2eb21091e63d2860a0ca910fe112e315.ppt
- Количество слайдов: 22
 Partnering with Pharmaceutical Leaders Active in Clinical Research
Partnering with Pharmaceutical Leaders Active in Clinical Research
 DO IT RIGHT THE FIRST TIME! Company Overview • Berlin-based CRO • Founded in 2004 • Founding member of MEDIS RESEARCH GROUP • Audited member of BVMA e. V. (in association with EUCROF) since 2009 • Specialist for study management and monitoring • Client base: international and regional biotechs, large pharma • Full-service in setting up and conducting clinical trials with our collaboration partners www. allied-clinical. com 2
DO IT RIGHT THE FIRST TIME! Company Overview • Berlin-based CRO • Founded in 2004 • Founding member of MEDIS RESEARCH GROUP • Audited member of BVMA e. V. (in association with EUCROF) since 2009 • Specialist for study management and monitoring • Client base: international and regional biotechs, large pharma • Full-service in setting up and conducting clinical trials with our collaboration partners www. allied-clinical. com 2
 DO IT RIGHT THE FIRST TIME! Academic Sourcing • Close relationship with academic institutions training clinical research professionals (Beuth Hochschule, Parexel Academy, Mibeg Institute) • Placements for practical training and thesis work • Early recruitment of top-of-their-class future professionals • Capacity for sudden increase of employees • High staff retention due to strong relationsships with employees www. allied-clinical. com 3
DO IT RIGHT THE FIRST TIME! Academic Sourcing • Close relationship with academic institutions training clinical research professionals (Beuth Hochschule, Parexel Academy, Mibeg Institute) • Placements for practical training and thesis work • Early recruitment of top-of-their-class future professionals • Capacity for sudden increase of employees • High staff retention due to strong relationsships with employees www. allied-clinical. com 3
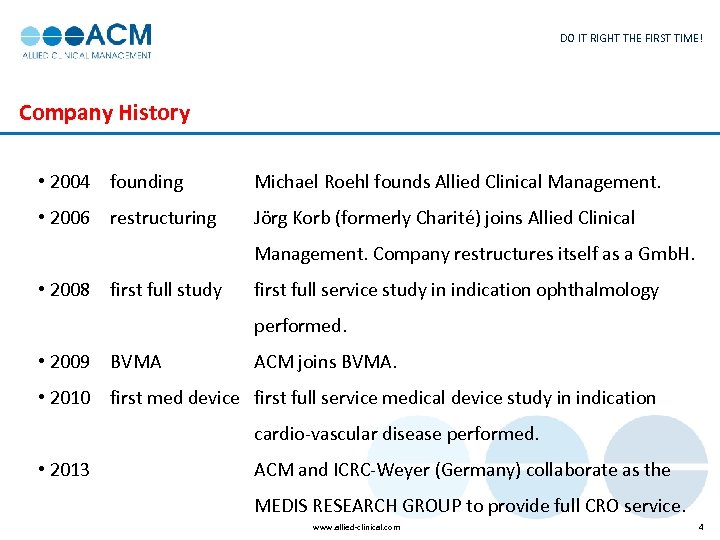 DO IT RIGHT THE FIRST TIME! Company History • 2004 founding Michael Roehl founds Allied Clinical Management. • 2006 restructuring Jörg Korb (formerly Charité) joins Allied Clinical Management. Company restructures itself as a Gmb. H. • 2008 first full study first full service study in indication ophthalmology performed. • 2009 BVMA ACM joins BVMA. • 2010 first med device first full service medical device study in indication cardio-vascular disease performed. • 2013 ACM and ICRC-Weyer (Germany) collaborate as the MEDIS RESEARCH GROUP to provide full CRO service. www. allied-clinical. com 4
DO IT RIGHT THE FIRST TIME! Company History • 2004 founding Michael Roehl founds Allied Clinical Management. • 2006 restructuring Jörg Korb (formerly Charité) joins Allied Clinical Management. Company restructures itself as a Gmb. H. • 2008 first full study first full service study in indication ophthalmology performed. • 2009 BVMA ACM joins BVMA. • 2010 first med device first full service medical device study in indication cardio-vascular disease performed. • 2013 ACM and ICRC-Weyer (Germany) collaborate as the MEDIS RESEARCH GROUP to provide full CRO service. www. allied-clinical. com 4
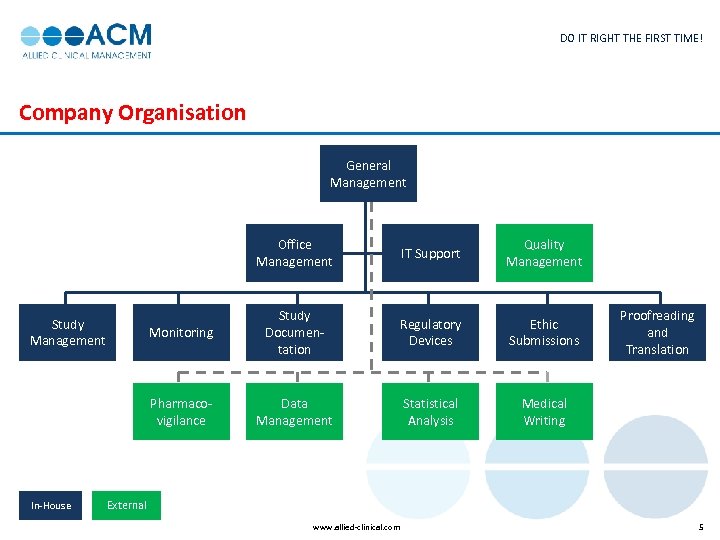 DO IT RIGHT THE FIRST TIME! Company Organisation General Management Office Management Study Documentation Regulatory Devices Ethic Submissions Pharmacovigilance In-House Quality Management Monitoring Study Management IT Support Data Management Statistical Analysis Medical Writing Proofreading and Translation External www. allied-clinical. com 5
DO IT RIGHT THE FIRST TIME! Company Organisation General Management Office Management Study Documentation Regulatory Devices Ethic Submissions Pharmacovigilance In-House Quality Management Monitoring Study Management IT Support Data Management Statistical Analysis Medical Writing Proofreading and Translation External www. allied-clinical. com 5
 DO IT RIGHT THE FIRST TIME! Quality • SOPs for all processes • Document management • Regular, documented GCP-Training for all employees • Regular system audits by an independent auditor every 18 months • Experience with CAPA-Processes and risk-based monitoring • ACM is an audited member of the German Federal Association of Medical CROs (in association with the European CRO Federation) since 2009 www. allied-clinical. com 6
DO IT RIGHT THE FIRST TIME! Quality • SOPs for all processes • Document management • Regular, documented GCP-Training for all employees • Regular system audits by an independent auditor every 18 months • Experience with CAPA-Processes and risk-based monitoring • ACM is an audited member of the German Federal Association of Medical CROs (in association with the European CRO Federation) since 2009 www. allied-clinical. com 6
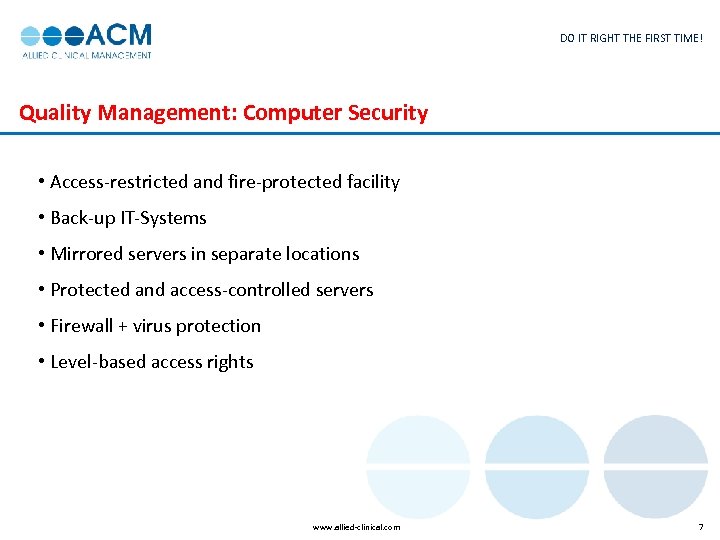 DO IT RIGHT THE FIRST TIME! Quality Management: Computer Security • Access-restricted and fire-protected facility • Back-up IT-Systems • Mirrored servers in separate locations • Protected and access-controlled servers • Firewall + virus protection • Level-based access rights www. allied-clinical. com 7
DO IT RIGHT THE FIRST TIME! Quality Management: Computer Security • Access-restricted and fire-protected facility • Back-up IT-Systems • Mirrored servers in separate locations • Protected and access-controlled servers • Firewall + virus protection • Level-based access rights www. allied-clinical. com 7
 DO IT RIGHT THE FIRST TIME! Our Services • Study Management • Study Monitoring • Study Documentation • Regulatory Services • Proofreading and translation →services can be contracted à la carte • Full Service as MEDIS RESEARCH GROUP www. allied-clinical. com 8
DO IT RIGHT THE FIRST TIME! Our Services • Study Management • Study Monitoring • Study Documentation • Regulatory Services • Proofreading and translation →services can be contracted à la carte • Full Service as MEDIS RESEARCH GROUP www. allied-clinical. com 8
 DO IT RIGHT THE FIRST TIME! Study Management • Oversight of study conduct and study monitoring • Budget management and tracking • Primary contact for the sponsor • Coordination of the study team • Oversight of quality and timelines • Risk assessment • Vendor management • Study set-up www. allied-clinical. com 9
DO IT RIGHT THE FIRST TIME! Study Management • Oversight of study conduct and study monitoring • Budget management and tracking • Primary contact for the sponsor • Coordination of the study team • Oversight of quality and timelines • Risk assessment • Vendor management • Study set-up www. allied-clinical. com 9
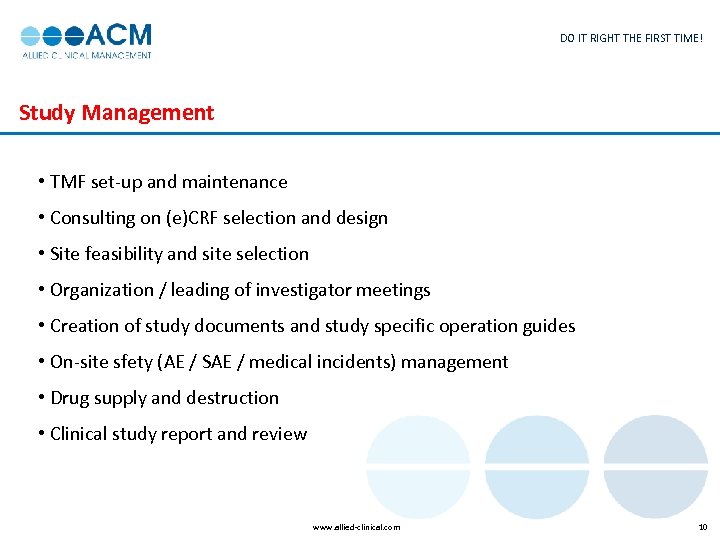 DO IT RIGHT THE FIRST TIME! Study Management • TMF set-up and maintenance • Consulting on (e)CRF selection and design • Site feasibility and site selection • Organization / leading of investigator meetings • Creation of study documents and study specific operation guides • On-site sfety (AE / SAE / medical incidents) management • Drug supply and destruction • Clinical study report and review www. allied-clinical. com 10
DO IT RIGHT THE FIRST TIME! Study Management • TMF set-up and maintenance • Consulting on (e)CRF selection and design • Site feasibility and site selection • Organization / leading of investigator meetings • Creation of study documents and study specific operation guides • On-site sfety (AE / SAE / medical incidents) management • Drug supply and destruction • Clinical study report and review www. allied-clinical. com 10
 DO IT RIGHT THE FIRST TIME! Study Monitoring • Full monitoring-service, on-site and remote • Risk-based monitoring • Site visits (selection, initiation, interim, close-out) • Source data verification • Check of study lists and documents • ISF set-up and maintenance • Training of site staff www. allied-clinical. com 11
DO IT RIGHT THE FIRST TIME! Study Monitoring • Full monitoring-service, on-site and remote • Risk-based monitoring • Site visits (selection, initiation, interim, close-out) • Source data verification • Check of study lists and documents • ISF set-up and maintenance • Training of site staff www. allied-clinical. com 11
 DO IT RIGHT THE FIRST TIME! Study Monitoring • Monitoring compliance with ICH-GCP, study protocol and regulatory requirements • AE / SAE management • Conduct of interim analysis • Query management • Investigational product accounting • Monitoring reports and follow-up letters www. allied-clinical. com 12
DO IT RIGHT THE FIRST TIME! Study Monitoring • Monitoring compliance with ICH-GCP, study protocol and regulatory requirements • AE / SAE management • Conduct of interim analysis • Query management • Investigational product accounting • Monitoring reports and follow-up letters www. allied-clinical. com 12
 DO IT RIGHT THE FIRST TIME! Study Documentation • TMF set-up and maintenace • ISF set-up and maintenance • Consultation on (e)CRF selection and design • Design of study documents (logs, worksheets, manuals, guidelines, • tracking lists etc. ) www. allied-clinical. com 13
DO IT RIGHT THE FIRST TIME! Study Documentation • TMF set-up and maintenace • ISF set-up and maintenance • Consultation on (e)CRF selection and design • Design of study documents (logs, worksheets, manuals, guidelines, • tracking lists etc. ) www. allied-clinical. com 13
 DO IT RIGHT THE FIRST TIME! Regulatory Services • Clinical trial applications • Marketing applications • Medical device applications • Collection of relevant documents • Submission package for study initiation • Submission of updated documents and changes • Completion of checklists • Correspondence with regulatory authorities and ethics committees • Regulatory advice • Quality review www. allied-clinical. com 14
DO IT RIGHT THE FIRST TIME! Regulatory Services • Clinical trial applications • Marketing applications • Medical device applications • Collection of relevant documents • Submission package for study initiation • Submission of updated documents and changes • Completion of checklists • Correspondence with regulatory authorities and ethics committees • Regulatory advice • Quality review www. allied-clinical. com 14
 DO IT RIGHT THE FIRST TIME! Proofreading and Translation • Proofreading and translation of study protocols, patient diaries, patient information and informed consent and other key clinical texts • Native English and German speakers www. allied-clinical. com 15
DO IT RIGHT THE FIRST TIME! Proofreading and Translation • Proofreading and translation of study protocols, patient diaries, patient information and informed consent and other key clinical texts • Native English and German speakers www. allied-clinical. com 15
 DO IT RIGHT THE FIRST TIME! Full Service MEDIS RESEARCH GROUP offers its clients full CRO services In addition to ACM services it offers: • Biostatistics • Data Management • Medical Writing • Pharmacovigilance • Scientific Consulting www. allied-clinical. com 16
DO IT RIGHT THE FIRST TIME! Full Service MEDIS RESEARCH GROUP offers its clients full CRO services In addition to ACM services it offers: • Biostatistics • Data Management • Medical Writing • Pharmacovigilance • Scientific Consulting www. allied-clinical. com 16
 DO IT RIGHT THE FIRST TIME! Experience with Remote Data Capture + Management Systems • Medidata RAVE system • RDC Oracle system • Initiator e. CRF system • Science and technology on efficient information systems and study planning tools (IMPACT, Cognos clinical trial planning system etc. ) and other clinical trial management systems • Cost tracking tools www. allied-clinical. com 17
DO IT RIGHT THE FIRST TIME! Experience with Remote Data Capture + Management Systems • Medidata RAVE system • RDC Oracle system • Initiator e. CRF system • Science and technology on efficient information systems and study planning tools (IMPACT, Cognos clinical trial planning system etc. ) and other clinical trial management systems • Cost tracking tools www. allied-clinical. com 17
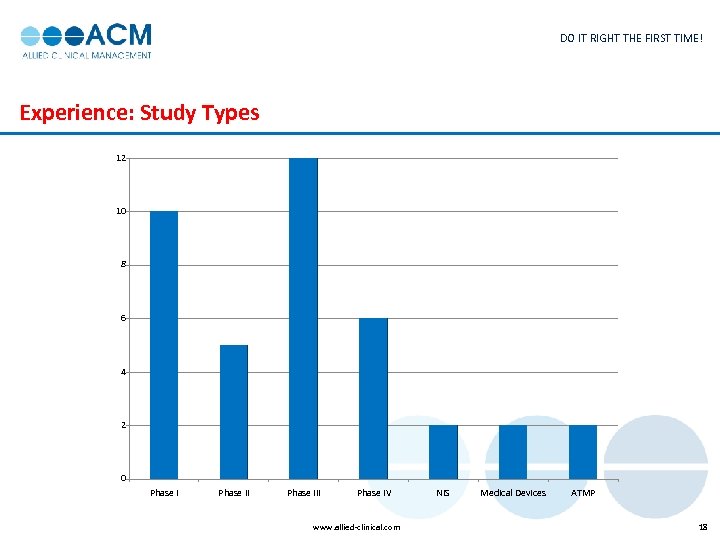 DO IT RIGHT THE FIRST TIME! Experience: Study Types 12 10 8 6 4 2 0 Phase III Phase IV www. allied-clinical. com NIS Medical Devices ATMP 18
DO IT RIGHT THE FIRST TIME! Experience: Study Types 12 10 8 6 4 2 0 Phase III Phase IV www. allied-clinical. com NIS Medical Devices ATMP 18
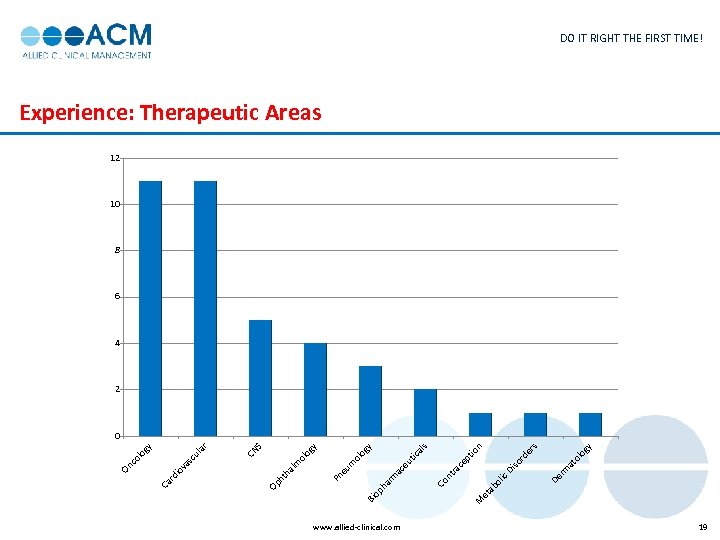 www. allied-clinical. com et M y og ol at s n er rd so Di rm De ic ol ab io pt ce ra s al y og ol eu tic ac nt Co rm ha op Bi m eu Pn y og ol m al ht h Op S CN ar ul sc va io rd Ca y og co l On DO IT RIGHT THE FIRST TIME! Experience: Therapeutic Areas 12 10 8 6 4 2 0 19
www. allied-clinical. com et M y og ol at s n er rd so Di rm De ic ol ab io pt ce ra s al y og ol eu tic ac nt Co rm ha op Bi m eu Pn y og ol m al ht h Op S CN ar ul sc va io rd Ca y og co l On DO IT RIGHT THE FIRST TIME! Experience: Therapeutic Areas 12 10 8 6 4 2 0 19
 DO IT RIGHT THE FIRST TIME! Countries ACM has monitored and performed oversight visits in the following countries: ● Austria ● Israel ● Czech Republic ● Latvia ● France ● Netherlands ● Germany ● Poland ● Great Britain ● Spain ● Hungary ● Switzerland ● Italy www. allied-clinical. com 20
DO IT RIGHT THE FIRST TIME! Countries ACM has monitored and performed oversight visits in the following countries: ● Austria ● Israel ● Czech Republic ● Latvia ● France ● Netherlands ● Germany ● Poland ● Great Britain ● Spain ● Hungary ● Switzerland ● Italy www. allied-clinical. com 20
 DO IT RIGHT THE FIRST TIME! Sponsors and Partners Sponsors ● Bayer Health Care ● GALENpharma ● Bayer Pharma AG ● Miltenyi Biotec Gmb. H ● Bio. MS Medical Corp ● Novartis ● Biotie Therapies ● Pluristem Ltd. ● Elbion AG ● Weleda AG Partners ● ICRC-Weyer (Germany) www. allied-clinical. com 21
DO IT RIGHT THE FIRST TIME! Sponsors and Partners Sponsors ● Bayer Health Care ● GALENpharma ● Bayer Pharma AG ● Miltenyi Biotec Gmb. H ● Bio. MS Medical Corp ● Novartis ● Biotie Therapies ● Pluristem Ltd. ● Elbion AG ● Weleda AG Partners ● ICRC-Weyer (Germany) www. allied-clinical. com 21
 DO IT RIGHT THE FIRST TIME! Contact Allied Clinical Management Gmb. H MEDIS RESEARCH GROUP Berlin office: Boyenstraße 41 10115 Berlin Tel: +49 30 - 240 47 88 0 +49 30 - 240 47 88 22 Fax: +49 30 - 240 47 88 29 22 info@allied-clinical. com info@medis-research. com www. allied-clinical. com 22
DO IT RIGHT THE FIRST TIME! Contact Allied Clinical Management Gmb. H MEDIS RESEARCH GROUP Berlin office: Boyenstraße 41 10115 Berlin Tel: +49 30 - 240 47 88 0 +49 30 - 240 47 88 22 Fax: +49 30 - 240 47 88 29 22 info@allied-clinical. com info@medis-research. com www. allied-clinical. com 22


