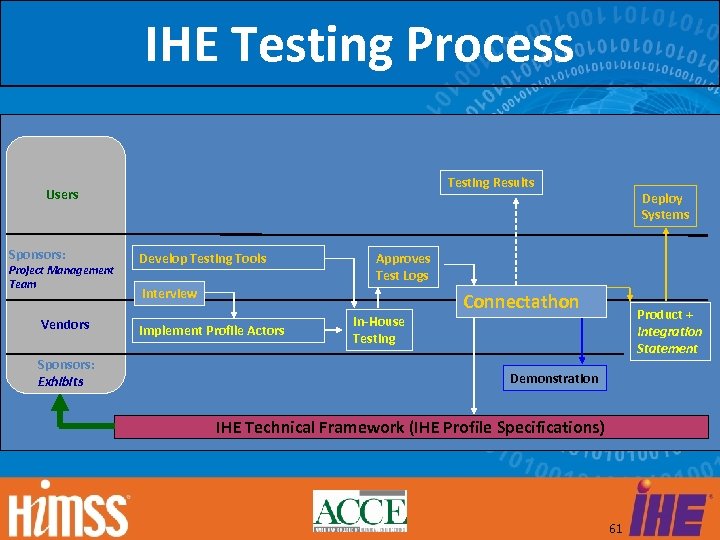
IHE Testing Process Testing Results Users Sponsors: Project Management Team Vendors Sponsors: Exhibits Develop Testing Tools Approves Test Logs Interview Implement Profile Actors Deploy Systems In-House Testing Connectathon Product + Integration Statement Demonstration IHE Technical Framework (IHE Profile Specifications) 61
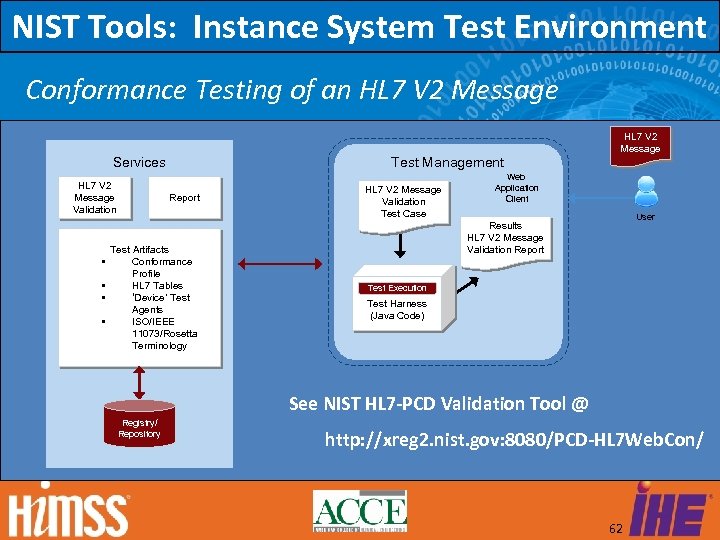
NIST Tools: Instance System Test Environment Conformance Testing of an HL 7 V 2 Message Services HL 7 V 2 Message Validation Test Management Report Test Artifacts Conformance Profile • HL 7 Tables • ‘Device’ Test Agents • ISO/IEEE 11073/Rosetta Terminology HL 7 V 2 Message Validation Test Case Web Application Client User Results HL 7 V 2 Message Validation Report • Test Execution Test Harness (Java Code) See NIST HL 7 -PCD Validation Tool @ Registry/ Repository http: //xreg 2. nist. gov: 8080/PCD-HL 7 Web. Con/ 62
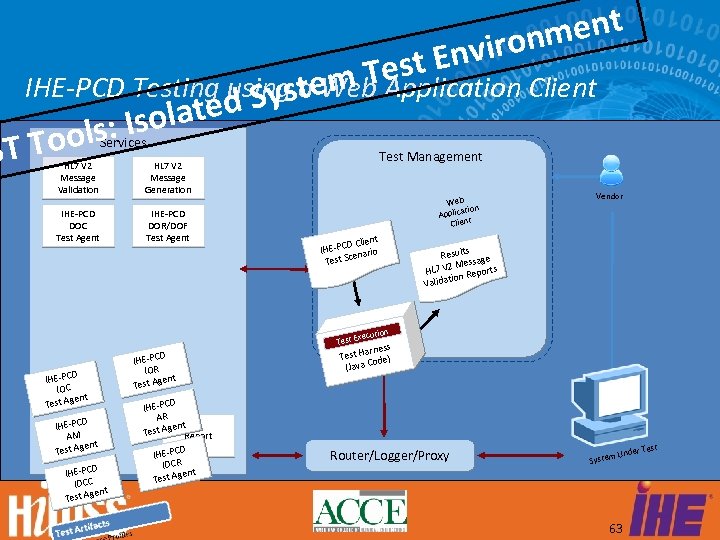
ment viron t En Tes IHE-PCD Testing using aem Application Client Syst Web lated ls: Iso Too ST Services HL 7 V 2 Message Validation IHE-PCD DOC Test Agent IHE-PCD DOR/DOF Test Agent Test Management HL 7 V 2 Message Generation Web ation Applic Client t D Clien IHE-PC nario e Test Sc Vendor s Result age ess M HL 7 V 2 Reports ation Valid n xecutio Test E D IHE-PC C IO gent Test A D IHE-PC M A gent Test A D IHE-PC IDCC gent Test A D IHE-PC IOR nt st Age Te D IHE-PC R A ent est Ag Report T D IHE-PC CR ID gent Test A arness Test H de) o (Java C Router/Logger/Proxy r Test Unde System 63
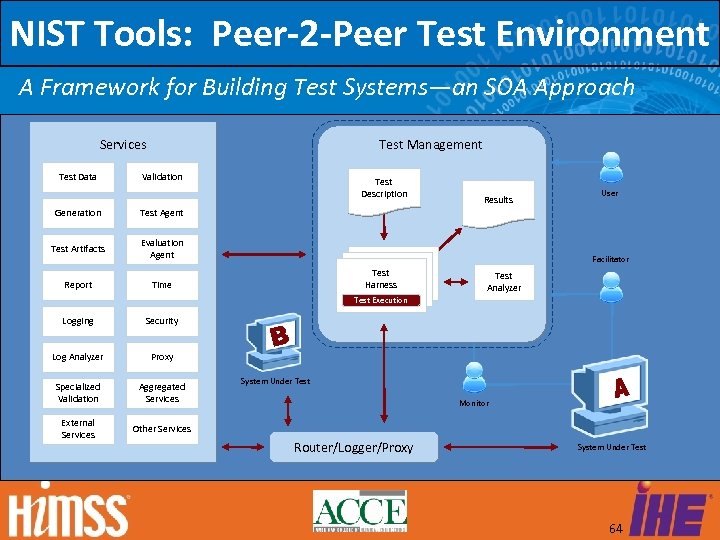
NIST Tools: Peer-2 -Peer Test Environment A Framework for Building Test Systems—an SOA Approach Services Test Data Test Management Validation Generation Evaluation Agent Report Time Results User Test Agent Test Artifacts Test Description Facilitator Test Harness Test Analyzer Test Execution Logging Security Log Analyzer Proxy Specialized Validation Aggregated Services External Services Other Services System Under Test Monitor Router/Logger/Proxy System Under Test 64

IHE Standards Adoption Process Develop technical specifications Testing at Connectathons IHE Demonstrations Products with IHE Identify available standards (e. g. HL 7, DICOM, IEEE, IETF) Document Use Case Requirements Improved safety, quality & efficiency! Easy to integrate products 65 65 65

PCD @ HIMSS 2010 66
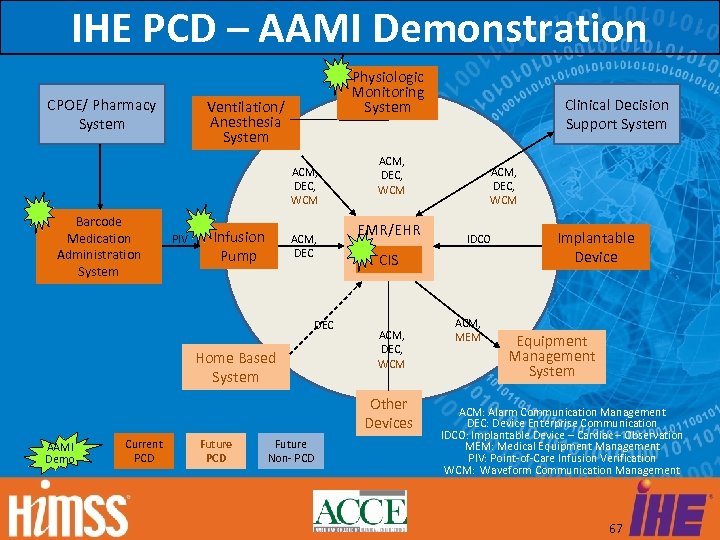
IHE PCD – AAMI Demonstration CPOE/ Pharmacy System Physiologic Monitoring System Ventilation/ Anesthesia System ACM, DEC, WCM Barcode Medication Administration System PIV Infusion Pump ACM, DEC Home Based System ACM, DEC, WCM EMR/EHR Current PCD Future Non- PCD ACM, DEC, WCM IDCO CIS ACM, DEC, WCM Other Devices AAMI Demo Clinical Decision Support System ACM, MEM Implantable Device Equipment Management System ACM: Alarm Communication Management DEC: Device Enterprise Communication IDCO: Implantable Device – Cardiac – Observation MEM: Medical Equipment Management PIV: Point-of-Care Infusion Verification WCM: Waveform Communication Management 67
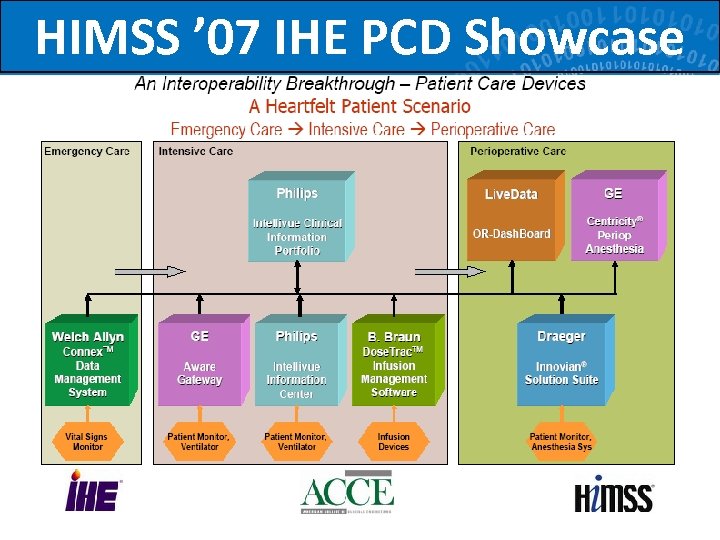
HIMSS ’ 07 IHE PCD Showcase 68
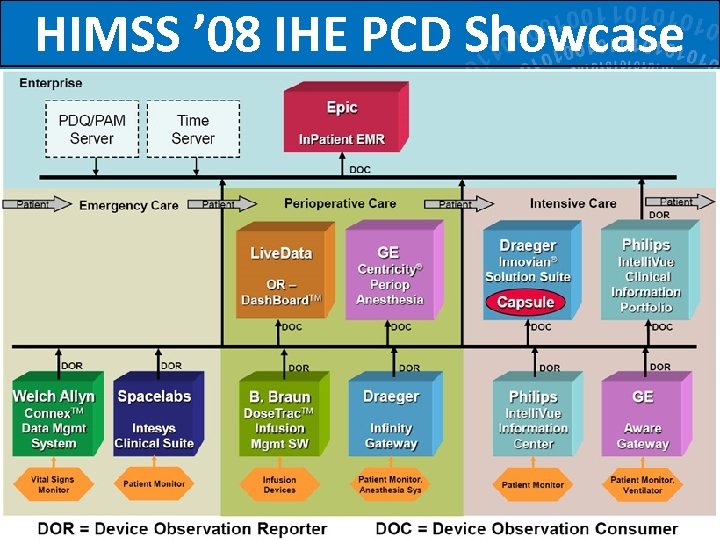
HIMSS ’ 08 IHE PCD Showcase 69
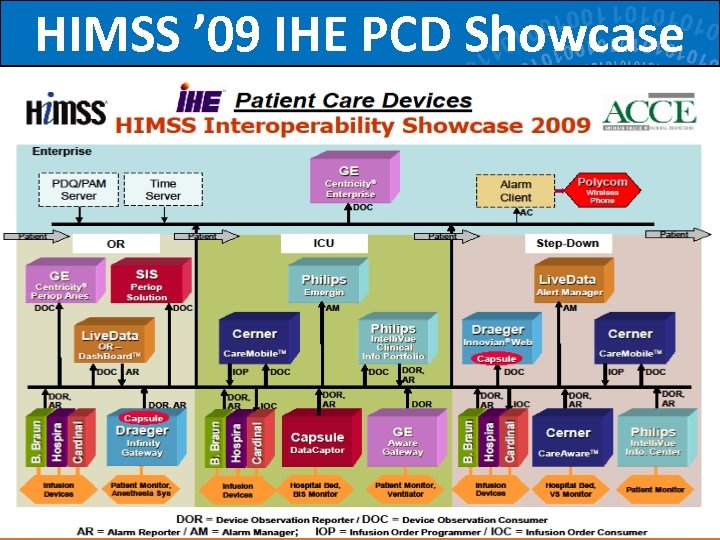
HIMSS ’ 09 IHE PCD Showcase 70
HIMSS ’ 10? Too big for a single diagram! HIMSS ’ 10 Atlanta Showcase Floorplan 71

IHE PCD – HIMSS ’ 10 Wall 1 72

IHE PCD – HIMSS ’ 10 Wall 2 73

IHE PCD – HIMSS ’ 10 Wall 3 74
IHE PCD – HIMSS ’ 10 Wall 4 75

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 76

Great Expectations? IHE PCD – Vendor value propositions… Simplify product development process Spend time innovating rather than supporting infrastucture work – again &. . . Facilitate clinical decision support - innovation - increased functionality Reduce regulatory impact/work Improve patient safety - reduce liability - make operations easier - device aware 77

The Dream … Deferred! Why has it not yet happened? Incomplete standards … but no longer the case! Uncoordinated & inconsistent demand from providers and other user stakeholders Undefined stakeholder value propositions Resources – esp. for standards–related projects Dr. Brailer: Infrastructure is a hard sell: Concentrated cost – Diffuse benefit A business issue – not a lack of technology! 78

IHE Patient Care Devices Overview and Update Devices and Interoperability Medical Devices - Not a Simple Problem!! IHE PCD – Overview IHE PCD – Profiles IHE PCD – Testing and Demonstrations Getting to Yes!! Questions & Answers 79

Q&A Find out more at www. ihe. net
49d6bffde1e5a4a1baec99d18da3909a.ppt