720aa14ea1bfad9b3a379d46e71003a8.ppt
- Количество слайдов: 54
Patentable and Non Patentable Biotech Inventions A Presentation By D. Calab Gabriel Senior Partner K & S Partners, Gurgaon
Scope • • • Patent Law What is non patentable Product patent Case studies –Product Patent Process Patent Case studies- Process Patent
Patent law Patents Act 1970 Patentable inventions any product or process which is : • novel • not obvious to a person skilled in the art • capable of industrial application
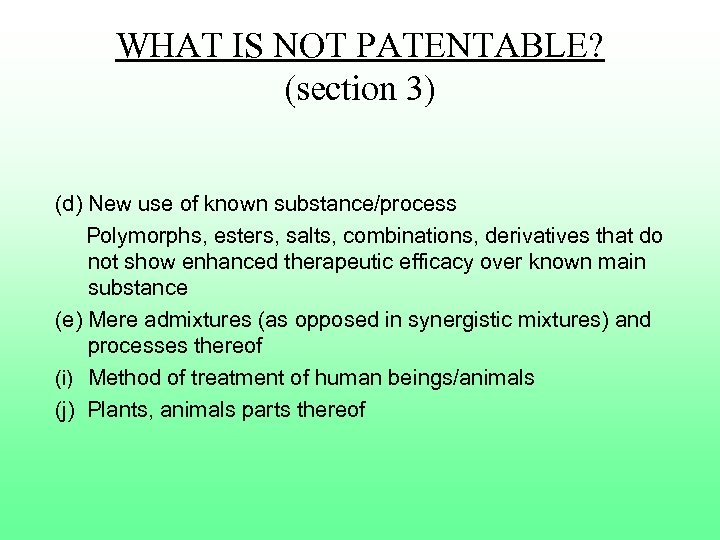
WHAT IS NOT PATENTABLE? (section 3) (d) New use of known substance/process Polymorphs, esters, salts, combinations, derivatives that do not show enhanced therapeutic efficacy over known main substance (e) Mere admixtures (as opposed in synergistic mixtures) and processes thereof (i) Method of treatment of human beings/animals (j) Plants, animals parts thereof

WHAT IS NOT PATENTABLE? (section 3) Section 3(d) The mere discovery of a new form of a known substance which does not result in the enhancement of the known efficacy of that substance or the mere discovery of any new property or new use for a known substance or of the mere use of a known process, machine or apparatus unless such known process results in a new product or employs at least one new reactant.

WHAT IS NOT PATENTABLE? (section 3) Section 3(d) (cont. ) Explanation: For the purpose of this clause, salts, esters, ethers, polymorphs, metabolites, pure from, particle size, isomers, mixtures of isomers, complexes, combinations and other derivatives of known substance shall be considered to be the same substance, unless they differ significantly in properties with regard to efficacy. n Examples: n n Toxic – non-toxic Stable – shelf life
WHAT IS NOT PATENTABLE? (section 3) (e) a substance obtained by a mere admixture resulting only in the aggregation of the properties of the components thereof
WHAT IS NOT PATENTABLE? (section 3) (i) any process for the medicinal / surgical / curative, prophylactic/diagnostic/therapeutic or other treatment of human beings or similar such process for treatment in animals. n IN VITRO methods?
WHAT IS NOT PATENTABLE? (section 3) (j) Plants and animals in whole or in part thereof other than microorganisms but including seeds, varieties and species and essentially biological processes for production or propagation of plants and animals. Cell per se?

SECOND MEDICAL USE
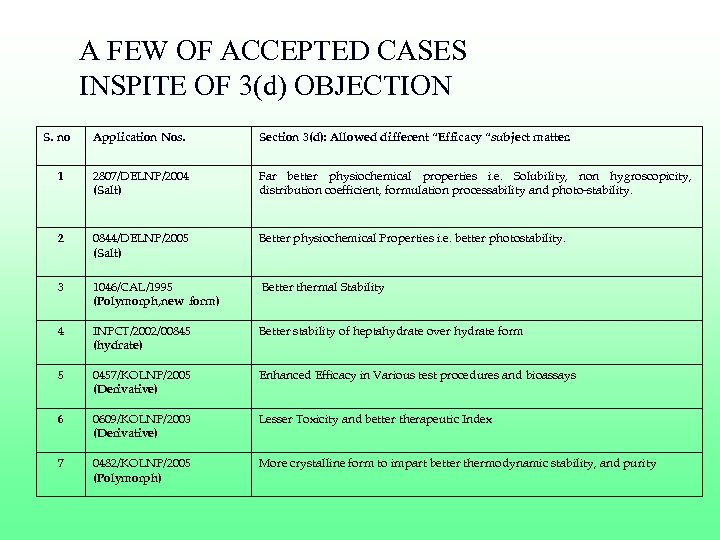
A FEW OF ACCEPTED CASES INSPITE OF 3(d) OBJECTION S. no Application Nos. Section 3(d): Allowed different “Efficacy “subject matter. 1 2807/DELNP/2004 (Salt) Far better physiochemical properties i. e. Solubility, non hygroscopicity, distribution coefficient, formulation processability and photo-stability. 2 0844/DELNP/2005 (Salt) Better physiochemical Properties i. e. better photostability. 3 1046/CAL/1995 (Polymorph, new form) Better thermal Stability 4 INPCT/2002/00845 (hydrate) Better stability of heptahydrate over hydrate form 5 0457/KOLNP/2005 (Derivative) Enhanced Efficacy in Various test procedures and bioassays 6 0609/KOLNP/2003 (Derivative) Lesser Toxicity and better therapeutic Index 7 0482/KOLNP/2005 (Polymorph) More crystalline form to impart better thermodynamic stability, and purity
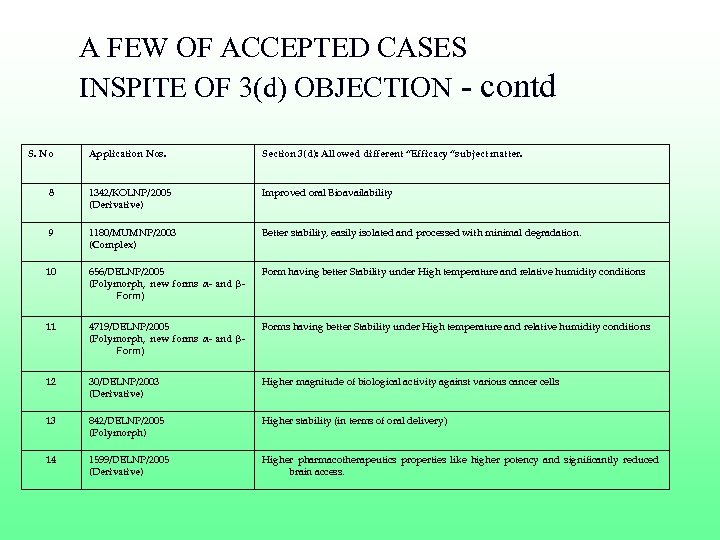
A FEW OF ACCEPTED CASES INSPITE OF 3(d) OBJECTION - contd S. No Application Nos. Section 3(d): Allowed different “Efficacy “subject matter. 8 1342/KOLNP/2005 (Derivative) Improved oral Bioavailability 9 1180/MUMNP/2003 (Complex) Better stability, easily isolated and processed with minimal degradation. 10 656/DELNP/2005 (Polymorph, new forms α- and βForm) Form having better Stability under High temperature and relative humidity conditions 11 4719/DELNP/2005 (Polymorph, new forms α- and βForm) Forms having better Stability under High temperature and relative humidity conditions 12 30/DELNP/2003 (Derivative) Higher magnitude of biological activity against various cancer cells 13 842/DELNP/2005 (Polymorph) Higher stability (in terms of oral delivery) 14 1599/DELNP/2005 (Derivative) Higher pharmacotherapeutics properties like higher potency and significantly reduced brain access.
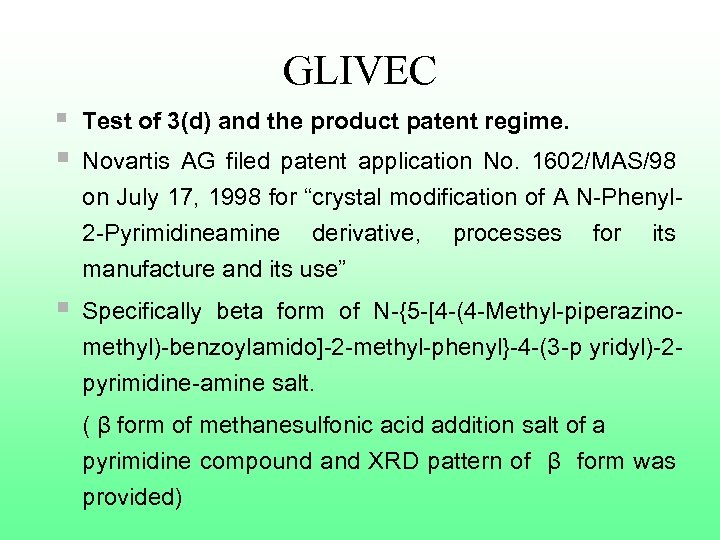
GLIVEC § Test of 3(d) and the product patent regime. § Novartis AG filed patent application No. 1602/MAS/98 on July 17, 1998 for “crystal modification of A N-Phenyl 2 -Pyrimidineamine derivative, manufacture and its use” § processes for its Specifically beta form of N-{5 -[4 -(4 -Methyl-piperazinomethyl)-benzoylamido]-2 -methyl-phenyl}-4 -(3 -p yridyl)-2 pyrimidine-amine salt. ( β form of methanesulfonic acid addition salt of a pyrimidine compound and XRD pattern of β form was provided)
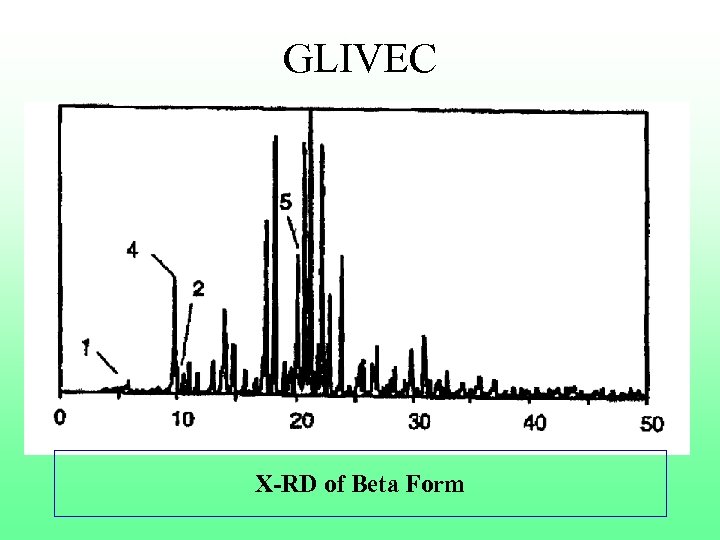
GLIVEC X-RD of Beta Form

GLIVEC n Notable statement in specification “It goes without saying that all the indicated inhibitory and pharmacological effects of β form are also found with the free base”. n No enhanced efficacy shown n Established β form was pre-existing n Application was rejected by the Patent Office n Limited appeal to DB Chennai, case rejected n Limited issue before IPAB-case rejected n Challenged IPAB decision by way of write in SC
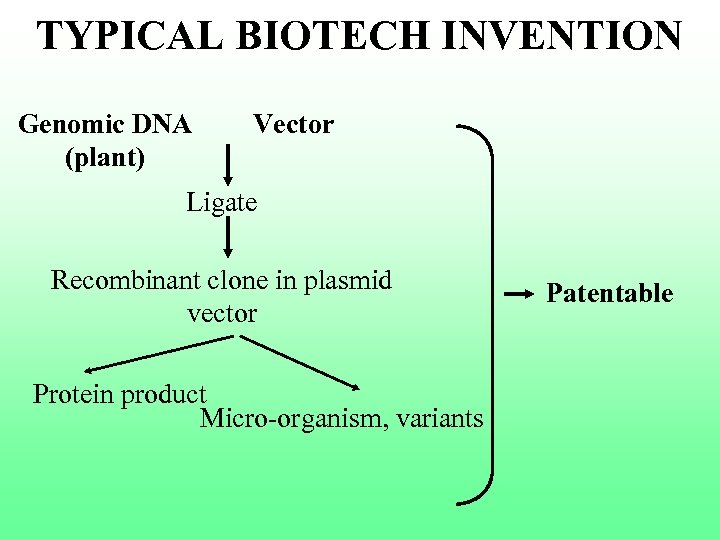
TYPICAL BIOTECH INVENTION Genomic DNA (plant) Vector Ligate Recombinant clone in plasmid vector Protein product Micro-organism, variants Patentable
PRODUCT PATENTS All products of molecular biology: whether for use as drug or food product § Novel micro-organism (isolated/genetically engineered) § Novel gene and peptide sequences § Promoter, Marker § Novel cassette, construct § Vaccine § New viral strain

Micro-organisms § Not defined by Act § Possibly includes yeast, bacteria, recombinants, DNA sequences, vectors § Are isolated microbes and colonies “mere discovery” ? ? § Isolates, if characterized, deposited in ID and utility found - patentable § Genetically modified organisms: patentable Source and origin of Biological material to be provided in specification n
Genes § Gene sequences if isolated and utility found, patentable § Are they ‘part of animal or human being’ ? § Antibodies, including chimeric antibodies are patentable
Nucleotide sequences Ø Nucleic Acids: Ø molecules containing A, G, C and T residues (DNA); Ø molecules containing A, G, C and U residues (RNA) Ø DNA is transcribed into RNA Ø RNA is translated into Proteins Ø Proteins are molecules containing up to 20 different amino acid residues: A (ala), C (cys), D (asp), E (glu), F (phe), G (gly), H (his), I (ile), K (lys), L (leu), M (met), N (asn), P (pro), Q (gln), R (arg), S (ser), T (thr), V (val), W (trp) Ø 3 nucleic acid residues code for one amino acid residue:
Isolated DNA: EP Position § What is a DNA sequence ? Can it be patented? § Held in RELAXIN CASE : Isolated DNA sequence is a chemical compound; can be patented
Isolated DNA: US Position patentable An isolated and purified DNA molecule, RNA molecule, or amino acid molecule n isolated chemical compounds n Full-Length Genetic Sequences patentable n Corresponding amino acid sequence patentable n
US position- Contd ESTs n Partial nucleic acid sequences if proved useful (eg: as encoding protein responsible for diagnosis of a specific disease) = patentable n DNA fragment encoding a full ORF n May be useful if homology to existing nucleic acids or proteins (with an established utility) is at least 95%
BOTTOM-LINE Nucleotide sequences n The sequence must be isolated and purified from its natural environment n raw sequences with no known use are not patentable n India: n Product patent regime just introduced; n So far few patents granted for nucleotide sequences n Sequences patentable provided: Isolated from natural surrounding n Utility proven n
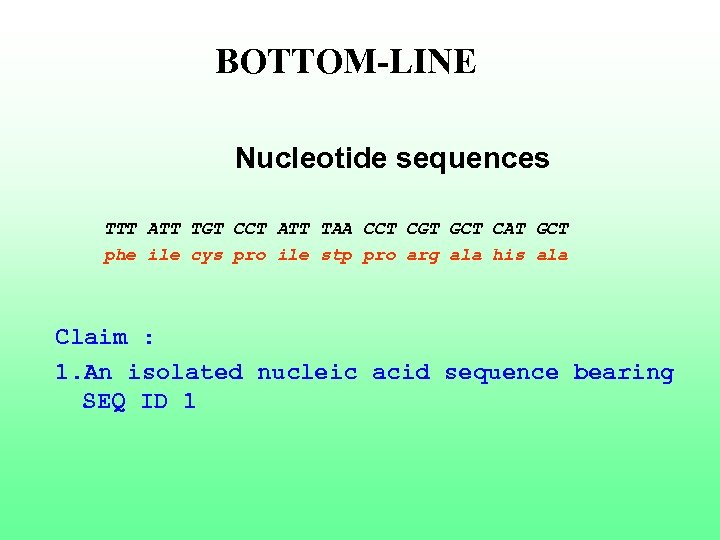
BOTTOM-LINE Nucleotide sequences TTT ATT TGT CCT ATT TAA CCT CGT GCT CAT GCT phe ile cys pro ile stp pro arg ala his ala Claim : 1. An isolated nucleic acid sequence bearing SEQ ID 1

Micro-organisms : EP position EP case T 396/93: Micro-organism includes bacteria, yeast, fungi, algae, protozoa, virus…. Biotechnology Directive : § Defines biological material not micro-organism § Includes microbes, cell lines, viruses…. § Excludes cell lines used for modifying germ line of human beings

Micro-organisms : US position n Diamond v. Chakrabarty, 447 U. S. 303 (1980) genetically engineered bacteria are patentable n “anything under the sun that is made by man” n n Patents granted for : n n n Yeast lines, Virus, hybridoma, oyester Harvard Mouse
Micro-organisms : Indian position § Section 3: § Plants, animals and human beings except microorganism not patentable § No distinction between isolated and genetically modified micro-organism § Many argue: Isolated micro-organism is no invention, only GM

ISOLATED MICROORGANISM § Micro-organism n Isolated ? n Mutant ? n Genetically modified ? § Deposit in international depository prior to date of Indian filing
LIFE SCIENCES § Micro-organism § Also describe source and origin of biological material § Bring out utility § If mutated: set out conditions, details
CHARECTERISATION OF YEAST STRAIN Ex: Mere mention of deposit of yeast strain in a depository without setting out its characteristics in the specification is “inadequate description” Pfizer’s Application 1974 RPC 689
LIFE SCIENCES n DNA/Protein Sequences n Describe fully how sequence was derived n If mutated, how? n Utility/applications of sequence n Products comprising sequence n Sequence listing to be submitted
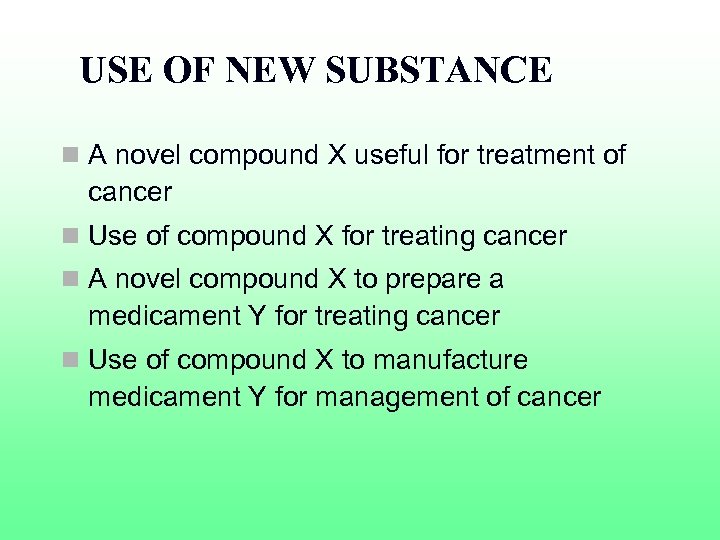
USE OF NEW SUBSTANCE n A novel compound X useful for treatment of cancer n Use of compound X for treating cancer n A novel compound X to prepare a medicament Y for treating cancer n Use of compound X to manufacture medicament Y for management of cancer

BIO-PRODUCTS § Indicate the class and specific chemicals used Ex: All the oxidants that would enable the invention, all alkali/acids that would help to work the invention

EXAMPLE

US PATENT NO. 6, 764, 824 n What is claimed is: 1. The primers of SEQ ID NO: 1 and 2 useful for screening human beings for a pre-disposition to schizophrenia. n 2. A method of screening human beings for a pre-disposition to schizophrenia by identifying nonsense mutation of codon TGG coding for amino acid tryptophan substituted with TAG, a non-sense codon, at nucleotide No. 825 from 5' end in exon 2 and its allelic variants in synaptogyrin 1 gene of chromosome 22 q 11 -13, said method comprising: (a) isolating DNA from blood leukocytes, (b) amplifying isolated DNA by PCR using primers of SEQ ID NO: 1 and/or 2 of enclosed sequence listing, specific for exons of synaptogyrin 1 gene, (c) sequencing the amplified DNA, (d) comparing the sequenced DNA with that of normal synaptogyrin 1 gene, (e) identifying the said mutation, (f) designing oligonucleotide primer and/or probe of SEQ ID NO: 3 of enclosed sequence listing with its 3' end extending up to penultimate position of said mutation, (g) screening for said non-sense mutation using primer and/or probe of step (f), and (h) screening of said allelic variation for said non-sense mutation using appropriate allele specific oligonucleotide probes and/or primers selected from the group consisting of SEQ ID NO: 4 to 7 of enclosed sequence listing.
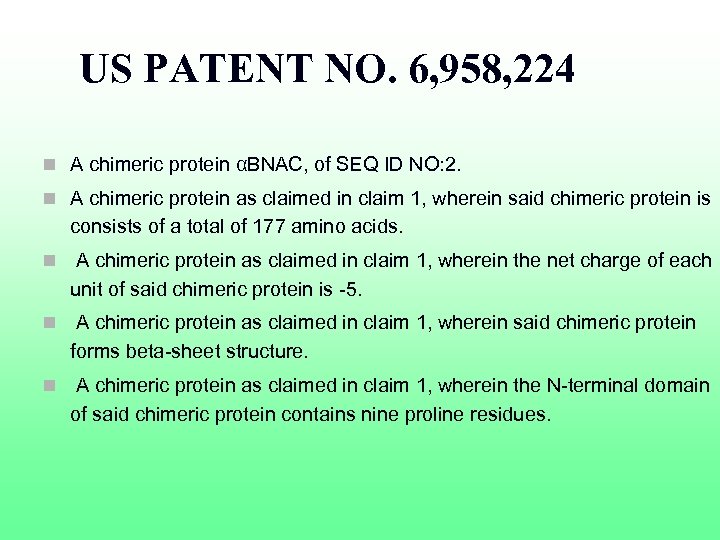
US PATENT NO. 6, 958, 224 n A chimeric protein αBNAC, of SEQ ID NO: 2. n A chimeric protein as claimed in claim 1, wherein said chimeric protein is consists of a total of 177 amino acids. n A chimeric protein as claimed in claim 1, wherein the net charge of each unit of said chimeric protein is -5. n A chimeric protein as claimed in claim 1, wherein said chimeric protein forms beta-sheet structure. n A chimeric protein as claimed in claim 1, wherein the N-terminal domain of said chimeric protein contains nine proline residues.

US PATENT NO. 6, 616, 950 n What is claimed is: 1. A fermented fruit based herbal health drink composition useful as an antioxidant, cardio-tonic, diuretic, digestive, choleretic, nervine relaxant and immuno-modulant, comprising extract from plant Andrographis with concentration ranging between 0. 5 to 10% in the total herbal health drink, extract from fruit, and ethanol ranging between 3 to 13% in the total herbal health drink, optionally extract from plant Tinospora and other additives.

US PATENT NO. 6, 558, 940 n A biologically pure culture of Streptomyces sp. CIMAP A. sub. 1 strain bearing ATCC Accession No. PTA-4131 and capable of inhibiting the growth of phytopathogenic fungi.

US PATENT NO. 6, 696, 284 n 1. A biological filter for the purification of waste gases, comprising a housing with at least one inlet and at least one outlet, and a bed of active micro-organisms contained in a carrier material consisting of pith extracted from coconut husks, the carrier material being provided in the housing such that the waste gases flowing in through the at least one inlet contact the bed of carrier material before exiting through the at least one outlet.

US PATENT NO. 6, 548, 746 1. A new and distinct high alkaloid producing Catharanthus roseus plant called `Dhawal` having NCIMB accession number 41147, and having the following combination of characteristics: (a) plant height of 65 -75 cm, (b) light green to grayish green (emerald green 758/1 color designation from the "Horticultural Colour Chart II") pubescent leaves with distinctly undulating leaf margin, (c) green stem, (d) white flowers, (e) field resistance to die back disease, (f) high leaf yield of 1352 to 2557 kg/ha, (g) 0. 89 to 1. 40% of total alkaloids in leaves, (h) 1. 60 to 2. 22% of total alkaloids in roots, (i) a randomly amplified polymorphic DNA (RAPD) profile distinct from the variety `Nirmal` when the DNA is polymerase chain reaction (PCR) amplified by four primers selected from the group consisting of: OPT 06, 09, 16 and 17, and (j) higher herbage and alkaloid yield as compared to the variety `Nirmal`.

PROCESS PATENTS § Any in vitro process § Method of protein purification, downstream processing § Process using micro-organisms to obtain chemicals

Screening Assays Usually patentable n Diagnostic assays: grey area n n Is it ex-vivo or in-vivo? Does the result of the method indicate that subject is suffering from a disorder? If yes, not patentable
Examples: PATENTABLE PROCESS n PCR process n Process using microbe … § Novel fermentation products, § novel techniques - RFLP, AFLP, fingerprinting etc
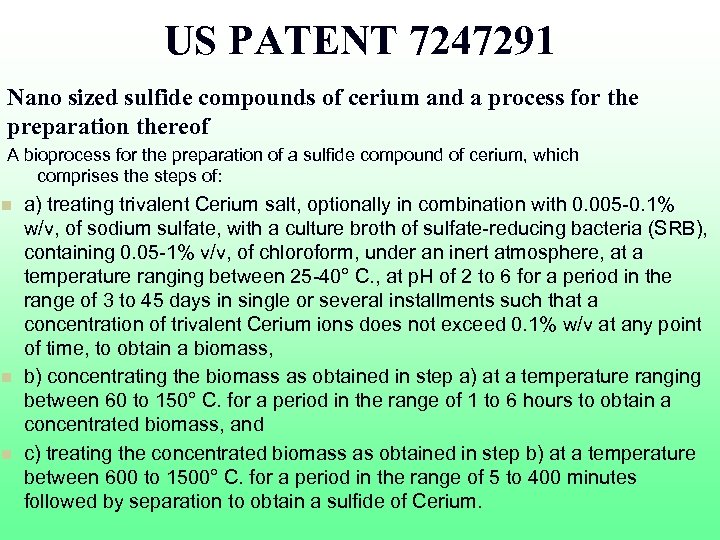
US PATENT 7247291 Nano sized sulfide compounds of cerium and a process for the preparation thereof A bioprocess for the preparation of a sulfide compound of cerium, which comprises the steps of: n n n a) treating trivalent Cerium salt, optionally in combination with 0. 005 -0. 1% w/v, of sodium sulfate, with a culture broth of sulfate-reducing bacteria (SRB), containing 0. 05 -1% v/v, of chloroform, under an inert atmosphere, at a temperature ranging between 25 -40° C. , at p. H of 2 to 6 for a period in the range of 3 to 45 days in single or several installments such that a concentration of trivalent Cerium ions does not exceed 0. 1% w/v at any point of time, to obtain a biomass, b) concentrating the biomass as obtained in step a) at a temperature ranging between 60 to 150° C. for a period in the range of 1 to 6 hours to obtain a concentrated biomass, and c) treating the concentrated biomass as obtained in step b) at a temperature between 600 to 1500° C. for a period in the range of 5 to 400 minutes followed by separation to obtain a sulfide of Cerium.

US PATENT 4960429 Chromium free process for the tanning of hides A chromium free process for the tanning of animal hides which includes, after the standard pretreatment of the hide, covalently modifying the skin collagen of said hide by a process ………. .

U. S. PATENT 6777219 PROCESS FOR THE PREPARATION OF ALKALINE PROTEASE A process for the preparation of alkaline protease which comprises growing a fungal strain Conidiobolus coronatus isolated from Anekal, Karnataka, India and deposited in American Type Cell Culture (ATCC) depository under Accession Number PTA-4132, in a medium having p. H 6. 0 to 9. 0, a carbon source and a nitrogen source under aerobic conditions in submerged culture, at a temperature ranging between 20 to 30 C. , for a period ranging between 2 to 6 days, harvesting the medium and separating the enzyme in liquid phase.

Australian Patent Application no: 2003217445 A method for unhairing animal skins or hides using a total lime and sulfide free enzymatic solution comprising: • preparing an enzymatic solution from animal or plant tissue, • optionally presoaking of skins or hides in water at 10°C to 60°C for 2 to 6 hours, • removing the soaking liquor, • applying the enzymatic solution by pasting or spraying on the flesh side of the skin or hide and left for 10 -24 hours at a temperature ranging between 10°C to 60°C, wherein the skins or hides are arranged flesh side to the flesh side or grain side to grain side, • floating the skins or hides in liquid comprising the enzymatic solution, vi. removing • the skins or hides from the liquid comprising enzymatic solution to produce an effluent and, • unhairing of the skins or hides either by scraping the hair with a curved knife on a wooden beam or by an using unhairing machine.
Method n for regeneration of organs A method for regenerating organs in humans using: n stem cells n from contiguous embryonic peritoneal layer n formation of mesodermal organs in vitro n avoids use of exogenous tissue n 5 organs regenerated

Method for regenerating organs F Patent Act, 1970 Fsec 3(i) “any process for the medical, surgical, …. treatment of human beings or …render them free of disease ……” Finvention in question hit by above provision F currently not patentable in India F US 6, 227, 202 obtained
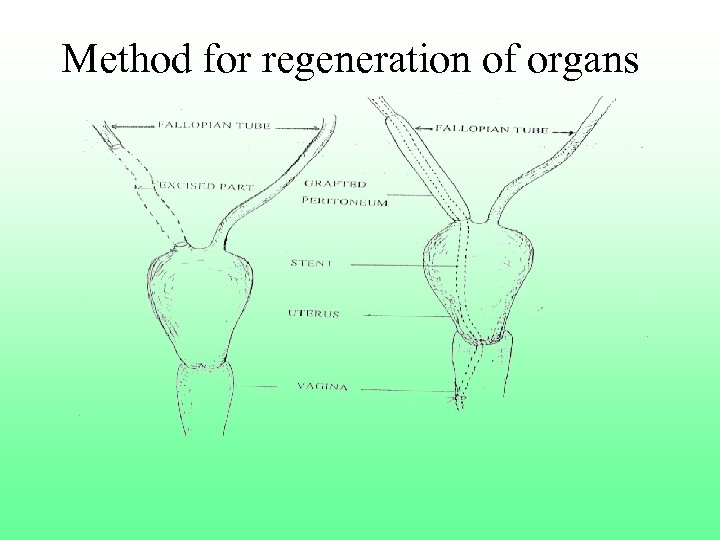
Method for regeneration of organs
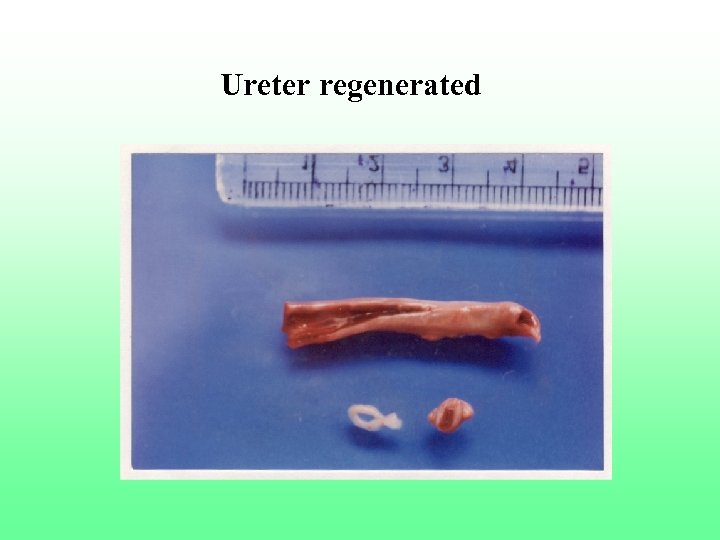
Ureter regenerated
?

720aa14ea1bfad9b3a379d46e71003a8.ppt