 Partnerships in Promoting Innovation and Managing Risk Scientific and Financial Innovation in AIDS Vaccines International AIDS Vaccine Initiative Labeeb M. Abboud General Counsel September 11, 2007
Partnerships in Promoting Innovation and Managing Risk Scientific and Financial Innovation in AIDS Vaccines International AIDS Vaccine Initiative Labeeb M. Abboud General Counsel September 11, 2007
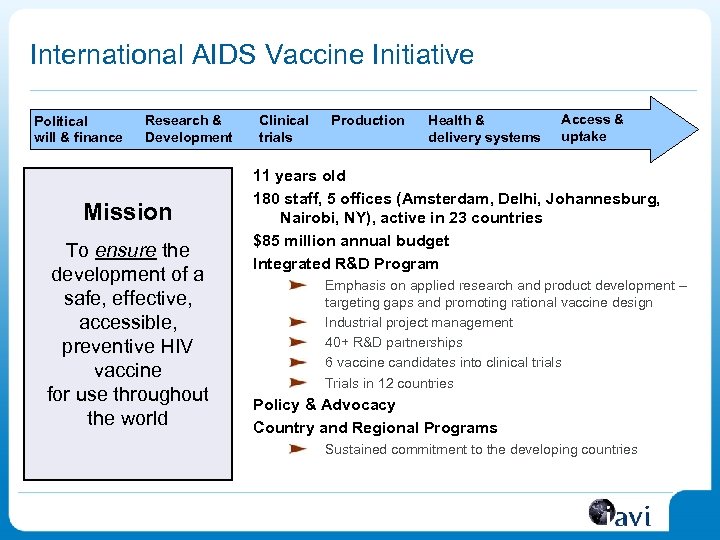 International AIDS Vaccine Initiative Political will & finance Research & Development Mission To ensure the development of a safe, effective, accessible, preventive HIV vaccine for use throughout the world Clinical trials Production Health & delivery systems Access & uptake 11 years old 180 staff, 5 offices (Amsterdam, Delhi, Johannesburg, Nairobi, NY), active in 23 countries $85 million annual budget Integrated R&D Program Emphasis on applied research and product development – targeting gaps and promoting rational vaccine design Industrial project management 40+ R&D partnerships 6 vaccine candidates into clinical trials Trials in 12 countries Policy & Advocacy Country and Regional Programs Sustained commitment to the developing countries
International AIDS Vaccine Initiative Political will & finance Research & Development Mission To ensure the development of a safe, effective, accessible, preventive HIV vaccine for use throughout the world Clinical trials Production Health & delivery systems Access & uptake 11 years old 180 staff, 5 offices (Amsterdam, Delhi, Johannesburg, Nairobi, NY), active in 23 countries $85 million annual budget Integrated R&D Program Emphasis on applied research and product development – targeting gaps and promoting rational vaccine design Industrial project management 40+ R&D partnerships 6 vaccine candidates into clinical trials Trials in 12 countries Policy & Advocacy Country and Regional Programs Sustained commitment to the developing countries
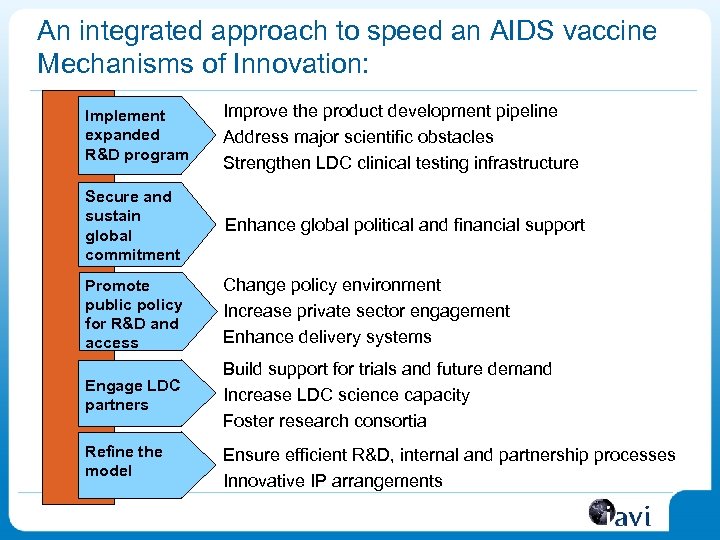 An integrated approach to speed an AIDS vaccine Mechanisms of Innovation: Implement expanded R&D program Improve the product development pipeline Address major scientific obstacles Strengthen LDC clinical testing infrastructure Secure and sustain global commitment Enhance global political and financial support Promote public policy for R&D and access Change policy environment Increase private sector engagement Enhance delivery systems Engage LDC partners Build support for trials and future demand Increase LDC science capacity Foster research consortia Refine the model Ensure efficient R&D, internal and partnership processes Innovative IP arrangements
An integrated approach to speed an AIDS vaccine Mechanisms of Innovation: Implement expanded R&D program Improve the product development pipeline Address major scientific obstacles Strengthen LDC clinical testing infrastructure Secure and sustain global commitment Enhance global political and financial support Promote public policy for R&D and access Change policy environment Increase private sector engagement Enhance delivery systems Engage LDC partners Build support for trials and future demand Increase LDC science capacity Foster research consortia Refine the model Ensure efficient R&D, internal and partnership processes Innovative IP arrangements
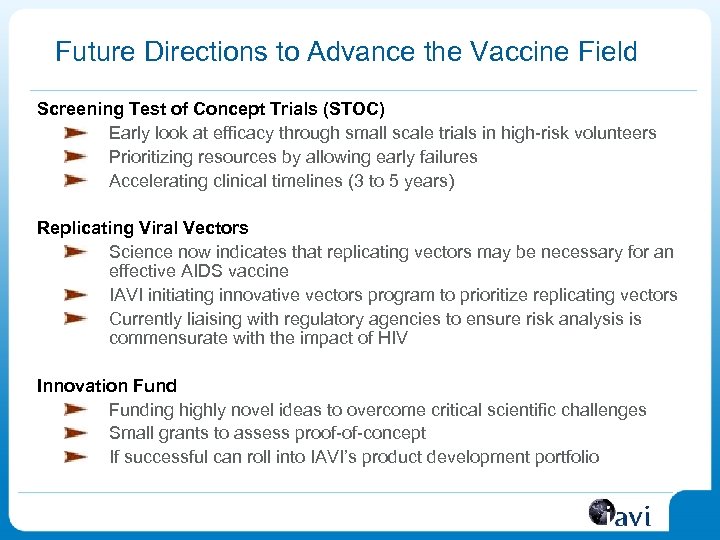 Future Directions to Advance the Vaccine Field Screening Test of Concept Trials (STOC) Early look at efficacy through small scale trials in high-risk volunteers Prioritizing resources by allowing early failures Accelerating clinical timelines (3 to 5 years) Replicating Viral Vectors Science now indicates that replicating vectors may be necessary for an effective AIDS vaccine IAVI initiating innovative vectors program to prioritize replicating vectors Currently liaising with regulatory agencies to ensure risk analysis is commensurate with the impact of HIV Innovation Funding highly novel ideas to overcome critical scientific challenges Small grants to assess proof-of-concept If successful can roll into IAVI’s product development portfolio
Future Directions to Advance the Vaccine Field Screening Test of Concept Trials (STOC) Early look at efficacy through small scale trials in high-risk volunteers Prioritizing resources by allowing early failures Accelerating clinical timelines (3 to 5 years) Replicating Viral Vectors Science now indicates that replicating vectors may be necessary for an effective AIDS vaccine IAVI initiating innovative vectors program to prioritize replicating vectors Currently liaising with regulatory agencies to ensure risk analysis is commensurate with the impact of HIV Innovation Funding highly novel ideas to overcome critical scientific challenges Small grants to assess proof-of-concept If successful can roll into IAVI’s product development portfolio
 IAVI gratefully acknowledges the support of our donors
IAVI gratefully acknowledges the support of our donors