64ccfbef39c1cdeddd6b95be0d91b8f1.ppt
- Количество слайдов: 53

Parkinson’s Disease Background o Best described as “shaking palsy” by James Parkinson in 1817 o “Involuntary tremulous motion, with lessened muscular power, in parts not in action and even when supported; with a propensity to bend the trunk forwards, and to pass from a walking to a running pace: the senses and intellects being uninjured. ” Olanow CW et al. Neurology. 2001; 56 (suppl 5): S 1 -S 88. National Parkinson Foundation Web site. www. parkinson. org. Marttila RJ, Rinne UK. Acta Neurol Scand. 1991; 84(suppl 136): 24 -28. De. Stefano AL et al. Am J Hum Genet. 2002; 70: 1089 -1095.
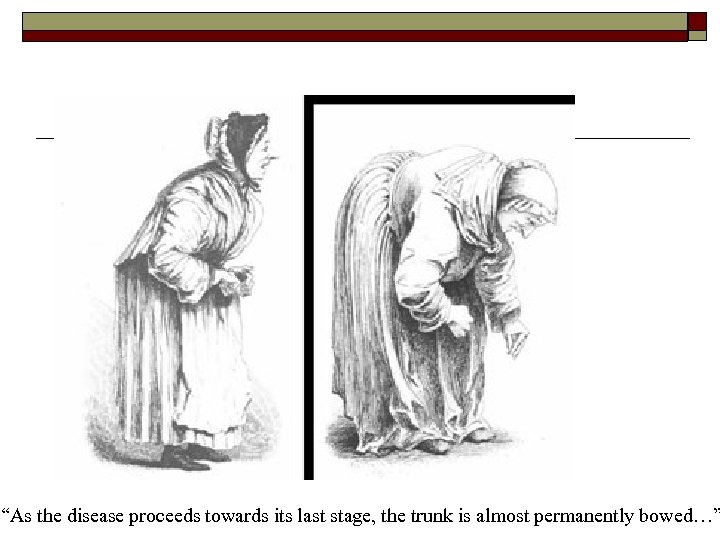
“As the disease proceeds towards its last stage, the trunk is almost permanently bowed…”
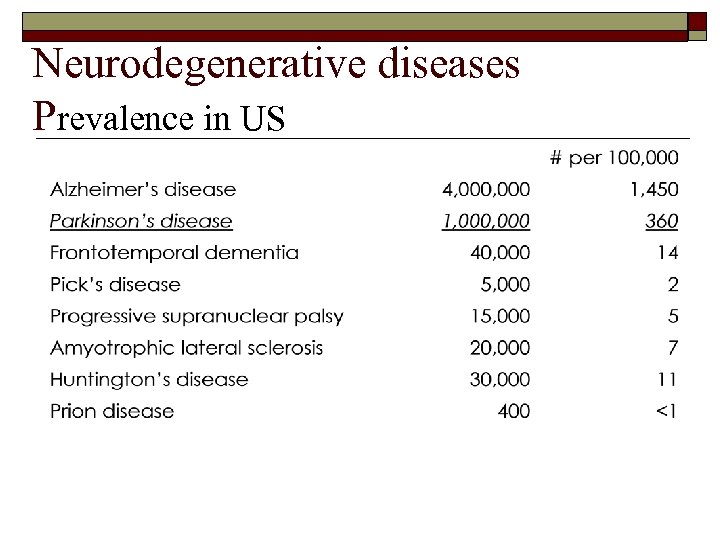
Neurodegenerative diseases Prevalence in US
Fast Facts about PD o Annual incidence: 60, 000 new cases/yr o Increase with age (3% population >65 years old) o Slightly more common in men o Mean age at onset: 60 years old o 85% of patients are over 65 years old

Risk of Parkinson’s Disease Increased risk o o o Age High Body Mass Index Male gender Family history Depression Environment factors n n rural living well-water drinking welding head injury Decreased risk o o o Caffeine intake Smoking cigarettes Anti-oxidants in diet

Motor Symptoms o o o o o Tremor at rest Bradykinesia Rigidity Postural instability Decreased arm swing when walking Micrographia Hypophonia Masked face Slow, shuffling gait Stooped posture Olanow CW, Watts RL, Koller WC. . Neurology. 2001; 56 (suppl 5): S 1 -S 88. . (suppl Waters CH. Diagnosis and Management of Parkinson’s Disease. 3 rd ed. 2002. National Parkinson Foundation. http: //www. parkinson. org.

“…the hand failing to answer with exactness to the dictates of the will. ”
Manifestations of PD Nutt JG, Wooten GF. N Engl J Med. 2005; 353: 1021 -1027. Parkinson’s Disease Foundation Web site. www. pdf. org. Additional Features o Cognitive, mood, and behavioral dysfunction o Olfactory disturbance o Sleep disturbance o Constipation o Seborrheic dermatitis o Pain o Autonomic disturbances
Diagnosing PD o o o United Kingdom Brain Bank Criteria Stage I Hoehn and Yahr (H&Y)- unilateral Stage II H&Y – bilateral Stage III H&Y – bilateral with loss of balance/falls Stage IV H&Y – all above and significant disability Stage V H&Y- bedbound
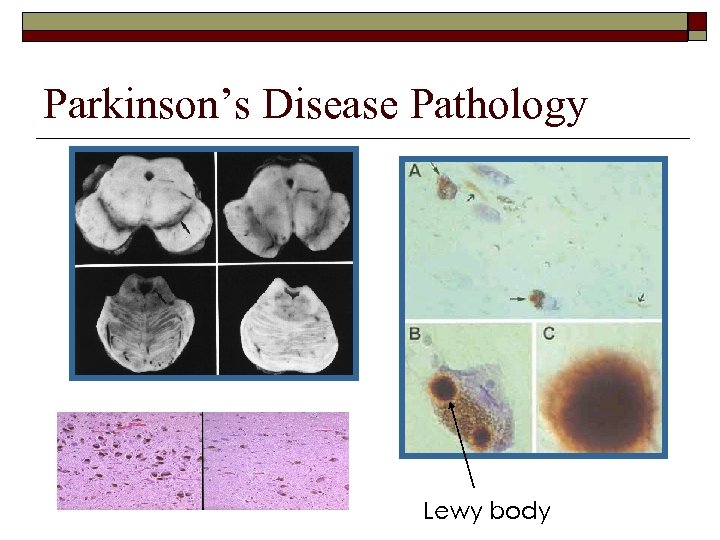
Parkinson’s Disease Pathology Lewy body
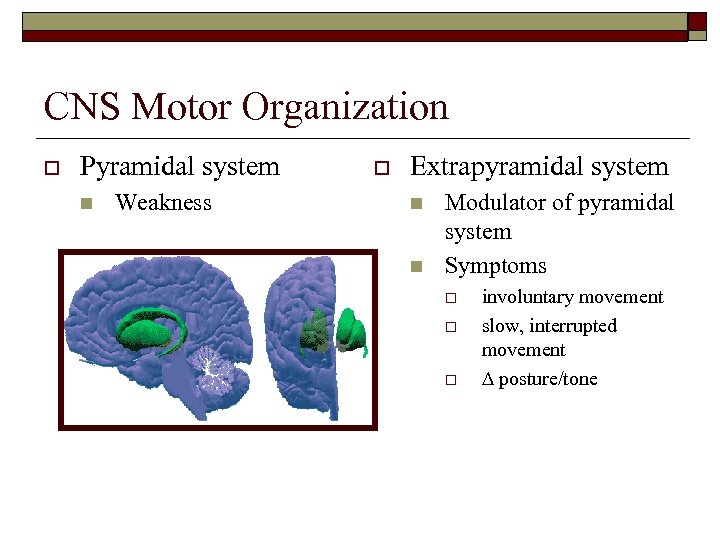
CNS Motor Organization o Pyramidal system n Weakness o Extrapyramidal system n n Modulator of pyramidal system Symptoms o o o involuntary movement slow, interrupted movement posture/tone
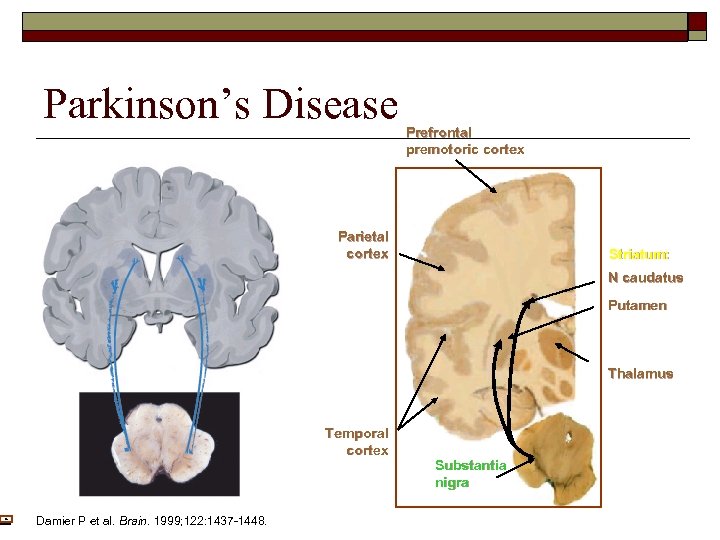
Parkinson’s Disease Prefrontal premotoric cortex Parietal cortex Striatum: N caudatus Putamen Thalamus Temporal cortex Damier P et al. Brain. 1999; 122: 1437 -1448. Substantia nigra
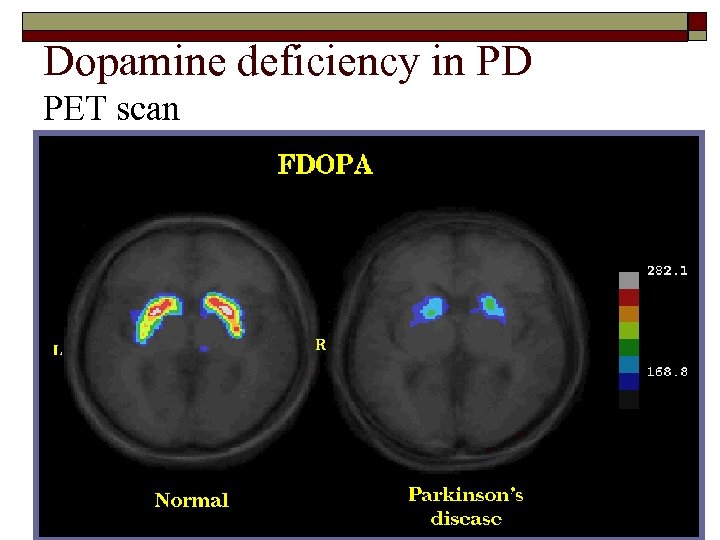
Dopamine deficiency in PD PET scan
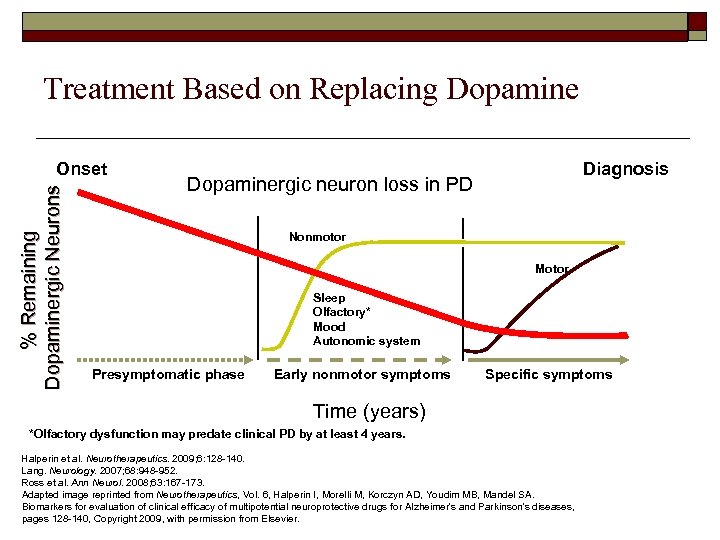
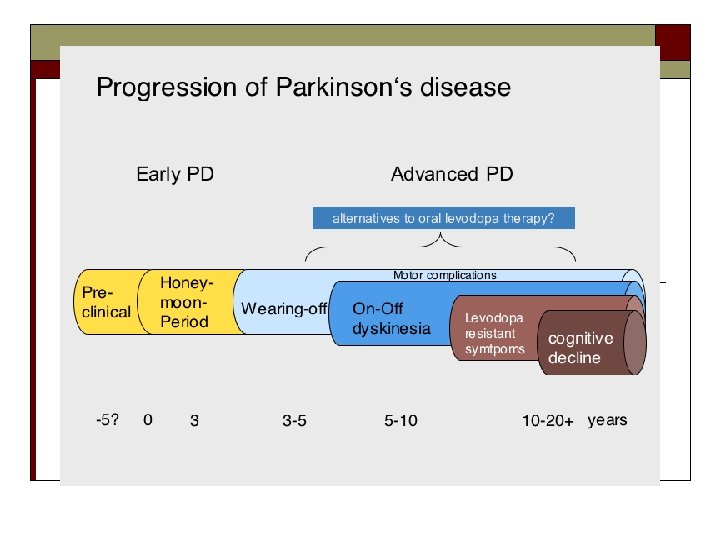
Treatment Based on Replacing Dopamine % Remaining Dopaminergic Neurons Onset Diagnosis Dopaminergic neuron loss in PD Nonmotor Motor Sleep Olfactory* Mood Autonomic system Presymptomatic phase Early nonmotor symptoms Specific symptoms Time (years) *Olfactory dysfunction may predate clinical PD by at least 4 years. Halperin et al. Neurotherapeutics. 2009; 6: 128 -140. Lang. Neurology. 2007; 68: 948 -952. Ross et al. Ann Neurol. 2008; 63: 167 -173. Adapted image reprinted from Neurotherapeutics, Vol. 6, Halperin I, Morelli M, Korczyn AD, Youdim MB, Mandel SA. Biomarkers for evaluation of clinical efficacy of multipotential neuroprotective drugs for Alzheimer's and Parkinson's diseases, pages 128 -140, Copyright 2009, with permission from Elsevier.
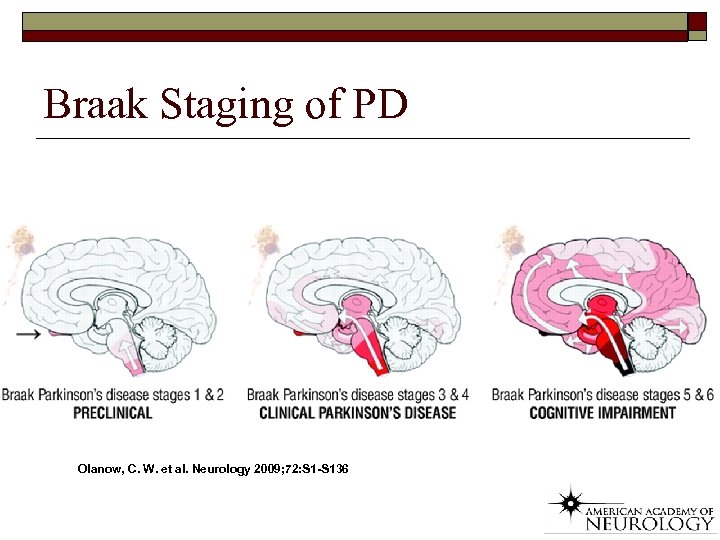
Braak Staging of PD Olanow, C. W. et al. Neurology 2009; 72: S 1 -S 136
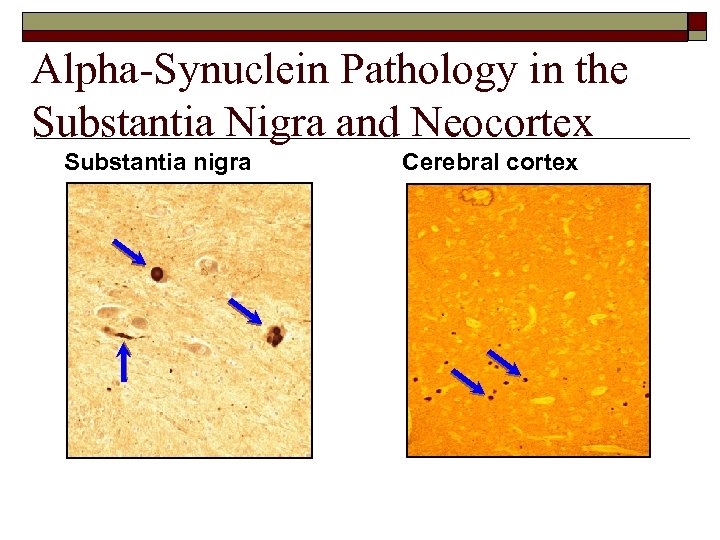
Alpha-Synuclein Pathology in the Substantia Nigra and Neocortex Substantia nigra Cerebral cortex
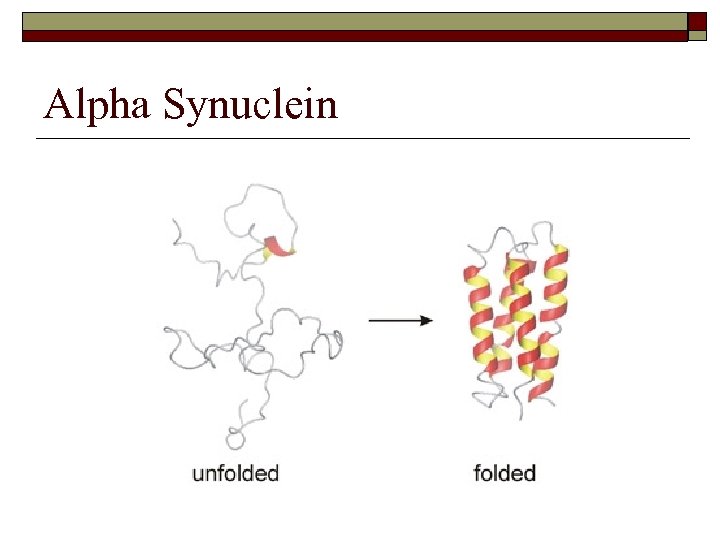
Alpha Synuclein
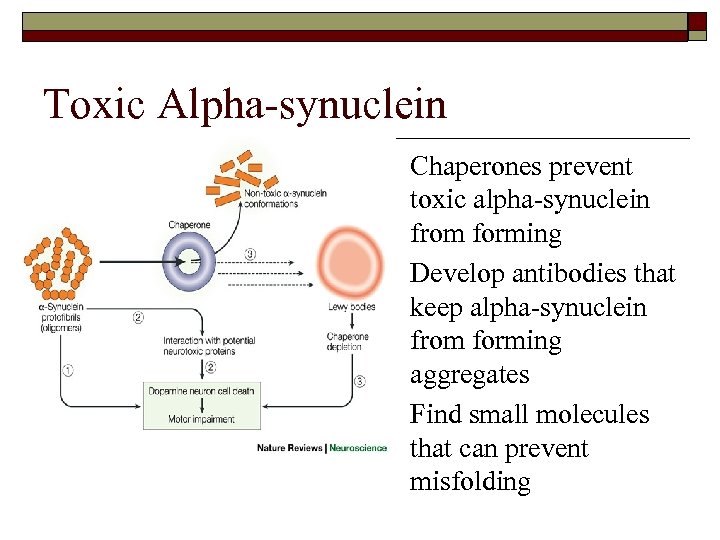
Toxic Alpha-synuclein o o o Chaperones prevent toxic alpha-synuclein from forming Develop antibodies that keep alpha-synuclein from forming aggregates Find small molecules that can prevent misfolding
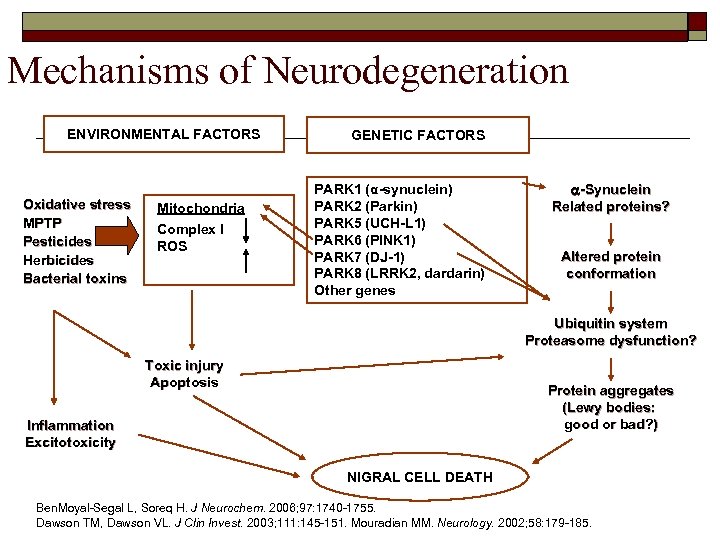
Mechanisms of Neurodegeneration ENVIRONMENTAL FACTORS Oxidative stress MPTP Pesticides Herbicides Bacterial toxins Mitochondria Complex I ROS GENETIC FACTORS PARK 1 (α-synuclein) PARK 2 (Parkin) PARK 5 (UCH-L 1) PARK 6 (PINK 1) PARK 7 (DJ-1) PARK 8 (LRRK 2, dardarin) Other genes a-Synuclein Related proteins? Altered protein conformation Ubiquitin system Proteasome dysfunction? Toxic injury Apoptosis Protein aggregates (Lewy bodies: good or bad? ) Inflammation Excitotoxicity NIGRAL CELL DEATH Ben. Moyal-Segal L, Soreq H. J Neurochem. 2006; 97: 1740 -1755. Dawson TM, Dawson VL. J Clin Invest. 2003; 111: 145 -151. Mouradian MM. Neurology. 2002; 58: 179 -185.
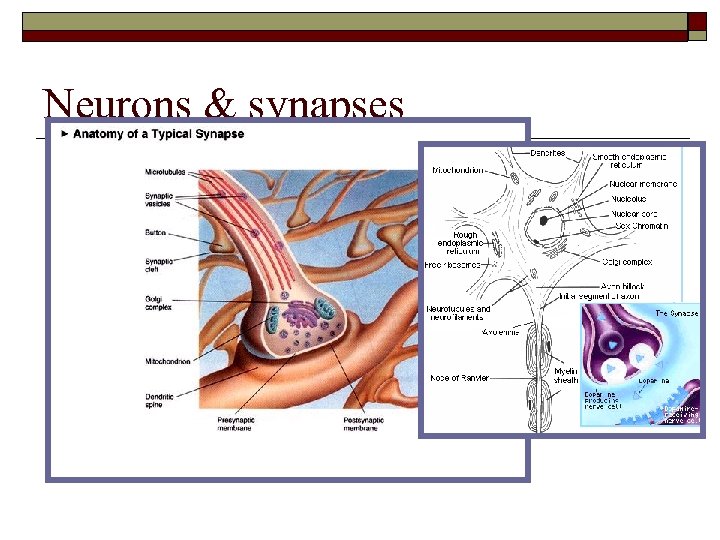
Neurons & synapses
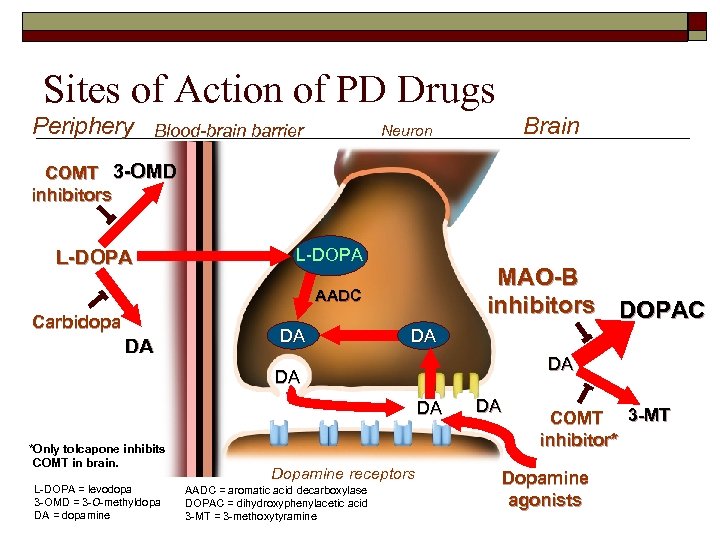
Sites of Action of PD Drugs Periphery Blood-brain barrier Brain Neuron COMT 3 -OMD inhibitors L-DOPA MAO-B inhibitors DOPAC AADC Carbidopa DA DA DA *Only tolcapone inhibits COMT in brain. L-DOPA = levodopa 3 -OMD = 3 -O-methyldopa DA = dopamine Dopamine receptors AADC = aromatic acid decarboxylase DOPAC = dihydroxyphenylacetic acid 3 -MT = 3 -methoxytyramine DA COMT 3 -MT inhibitor* Dopamine agonists
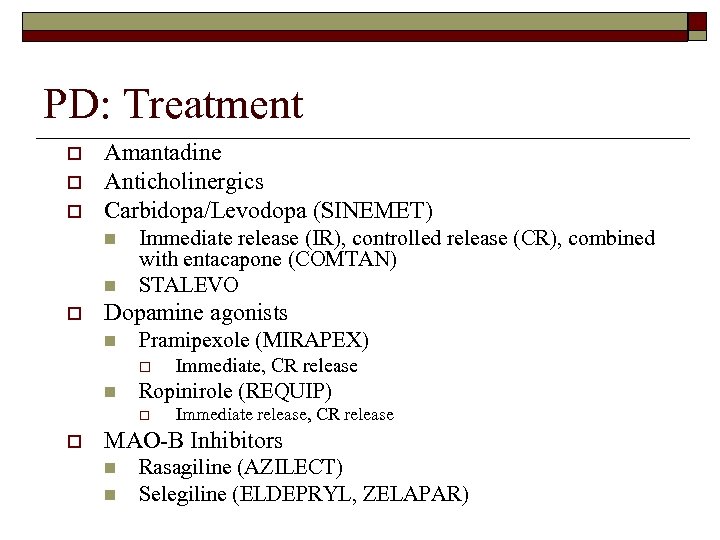
PD: Treatment o o o Amantadine Anticholinergics Carbidopa/Levodopa (SINEMET) n n o Immediate release (IR), controlled release (CR), combined with entacapone (COMTAN) STALEVO Dopamine agonists n Pramipexole (MIRAPEX) o n Ropinirole (REQUIP) o o Immediate, CR release Immediate release, CR release MAO-B Inhibitors n n Rasagiline (AZILECT) Selegiline (ELDEPRYL, ZELAPAR)
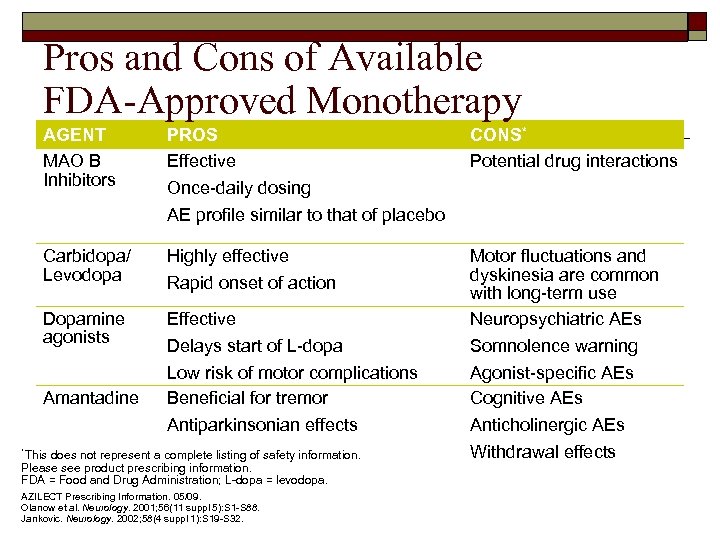
Pros and Cons of Available FDA-Approved Monotherapy AGENT MAO B Inhibitors PROS Effective Carbidopa/ Levodopa Highly effective Dopamine agonists Effective Motor fluctuations and dyskinesia are common with long-term use Neuropsychiatric AEs Delays start of L-dopa Low risk of motor complications Beneficial for tremor Somnolence warning Agonist-specific AEs Cognitive AEs Antiparkinsonian effects Anticholinergic AEs Withdrawal effects Amantadine CONS* Potential drug interactions Once-daily dosing AE profile similar to that of placebo Rapid onset of action *This does not represent a complete listing of safety information. Please see product prescribing information. FDA = Food and Drug Administration; L-dopa = levodopa. AZILECT Prescribing Information. 05/09. Olanow et al. Neurology. 2001; 56(11 suppl 5): S 1 -S 88. Jankovic. Neurology. 2002; 58(4 suppl 1): S 19 -S 32.
Levodopa: The Cornerstone of PD Therapy o Levodopa provides substantial antiparkinsonian symptom control, and significantly improves patient quality of life 1 o Levodopa is the most efficacious antiparkinsonian medication in moderate and advanced disease o Levodopa provides relatively rapid symptomatic benefits 2, 3 o Levodopa is generally well tolerated with few initial side effects o Levodopa continues to provide antiparkinsonian benefits through the course of the illness o All PD patients eventually require levodopa therapy 1. Louis ED, et al. Arch Neurol. 1997; 54: 260 -264. 2. Olanow CW, et al. Neurology. 2001; 56: S 1 -S 86. 3. Agidy et al. Lancet. 2002; 360: 575.
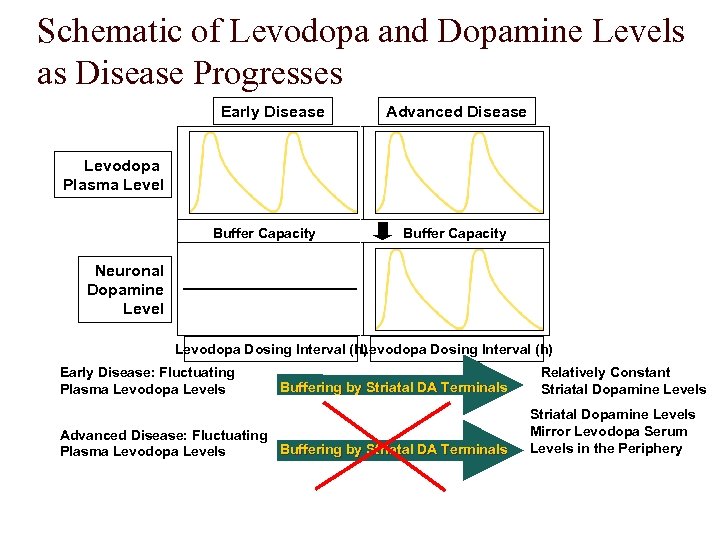
Schematic of Levodopa and Dopamine Levels as Disease Progresses Early Disease Advanced Disease Levodopa Plasma Level Buffer Capacity Neuronal Dopamine Level Levodopa Dosing Interval (h) Early Disease: Fluctuating Plasma Levodopa Levels Buffering by Striatal DA Terminals Advanced Disease: Fluctuating Buffering by Striatal DA Terminals Plasma Levodopa Levels Relatively Constant Striatal Dopamine Levels Mirror Levodopa Serum Levels in the Periphery

Wearing Off o o o Most common motor fluctuation Occurs toward end of dose “wear down” Regular and predictable Adjust dose
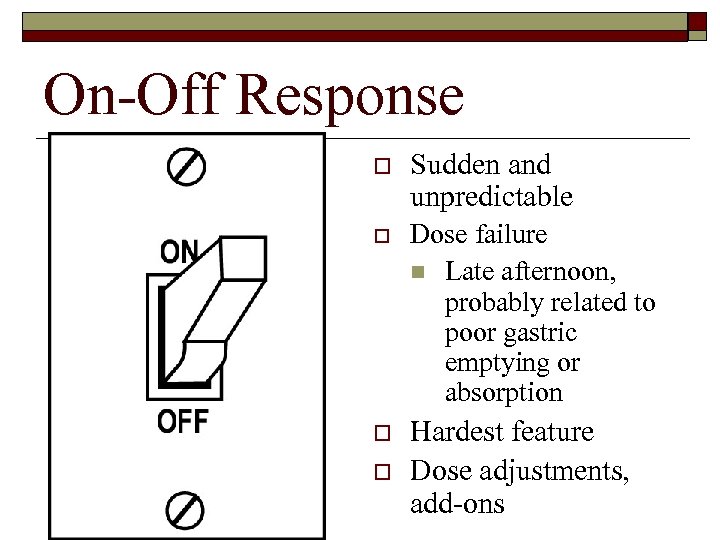
On-Off Response o Sudden and unpredictable o Dose failure n Late afternoon, probably related to poor gastric emptying or absorption o Hardest feature Dose adjustments, add-ons o

Dyskinesia

Off Freezing
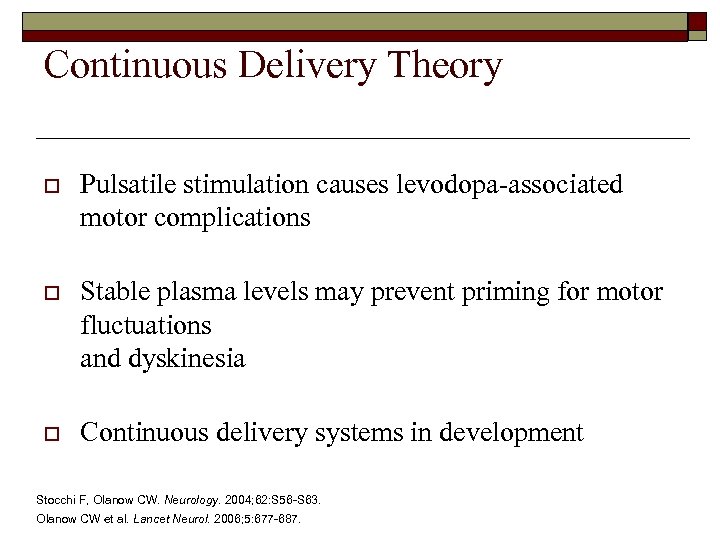
Continuous Delivery Theory o Pulsatile stimulation causes levodopa-associated motor complications o Stable plasma levels may prevent priming for motor fluctuations and dyskinesia o Continuous delivery systems in development Stocchi F, Olanow CW. Neurology. 2004; 62: S 56 -S 63. Olanow CW et al. Lancet Neurol. 2006; 5: 677 -687.
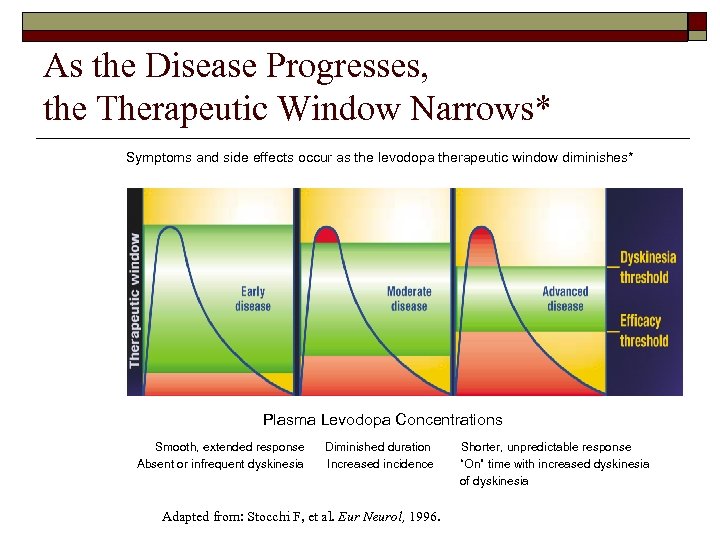
As the Disease Progresses, the Therapeutic Window Narrows* Symptoms and side effects occur as the levodopa therapeutic window diminishes* Plasma Levodopa Concentrations Smooth, extended response Absent or infrequent dyskinesia Diminished duration Increased incidence Adapted from: Stocchi F, et al. Eur Neurol, 1996. Shorter, unpredictable response “On” time with increased dyskinesia of dyskinesia
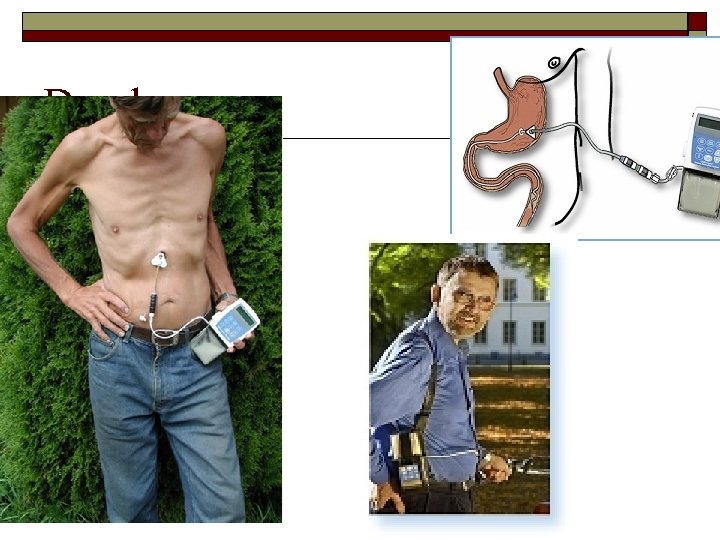
Duodopa o Du
Key Points: Early but No Impairment o Early patients- no functional impairment n n Easiest treatment category ADAGIO, TEMPO (rasagiline) and ELLDOPA trial indicate earlier treatment may be better o n Consider rasagiline (Azilect), selegiline (Eldepryl, Zelapar) Refer for potential neuroprotective trials o Coenzyme Q 10, selegiline, rasagiline, creatine
Key Points: Early with Impairment o o Early patient-functional impairment n Bothersome tremor, stiffness, slowness, decrease in dexterity interfering with ADLs or job AAN guidelines 2002 n MAO B inhibitors provide some benefit n Dopamine agonists n Levodopa If the patient is chronologically or physiologically young (<70) try a dopamine agonist as the first robust treatment If older, or cognitively impaired, use levodopa first
Key Points: Middle Stage Patients o o o Starting to have wearing off of drug benefit prior to next dose Goal is to enhance dopamine system in the brain, since these medications have different mechanisms of action=Polypharmacy is expected! Layer on medications and adjust to best benefit
Key Points: Later Mid-Stage Patient o o Experiencing fluctuation in motor control to include significant wearing off with poor mobility and dyskinesias n Have patients keep diaries of motor control n Add additional medications n Consider smaller, more frequent doses of medications to minimize “off” time and dyskinesia Onset or worsening of many non-levodopa responsive symptoms, such as falling, worsening cognition, dysphagia, autonomic dysfunction

Key Points: Advanced Parkinson’s Disease o o Treatment is made difficult by the worsening of motor complications, cognitive, psychiatric and autonomic disturbances Medications may need to be streamlined (reduced or eliminated) because of confusion or psychosis
Surgical Treatment for PD o o o Patient selection is KEY Patients who are very sensitive to levodopa are the best candidates Patients should have motor fluctuations including dyskinesias Patients must be free of significant cognitive disease Usually, consideration after 10 years of disease, but trend toward earlier use in several trials
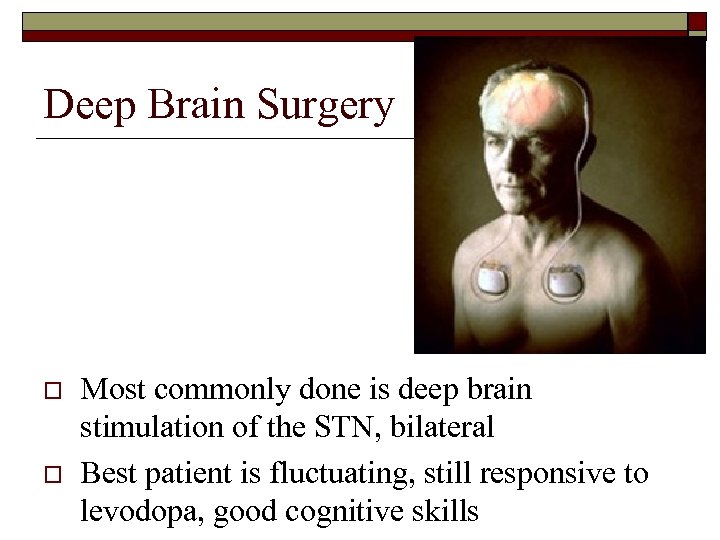
Deep Brain Surgery o o Most commonly done is deep brain stimulation of the STN, bilateral Best patient is fluctuating, still responsive to levodopa, good cognitive skills
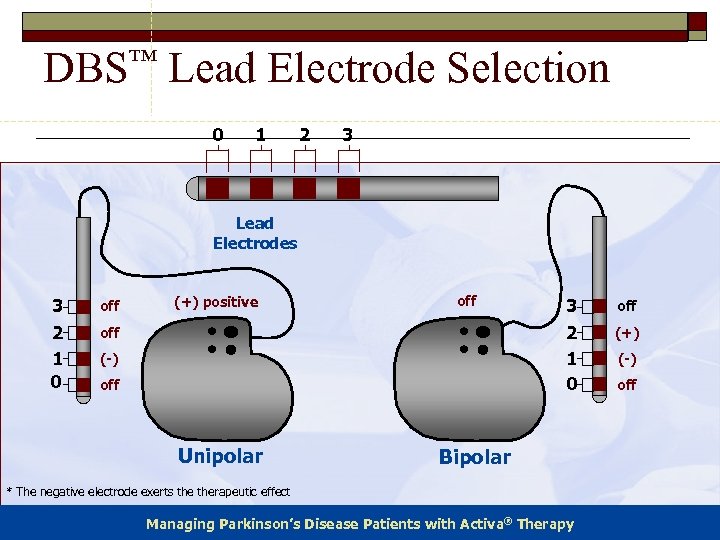
DBS™ Lead Electrode Selection 0 1 2 3 Lead Electrodes 3 2 1 0 off (+) positive off (-) off Unipolar 3 2 1 0 Bipolar * The negative electrode exerts therapeutic effect Managing Parkinson’s Disease Patients with Activa® Therapy off (+) (-) off

DBS in PD

Parkinsonian Syndromes o o Patient has bilateral onset of a gait disorder Patient has early falling Patient does not respond well to L-Dopa Patient has prominent dementia or autonomic nervous system dysfunction
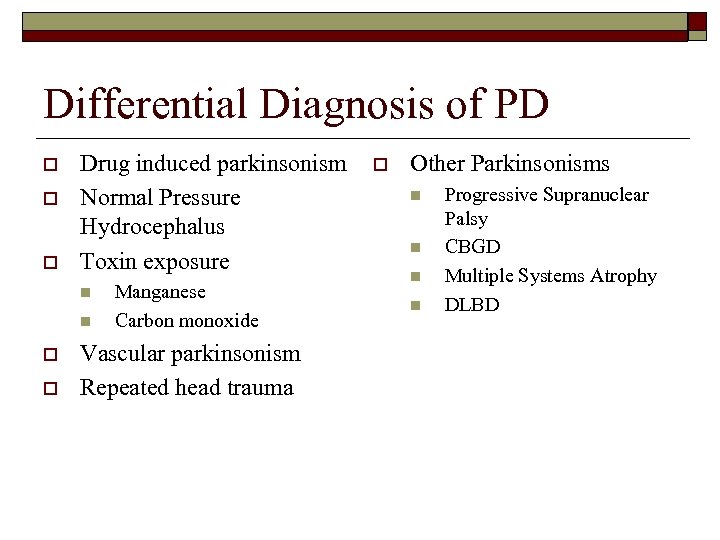
Differential Diagnosis of PD o o o Drug induced parkinsonism Normal Pressure Hydrocephalus Toxin exposure n n o o Manganese Carbon monoxide Vascular parkinsonism Repeated head trauma o Other Parkinsonisms n n Progressive Supranuclear Palsy CBGD Multiple Systems Atrophy DLBD
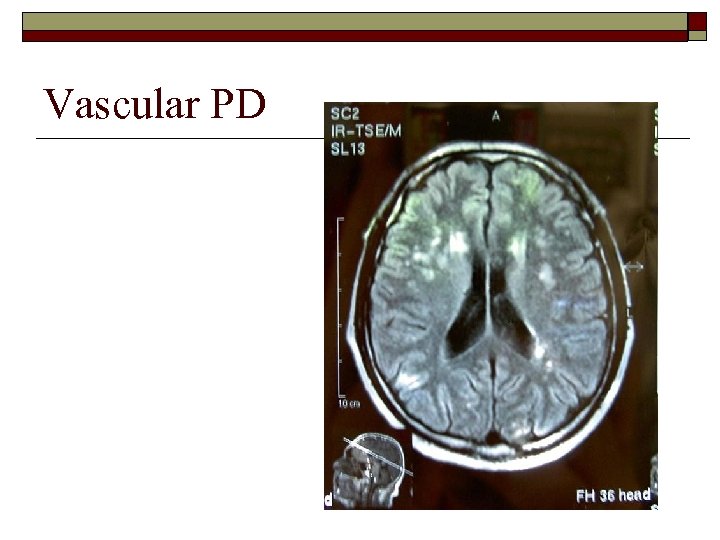
Vascular PD
Multiple Systems Atrophy o o o Combination disorder with parkinsonism, ataxia and signs of autonomic nervous system dysfunction Pathologically distinct from Parkinson’s disease Three types n MSA-P (SND), MSA-C (OPCA), PAF (SDS)
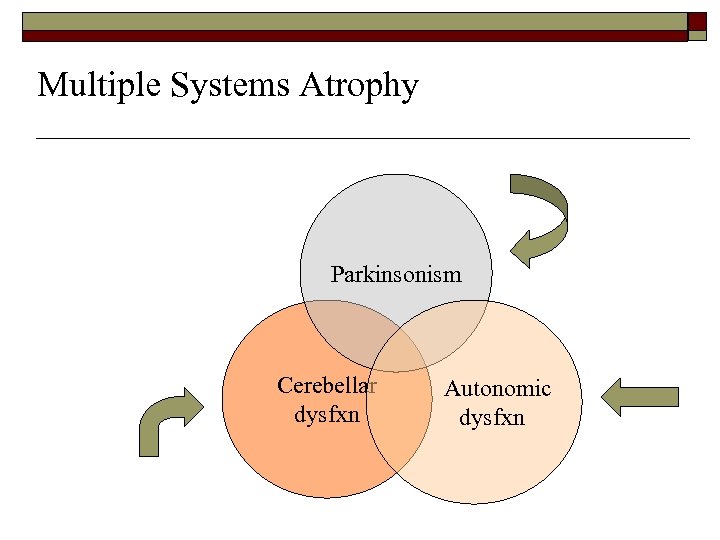
Multiple Systems Atrophy Parkinsonism Cerebellar dysfxn Autonomic dysfxn

Early CBDG

PSP o Video
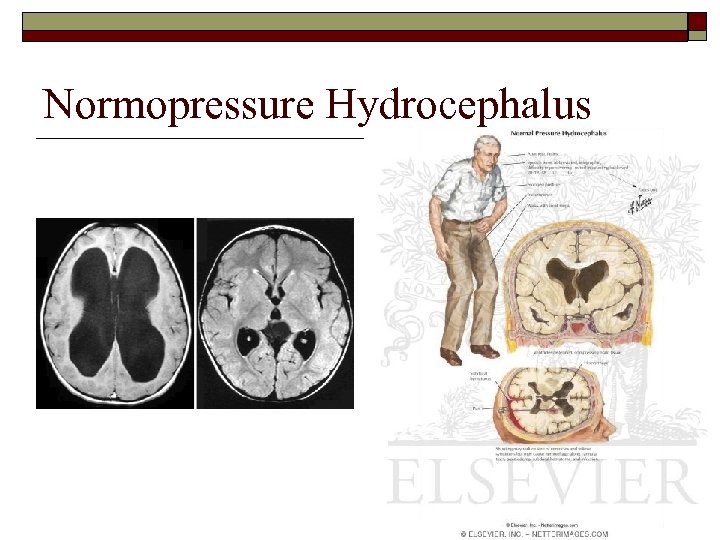
Normopressure Hydrocephalus

Magnetic Gait
64ccfbef39c1cdeddd6b95be0d91b8f1.ppt