f219f3a37667bbcae50f7c71be50a9e8.ppt
- Количество слайдов: 112
 Pandemic Influenza Preparedness Response Guidance for Healthcare Workers and Healthcare Employers Educational material developed through funding from OSHA Susan Harwood Grant #SH-16624 -07 -60 -F-54 1
Pandemic Influenza Preparedness Response Guidance for Healthcare Workers and Healthcare Employers Educational material developed through funding from OSHA Susan Harwood Grant #SH-16624 -07 -60 -F-54 1
 Introduction n Any Pandemic Disease is a global disease outbreak n occurs when a new virus emerges n spreads where people have no immunity n is a disease for which there is no vaccine n spreads easily person-to-person n causes serious illness n sweeps around the world in a short time n 2
Introduction n Any Pandemic Disease is a global disease outbreak n occurs when a new virus emerges n spreads where people have no immunity n is a disease for which there is no vaccine n spreads easily person-to-person n causes serious illness n sweeps around the world in a short time n 2
 Initial Control Measures n n After a pandemic disease starts, everyone in the world is at risk Countries might try to delay or stop the arrival of the virus through border closures n travel restrictions n quarantines n 3
Initial Control Measures n n After a pandemic disease starts, everyone in the world is at risk Countries might try to delay or stop the arrival of the virus through border closures n travel restrictions n quarantines n 3
 Effects of A Severe Pandemic n Everyday life would come to a standstill due to n n n high levels of illness, death, social disruption, and economic loss everyone being ill at the same time interruptions of basic services such as public transportation and food delivery 4
Effects of A Severe Pandemic n Everyday life would come to a standstill due to n n n high levels of illness, death, social disruption, and economic loss everyone being ill at the same time interruptions of basic services such as public transportation and food delivery 4
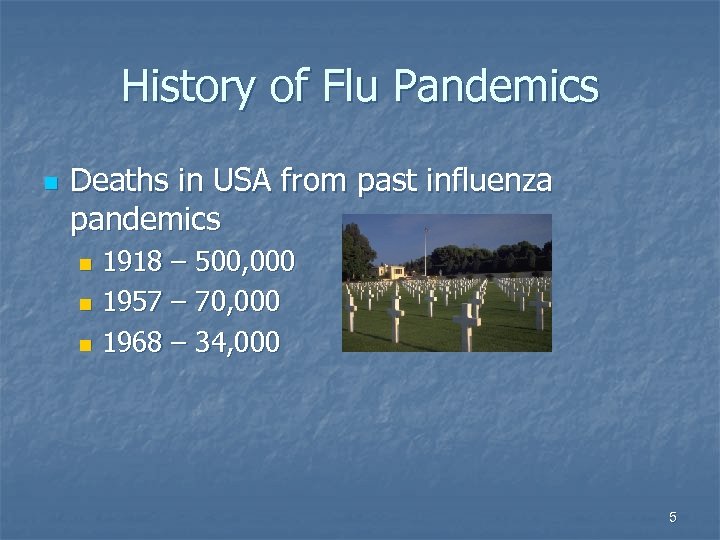 History of Flu Pandemics n Deaths in USA from past influenza pandemics 1918 – 500, 000 n 1957 – 70, 000 n 1968 – 34, 000 n 5
History of Flu Pandemics n Deaths in USA from past influenza pandemics 1918 – 500, 000 n 1957 – 70, 000 n 1968 – 34, 000 n 5
 The Public Health Service n n By 1918, most PHS officers understood how diseases spread Without antibiotics, PHS officers were limited in their ability to fight disease 6
The Public Health Service n n By 1918, most PHS officers understood how diseases spread Without antibiotics, PHS officers were limited in their ability to fight disease 6
 Devastation of 1918 Avian Flu The Influenza Pandemic occurred in three waves in the United States throughout 1918 and 1919. http: //www. pandemicflu. gov/ 7
Devastation of 1918 Avian Flu The Influenza Pandemic occurred in three waves in the United States throughout 1918 and 1919. http: //www. pandemicflu. gov/ 7
 1918 – Rapid-paced Outbreaks n n The 1918 influenza pandemic occurred too rapidly for the PHS to develop a detailed study of the pandemic After the pandemic, they developed a map with approximate dates of the outbreak 8
1918 – Rapid-paced Outbreaks n n The 1918 influenza pandemic occurred too rapidly for the PHS to develop a detailed study of the pandemic After the pandemic, they developed a map with approximate dates of the outbreak 8
![[Credit: Office of the Public Health Service Historian] 9 [Credit: Office of the Public Health Service Historian] 9](https://present5.com/presentation/f219f3a37667bbcae50f7c71be50a9e8/image-9.jpg) [Credit: Office of the Public Health Service Historian] 9
[Credit: Office of the Public Health Service Historian] 9
 Effects on Healthcare System n Healthcare facilities would be overwhelmed including shortage of hospital staff n beds n ventilators n supplies n 10
Effects on Healthcare System n Healthcare facilities would be overwhelmed including shortage of hospital staff n beds n ventilators n supplies n 10
 Healthcare Worker Demands n Healthcare will be affected since healthcare workers will be ill, too n (including first responders, nurses, physicians, pharmacists, technicians and aides, building maintenance, security and administrative personnel, social workers, laboratory employees, food service, housekeeping, and mortuary personnel) 11
Healthcare Worker Demands n Healthcare will be affected since healthcare workers will be ill, too n (including first responders, nurses, physicians, pharmacists, technicians and aides, building maintenance, security and administrative personnel, social workers, laboratory employees, food service, housekeeping, and mortuary personnel) 11
 Healthcare Facility Demands n Healthcare will be affected since healthcare resources will be expected to meet nonpandemic associated healthcare needs in a variety of workplace settings n (including medical and dental offices, schools, physical and rehabilitation therapy centers, health departments, occupational health clinics, and prisons, free-standing ambulatory care and surgical facilities, and emergency response settings) 12
Healthcare Facility Demands n Healthcare will be affected since healthcare resources will be expected to meet nonpandemic associated healthcare needs in a variety of workplace settings n (including medical and dental offices, schools, physical and rehabilitation therapy centers, health departments, occupational health clinics, and prisons, free-standing ambulatory care and surgical facilities, and emergency response settings) 12
 Cornerstones of Preparedness n Cornerstones of effective pandemic influenza preparedness and response Risk Assessment n Policy Development n Procedure Execution n 13
Cornerstones of Preparedness n Cornerstones of effective pandemic influenza preparedness and response Risk Assessment n Policy Development n Procedure Execution n 13
 Each Facility is Unique n To insure adequate preparation of healthcare workers make preparations for each facility n collaborate with local, state, and federal partners n follow related standards and guidelines n 14
Each Facility is Unique n To insure adequate preparation of healthcare workers make preparations for each facility n collaborate with local, state, and federal partners n follow related standards and guidelines n 14
 Specific Areas for Planning n n n n Infection Control Plans Risk Communication Tools Self-triage Instructions for Home Care of Flu Patients Diagnosis and Treatment of Staff During a Pandemic Technical Information Available Through Internet Sources Supply Checklists 15
Specific Areas for Planning n n n n Infection Control Plans Risk Communication Tools Self-triage Instructions for Home Care of Flu Patients Diagnosis and Treatment of Staff During a Pandemic Technical Information Available Through Internet Sources Supply Checklists 15
 Permission of Gary Brookins and the Richmond Times-Dispatch 16
Permission of Gary Brookins and the Richmond Times-Dispatch 16
 Summit for Pandemic Planning n January 12, 2006 Pandemic planning summit n n The state of West Virginia HHS and other federal agencies Public health officials Emergency management and response leaders 17
Summit for Pandemic Planning n January 12, 2006 Pandemic planning summit n n The state of West Virginia HHS and other federal agencies Public health officials Emergency management and response leaders 17
 North America n n The United States Northern Command has a role in protecting our health Resources are available at their website n n n Information Sheet Personal Training Brief Readiness Guide Newsletter Department of Defense Influenza Watchboard http: //www. northcom. mil/Avian%20 Flu/index. html 18
North America n n The United States Northern Command has a role in protecting our health Resources are available at their website n n n Information Sheet Personal Training Brief Readiness Guide Newsletter Department of Defense Influenza Watchboard http: //www. northcom. mil/Avian%20 Flu/index. html 18
 Pandemic. Flu. gov State Pandemic Plans http: //www. pandemicflu. gov/plan/states/sta teplans. html n n Site contains list of pandemic plans that are currently available on state websites 19
Pandemic. Flu. gov State Pandemic Plans http: //www. pandemicflu. gov/plan/states/sta teplans. html n n Site contains list of pandemic plans that are currently available on state websites 19
 WV Pandemic Planning n Agreement - WV & U. S. Dept. of Health and Human Services n January 12, 2006 HHS Secretary Mike Leavitt and Governor Joe Manchin III signed a Planning Resolution detailing HHS‘s and West Virginia's shared and independent responsibilities for pandemic planning 20
WV Pandemic Planning n Agreement - WV & U. S. Dept. of Health and Human Services n January 12, 2006 HHS Secretary Mike Leavitt and Governor Joe Manchin III signed a Planning Resolution detailing HHS‘s and West Virginia's shared and independent responsibilities for pandemic planning 20
 WV n for Pandemic Planning WV Federal Funding - 2006 $940, 502 - Phase One funding from U. S. Dept. of Health and Human Services (HHS) for pandemic planning activities n $1, 688, 192 - revised Phase Two of Health and Human Services’ local and state allocations n $620, 408 - awarded to help strengthen the state's capacity to respond to a pandemic influenza outbreak. n 21
WV n for Pandemic Planning WV Federal Funding - 2006 $940, 502 - Phase One funding from U. S. Dept. of Health and Human Services (HHS) for pandemic planning activities n $1, 688, 192 - revised Phase Two of Health and Human Services’ local and state allocations n $620, 408 - awarded to help strengthen the state's capacity to respond to a pandemic influenza outbreak. n 21
 WV Agencies Involved in Planning n n n Bureau for Public Health and Human Resources WV Department of Military Affairs and Public 22
WV Agencies Involved in Planning n n n Bureau for Public Health and Human Resources WV Department of Military Affairs and Public 22
 n n n WV Pandemic Flu Website http: //www. wvflu. org/ West Virginia Bureau for Public Health - Threat Preparedness 505 Capitol Street • Suite 200 • Charleston, WV 25301 Phone: (304) 558 -6900 ext. 2005 Fax: (304) 558 -0464 23
n n n WV Pandemic Flu Website http: //www. wvflu. org/ West Virginia Bureau for Public Health - Threat Preparedness 505 Capitol Street • Suite 200 • Charleston, WV 25301 Phone: (304) 558 -6900 ext. 2005 Fax: (304) 558 -0464 23
 24
24
 WV Pandemic Flu Brochure 25
WV Pandemic Flu Brochure 25
 Influenza 26
Influenza 26
 Influenza - History n Clinical Background n n Three pandemics in the 20 th Century Three types: A, B, & C Only Type A influenza viruses cause pandemics Influenza A virus variations n n Virulence Infectivity to specific hosts Modes of transmission Clinical presentation of infection 27
Influenza - History n Clinical Background n n Three pandemics in the 20 th Century Three types: A, B, & C Only Type A influenza viruses cause pandemics Influenza A virus variations n n Virulence Infectivity to specific hosts Modes of transmission Clinical presentation of infection 27
 Influenza Type A: Subtypes n Only Type A is divided into subtypes n Based on presence of two viral surface proteins (antigens) Hemagglutin (H) n Neuraminidase (N) n n Number of surface proteins identified in influenza A viruses 16 hemagglutinin n 9 neuraminidase n 28
Influenza Type A: Subtypes n Only Type A is divided into subtypes n Based on presence of two viral surface proteins (antigens) Hemagglutin (H) n Neuraminidase (N) n n Number of surface proteins identified in influenza A viruses 16 hemagglutinin n 9 neuraminidase n 28
 Pandemic Subtypes n In the 20 th Century, 3 different subtypes have caused pandemics H 1 N 1 n H 2 N 2 n H 3 N 2 n n Subtypes are designated as H protein type (1 -16) solely, OR followed by the N protein type (1 -9) 29
Pandemic Subtypes n In the 20 th Century, 3 different subtypes have caused pandemics H 1 N 1 n H 2 N 2 n H 3 N 2 n n Subtypes are designated as H protein type (1 -16) solely, OR followed by the N protein type (1 -9) 29
 Terminology n n Avian (bird) flu is caused by influenza A viruses that occur naturally among birds Different subtypes of these viruses exist because of changes in certain proteins on the surface of the influenza A virus and the way the proteins combine n n n hemagglutinin [HA] neuraminidase [NA] Each combination represents a different subtype All known subtypes of influenza A viruses can be found in birds The avian flu currently of concern is the H 5 N 1 subtype 30
Terminology n n Avian (bird) flu is caused by influenza A viruses that occur naturally among birds Different subtypes of these viruses exist because of changes in certain proteins on the surface of the influenza A virus and the way the proteins combine n n n hemagglutinin [HA] neuraminidase [NA] Each combination represents a different subtype All known subtypes of influenza A viruses can be found in birds The avian flu currently of concern is the H 5 N 1 subtype 30
 H 5 N 1 Virus n H 5 N 1 n n n n http: //en. wikipedia. org/wiki/H 5 n 1 spreading rapidly first appeared in Asia epizootic (an epidemic in nonhumans) panzootic (affecting animals of many species, especially over a wide area) killing tens of millions of birds spurring the culling of hundreds of millions of birds to stem its spread Most references to "bird flu" and H 5 N 1 in the popular media refer to this strain 31
H 5 N 1 Virus n H 5 N 1 n n n n http: //en. wikipedia. org/wiki/H 5 n 1 spreading rapidly first appeared in Asia epizootic (an epidemic in nonhumans) panzootic (affecting animals of many species, especially over a wide area) killing tens of millions of birds spurring the culling of hundreds of millions of birds to stem its spread Most references to "bird flu" and H 5 N 1 in the popular media refer to this strain 31
 Seasonal Influenza n n Refers to periodic outbreaks of acute onset viral respiratory infection caused by circulating strains of human influenza A and B viruses Between 5– 20 percent of the population may be infected annually Most people have some immunity to the currently circulating strains of influenza virus Thus, the severity and impact of seasonal influenza is substantially less than during pandemics 32
Seasonal Influenza n n Refers to periodic outbreaks of acute onset viral respiratory infection caused by circulating strains of human influenza A and B viruses Between 5– 20 percent of the population may be infected annually Most people have some immunity to the currently circulating strains of influenza virus Thus, the severity and impact of seasonal influenza is substantially less than during pandemics 32
 Avian Influenza: Bird Flu n n n Caused by type A influenza viruses that infect wild birds and domestic poultry Some forms of the avian influenza are worse than others Generally divided into two groups n n low pathogenic avian influenza highly pathogenic avian influenza 33
Avian Influenza: Bird Flu n n n Caused by type A influenza viruses that infect wild birds and domestic poultry Some forms of the avian influenza are worse than others Generally divided into two groups n n low pathogenic avian influenza highly pathogenic avian influenza 33
 Low Pathogenic Avian Influenza n n n Naturally occurs in wild birds and can spread to domestic birds In general, poses little threat to human health Has the potential to mutate into highly pathogenic avian influenza and is, therefore, closely monitored 34
Low Pathogenic Avian Influenza n n n Naturally occurs in wild birds and can spread to domestic birds In general, poses little threat to human health Has the potential to mutate into highly pathogenic avian influenza and is, therefore, closely monitored 34
 High Pathogenic Avian Influenza n n n Can spread rapidly Has a high death rate in birds H 5 N 1 n now rapidly spreading in birds in some parts of the world one of the few avian influenza viruses to have crossed the species barrier to infect humans the most deadly of those viruses that have crossed the barrier 35
High Pathogenic Avian Influenza n n n Can spread rapidly Has a high death rate in birds H 5 N 1 n now rapidly spreading in birds in some parts of the world one of the few avian influenza viruses to have crossed the species barrier to infect humans the most deadly of those viruses that have crossed the barrier 35
 Humans and H 5 N 1 n n Most cases of H 5 N 1 infections in humans have resulted from contact with infected poultry or surfaces contaminated with secretion/excretions from infected birds Spread of H 5 N 1 from person to person has been limited to rare, sporadic cases H 5 N 1 does not commonly infect humans In humans, there is little or no immune protection against H 5 N 1 (Information from November 2006) 36
Humans and H 5 N 1 n n Most cases of H 5 N 1 infections in humans have resulted from contact with infected poultry or surfaces contaminated with secretion/excretions from infected birds Spread of H 5 N 1 from person to person has been limited to rare, sporadic cases H 5 N 1 does not commonly infect humans In humans, there is little or no immune protection against H 5 N 1 (Information from November 2006) 36
 Influenza Pandemic Patterns n n n Many scientists believe that since no pandemic has occurred since 1968, it is only a matter of time before another pandemic occurs A pandemic may occur in waves of outbreaks with each wave in a community lasting 8 to 12 weeks One-to-three waves may occur 37
Influenza Pandemic Patterns n n n Many scientists believe that since no pandemic has occurred since 1968, it is only a matter of time before another pandemic occurs A pandemic may occur in waves of outbreaks with each wave in a community lasting 8 to 12 weeks One-to-three waves may occur 37
 Critical Response Elements n n n Rapid detection of unusual influenza outbreaks Isolation of possible pandemic viruses Immediate notification of national and international health authorities 38
Critical Response Elements n n n Rapid detection of unusual influenza outbreaks Isolation of possible pandemic viruses Immediate notification of national and international health authorities 38
 n n n URL: http: //www. who. int/en/ WHO is the directing and coordinating authority for health within the United Nations system. It is responsible for providing leadership on global health matters, shaping the health research agenda, setting norms and standards, articulating evidence-based policy options, providing technical support to countries and monitoring and assessing health trends. WHO URL for Avian Influenza http: //www. who. int/topics/avian_influenza/en/ 39
n n n URL: http: //www. who. int/en/ WHO is the directing and coordinating authority for health within the United Nations system. It is responsible for providing leadership on global health matters, shaping the health research agenda, setting norms and standards, articulating evidence-based policy options, providing technical support to countries and monitoring and assessing health trends. WHO URL for Avian Influenza http: //www. who. int/topics/avian_influenza/en/ 39
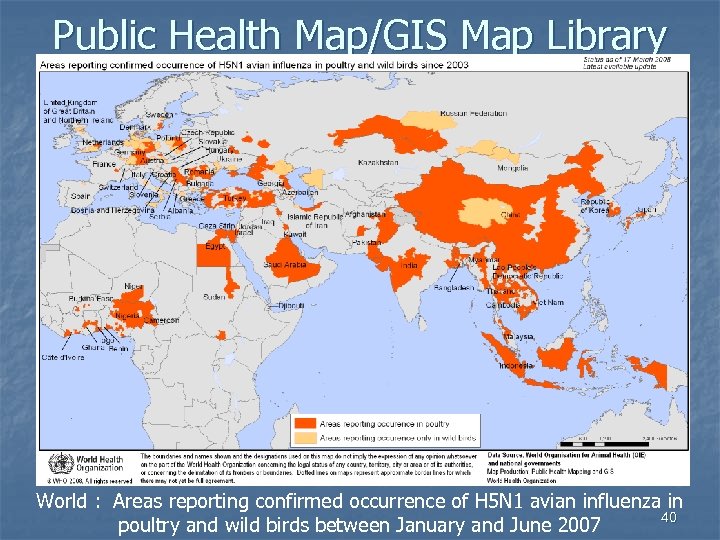 Public Health Map/GIS Map Library World : Areas reporting confirmed occurrence of H 5 N 1 avian influenza in 40 poultry and wild birds between January and June 2007
Public Health Map/GIS Map Library World : Areas reporting confirmed occurrence of H 5 N 1 avian influenza in 40 poultry and wild birds between January and June 2007
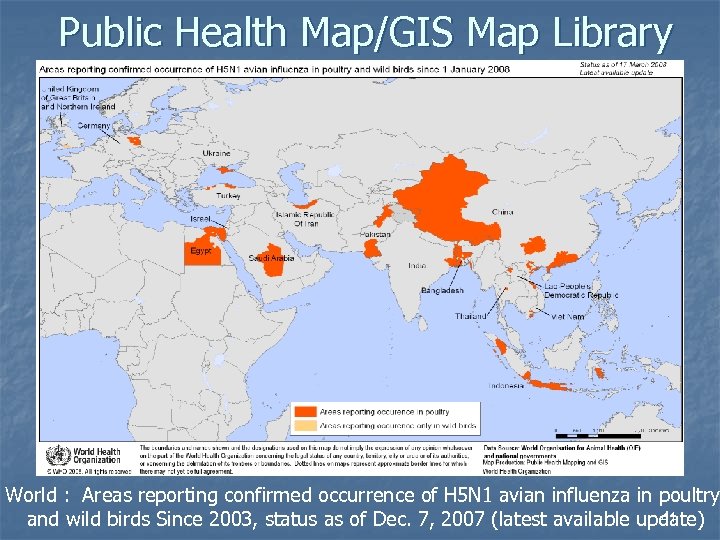 Public Health Map/GIS Map Library World : Areas reporting confirmed occurrence of H 5 N 1 avian influenza in poultry 41 and wild birds Since 2003, status as of Dec. 7, 2007 (latest available update)
Public Health Map/GIS Map Library World : Areas reporting confirmed occurrence of H 5 N 1 avian influenza in poultry 41 and wild birds Since 2003, status as of Dec. 7, 2007 (latest available update)
 WHO Pandemic Alert & Response System n n URL: http: //www. who. int/csr/en/ Purpose: The world requires a global system that can rapidly identify and contain public health emergencies and reduce unneeded panic and disruption of trade, travel and society in general 42
WHO Pandemic Alert & Response System n n URL: http: //www. who. int/csr/en/ Purpose: The world requires a global system that can rapidly identify and contain public health emergencies and reduce unneeded panic and disruption of trade, travel and society in general 42
 WHO Pandemic Alert System n Phase 1 & 2 n n n The “Inter-Pandemic Period” There is a novel influenza A virus in animals but no human cases have been observed Phase 2 indicates that an animal influenza subtype that poses a risk to humans has been detected 43
WHO Pandemic Alert System n Phase 1 & 2 n n n The “Inter-Pandemic Period” There is a novel influenza A virus in animals but no human cases have been observed Phase 2 indicates that an animal influenza subtype that poses a risk to humans has been detected 43
 WHO Pandemic Alert System n Phase 3, 4 & 5 n n The “Pandemic Alert Period” A novel influenza virus causes human infection with a new subtype, but does not exhibit efficient and sustained human-to-human transmission 44
WHO Pandemic Alert System n Phase 3, 4 & 5 n n The “Pandemic Alert Period” A novel influenza virus causes human infection with a new subtype, but does not exhibit efficient and sustained human-to-human transmission 44
 WHO Pandemic Alert System n Phase 6 n n n The “Pandemic Period” A new influenza A virus develops the capacity for efficient and sustained human-to-human transmission in the general population The WHO declares that an influenza pandemic is in progress 45
WHO Pandemic Alert System n Phase 6 n n n The “Pandemic Period” A new influenza A virus develops the capacity for efficient and sustained human-to-human transmission in the general population The WHO declares that an influenza pandemic is in progress 45
 Sentinel Provider Network n n Operated by the CDC (Centers for Disease Control and Prevention URL: http: //www. cdc. gov/ 46
Sentinel Provider Network n n Operated by the CDC (Centers for Disease Control and Prevention URL: http: //www. cdc. gov/ 46
 CLINICAL DIAGNOSIS 47
CLINICAL DIAGNOSIS 47
 Clinical Presentation of Influenza n Varies from “no symptoms” at all in seasonal influenza to “fulminant” (fully “ symptomatic) disease in pandemic strains that result in severe illness and death (even among previously healthy adults and children) No Symptoms ----------- > Fulminant 48
Clinical Presentation of Influenza n Varies from “no symptoms” at all in seasonal influenza to “fulminant” (fully “ symptomatic) disease in pandemic strains that result in severe illness and death (even among previously healthy adults and children) No Symptoms ----------- > Fulminant 48
 Clinical Diagnosis n Clinical Diagnosis of Seasonal Influenza n n n n n sudden onset of fever respiratory illness muscle aches headaches nonproductive cough sore throat runny nose ear infections gastrointestinal symptoms 49
Clinical Diagnosis n Clinical Diagnosis of Seasonal Influenza n n n n n sudden onset of fever respiratory illness muscle aches headaches nonproductive cough sore throat runny nose ear infections gastrointestinal symptoms 49
 Accuracy of Diagnosis n It has been reported that the use of the influenza-like case definition is n n 63 to 78% accurate in identifying culture-confirmed cases of influenza (a sensitivity of 63 to 78%) 55 to 71% accurate in excluding influenza (specificity of 55 to 71%) 50
Accuracy of Diagnosis n It has been reported that the use of the influenza-like case definition is n n 63 to 78% accurate in identifying culture-confirmed cases of influenza (a sensitivity of 63 to 78%) 55 to 71% accurate in excluding influenza (specificity of 55 to 71%) 50
 Clinical Signs & Symptoms n n Sensitivity - The likelihood of a clinical sign or symptom to accurately detect influenza infection in a group of patients Specificity - The likelihood of a clinical sign T or symptom to exclude influenza infection in a group of patients who do not have influenza 51
Clinical Signs & Symptoms n n Sensitivity - The likelihood of a clinical sign or symptom to accurately detect influenza infection in a group of patients Specificity - The likelihood of a clinical sign T or symptom to exclude influenza infection in a group of patients who do not have influenza 51
 Laboratory Diagnosis Seasonal Influenza n n n n Commercial rapid testing can detect seasonal influenza virus in less than 30 minutes Some tests not very sensitive False negative results are common Not all of these tests can distinguish between influenza A and B viruses HHS/CDC Influenza Laboratory Diagnostic Procedures http: //www. cdc. gov/flu/professionals/labdiagnosis. htm 52
Laboratory Diagnosis Seasonal Influenza n n n n Commercial rapid testing can detect seasonal influenza virus in less than 30 minutes Some tests not very sensitive False negative results are common Not all of these tests can distinguish between influenza A and B viruses HHS/CDC Influenza Laboratory Diagnostic Procedures http: //www. cdc. gov/flu/professionals/labdiagnosis. htm 52
 Clinical Diagnosis Pandemic Influenza n n Patients will likely have clinical signs and symptoms similar to seasonal influenza Clinical presentation and course of illness may be severe in a higher percentage of the cases of pandemic influenza 53
Clinical Diagnosis Pandemic Influenza n n Patients will likely have clinical signs and symptoms similar to seasonal influenza Clinical presentation and course of illness may be severe in a higher percentage of the cases of pandemic influenza 53
 Laboratory Diagnosis Avian Influenza n n n HHS/CDC developed a 4 -hour RT-PCR assay to detect gene coding for H 5 surface protein of Asian lineage of the highly pathogenic H 5 N 1 avian influenza virus RT-PCR reagents distributed to approximately 140 designated laboratories of the Laboratory Response Network (LRN) Laboratories located in all 50 states 54
Laboratory Diagnosis Avian Influenza n n n HHS/CDC developed a 4 -hour RT-PCR assay to detect gene coding for H 5 surface protein of Asian lineage of the highly pathogenic H 5 N 1 avian influenza virus RT-PCR reagents distributed to approximately 140 designated laboratories of the Laboratory Response Network (LRN) Laboratories located in all 50 states 54
 Modes of Transmission Seasonal Influenza n 1 - Droplet Transmission n 2 - Airborne Transmission n n coughing, sneezing, or talking therapeutic manipulations i. e. suctioning or bronchoscopy disseminated by air currents to susceptible individuals can travel significant distances penetrate deep into the lung to the alveoli can establish an infection 3 - Contact Transmission n Direct contact - touching skin-to- skin 55
Modes of Transmission Seasonal Influenza n 1 - Droplet Transmission n 2 - Airborne Transmission n n coughing, sneezing, or talking therapeutic manipulations i. e. suctioning or bronchoscopy disseminated by air currents to susceptible individuals can travel significant distances penetrate deep into the lung to the alveoli can establish an infection 3 - Contact Transmission n Direct contact - touching skin-to- skin 55
 Modes of Transmission Pandemic Influenza n Transmission - Past Pandemics n n n Person-to-person Airborne Transmission - Future Pandemics n May be possible by n n n contact with blood CSF (cerebrospinal fluid) feces respiratory secretions mucous membranes of the eye 56
Modes of Transmission Pandemic Influenza n Transmission - Past Pandemics n n n Person-to-person Airborne Transmission - Future Pandemics n May be possible by n n n contact with blood CSF (cerebrospinal fluid) feces respiratory secretions mucous membranes of the eye 56
 Treatment and Prevention Seasonal Influenza n n Antiviral medications Vaccinations n n Intranasal live attenuated vaccine Injectable, inactivated trivalent vaccine 57
Treatment and Prevention Seasonal Influenza n n Antiviral medications Vaccinations n n Intranasal live attenuated vaccine Injectable, inactivated trivalent vaccine 57
 Treatment and Prevention Pandemic Influenza n n Antiviral medications HHS recommends use of neuraminidase inhibitors zanamivir and oseltamivir because of influenza resistance to amantadine and rimantadine 58
Treatment and Prevention Pandemic Influenza n n Antiviral medications HHS recommends use of neuraminidase inhibitors zanamivir and oseltamivir because of influenza resistance to amantadine and rimantadine 58
 Poster n A printable poster on the sequences for putting on and taking off PPE, which can be used for employee training and can be posted outside respiratory isolation rooms, is available at http: //www. cdc. gov/nc idod/sars/ic. htm 59
Poster n A printable poster on the sequences for putting on and taking off PPE, which can be used for employee training and can be posted outside respiratory isolation rooms, is available at http: //www. cdc. gov/nc idod/sars/ic. htm 59
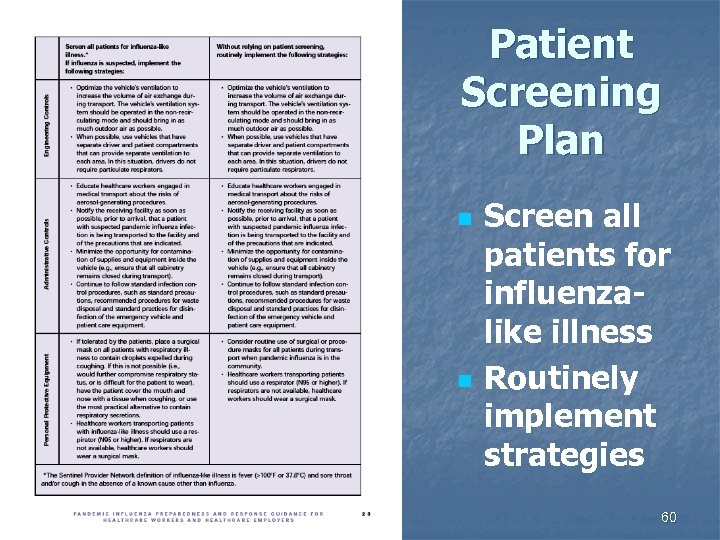 Patient Screening Plan n n Screen all patients for influenzalike illness Routinely implement strategies 60
Patient Screening Plan n n Screen all patients for influenzalike illness Routinely implement strategies 60
 Surveillance Activities Healthcare Workers n Keep a register of healthcare workers who have n n provided care for pandemic influenza-infected patients recovered from pandemic influenza Encourage self-reporting by symptomatic healthcare workers Exclude symptomatic healthcare workers from duty 61
Surveillance Activities Healthcare Workers n Keep a register of healthcare workers who have n n provided care for pandemic influenza-infected patients recovered from pandemic influenza Encourage self-reporting by symptomatic healthcare workers Exclude symptomatic healthcare workers from duty 61
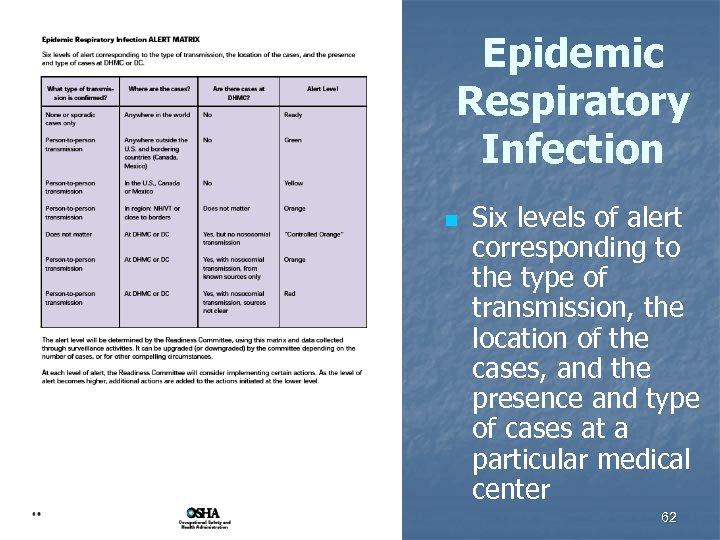 Epidemic Respiratory Infection n Six levels of alert corresponding to the type of transmission, the location of the cases, and the presence and type of cases at a particular medical center 62
Epidemic Respiratory Infection n Six levels of alert corresponding to the type of transmission, the location of the cases, and the presence and type of cases at a particular medical center 62
 Alert Levels n Determined by Readiness Committee n n Can be upgraded (or downgraded) by the committee depending on n n use matrix and data collected through surveillance activities number of cases other compelling circumstances At each level of alert Readiness Committee will consider implementing certain actions As level of alert becomes higher, additional actions are added to actions initiated at lower level 63
Alert Levels n Determined by Readiness Committee n n Can be upgraded (or downgraded) by the committee depending on n n use matrix and data collected through surveillance activities number of cases other compelling circumstances At each level of alert Readiness Committee will consider implementing certain actions As level of alert becomes higher, additional actions are added to actions initiated at lower level 63
 Level: READY n n Baseline activities to ensure preparedness in the absence of known active epidemic of ERI in the world ERI = Epidemic Respiratory Infection 64
Level: READY n n Baseline activities to ensure preparedness in the absence of known active epidemic of ERI in the world ERI = Epidemic Respiratory Infection 64
 Level: GREEN n Confirmed efficient human-to-human transmission of potentially epidemic contagious respiratory infection present outside the U. S. and bordering countries (Canada and Mexico) 65
Level: GREEN n Confirmed efficient human-to-human transmission of potentially epidemic contagious respiratory infection present outside the U. S. and bordering countries (Canada and Mexico) 65
 Level: YELLOW n Confirmed human-tohuman transmission of potentially epidemic contagious respiratory infection (ERI) documented in the U. S. or bordering countries (Canada or Mexico) 66
Level: YELLOW n Confirmed human-tohuman transmission of potentially epidemic contagious respiratory infection (ERI) documented in the U. S. or bordering countries (Canada or Mexico) 66
 Level: CONTROLLED ORANGE n A case of ERI has been diagnosed at a particular medical center or in an inpatient at a medical center but there has been no documented nosocomial or community spread from this person to others 67
Level: CONTROLLED ORANGE n A case of ERI has been diagnosed at a particular medical center or in an inpatient at a medical center but there has been no documented nosocomial or community spread from this person to others 67
 Level: ORANGE n There is evidence of nosocomial transmission of ERI from known infected patients to other patients, employees, or visitors at a particular medical center, OR there is human-to-human transmission in specified region, or nearby 68
Level: ORANGE n There is evidence of nosocomial transmission of ERI from known infected patients to other patients, employees, or visitors at a particular medical center, OR there is human-to-human transmission in specified region, or nearby 68
 Level: RED n There is evidence of untraceable or uncontrolled nosocomial transmission of ERI OR there is widespread human-to-human transmission in a particular region or nearby 69
Level: RED n There is evidence of untraceable or uncontrolled nosocomial transmission of ERI OR there is widespread human-to-human transmission in a particular region or nearby 69
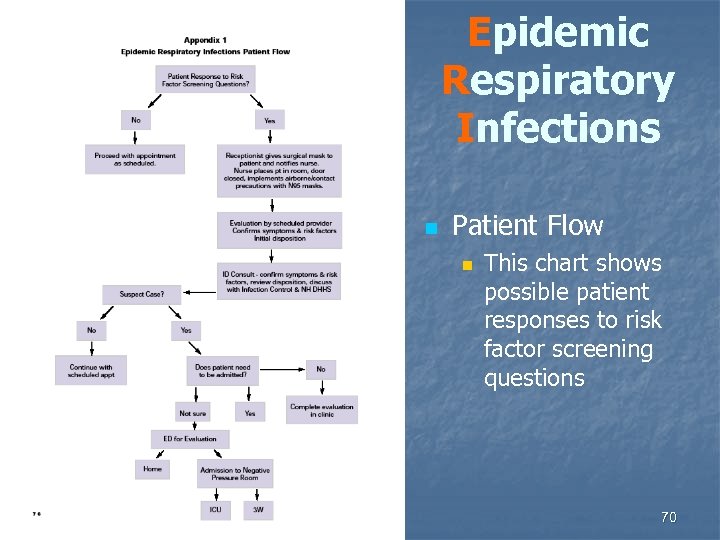 Epidemic Respiratory Infections n Patient Flow n This chart shows possible patient responses to risk factor screening questions 70
Epidemic Respiratory Infections n Patient Flow n This chart shows possible patient responses to risk factor screening questions 70
 ERI Outpatient Management Protocol n n For patients with new cough and risk factors associated with epidemic respiratory infection (ERI) Key points n n n Give patient a mask Require PPE for anyone visiting patient Evaluate risk factors n n n Consult with physician Move patient to room with negative air pressure Administer tests Coordinate medical followup if patient is released Activate ERI plan if patient is admitted 71
ERI Outpatient Management Protocol n n For patients with new cough and risk factors associated with epidemic respiratory infection (ERI) Key points n n n Give patient a mask Require PPE for anyone visiting patient Evaluate risk factors n n n Consult with physician Move patient to room with negative air pressure Administer tests Coordinate medical followup if patient is released Activate ERI plan if patient is admitted 71
 Principles Care of ERI Patients n n n Minimize Health Care Workers (HCW) contact with the patient Protect HCWs during contact with patient Minimize opportunities for exposure to other patients or visitors 72
Principles Care of ERI Patients n n n Minimize Health Care Workers (HCW) contact with the patient Protect HCWs during contact with patient Minimize opportunities for exposure to other patients or visitors 72
 ERI Inpatient Management Protocol n This plan will be put into effect when a patient is believed to n n meet the criteria for an Epidemic Respiratory Infection by one of the Infectious Disease Physicians, and needs hospitalization 73
ERI Inpatient Management Protocol n This plan will be put into effect when a patient is believed to n n meet the criteria for an Epidemic Respiratory Infection by one of the Infectious Disease Physicians, and needs hospitalization 73
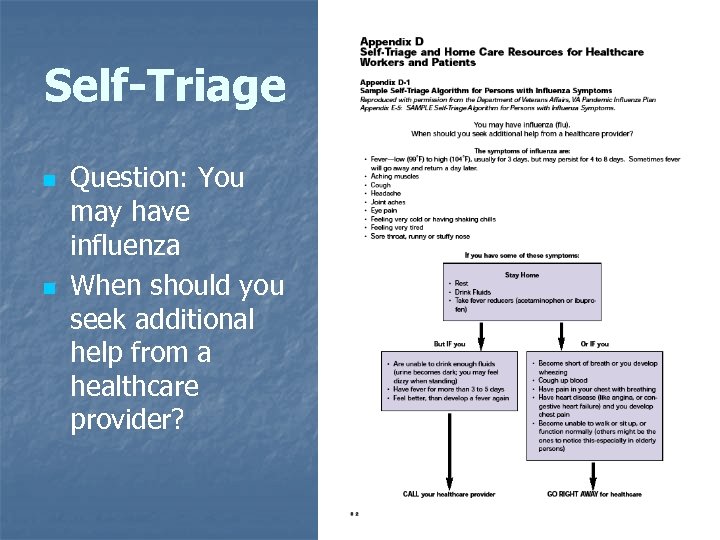 Self-Triage n n Question: You may have influenza When should you seek additional help from a healthcare provider? 74
Self-Triage n n Question: You may have influenza When should you seek additional help from a healthcare provider? 74
 Home Care Guide for Influenza n n Common symptoms Supplies to have on hand Caring for a person with influenza When to seek additional medical advice 75
Home Care Guide for Influenza n n Common symptoms Supplies to have on hand Caring for a person with influenza When to seek additional medical advice 75
 Symptom and Care Log for Home Care n Copy, fill out, and bring log sheets to healthcare provider visits 76
Symptom and Care Log for Home Care n Copy, fill out, and bring log sheets to healthcare provider visits 76
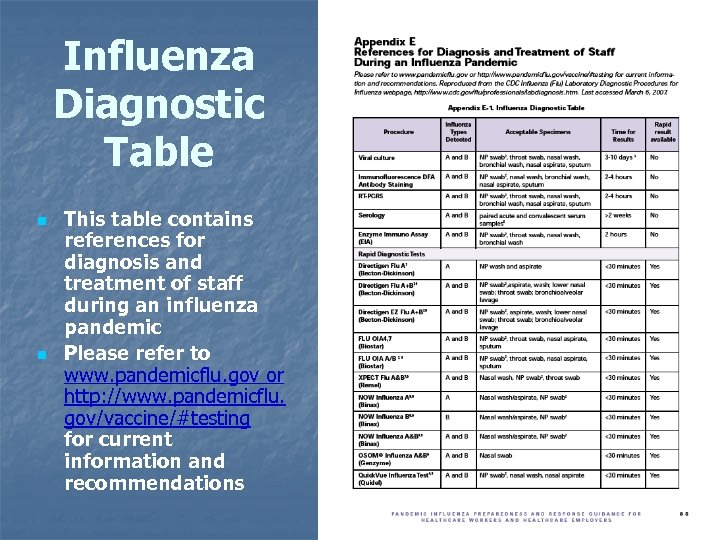 Influenza Diagnostic Table n n This table contains references for diagnosis and treatment of staff during an influenza pandemic Please refer to www. pandemicflu. gov or http: //www. pandemicflu. gov/vaccine/#testing for current information and recommendations 77
Influenza Diagnostic Table n n This table contains references for diagnosis and treatment of staff during an influenza pandemic Please refer to www. pandemicflu. gov or http: //www. pandemicflu. gov/vaccine/#testing for current information and recommendations 77
 Planning Checklists and Example Plans n Many government and private organizations have developed viable resources including n n n planning checklists example plans communication plans 78
Planning Checklists and Example Plans n Many government and private organizations have developed viable resources including n n n planning checklists example plans communication plans 78
 Assistance Available from n Safety and Health Program Management Guidelines n n Management leadership and employee involvement Worksite analysis Hazard prevention and control Safety and health training 79
Assistance Available from n Safety and Health Program Management Guidelines n n Management leadership and employee involvement Worksite analysis Hazard prevention and control Safety and health training 79
 INFECTION CONTROL 80
INFECTION CONTROL 80
 Infection Control n Use same strategies implemented for any infectious agent n facility and environmental controls n n engineering controls standard operating procedures administrative controls n personal protective clothing and equipment n safe work practices n 81
Infection Control n Use same strategies implemented for any infectious agent n facility and environmental controls n n engineering controls standard operating procedures administrative controls n personal protective clothing and equipment n safe work practices n 81
 Standard Precautions n Apply to n n blood all body fluids, secretions, and excretions except sweat, regardless of whether or not they contain visible blood non-intact skin mucous membranes 82
Standard Precautions n Apply to n n blood all body fluids, secretions, and excretions except sweat, regardless of whether or not they contain visible blood non-intact skin mucous membranes 82
 First, Conduct Risk Assessment n n Conduct to determine necessary PPE and work practices to avoid contact with blood, body fluids, excretions, and secretions Will help to customize standard precautions to the healthcare setting of interest 83
First, Conduct Risk Assessment n n Conduct to determine necessary PPE and work practices to avoid contact with blood, body fluids, excretions, and secretions Will help to customize standard precautions to the healthcare setting of interest 83
 Next, Implement Procedures n Include n n n Gloves and facial (nose, mouth, and eye) protection Hand hygiene before and after patient contact, and after removing gloves or other PPE Handling and disinfection of patient care equipment, patient rooms, and soiled linen 84
Next, Implement Procedures n Include n n n Gloves and facial (nose, mouth, and eye) protection Hand hygiene before and after patient contact, and after removing gloves or other PPE Handling and disinfection of patient care equipment, patient rooms, and soiled linen 84
 Then, Use Precautions n Contact Precautions n n Droplet Precautions n n Use PPE, dedicated patient care equipment, limitation of patient movement, private rooms Use surgical masks within 3 feet of a patient Airborne Precautions n Place patient in a negative pressure room and follow associated precautions 85
Then, Use Precautions n Contact Precautions n n Droplet Precautions n n Use PPE, dedicated patient care equipment, limitation of patient movement, private rooms Use surgical masks within 3 feet of a patient Airborne Precautions n Place patient in a negative pressure room and follow associated precautions 85
 Compliance with Infection Control n “Weak Links” n n Adherence to hand hygiene Consistent and proper use of PPE Influenza vaccination of healthcare workers Perform serologic and other testing for pandemic influenza on healthcare workers with influenza-like illness and who have had likely exposures to pandemic influenza-infected patients 86
Compliance with Infection Control n “Weak Links” n n Adherence to hand hygiene Consistent and proper use of PPE Influenza vaccination of healthcare workers Perform serologic and other testing for pandemic influenza on healthcare workers with influenza-like illness and who have had likely exposures to pandemic influenza-infected patients 86
 Personal Protective Equipment Gloves n HHS recommends the use of gloves when there is contact with blood and bodily fluids, including respiratory secretions n n latex vinyl nitrile other synthetic materials 87
Personal Protective Equipment Gloves n HHS recommends the use of gloves when there is contact with blood and bodily fluids, including respiratory secretions n n latex vinyl nitrile other synthetic materials 87
 Personal Protective Equipment Gowns n Healthcare workers should wear an isolation gown when clothes will come into contact with blood or other bodily fluids (respiratory secretions) n n n during procedures such as intubation when closely holding a pediatric patient Isolation gowns can be n n disposable and made of synthetic material reusable and made of washable cloth 88
Personal Protective Equipment Gowns n Healthcare workers should wear an isolation gown when clothes will come into contact with blood or other bodily fluids (respiratory secretions) n n n during procedures such as intubation when closely holding a pediatric patient Isolation gowns can be n n disposable and made of synthetic material reusable and made of washable cloth 88
 Personal Protective Equipment Goggles/Face Shields n Wear goggles and/or face shields if n n sprays or splatters of infectious material are likely if a pandemic influenza patient is coughing For additional information about eye protection for infection control, visit NIOSH’s website at http: //www. cdc. gov/niosh/topics/eyeinfectious. html 89
Personal Protective Equipment Goggles/Face Shields n Wear goggles and/or face shields if n n sprays or splatters of infectious material are likely if a pandemic influenza patient is coughing For additional information about eye protection for infection control, visit NIOSH’s website at http: //www. cdc. gov/niosh/topics/eyeinfectious. html 89
 Personal Protective Equipment Respiratory Protection n Comply with OSHA’s Respiratory Protection standard (29 CFR 1910. 134) to achieve high levels of protection All respirators used by employees are required to be tested and certified by NIOSH For a list of all NIOSH-certified respirators (the Certified Equipment List), see http: //www. cdc. gov/niosh/celintro. html 90
Personal Protective Equipment Respiratory Protection n Comply with OSHA’s Respiratory Protection standard (29 CFR 1910. 134) to achieve high levels of protection All respirators used by employees are required to be tested and certified by NIOSH For a list of all NIOSH-certified respirators (the Certified Equipment List), see http: //www. cdc. gov/niosh/celintro. html 90
 Personal Protective Equipment NIOSH-Certified Respirators n NIOSH-certified respirators are marked with the n n n This information is printed on the n n manufacturer’s name part number protection provided by the filter (e. g. , N 95) “NIOSH” facepiece exhalation valve cover, or head straps If a respirator does not have these markings and does not appear on the Certified Equipment List, it has not been certified by NIOSH 91
Personal Protective Equipment NIOSH-Certified Respirators n NIOSH-certified respirators are marked with the n n n This information is printed on the n n manufacturer’s name part number protection provided by the filter (e. g. , N 95) “NIOSH” facepiece exhalation valve cover, or head straps If a respirator does not have these markings and does not appear on the Certified Equipment List, it has not been certified by NIOSH 91
 Required Elements of an OSHA Respirator Program 1. 2. 3. 4. 5. 6. 7. 8. Selection Medical evaluation Fit testing Use Maintenance and care Breathing air quality and use Training Program evaluation 92
Required Elements of an OSHA Respirator Program 1. 2. 3. 4. 5. 6. 7. 8. Selection Medical evaluation Fit testing Use Maintenance and care Breathing air quality and use Training Program evaluation 92
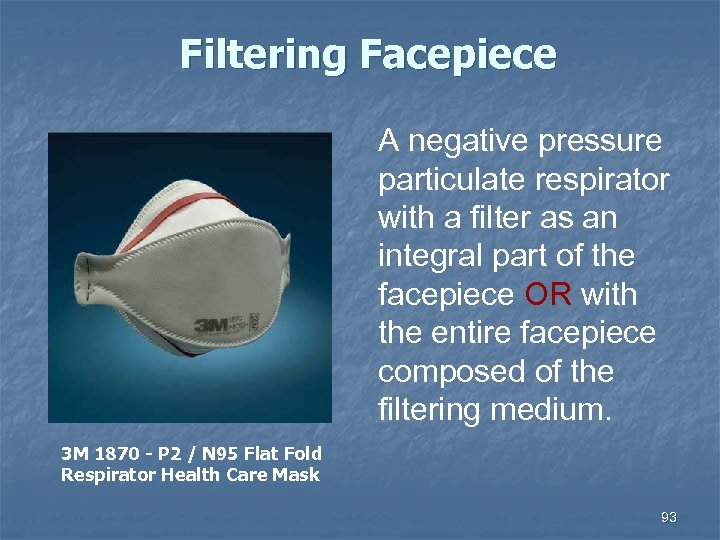 Filtering Facepiece A negative pressure particulate respirator with a filter as an integral part of the facepiece OR with the entire facepiece composed of the filtering medium. 3 M 1870 - P 2 / N 95 Flat Fold Respirator Health Care Mask 93
Filtering Facepiece A negative pressure particulate respirator with a filter as an integral part of the facepiece OR with the entire facepiece composed of the filtering medium. 3 M 1870 - P 2 / N 95 Flat Fold Respirator Health Care Mask 93
 Medical Evaluation If these masks are required, then: n Provide a medical evaluation according to the OSHA standard n n Required to determine employee’s ability to use a respirator before fit testing and use Identify a PLHCP n Required to perform medical evaluations using a medical questionnaire OR an initial medical examination that obtains the same information 94
Medical Evaluation If these masks are required, then: n Provide a medical evaluation according to the OSHA standard n n Required to determine employee’s ability to use a respirator before fit testing and use Identify a PLHCP n Required to perform medical evaluations using a medical questionnaire OR an initial medical examination that obtains the same information 94
 PLHCP Physician or Other Licensed Health Care Professional An individual whose legally permitted scope of practice (i. e. , license, registration, or certification) allows him / her to independently provide, or be delegated the responsibility to provide, some or all of the health care services required by paragraph (e) Medical evaluation 95
PLHCP Physician or Other Licensed Health Care Professional An individual whose legally permitted scope of practice (i. e. , license, registration, or certification) allows him / her to independently provide, or be delegated the responsibility to provide, some or all of the health care services required by paragraph (e) Medical evaluation 95
 Medical Evaluation n n Must obtain information requested by questionnaire in Sections 1 and 2, Part A of Appendix C Follow-up medical examination n Required for employee who gives a positive response to any question among questions #1 through #8 in Section 2, Part A of App. C OR whose initial medical examination demonstrates the need for a follow-up medical examination 96
Medical Evaluation n n Must obtain information requested by questionnaire in Sections 1 and 2, Part A of Appendix C Follow-up medical examination n Required for employee who gives a positive response to any question among questions #1 through #8 in Section 2, Part A of App. C OR whose initial medical examination demonstrates the need for a follow-up medical examination 96
 OSHA Respirator Medical Evaluation Questionnaire Page 1 of 10 97
OSHA Respirator Medical Evaluation Questionnaire Page 1 of 10 97
 Medical Evaluation n n Annual review of medical status is not required At a minimum, employer must provide additional medical evaluations if: Employee reports medical signs or symptoms n PLHCP, supervisor, or program administrator indicates an employee needs to be reevaluated n Change occurs in workplace conditions that may increase the burden on an employee n 98
Medical Evaluation n n Annual review of medical status is not required At a minimum, employer must provide additional medical evaluations if: Employee reports medical signs or symptoms n PLHCP, supervisor, or program administrator indicates an employee needs to be reevaluated n Change occurs in workplace conditions that may increase the burden on an employee n 98
 Fit Testing n Employees using tight-fitting facepiece respirators must pass an appropriate qualitative fit test (QLFT) or quantitative fit test (QNFT) prior to initial use n whenever a different respirator facepiece (size, style, model or make) is used, and n at least annually thereafter n 99
Fit Testing n Employees using tight-fitting facepiece respirators must pass an appropriate qualitative fit test (QLFT) or quantitative fit test (QNFT) prior to initial use n whenever a different respirator facepiece (size, style, model or make) is used, and n at least annually thereafter n 99
 Fit Testing The respirator used for fit testing MUST be the same make, model, style, and size of respirator that will be used by the employee. 100
Fit Testing The respirator used for fit testing MUST be the same make, model, style, and size of respirator that will be used by the employee. 100
 Quantitative Fit Test - QNFT An assessment of the adequacy of respirator fit by numerically measuring the amount of leakage into the respirator. QNFT Protocols –Generated Aerosol (corn oil, salt, DEHP) –Condensation Nuclei Counter (Porta. Count) –Controlled Negative Pressure (Dynatech Fit. Tester 3000) 101
Quantitative Fit Test - QNFT An assessment of the adequacy of respirator fit by numerically measuring the amount of leakage into the respirator. QNFT Protocols –Generated Aerosol (corn oil, salt, DEHP) –Condensation Nuclei Counter (Porta. Count) –Controlled Negative Pressure (Dynatech Fit. Tester 3000) 101
 Qualiitative Fit Test - QLFT A pass/fail fit test to assess the adequacy of respirator fit that relies on the individual’s response to the test agent. n Isoamyl acetate n Saccharin n Bitrex n Irritant smoke Saccharin Fit Test Kit 102
Qualiitative Fit Test - QLFT A pass/fail fit test to assess the adequacy of respirator fit that relies on the individual’s response to the test agent. n Isoamyl acetate n Saccharin n Bitrex n Irritant smoke Saccharin Fit Test Kit 102
 Training and Information n Employees who are required to use respirators must be trained such that they can demonstrate knowledge of at least why the respirator is necessary n limitations and capabilities of the respirator n effective use in emergency situations n how to don the respirator n n How to perform user seal check (each time) recognition of medical signs and symptoms n Proper disposal of the respirator n 103
Training and Information n Employees who are required to use respirators must be trained such that they can demonstrate knowledge of at least why the respirator is necessary n limitations and capabilities of the respirator n effective use in emergency situations n how to don the respirator n n How to perform user seal check (each time) recognition of medical signs and symptoms n Proper disposal of the respirator n 103
 Personal Protective Equipment Putting on and Removing PPE n HHS/CDC recommends that personal protective equipment be put on in the following order n n n Gown Respirator (or mask, when appropriate) Face shield or goggles Gloves Upon leaving the room, HHS/CDC recommends that PPE be removed in a way to avoid selfcontamination, as follows n n Gloves Faceshield or goggles Gown Respirator or mask 104
Personal Protective Equipment Putting on and Removing PPE n HHS/CDC recommends that personal protective equipment be put on in the following order n n n Gown Respirator (or mask, when appropriate) Face shield or goggles Gloves Upon leaving the room, HHS/CDC recommends that PPE be removed in a way to avoid selfcontamination, as follows n n Gloves Faceshield or goggles Gown Respirator or mask 104
 User Seal Check n n n Check the seal each time you don the respirator Place one or both hands completely over the middle panel Inhale and exhale sharply n n If air leaks around your nose, readjust the nosepiece If air leaks between the face and face seal of the respirator, reposition it and adjust straps If you cannot achieve a proper seal, do not enter the contaminated area See your supervisor
User Seal Check n n n Check the seal each time you don the respirator Place one or both hands completely over the middle panel Inhale and exhale sharply n n If air leaks around your nose, readjust the nosepiece If air leaks between the face and face seal of the respirator, reposition it and adjust straps If you cannot achieve a proper seal, do not enter the contaminated area See your supervisor
 Time / Use Limitation n 3 M #1860 N 95 HEALTH CARE RESPIRATOR n If the respirator becomes damaged, soiled, or breathing becomes difficult, leave the contaminated area and replace the respirator Wear a respirator only once Remove and dispose of a respirator in an appropriate trash receptacle Upon re-entry, don a new respirator
Time / Use Limitation n 3 M #1860 N 95 HEALTH CARE RESPIRATOR n If the respirator becomes damaged, soiled, or breathing becomes difficult, leave the contaminated area and replace the respirator Wear a respirator only once Remove and dispose of a respirator in an appropriate trash receptacle Upon re-entry, don a new respirator
 Healthcare Respirator n n 3 M™ Health Care Particulate Respirator and Surgical Mask 1870 NIOSH approved as an N 95 particulate filter respirator Designed to be fluid resistant to splash and spatter of blood and other infectious materials Intended for use against both mechanically generated particulates and thermally generated fumes, plumes and smokes Applications include n OR Laser Surgery n Electrocautery n Other powered medical instruments n Exposure to Mycobacterium tuberculosis n Aerosol droplet transfer, e. g. working with SARS patients n Dental care where aerosol and particle exposures are possible n Aerosols created during use and manipulation of chemotherapy solutions n Removal of casts or other dust producing activity 107
Healthcare Respirator n n 3 M™ Health Care Particulate Respirator and Surgical Mask 1870 NIOSH approved as an N 95 particulate filter respirator Designed to be fluid resistant to splash and spatter of blood and other infectious materials Intended for use against both mechanically generated particulates and thermally generated fumes, plumes and smokes Applications include n OR Laser Surgery n Electrocautery n Other powered medical instruments n Exposure to Mycobacterium tuberculosis n Aerosol droplet transfer, e. g. working with SARS patients n Dental care where aerosol and particle exposures are possible n Aerosols created during use and manipulation of chemotherapy solutions n Removal of casts or other dust producing activity 107
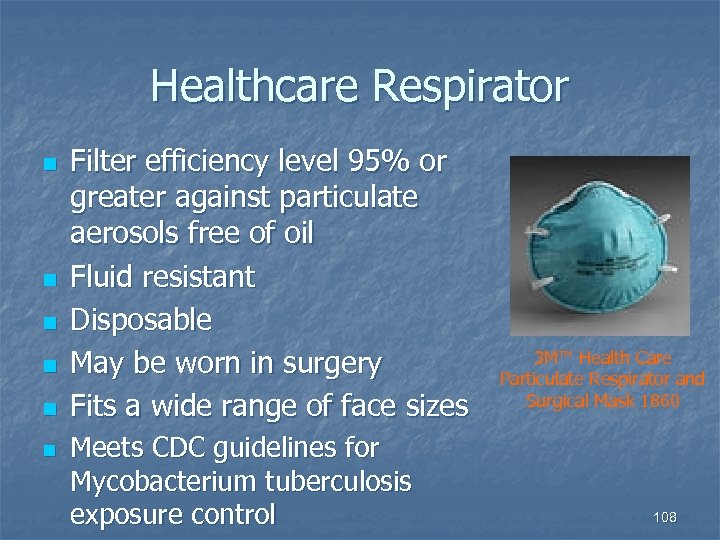 Healthcare Respirator n n n Filter efficiency level 95% or greater against particulate aerosols free of oil Fluid resistant Disposable May be worn in surgery Fits a wide range of face sizes Meets CDC guidelines for Mycobacterium tuberculosis exposure control 3 M™ Health Care Particulate Respirator and Surgical Mask 1860 108
Healthcare Respirator n n n Filter efficiency level 95% or greater against particulate aerosols free of oil Fluid resistant Disposable May be worn in surgery Fits a wide range of face sizes Meets CDC guidelines for Mycobacterium tuberculosis exposure control 3 M™ Health Care Particulate Respirator and Surgical Mask 1860 108
 Healthcare Respirator n n n Kimberly-Clark Fluidshield PFR 95 N 95 Particulate Filter Respirator and Surgical Mask n NIOSH approved as an N 95 particulate filter respirator Intended for use by operating room personnel and health care workers Meets CDC guidelines for TB control Helps protect patients and health care workers from the transfer of microorganisms, blood and bodily fluids 109
Healthcare Respirator n n n Kimberly-Clark Fluidshield PFR 95 N 95 Particulate Filter Respirator and Surgical Mask n NIOSH approved as an N 95 particulate filter respirator Intended for use by operating room personnel and health care workers Meets CDC guidelines for TB control Helps protect patients and health care workers from the transfer of microorganisms, blood and bodily fluids 109
 Healthcare Respirator n Moldex 3200 Series N 95 Particulate Respirators Both an N 95 respirator and surgical mask n Latex free n First and only NIOSHapproved single strap N 95 respirator n Meets CDC guidelines for TB exposure control standards n Moldex 3200 Series N 95 Particulate Respirator 110
Healthcare Respirator n Moldex 3200 Series N 95 Particulate Respirators Both an N 95 respirator and surgical mask n Latex free n First and only NIOSHapproved single strap N 95 respirator n Meets CDC guidelines for TB exposure control standards n Moldex 3200 Series N 95 Particulate Respirator 110
 SUMMARY This course has covered Definition of Pandemic Influenza n Transmission Routes n Clinical Diagnosis n Control n Prevention n 111
SUMMARY This course has covered Definition of Pandemic Influenza n Transmission Routes n Clinical Diagnosis n Control n Prevention n 111
 Questions? 112
Questions? 112


