d50eee2d4afb269057682e06cba29704.ppt
- Количество слайдов: 12
 Parstatin: a novel pharmacological target for the development of new agents in the treatment of cardiovascular diseases Nikos E. Tsopanoglou Assistant Professor Department of Pharmacology, Medical School, University of Patras
Parstatin: a novel pharmacological target for the development of new agents in the treatment of cardiovascular diseases Nikos E. Tsopanoglou Assistant Professor Department of Pharmacology, Medical School, University of Patras
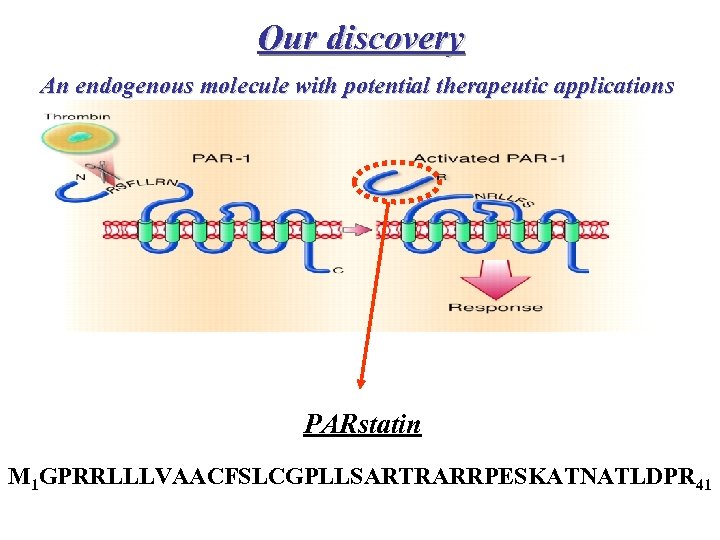 Our discovery An endogenous molecule with potential therapeutic applications Human peptide (MW: 4468 Da) PARstatin M 1 GPRRLLLVAACFSLCGPLLSARTRARRPESKATNATLDPR 41
Our discovery An endogenous molecule with potential therapeutic applications Human peptide (MW: 4468 Da) PARstatin M 1 GPRRLLLVAACFSLCGPLLSARTRARRPESKATNATLDPR 41
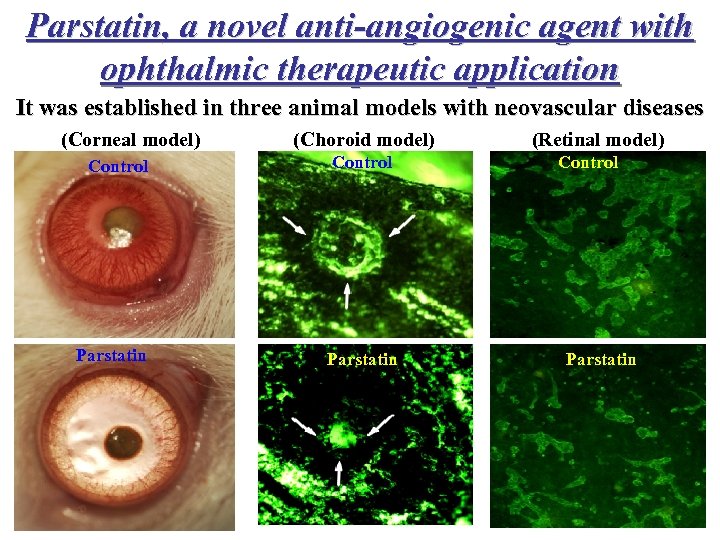 Parstatin, a novel anti-angiogenic agent with ophthalmic therapeutic application It was established in three animal models with neovascular diseases (Corneal model) Control Parstatin (Choroid model) Control Parstatin 2 x 75μg (Retinal model) Control Parstatin 2 x 100μg
Parstatin, a novel anti-angiogenic agent with ophthalmic therapeutic application It was established in three animal models with neovascular diseases (Corneal model) Control Parstatin (Choroid model) Control Parstatin 2 x 75μg (Retinal model) Control Parstatin 2 x 100μg
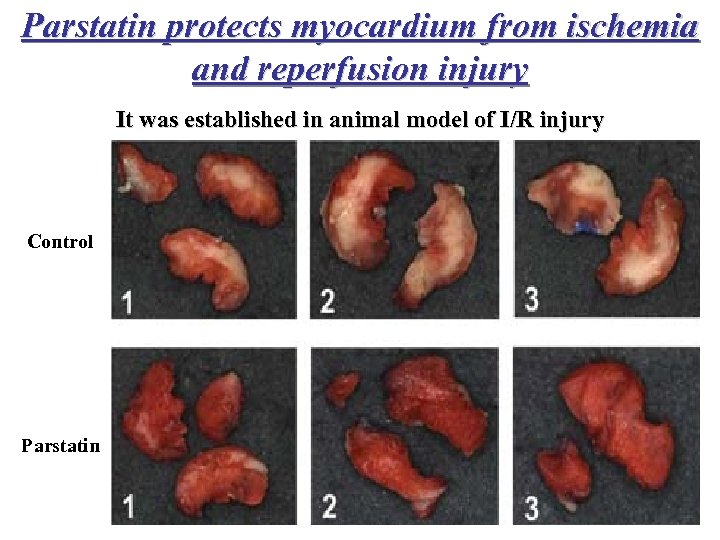 Parstatin protects myocardium from ischemia and reperfusion injury It was established in animal model of I/R injury Control Parstatin
Parstatin protects myocardium from ischemia and reperfusion injury It was established in animal model of I/R injury Control Parstatin
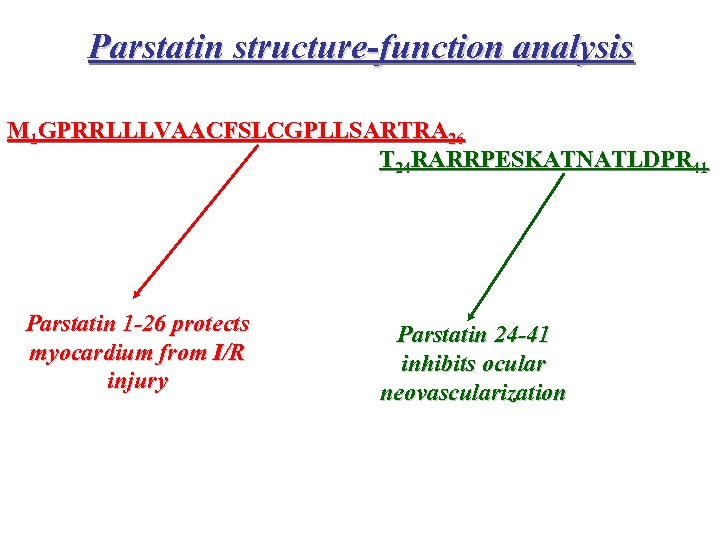 Parstatin structure-function analysis M 1 GPRRLLLVAACFSLCGPLLSARTRA 26 T 24 RARRPESKATNATLDPR 41 Parstatin 1 -26 protects myocardium from I/R injury Parstatin 24 -41 inhibits ocular neovascularization
Parstatin structure-function analysis M 1 GPRRLLLVAACFSLCGPLLSARTRA 26 T 24 RARRPESKATNATLDPR 41 Parstatin 1 -26 protects myocardium from I/R injury Parstatin 24 -41 inhibits ocular neovascularization
 The opportunity in neovascular ocular diseases - more than 35 million people currently suffer from ocular diseases in the USA - the annual cost of vision impairment from eye diseases in the USA alone is $68 billion Approved treatment only for age-related macular degeneration Ø Macugen® (Eyetech/Pfizer). - The average patient continues to lose vision and only a very small percent (5%) gained vision Ø Lucentis® (Genentech) - It is effective only in 35 -40% of the patients - It cost $23. 400/treatment and - the sales for 2008 for Lucentis were $880 million - the projected future sales are estimated to reach $9 billion/year
The opportunity in neovascular ocular diseases - more than 35 million people currently suffer from ocular diseases in the USA - the annual cost of vision impairment from eye diseases in the USA alone is $68 billion Approved treatment only for age-related macular degeneration Ø Macugen® (Eyetech/Pfizer). - The average patient continues to lose vision and only a very small percent (5%) gained vision Ø Lucentis® (Genentech) - It is effective only in 35 -40% of the patients - It cost $23. 400/treatment and - the sales for 2008 for Lucentis were $880 million - the projected future sales are estimated to reach $9 billion/year
 The opportunity in cardiovascular and renal diseases Ø Essential medical conditions in which I/R injury is playing a key role are: • In cardiology – myocardial infarctions, cardiac and thoracic surgery and neurosurgery • In neurology – stroke and brain traumas • In transplantations – after every organ transplantation Ø There are no available approved treatments Ø It remains an unmet medical need for novel agents to treat, prevent or protect myocardium from the onset or severity of these conditions.
The opportunity in cardiovascular and renal diseases Ø Essential medical conditions in which I/R injury is playing a key role are: • In cardiology – myocardial infarctions, cardiac and thoracic surgery and neurosurgery • In neurology – stroke and brain traumas • In transplantations – after every organ transplantation Ø There are no available approved treatments Ø It remains an unmet medical need for novel agents to treat, prevent or protect myocardium from the onset or severity of these conditions.
 Publications 1. Zania P, et al. Parstatin, the cleaved peptide on proteinase-activated receptor 1 activation, is a potent inhibitor of angiogenesis. J Pharmacol Exp Ther, 2009, 328: 378389. 2. Strande JL, et al. Parstatin: a cryptic peptide involved in cardioprotection after ischemia and reperfusion injury. Cardiovasc Res, 2009, 83: 325 -34. 3. Routhu KV, et al. Parstatin (1 -26): The putative signal peptide of PAR 1 confers potent protection from myocardial ischemia-reperfusion injury. J Pharmacol Exp Ther, 2010, 332: 898 -905. 4. Huang H, et al. Parstatin suppresses ocular neovascularization and inflammation. Invest Ophthalmol Vis Sci, 2010, 51: 5825 -5832. 5. Zampatis ED, et al. The protease-activated receptor 1 possesses a functional and cleavable signal peptide which is necessary for receptor expression. FEBS Lett, 2012, 586: 2351 -2359. 6. Diamantopoulos A, et al. Parstatin prevents renal injury following ischemia/reperfusion and radiocontrast administration. Am J Nephrol, 2012, 36: 278 -286
Publications 1. Zania P, et al. Parstatin, the cleaved peptide on proteinase-activated receptor 1 activation, is a potent inhibitor of angiogenesis. J Pharmacol Exp Ther, 2009, 328: 378389. 2. Strande JL, et al. Parstatin: a cryptic peptide involved in cardioprotection after ischemia and reperfusion injury. Cardiovasc Res, 2009, 83: 325 -34. 3. Routhu KV, et al. Parstatin (1 -26): The putative signal peptide of PAR 1 confers potent protection from myocardial ischemia-reperfusion injury. J Pharmacol Exp Ther, 2010, 332: 898 -905. 4. Huang H, et al. Parstatin suppresses ocular neovascularization and inflammation. Invest Ophthalmol Vis Sci, 2010, 51: 5825 -5832. 5. Zampatis ED, et al. The protease-activated receptor 1 possesses a functional and cleavable signal peptide which is necessary for receptor expression. FEBS Lett, 2012, 586: 2351 -2359. 6. Diamantopoulos A, et al. Parstatin prevents renal injury following ischemia/reperfusion and radiocontrast administration. Am J Nephrol, 2012, 36: 278 -286
 Patent Applications 1. Tsopanoglou N. E and Maragoudakis M. E. Bioactive peptides and methods of use. Patent No: US 8, 227, 412 B 2, filed March 25, 2008. 2. Tsopanoglou et al. Parstatin peptides and uses thereof. U. S. A. Patent Continuation-in-Part Application, No: 12/645, 991, filed December 23, 2009. 3. Tsopanoglou et al. Peptides. International Patent Application (PCT), No: WO 2011/039584, filed August 5, 2010. 4. Tsopanoglou et al. Peptides derivatives. U. S. A. Patent Application, No: 13/499, 481, filed April 1, 2012. 5. Tsopanoglou et al. Peptides derivatives. European Patent Application, No: EP 10755236. 6, filed April 1, 2012. 6. Tsopanoglou et al. Peptides derivatives. Australian Patent Application, No: 2010302383, filed April 1, 2012. 7. Tsopanoglou et al. Peptides derivatives. Canadian Patent Application, No: 2, 776, 778, filed April 1, 2012.
Patent Applications 1. Tsopanoglou N. E and Maragoudakis M. E. Bioactive peptides and methods of use. Patent No: US 8, 227, 412 B 2, filed March 25, 2008. 2. Tsopanoglou et al. Parstatin peptides and uses thereof. U. S. A. Patent Continuation-in-Part Application, No: 12/645, 991, filed December 23, 2009. 3. Tsopanoglou et al. Peptides. International Patent Application (PCT), No: WO 2011/039584, filed August 5, 2010. 4. Tsopanoglou et al. Peptides derivatives. U. S. A. Patent Application, No: 13/499, 481, filed April 1, 2012. 5. Tsopanoglou et al. Peptides derivatives. European Patent Application, No: EP 10755236. 6, filed April 1, 2012. 6. Tsopanoglou et al. Peptides derivatives. Australian Patent Application, No: 2010302383, filed April 1, 2012. 7. Tsopanoglou et al. Peptides derivatives. Canadian Patent Application, No: 2, 776, 778, filed April 1, 2012.
 Our Mission and objectives üSecure funds to develop Parstatin peptides to IND stage for at least one (1) indication (e. g. AMD) in a two year period üTo bring to the clinic novel drugs for the treatment of: - ocular angiogenesis-based diseases - ischemia/reperfusion injury-based diseases
Our Mission and objectives üSecure funds to develop Parstatin peptides to IND stage for at least one (1) indication (e. g. AMD) in a two year period üTo bring to the clinic novel drugs for the treatment of: - ocular angiogenesis-based diseases - ischemia/reperfusion injury-based diseases
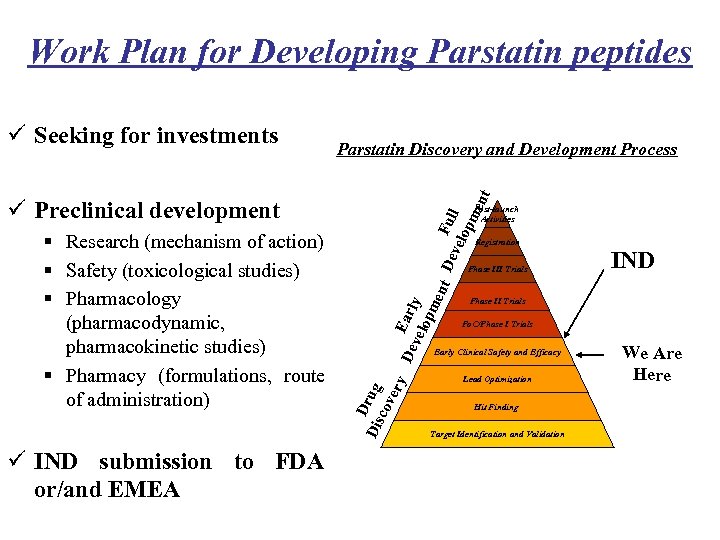 Work Plan for Developing Parstatin peptides Parstatin Discovery and Development Process F vel ull opm ent ü Seeking for investments ü Preclinical development ü IND submission to FDA or/and EMEA De Early vel opm ent De Registration Phase III Trials IND Phase II Trials Po. C/Phase I Trials Early Clinical Safety and Efficacy D Dis rug cov ery § Research (mechanism of action) § Safety (toxicological studies) § Pharmacology (pharmacodynamic, pharmacokinetic studies) § Pharmacy (formulations, route of administration) Post-launch Activities Lead Optimization Hit Finding Target Identification and Validation We Are Here
Work Plan for Developing Parstatin peptides Parstatin Discovery and Development Process F vel ull opm ent ü Seeking for investments ü Preclinical development ü IND submission to FDA or/and EMEA De Early vel opm ent De Registration Phase III Trials IND Phase II Trials Po. C/Phase I Trials Early Clinical Safety and Efficacy D Dis rug cov ery § Research (mechanism of action) § Safety (toxicological studies) § Pharmacology (pharmacodynamic, pharmacokinetic studies) § Pharmacy (formulations, route of administration) Post-launch Activities Lead Optimization Hit Finding Target Identification and Validation We Are Here
 Advantage of Parstatin Project ü Solid scientific background of inventors with international collaborations ü Innovative, First in Class Product ü Well-thought Strategy of Development ü Intellectual property protection (USA, PCT patents) ü Unmet medical needs with a very large potential market ü Experienced, Balanced & Motivated Team which will proactively mitigate risks
Advantage of Parstatin Project ü Solid scientific background of inventors with international collaborations ü Innovative, First in Class Product ü Well-thought Strategy of Development ü Intellectual property protection (USA, PCT patents) ü Unmet medical needs with a very large potential market ü Experienced, Balanced & Motivated Team which will proactively mitigate risks


