922203590a93ba680c3220cf6b8a836f.ppt
- Количество слайдов: 51
 Part II • XAFS: Principles • XANES/NEXAFS • Applications 1
Part II • XAFS: Principles • XANES/NEXAFS • Applications 1
 X-ray Absorption Spectroscopy (XAS) X-ray Absorption spectroscopy is often referred to as - NEXAFS for low Z elements (C, N, O, F, etc. K-edge, Si, P, S, L-edges) or - XAFS (XANES and EXAFS) for intermediate Z and high Z elements. 2
X-ray Absorption Spectroscopy (XAS) X-ray Absorption spectroscopy is often referred to as - NEXAFS for low Z elements (C, N, O, F, etc. K-edge, Si, P, S, L-edges) or - XAFS (XANES and EXAFS) for intermediate Z and high Z elements. 2
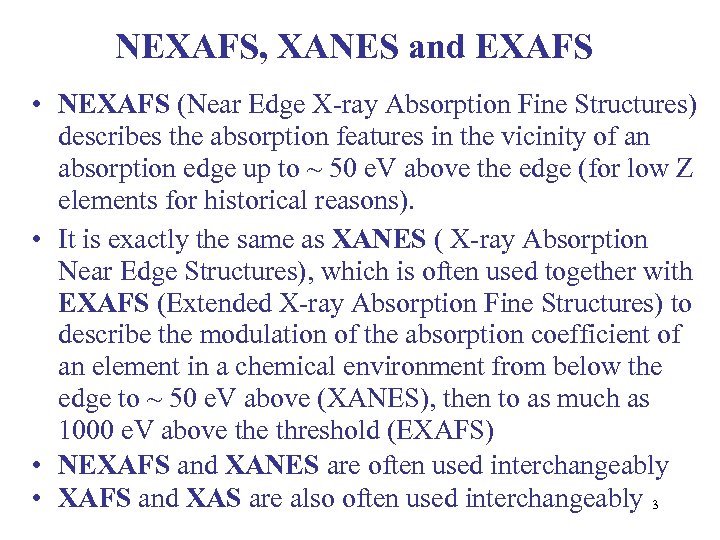 NEXAFS, XANES and EXAFS • NEXAFS (Near Edge X-ray Absorption Fine Structures) describes the absorption features in the vicinity of an absorption edge up to ~ 50 e. V above the edge (for low Z elements for historical reasons). • It is exactly the same as XANES ( X-ray Absorption Near Edge Structures), which is often used together with EXAFS (Extended X-ray Absorption Fine Structures) to describe the modulation of the absorption coefficient of an element in a chemical environment from below the edge to ~ 50 e. V above (XANES), then to as much as 1000 e. V above threshold (EXAFS) • NEXAFS and XANES are often used interchangeably • XAFS and XAS are also often used interchangeably 3
NEXAFS, XANES and EXAFS • NEXAFS (Near Edge X-ray Absorption Fine Structures) describes the absorption features in the vicinity of an absorption edge up to ~ 50 e. V above the edge (for low Z elements for historical reasons). • It is exactly the same as XANES ( X-ray Absorption Near Edge Structures), which is often used together with EXAFS (Extended X-ray Absorption Fine Structures) to describe the modulation of the absorption coefficient of an element in a chemical environment from below the edge to ~ 50 e. V above (XANES), then to as much as 1000 e. V above threshold (EXAFS) • NEXAFS and XANES are often used interchangeably • XAFS and XAS are also often used interchangeably 3
 XAFS of free atom In rare gases, the pre-edge region exhibits a series of sharp peaks arising from bound to bound transitions (dipole: 1 s to np etc. ) called Rydberg transitions 4
XAFS of free atom In rare gases, the pre-edge region exhibits a series of sharp peaks arising from bound to bound transitions (dipole: 1 s to np etc. ) called Rydberg transitions 4
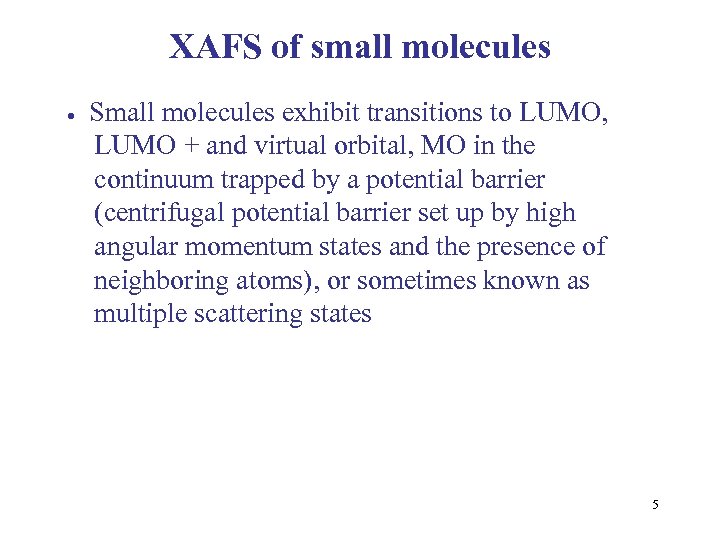 XAFS of small molecules Small molecules exhibit transitions to LUMO, LUMO + and virtual orbital, MO in the continuum trapped by a potential barrier (centrifugal potential barrier set up by high angular momentum states and the presence of neighboring atoms), or sometimes known as multiple scattering states 5
XAFS of small molecules Small molecules exhibit transitions to LUMO, LUMO + and virtual orbital, MO in the continuum trapped by a potential barrier (centrifugal potential barrier set up by high angular momentum states and the presence of neighboring atoms), or sometimes known as multiple scattering states 5
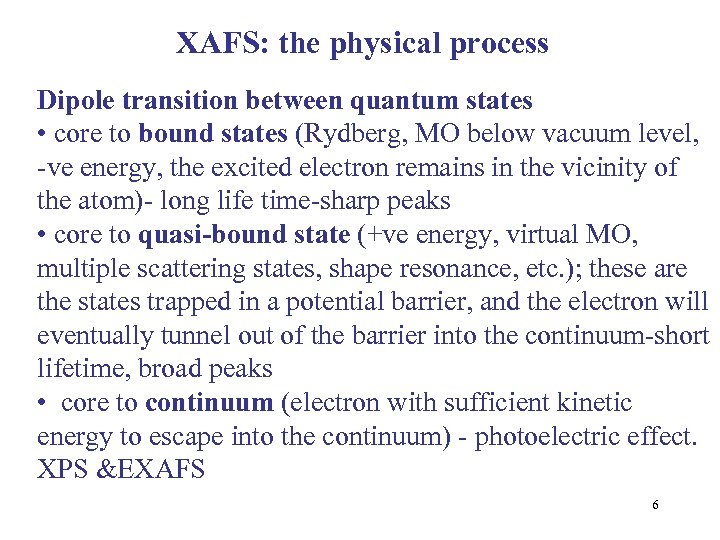 XAFS: the physical process Dipole transition between quantum states • core to bound states (Rydberg, MO below vacuum level, -ve energy, the excited electron remains in the vicinity of the atom)- long life time-sharp peaks • core to quasi-bound state (+ve energy, virtual MO, multiple scattering states, shape resonance, etc. ); these are the states trapped in a potential barrier, and the electron will eventually tunnel out of the barrier into the continuum-short lifetime, broad peaks • core to continuum (electron with sufficient kinetic energy to escape into the continuum) - photoelectric effect. XPS &EXAFS 6
XAFS: the physical process Dipole transition between quantum states • core to bound states (Rydberg, MO below vacuum level, -ve energy, the excited electron remains in the vicinity of the atom)- long life time-sharp peaks • core to quasi-bound state (+ve energy, virtual MO, multiple scattering states, shape resonance, etc. ); these are the states trapped in a potential barrier, and the electron will eventually tunnel out of the barrier into the continuum-short lifetime, broad peaks • core to continuum (electron with sufficient kinetic energy to escape into the continuum) - photoelectric effect. XPS &EXAFS 6
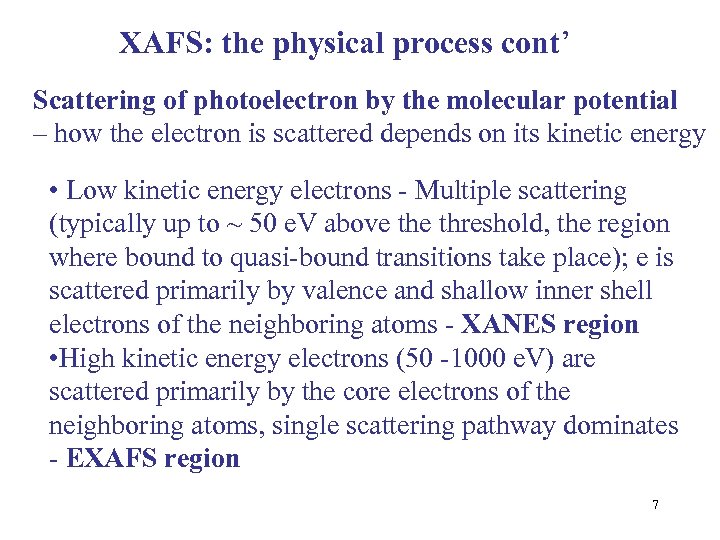 XAFS: the physical process cont’ Scattering of photoelectron by the molecular potential – how the electron is scattered depends on its kinetic energy • Low kinetic energy electrons - Multiple scattering (typically up to ~ 50 e. V above threshold, the region where bound to quasi-bound transitions take place); e is scattered primarily by valence and shallow inner shell electrons of the neighboring atoms - XANES region • High kinetic energy electrons (50 -1000 e. V) are scattered primarily by the core electrons of the neighboring atoms, single scattering pathway dominates - EXAFS region 7
XAFS: the physical process cont’ Scattering of photoelectron by the molecular potential – how the electron is scattered depends on its kinetic energy • Low kinetic energy electrons - Multiple scattering (typically up to ~ 50 e. V above threshold, the region where bound to quasi-bound transitions take place); e is scattered primarily by valence and shallow inner shell electrons of the neighboring atoms - XANES region • High kinetic energy electrons (50 -1000 e. V) are scattered primarily by the core electrons of the neighboring atoms, single scattering pathway dominates - EXAFS region 7
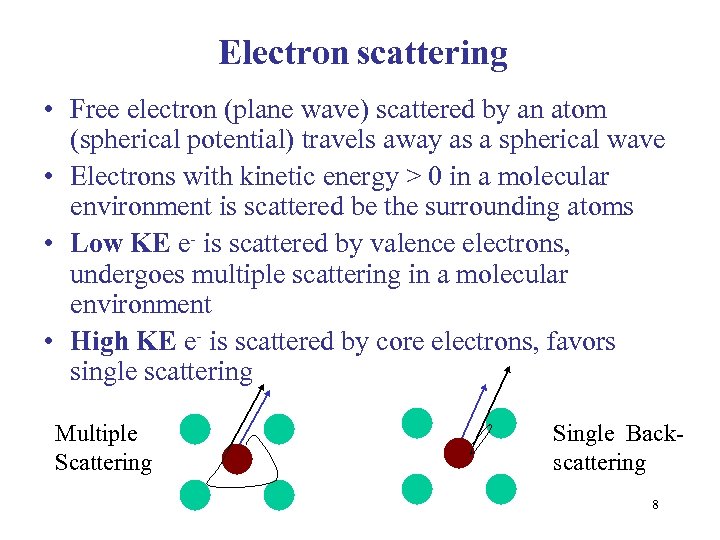 Electron scattering • Free electron (plane wave) scattered by an atom (spherical potential) travels away as a spherical wave • Electrons with kinetic energy > 0 in a molecular environment is scattered be the surrounding atoms • Low KE e- is scattered by valence electrons, undergoes multiple scattering in a molecular environment • High KE e- is scattered by core electrons, favors single scattering Multiple Scattering Single Backscattering 8
Electron scattering • Free electron (plane wave) scattered by an atom (spherical potential) travels away as a spherical wave • Electrons with kinetic energy > 0 in a molecular environment is scattered be the surrounding atoms • Low KE e- is scattered by valence electrons, undergoes multiple scattering in a molecular environment • High KE e- is scattered by core electrons, favors single scattering Multiple Scattering Single Backscattering 8
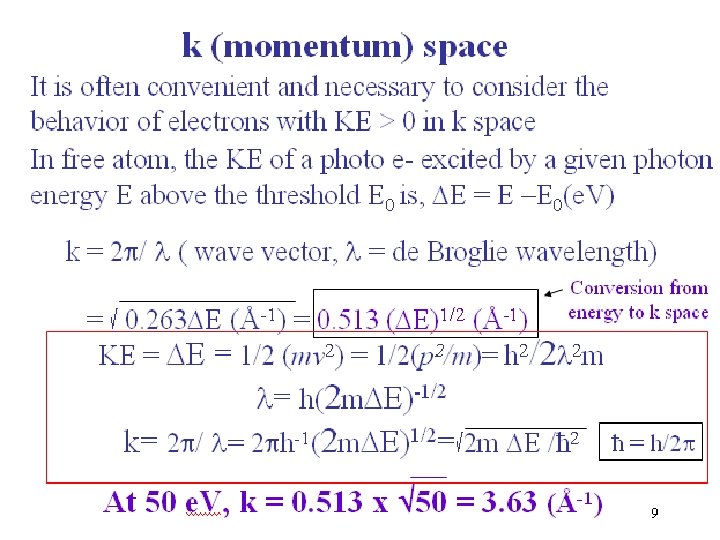 9
9
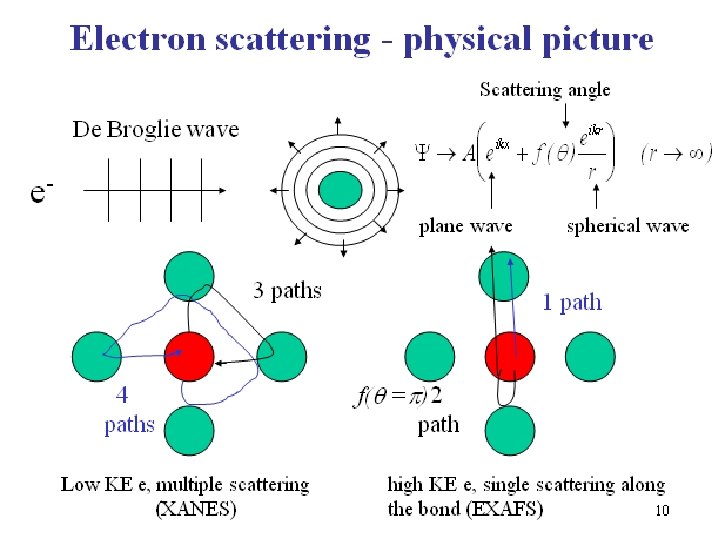 10
10
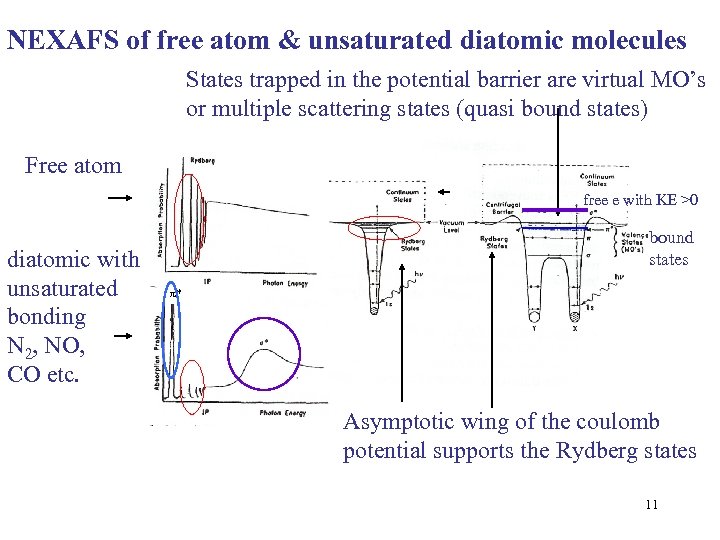 NEXAFS of free atom & unsaturated diatomic molecules States trapped in the potential barrier are virtual MO’s or multiple scattering states (quasi bound states) Free atom free e with KE >0 diatomic with unsaturated bonding N 2, NO, CO etc. bound states * Asymptotic wing of the coulomb potential supports the Rydberg states 11
NEXAFS of free atom & unsaturated diatomic molecules States trapped in the potential barrier are virtual MO’s or multiple scattering states (quasi bound states) Free atom free e with KE >0 diatomic with unsaturated bonding N 2, NO, CO etc. bound states * Asymptotic wing of the coulomb potential supports the Rydberg states 11
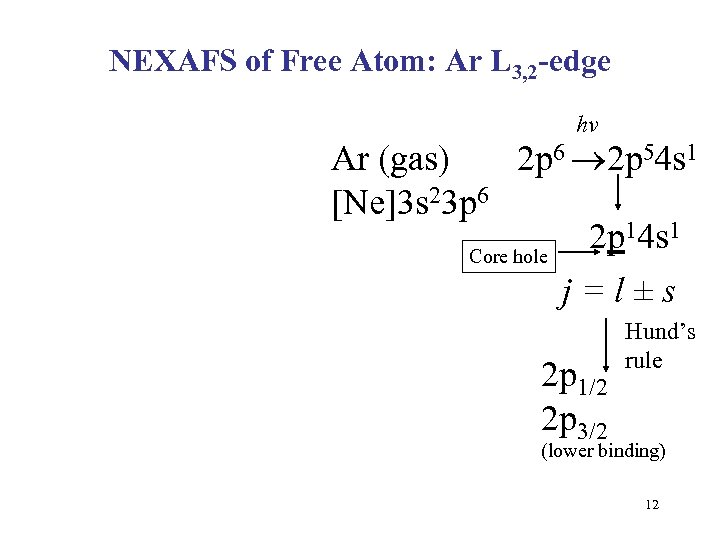 NEXAFS of Free Atom: Ar L 3, 2 -edge hv Ar (gas) 2 p 6 2 p 54 s 1 [Ne]3 s 23 p 6 2 p 14 s 1 Core hole j=l±s 2 p 1/2 2 p 3/2 Hund’s rule (lower binding) 12
NEXAFS of Free Atom: Ar L 3, 2 -edge hv Ar (gas) 2 p 6 2 p 54 s 1 [Ne]3 s 23 p 6 2 p 14 s 1 Core hole j=l±s 2 p 1/2 2 p 3/2 Hund’s rule (lower binding) 12
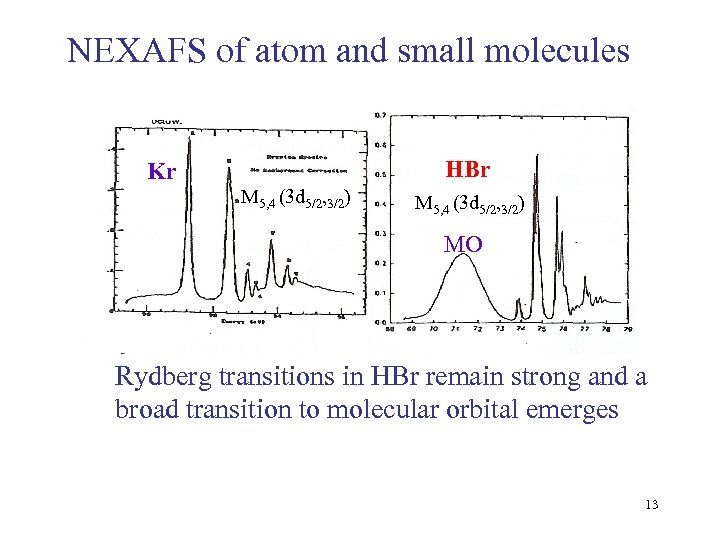 NEXAFS of atom and small molecules HBr Kr M 5, 4 (3 d 5/2, 3/2) MO Rydberg transitions in HBr remain strong and a broad transition to molecular orbital emerges 13
NEXAFS of atom and small molecules HBr Kr M 5, 4 (3 d 5/2, 3/2) MO Rydberg transitions in HBr remain strong and a broad transition to molecular orbital emerges 13
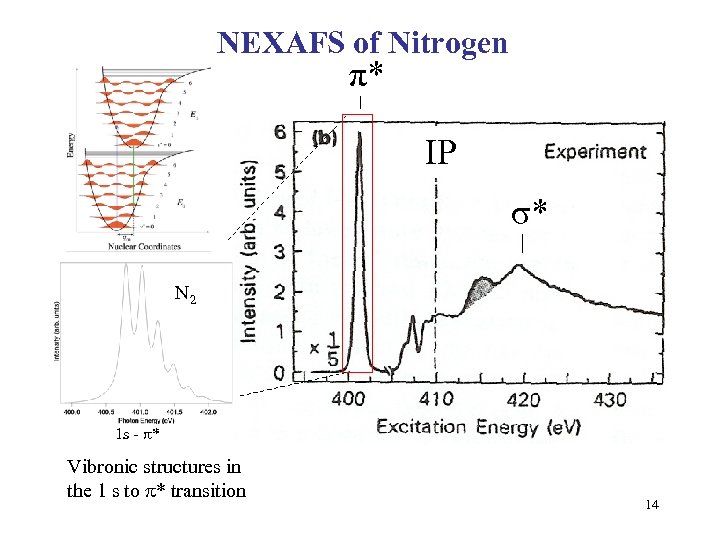 NEXAFS of Nitrogen π* IP σ* N 2 1 s - * Vibronic structures in the 1 s to * transition 14
NEXAFS of Nitrogen π* IP σ* N 2 1 s - * Vibronic structures in the 1 s to * transition 14
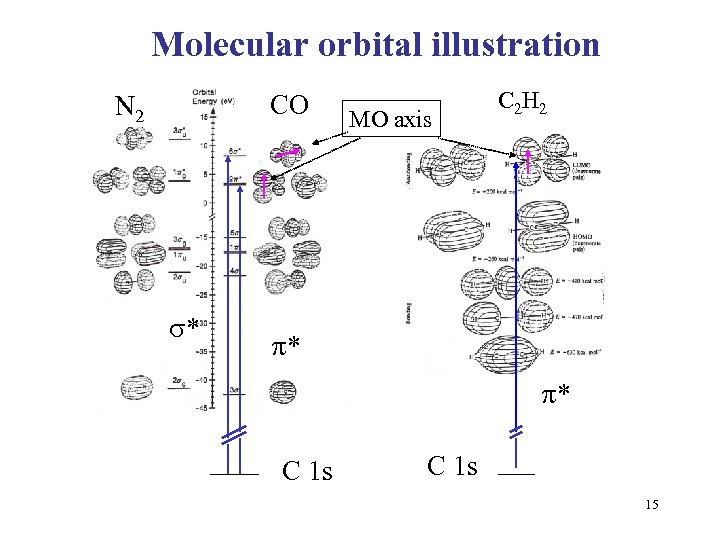 Molecular orbital illustration CO N 2 * MO axis C 2 H 2 * * C 1 s 15
Molecular orbital illustration CO N 2 * MO axis C 2 H 2 * * C 1 s 15
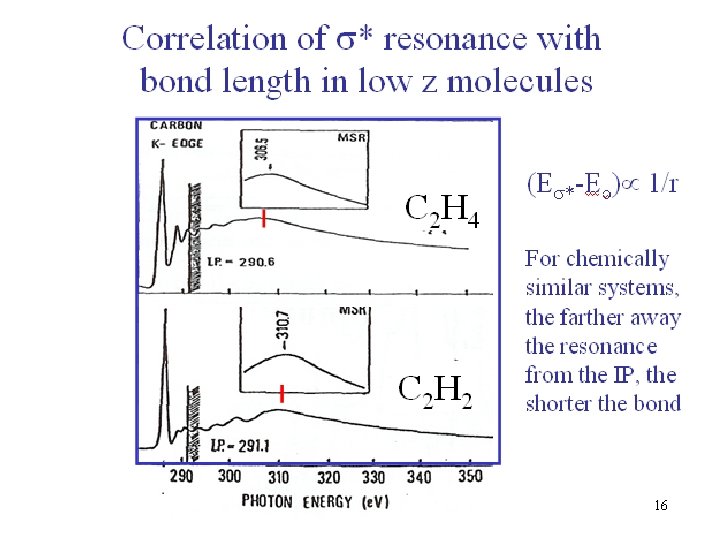 16
16
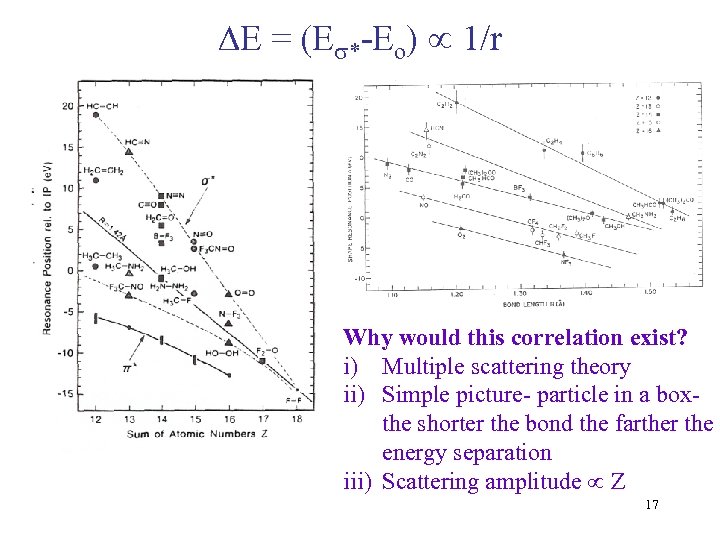 E = (E *-Eo) 1/r Why would this correlation exist? i) Multiple scattering theory ii) Simple picture- particle in a boxthe shorter the bond the farther the energy separation iii) Scattering amplitude Z 17
E = (E *-Eo) 1/r Why would this correlation exist? i) Multiple scattering theory ii) Simple picture- particle in a boxthe shorter the bond the farther the energy separation iii) Scattering amplitude Z 17
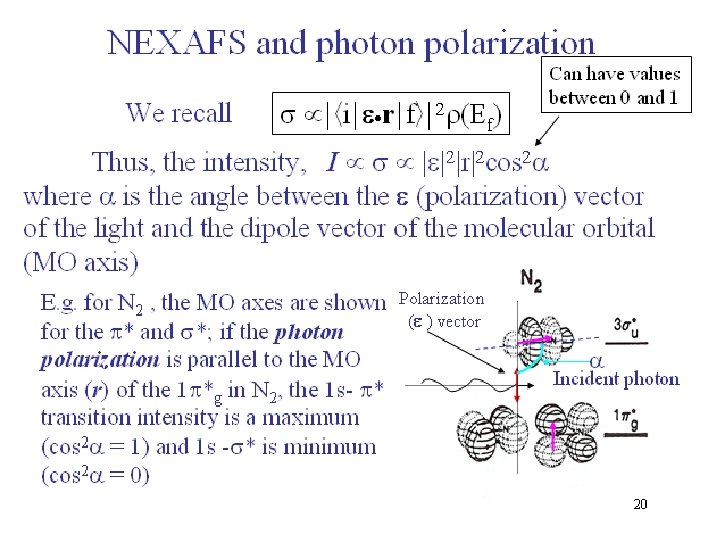 18
18
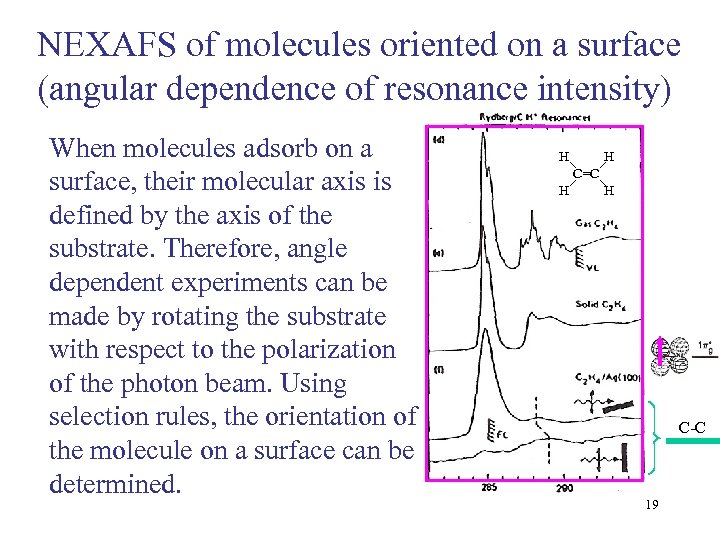 NEXAFS of molecules oriented on a surface (angular dependence of resonance intensity) When molecules adsorb on a surface, their molecular axis is defined by the axis of the substrate. Therefore, angle dependent experiments can be made by rotating the substrate with respect to the polarization of the photon beam. Using selection rules, the orientation of the molecule on a surface can be determined. H H C=C H H C-C 19
NEXAFS of molecules oriented on a surface (angular dependence of resonance intensity) When molecules adsorb on a surface, their molecular axis is defined by the axis of the substrate. Therefore, angle dependent experiments can be made by rotating the substrate with respect to the polarization of the photon beam. Using selection rules, the orientation of the molecule on a surface can be determined. H H C=C H H C-C 19
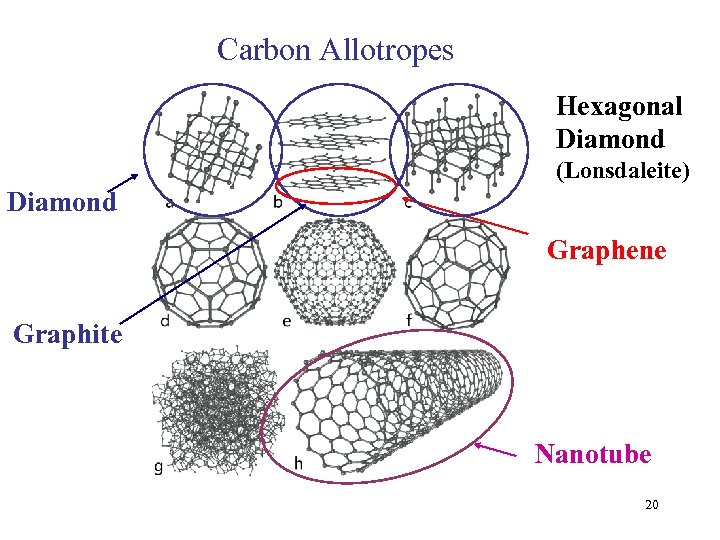 Carbon Allotropes Hexagonal Diamond (Lonsdaleite) Diamond Graphene Graphite Nanotube 20
Carbon Allotropes Hexagonal Diamond (Lonsdaleite) Diamond Graphene Graphite Nanotube 20
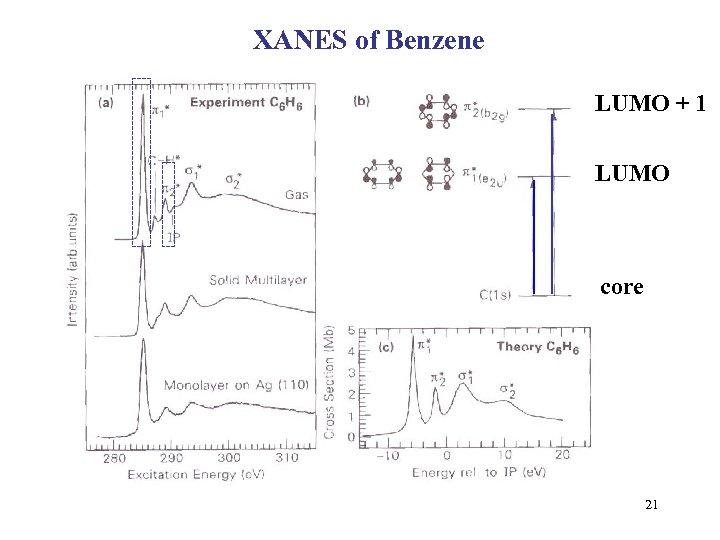 XANES of Benzene LUMO + 1 LUMO core 21
XANES of Benzene LUMO + 1 LUMO core 21
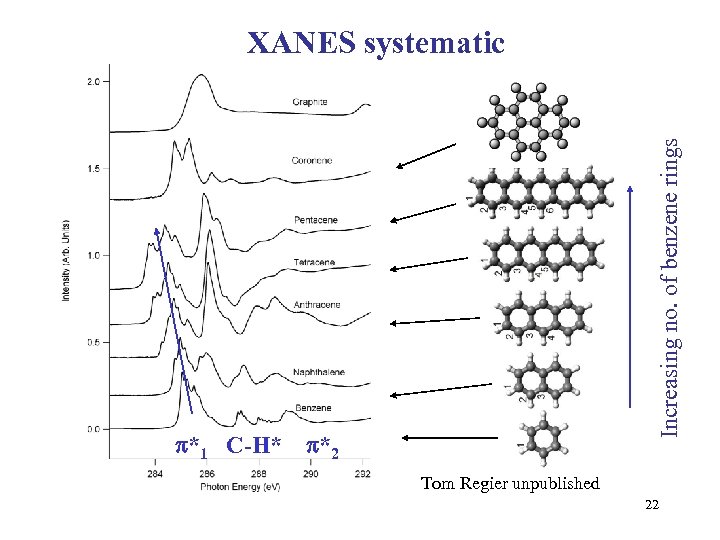 *1 C-H* Increasing no. of benzene rings XANES systematic *2 Tom Regier unpublished 22
*1 C-H* Increasing no. of benzene rings XANES systematic *2 Tom Regier unpublished 22
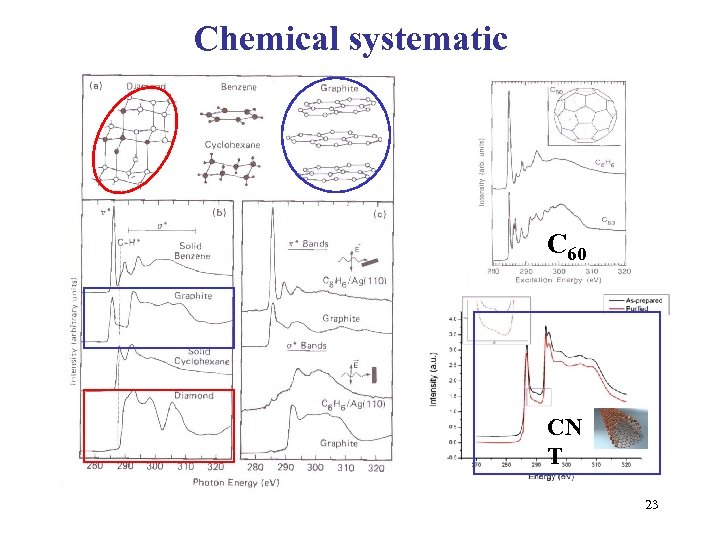 Chemical systematic C 60 CN T 23
Chemical systematic C 60 CN T 23
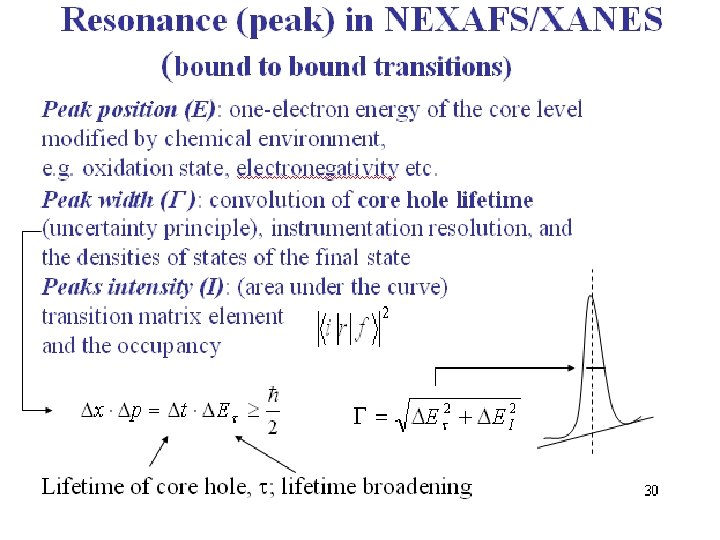 24
24
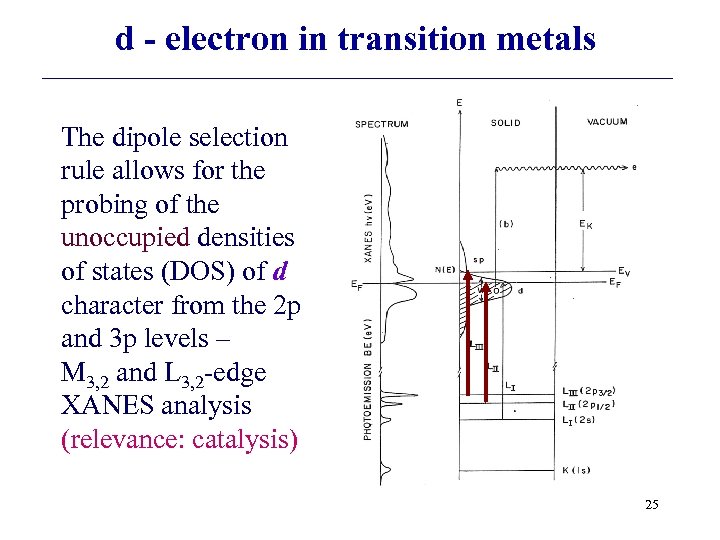 d - electron in transition metals The dipole selection rule allows for the probing of the unoccupied densities of states (DOS) of d character from the 2 p and 3 p levels – M 3, 2 and L 3, 2 -edge XANES analysis (relevance: catalysis) 25
d - electron in transition metals The dipole selection rule allows for the probing of the unoccupied densities of states (DOS) of d character from the 2 p and 3 p levels – M 3, 2 and L 3, 2 -edge XANES analysis (relevance: catalysis) 25
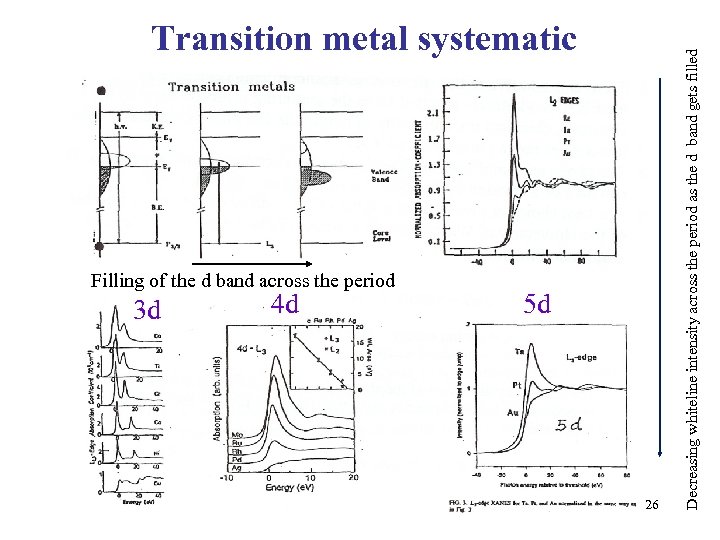 Filling of the d band across the period 3 d 4 d 5 d 26 Decreasing whiteline intensity across the period as the d band gets filled Transition metal systematic
Filling of the d band across the period 3 d 4 d 5 d 26 Decreasing whiteline intensity across the period as the d band gets filled Transition metal systematic
 L 3, 2/M 3, 2 Whiteline and unoccupied densities of d states • 3 d metal: the L-edge WL for early 3 d metals is most complex due to the proximity of the L 3, L 2 edges as well as crystal field effect; the 3 d spin-orbit is negligible • 4 d metals: the L 3, L 2 edges are further apart, WL intensity is a good measure of 4 d hole population • 5 d metals: The L 3 and L 2 are well separated but the spin orbit splitting of the 5 d orbital becomes important, j is a better quantum number than l, WL intensity is a good measure of d 5/3 and d 3/2 hole populations 27
L 3, 2/M 3, 2 Whiteline and unoccupied densities of d states • 3 d metal: the L-edge WL for early 3 d metals is most complex due to the proximity of the L 3, L 2 edges as well as crystal field effect; the 3 d spin-orbit is negligible • 4 d metals: the L 3, L 2 edges are further apart, WL intensity is a good measure of 4 d hole population • 5 d metals: The L 3 and L 2 are well separated but the spin orbit splitting of the 5 d orbital becomes important, j is a better quantum number than l, WL intensity is a good measure of d 5/3 and d 3/2 hole populations 27
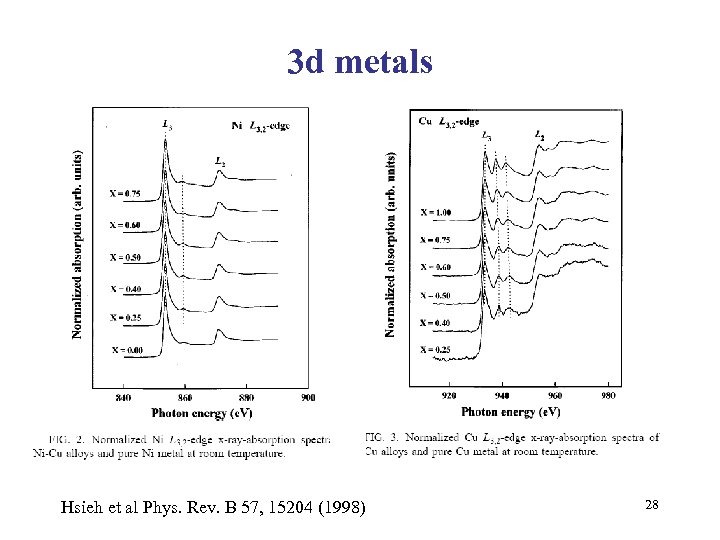 3 d metals Hsieh et al Phys. Rev. B 57, 15204 (1998) 28
3 d metals Hsieh et al Phys. Rev. B 57, 15204 (1998) 28
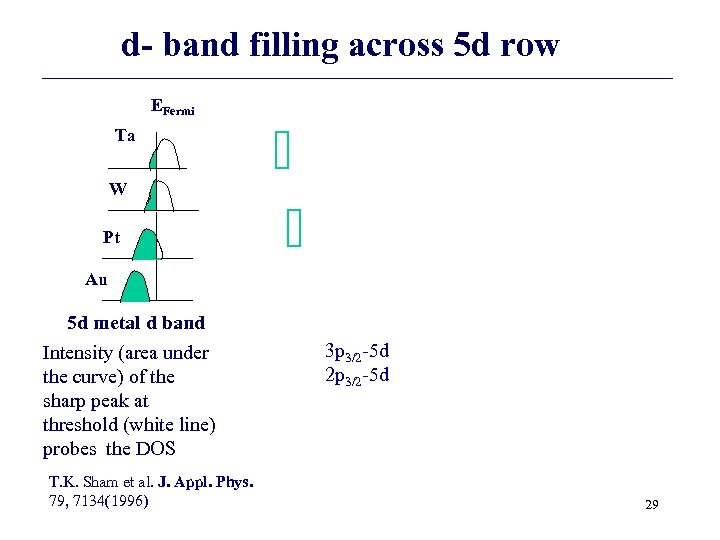 d- band filling across 5 d row EFermi Ta W Pt Au 5 d metal d band Intensity (area under the curve) of the sharp peak at threshold (white line) probes the DOS T. K. Sham et al. J. Appl. Phys. 79, 7134(1996) 3 p 3/2 -5 d 29
d- band filling across 5 d row EFermi Ta W Pt Au 5 d metal d band Intensity (area under the curve) of the sharp peak at threshold (white line) probes the DOS T. K. Sham et al. J. Appl. Phys. 79, 7134(1996) 3 p 3/2 -5 d 29
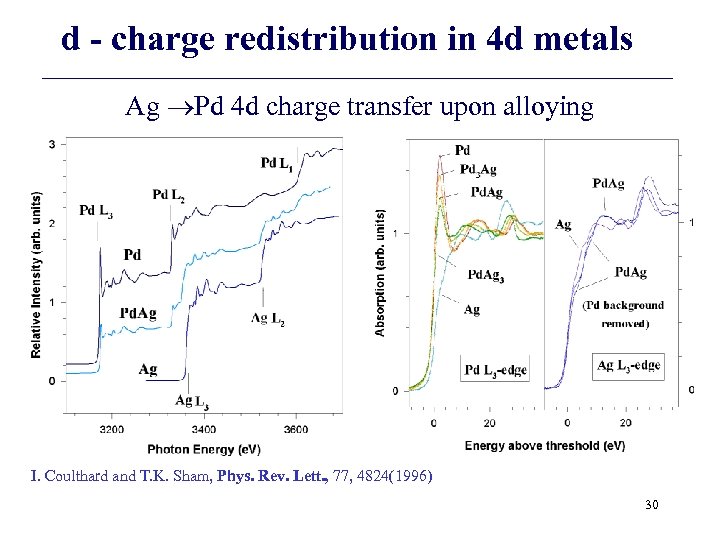 d - charge redistribution in 4 d metals Ag Pd 4 d charge transfer upon alloying I. Coulthard and T. K. Sham, Phys. Rev. Lett. , 77, 4824(1996) 30
d - charge redistribution in 4 d metals Ag Pd 4 d charge transfer upon alloying I. Coulthard and T. K. Sham, Phys. Rev. Lett. , 77, 4824(1996) 30
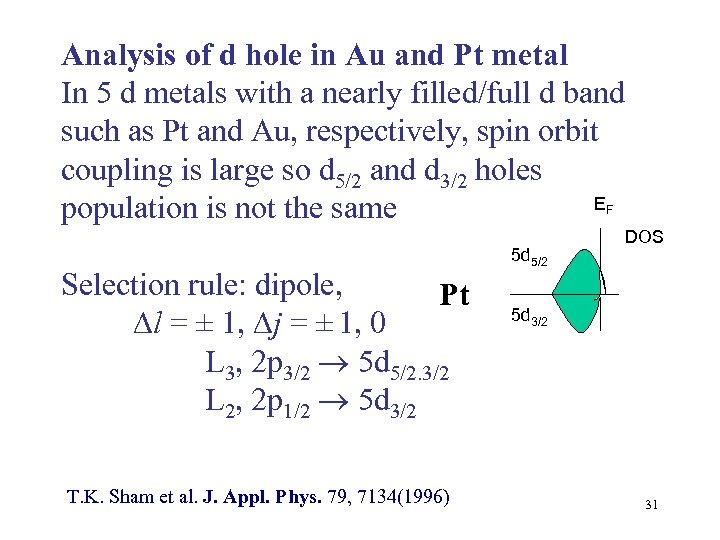 Analysis of d hole in Au and Pt metal In 5 d metals with a nearly filled/full d band such as Pt and Au, respectively, spin orbit coupling is large so d 5/2 and d 3/2 holes E population is not the same F Selection rule: dipole, Pt l = ± 1, j = ± 1, 0 L 3, 2 p 3/2 5 d 5/2. 3/2 L 2, 2 p 1/2 5 d 3/2 T. K. Sham et al. J. Appl. Phys. 79, 7134(1996) 5 d 5/2 DOS 5 d 3/2 31
Analysis of d hole in Au and Pt metal In 5 d metals with a nearly filled/full d band such as Pt and Au, respectively, spin orbit coupling is large so d 5/2 and d 3/2 holes E population is not the same F Selection rule: dipole, Pt l = ± 1, j = ± 1, 0 L 3, 2 p 3/2 5 d 5/2. 3/2 L 2, 2 p 1/2 5 d 3/2 T. K. Sham et al. J. Appl. Phys. 79, 7134(1996) 5 d 5/2 DOS 5 d 3/2 31
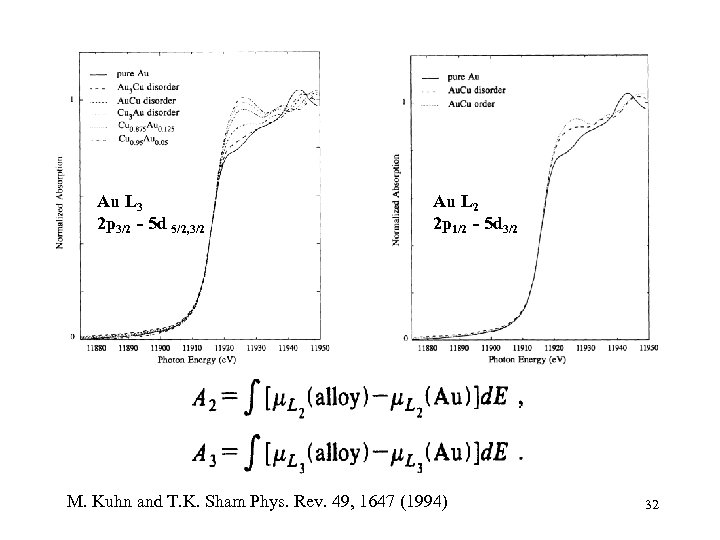 Au L 3 2 p 3/2 - 5 d 5/2, 3/2 Au L 2 2 p 1/2 - 5 d 3/2 M. Kuhn and T. K. Sham Phys. Rev. 49, 1647 (1994) 32
Au L 3 2 p 3/2 - 5 d 5/2, 3/2 Au L 2 2 p 1/2 - 5 d 3/2 M. Kuhn and T. K. Sham Phys. Rev. 49, 1647 (1994) 32
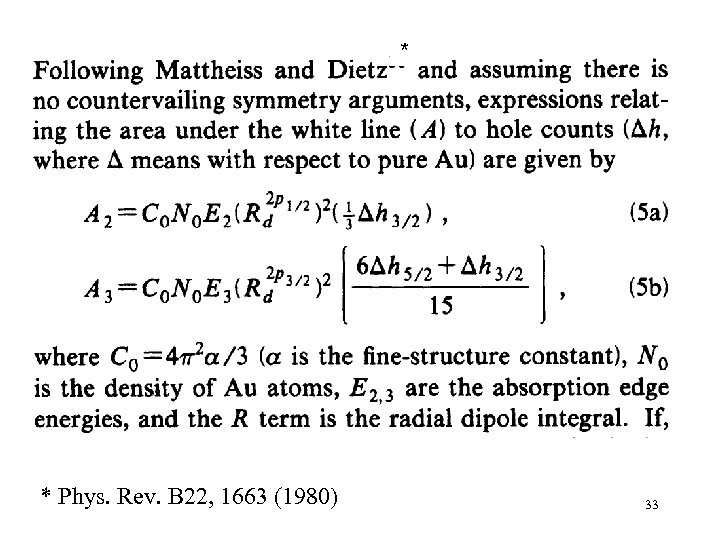 * * Phys. Rev. B 22, 1663 (1980) 33
* * Phys. Rev. B 22, 1663 (1980) 33
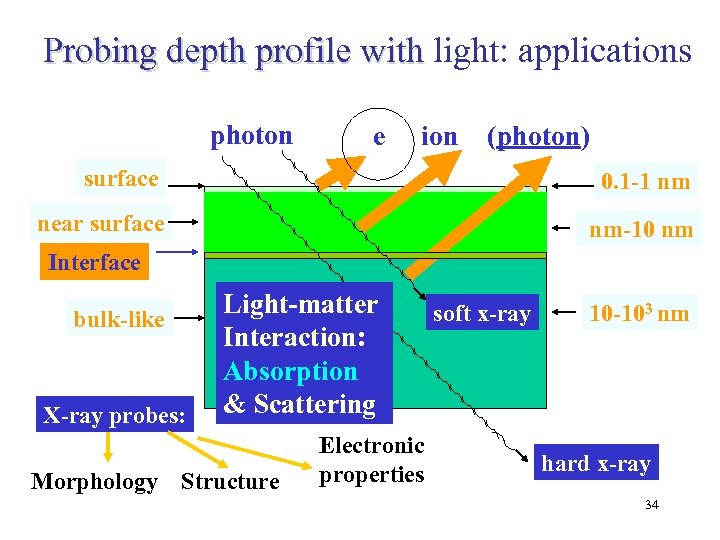 Probing depth profile with light: applications photon e ion (photon) surface 0. 1 -1 nm near surface nm-10 nm Interface bulk-like X-ray probes: Light-matter Interaction: Absorption & Scattering Morphology Structure Electronic properties soft x-ray 10 -103 nm hard x-ray 34
Probing depth profile with light: applications photon e ion (photon) surface 0. 1 -1 nm near surface nm-10 nm Interface bulk-like X-ray probes: Light-matter Interaction: Absorption & Scattering Morphology Structure Electronic properties soft x-ray 10 -103 nm hard x-ray 34
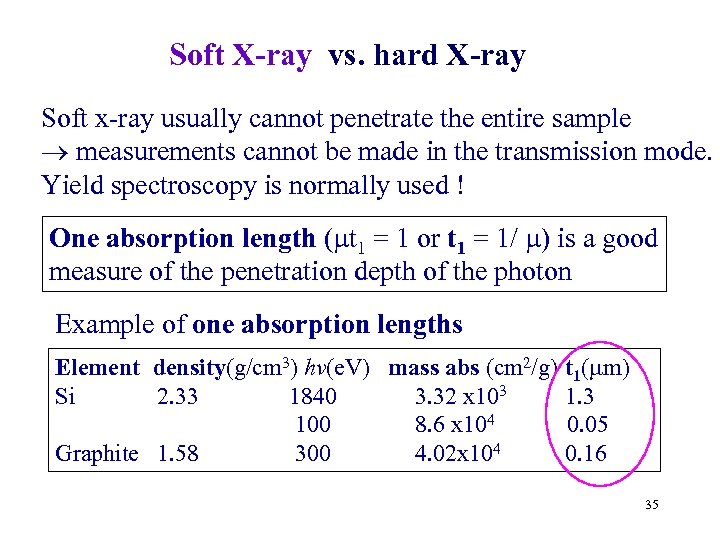 Soft X-ray vs. hard X-ray Soft x-ray usually cannot penetrate the entire sample measurements cannot be made in the transmission mode. Yield spectroscopy is normally used ! One absorption length ( t 1 = 1 or t 1 = 1/ ) is a good measure of the penetration depth of the photon Example of one absorption lengths Element density(g/cm 3) hv(e. V) mass abs (cm 2/g) Si 2. 33 1840 3. 32 x 103 100 8. 6 x 104 Graphite 1. 58 300 4. 02 x 104 t 1( m) 1. 3 0. 05 0. 16 35
Soft X-ray vs. hard X-ray Soft x-ray usually cannot penetrate the entire sample measurements cannot be made in the transmission mode. Yield spectroscopy is normally used ! One absorption length ( t 1 = 1 or t 1 = 1/ ) is a good measure of the penetration depth of the photon Example of one absorption lengths Element density(g/cm 3) hv(e. V) mass abs (cm 2/g) Si 2. 33 1840 3. 32 x 103 100 8. 6 x 104 Graphite 1. 58 300 4. 02 x 104 t 1( m) 1. 3 0. 05 0. 16 35
 Soft x-ray spectroscopy: unique features • XAFS measurement in the soft x-ray (short absorption length) region is often made using yield spectroscopy Electron yield (total, partial, Auger) X-ray fluorescence yield (total, selected wavelength) Photoluminescence yield (visible, light emitting materials) XEOL (X-ray excited optical luminescence) and TRXEOL (Time-resolved X-ray Excited Optical Luminescence) • Since soft X - ray associates with the excitation of shallow core levels, the near-edge region (XANES/ NEXAFS) is the focus of interest for low z elements and shallow cores 36
Soft x-ray spectroscopy: unique features • XAFS measurement in the soft x-ray (short absorption length) region is often made using yield spectroscopy Electron yield (total, partial, Auger) X-ray fluorescence yield (total, selected wavelength) Photoluminescence yield (visible, light emitting materials) XEOL (X-ray excited optical luminescence) and TRXEOL (Time-resolved X-ray Excited Optical Luminescence) • Since soft X - ray associates with the excitation of shallow core levels, the near-edge region (XANES/ NEXAFS) is the focus of interest for low z elements and shallow cores 36
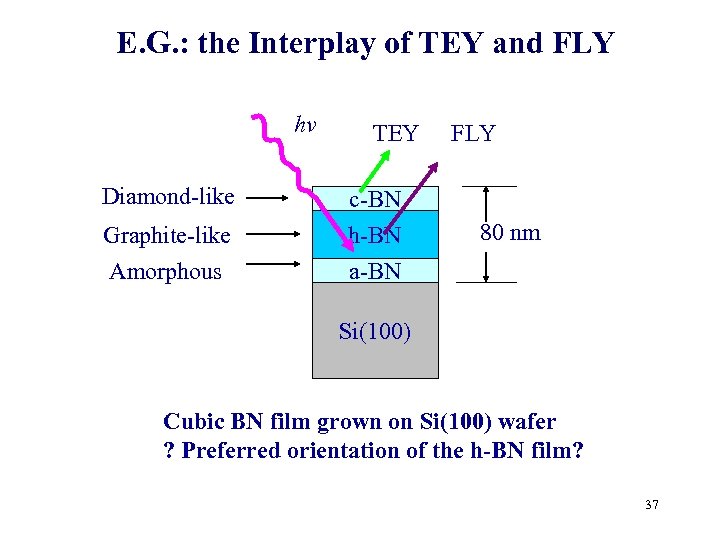 E. G. : the Interplay of TEY and FLY hv Diamond-like Graphite-like Amorphous TEY c-BN h-BN FLY 80 nm a-BN Si(100) Cubic BN film grown on Si(100) wafer ? Preferred orientation of the h-BN film? 37
E. G. : the Interplay of TEY and FLY hv Diamond-like Graphite-like Amorphous TEY c-BN h-BN FLY 80 nm a-BN Si(100) Cubic BN film grown on Si(100) wafer ? Preferred orientation of the h-BN film? 37
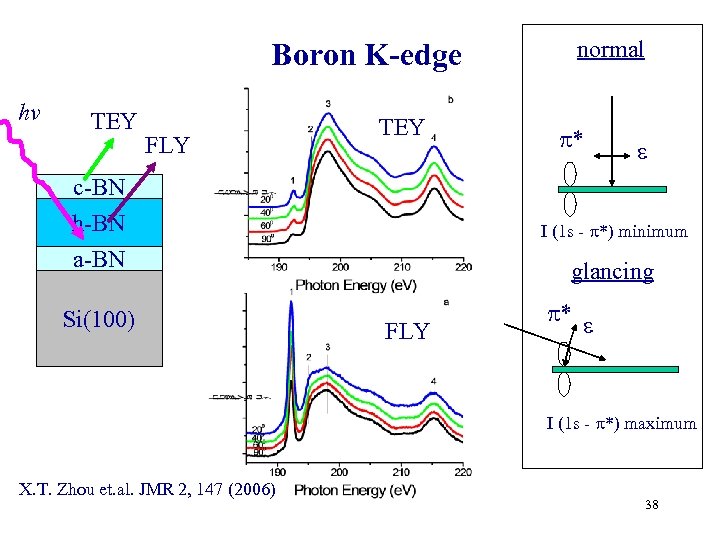 normal Boron K-edge hv TEY FLY TEY c-BN h-BN a-BN Si(100) * I (1 s - *) minimum glancing FLY * I (1 s - *) maximum X. T. Zhou et. al. JMR 2, 147 (2006) 38
normal Boron K-edge hv TEY FLY TEY c-BN h-BN a-BN Si(100) * I (1 s - *) minimum glancing FLY * I (1 s - *) maximum X. T. Zhou et. al. JMR 2, 147 (2006) 38
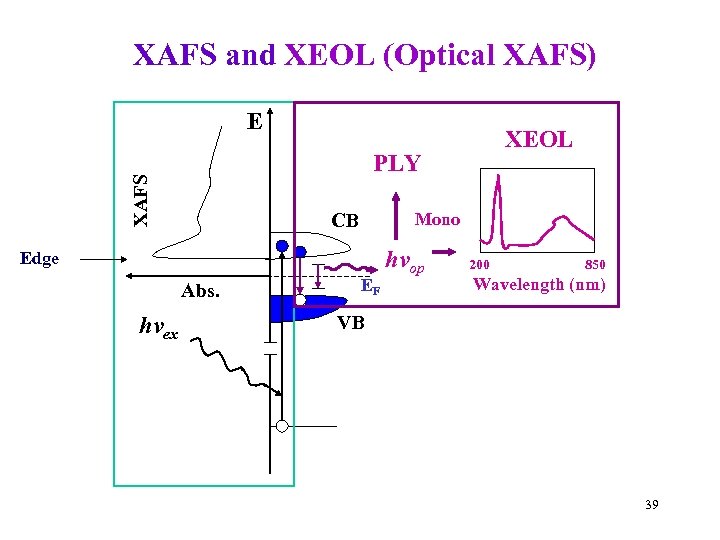 XAFS and XEOL (Optical XAFS) E XAFS PLY Mono CB Edge Abs. hvex XEOL EF hvop 200 850 Wavelength (nm) VB 39
XAFS and XEOL (Optical XAFS) E XAFS PLY Mono CB Edge Abs. hvex XEOL EF hvop 200 850 Wavelength (nm) VB 39
 XEOL - Energy Domain • X-ray photons in, optical photons out Illustrations BN nanowires Si nanowires Zn. S nanoribbons 40 40
XEOL - Energy Domain • X-ray photons in, optical photons out Illustrations BN nanowires Si nanowires Zn. S nanoribbons 40 40
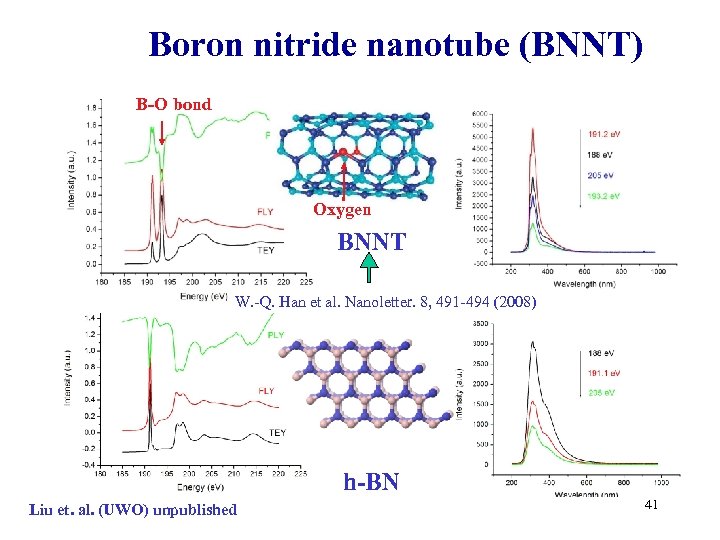 Boron nitride nanotube (BNNT) B-O bond Oxygen BNNT W. -Q. Han et al. Nanoletter. 8, 491 -494 (2008) h-BN Liu et. al. (UWO) unpublished 41
Boron nitride nanotube (BNNT) B-O bond Oxygen BNNT W. -Q. Han et al. Nanoletter. 8, 491 -494 (2008) h-BN Liu et. al. (UWO) unpublished 41
 Si K-edge XEOL of silicon nanowires T. K. Sham et al. Phys. Rev. B 70, 045313 (2004) 42 42
Si K-edge XEOL of silicon nanowires T. K. Sham et al. Phys. Rev. B 70, 045313 (2004) 42 42
 Photoluminescence: Si K-edge 43 43
Photoluminescence: Si K-edge 43 43
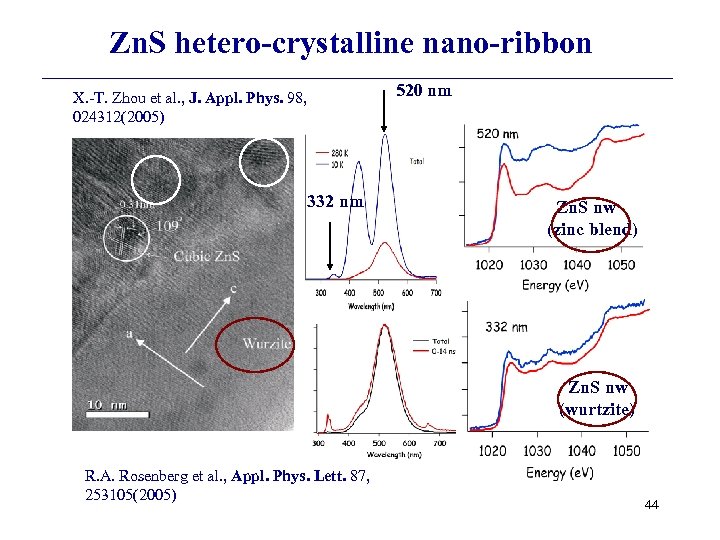 Zn. S hetero-crystalline nano-ribbon X. -T. Zhou et al. , J. Appl. Phys. 98, 024312(2005) 332 nm 520 nm Zn. S nw (zinc blend) Zn. S nw (wurtzite) R. A. Rosenberg et al. , Appl. Phys. Lett. 87, 253105(2005) 44
Zn. S hetero-crystalline nano-ribbon X. -T. Zhou et al. , J. Appl. Phys. 98, 024312(2005) 332 nm 520 nm Zn. S nw (zinc blend) Zn. S nw (wurtzite) R. A. Rosenberg et al. , Appl. Phys. Lett. 87, 253105(2005) 44
 XEOL - Time Domain • Timing - resolved XEOL (TRXEOL) • Illustrations Zn. O: Nanodeedle vs Nanowire Cd. Se-Si: Hetero nanostructures 45 45
XEOL - Time Domain • Timing - resolved XEOL (TRXEOL) • Illustrations Zn. O: Nanodeedle vs Nanowire Cd. Se-Si: Hetero nanostructures 45 45
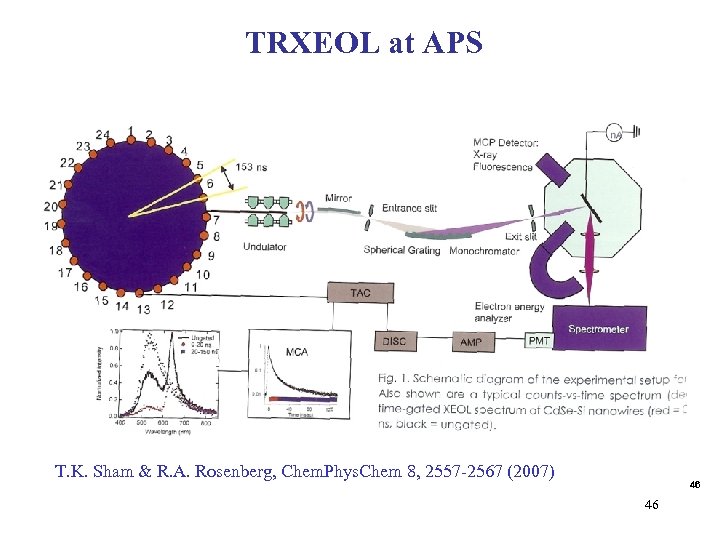 TRXEOL at APS T. K. Sham & R. A. Rosenberg, Chem. Phys. Chem 8, 2557 -2567 (2007) 46 46
TRXEOL at APS T. K. Sham & R. A. Rosenberg, Chem. Phys. Chem 8, 2557 -2567 (2007) 46 46
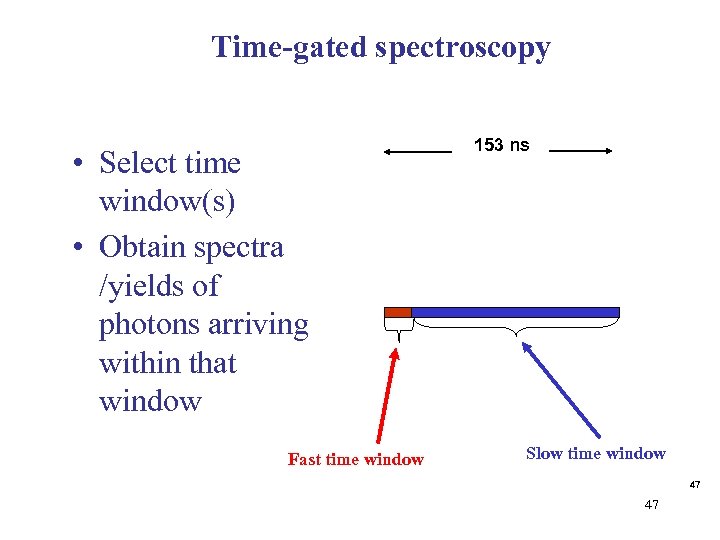 Time-gated spectroscopy • Select time window(s) • Obtain spectra /yields of photons arriving within that window Fast time window 153 ns Slow time window 47 47
Time-gated spectroscopy • Select time window(s) • Obtain spectra /yields of photons arriving within that window Fast time window 153 ns Slow time window 47 47
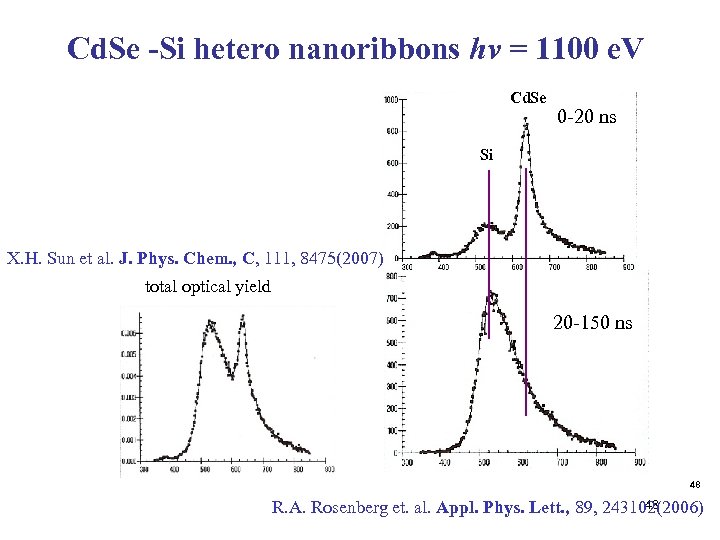 Cd. Se -Si hetero nanoribbons hv = 1100 e. V Cd. Se 0 -20 ns Si X. H. Sun et al. J. Phys. Chem. , C, 111, 8475(2007) total optical yield 20 -150 ns 48 48 R. A. Rosenberg et. al. Appl. Phys. Lett. , 89, 243102(2006)
Cd. Se -Si hetero nanoribbons hv = 1100 e. V Cd. Se 0 -20 ns Si X. H. Sun et al. J. Phys. Chem. , C, 111, 8475(2007) total optical yield 20 -150 ns 48 48 R. A. Rosenberg et. al. Appl. Phys. Lett. , 89, 243102(2006)
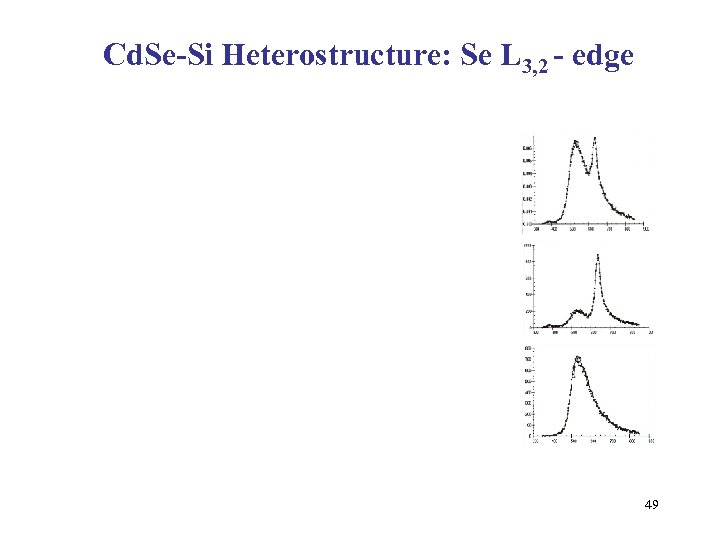 Cd. Se-Si Heterostructure: Se L 3, 2 - edge 49
Cd. Se-Si Heterostructure: Se L 3, 2 - edge 49
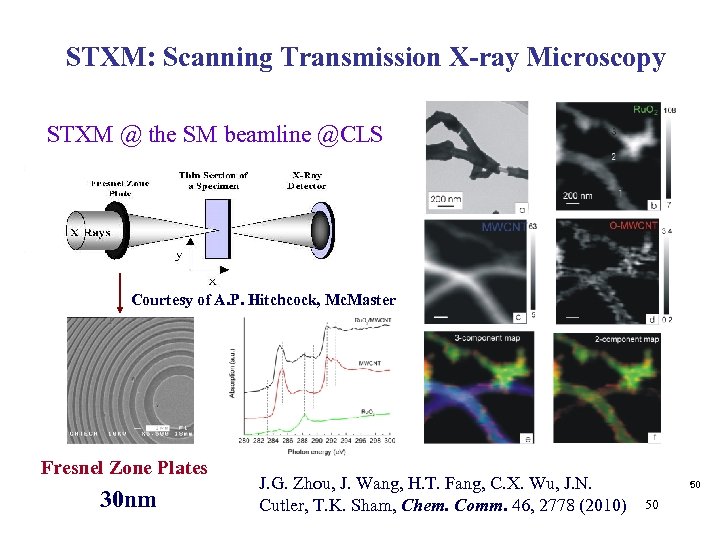 STXM: Scanning Transmission X-ray Microscopy STXM @ the SM beamline @CLS Courtesy of A. P. Hitchcock, Mc. Master Fresnel Zone Plates 30 nm J. G. Zhou, J. Wang, H. T. Fang, C. X. Wu, J. N. Cutler, T. K. Sham, Chem. Comm. 46, 2778 (2010) 50 50
STXM: Scanning Transmission X-ray Microscopy STXM @ the SM beamline @CLS Courtesy of A. P. Hitchcock, Mc. Master Fresnel Zone Plates 30 nm J. G. Zhou, J. Wang, H. T. Fang, C. X. Wu, J. N. Cutler, T. K. Sham, Chem. Comm. 46, 2778 (2010) 50 50
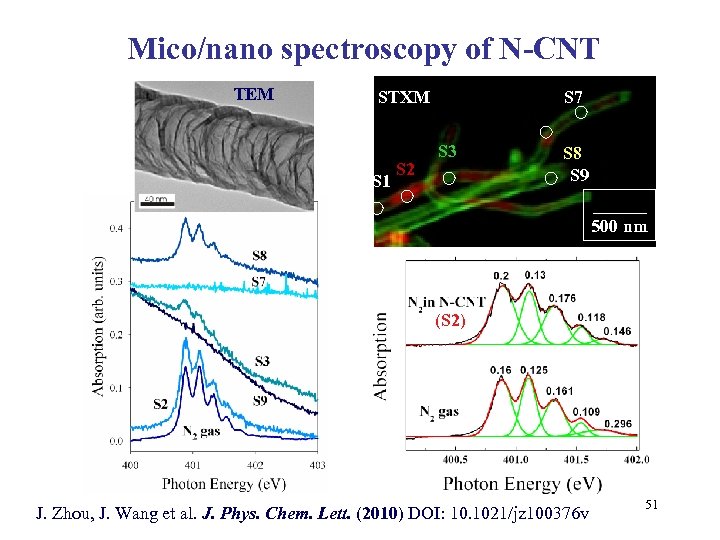 Mico/nano spectroscopy of N-CNT TEM STXM S 1 S 2 S 7 S 3 S 8 S 9 ______ 500 nm (S 2) J. Zhou, J. Wang et al. J. Phys. Chem. Lett. (2010) DOI: 10. 1021/jz 100376 v 51
Mico/nano spectroscopy of N-CNT TEM STXM S 1 S 2 S 7 S 3 S 8 S 9 ______ 500 nm (S 2) J. Zhou, J. Wang et al. J. Phys. Chem. Lett. (2010) DOI: 10. 1021/jz 100376 v 51


