4189cbfa215ce4720db9139bf5f72091.ppt
- Количество слайдов: 23
 OVERVIEW OF MQSA INSPECTION FINDINGS 8/22/01 Walid G. Mourad, Ph. D. , CSO Inspection Support Branch DMQRP/OHIP/CDRH/FDA
OVERVIEW OF MQSA INSPECTION FINDINGS 8/22/01 Walid G. Mourad, Ph. D. , CSO Inspection Support Branch DMQRP/OHIP/CDRH/FDA
 OVERVIEW OF MQSA INSPECTION FINDINGS · BACKGROUND · FINDING LEVEL DISTRIBUTION - Historical Perspective · INSPECTION FINDINGS - Final Regulations · L 1 & L 2 FINDINGS - The last two years · PROGRAMS UNDERWAY
OVERVIEW OF MQSA INSPECTION FINDINGS · BACKGROUND · FINDING LEVEL DISTRIBUTION - Historical Perspective · INSPECTION FINDINGS - Final Regulations · L 1 & L 2 FINDINGS - The last two years · PROGRAMS UNDERWAY
 BACKGROUND – MQSA (10/92) • Interim Regulations (10/94) • Final Regulations (published 10/97, effective 4/99) – MQSRA (10/98) • Lay summary to all women • Release of original mammogram • Demonstration Program – Inspection Frequency
BACKGROUND – MQSA (10/92) • Interim Regulations (10/94) • Final Regulations (published 10/97, effective 4/99) – MQSRA (10/98) • Lay summary to all women • Release of original mammogram • Demonstration Program – Inspection Frequency
 Inspection Scope • Equipment performance including dose, phantom image, processing, & darkroom fog • Quality assurance & quality control test records (including survey report & mammography equipment evaluations) • • Consumer complaint mechanism Personnel qualifications Medical reports and lay summaries Medical outcomes audit
Inspection Scope • Equipment performance including dose, phantom image, processing, & darkroom fog • Quality assurance & quality control test records (including survey report & mammography equipment evaluations) • • Consumer complaint mechanism Personnel qualifications Medical reports and lay summaries Medical outcomes audit
 MQSA Finding Levels • Level 1 – Serious - Warning Letter within 15 days & subsequent facility response within 15 days • Level 2 – Moderate - 30 day response • Level 3 – Minor - Follow up at next inspection
MQSA Finding Levels • Level 1 – Serious - Warning Letter within 15 days & subsequent facility response within 15 days • Level 2 – Moderate - 30 day response • Level 3 – Minor - Follow up at next inspection
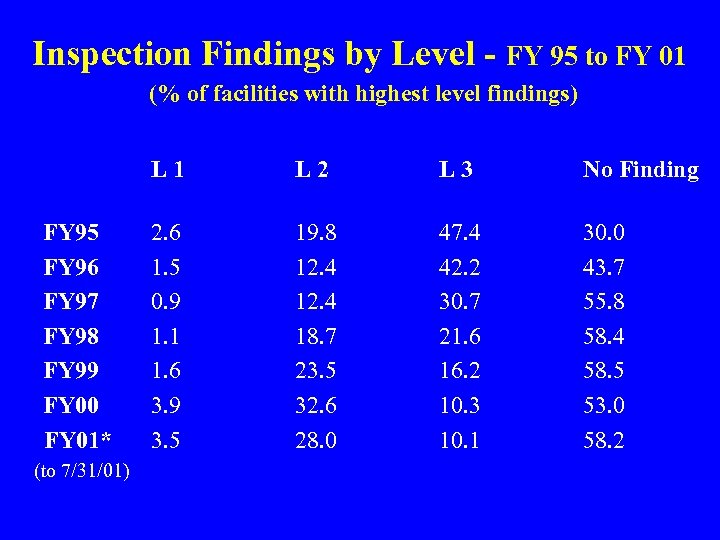 Inspection Findings by Level - FY 95 to FY 01 (% of facilities with highest level findings) L 1 FY 95 FY 96 FY 97 FY 98 FY 99 FY 00 FY 01* (to 7/31/01) L 2 L 3 No Finding 2. 6 1. 5 0. 9 1. 1 1. 6 3. 9 3. 5 19. 8 12. 4 18. 7 23. 5 32. 6 28. 0 47. 4 42. 2 30. 7 21. 6 16. 2 10. 3 10. 1 30. 0 43. 7 55. 8 58. 4 58. 5 53. 0 58. 2
Inspection Findings by Level - FY 95 to FY 01 (% of facilities with highest level findings) L 1 FY 95 FY 96 FY 97 FY 98 FY 99 FY 00 FY 01* (to 7/31/01) L 2 L 3 No Finding 2. 6 1. 5 0. 9 1. 1 1. 6 3. 9 3. 5 19. 8 12. 4 18. 7 23. 5 32. 6 28. 0 47. 4 42. 2 30. 7 21. 6 16. 2 10. 3 10. 1 30. 0 43. 7 55. 8 58. 4 58. 5 53. 0 58. 2
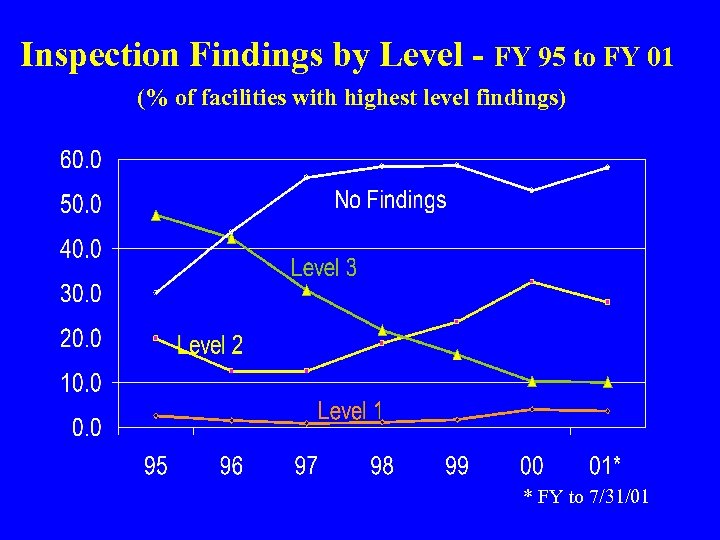 Inspection Findings by Level - FY 95 to FY 01 (% of facilities with highest level findings) * FY to 7/31/01
Inspection Findings by Level - FY 95 to FY 01 (% of facilities with highest level findings) * FY to 7/31/01
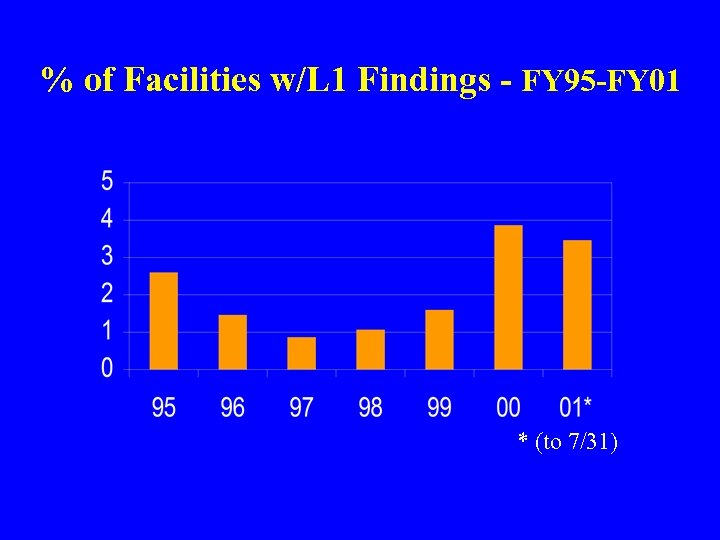 % of Facilities w/L 1 Findings - FY 95 -FY 01 * (to 7/31)
% of Facilities w/L 1 Findings - FY 95 -FY 01 * (to 7/31)
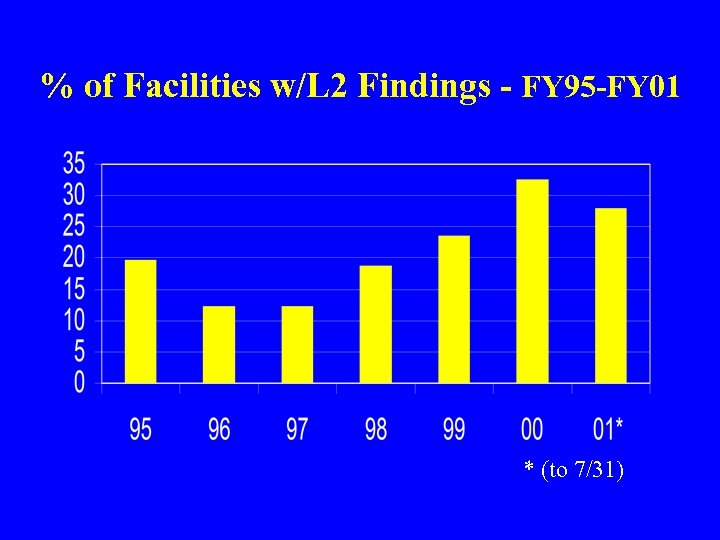 % of Facilities w/L 2 Findings - FY 95 -FY 01 * (to 7/31)
% of Facilities w/L 2 Findings - FY 95 -FY 01 * (to 7/31)
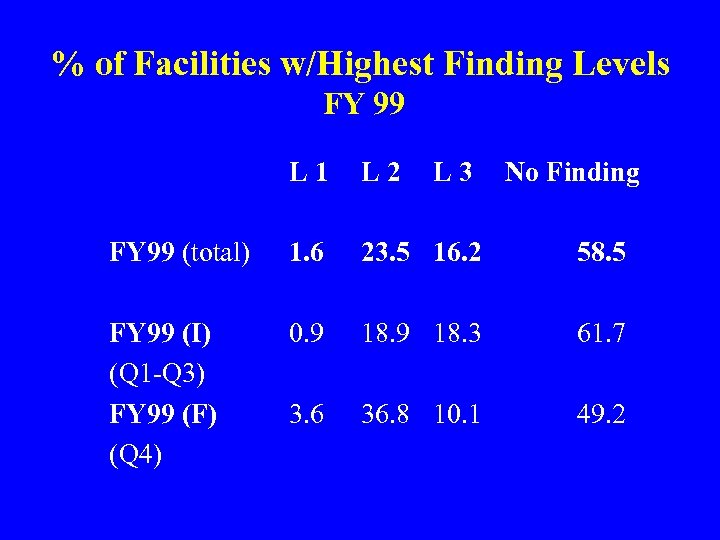 % of Facilities w/Highest Finding Levels FY 99 L 1 L 2 L 3 No Finding FY 99 (total) 1. 6 23. 5 16. 2 58. 5 FY 99 (I) (Q 1 -Q 3) FY 99 (F) (Q 4) 0. 9 18. 3 61. 7 3. 6 36. 8 10. 1 49. 2
% of Facilities w/Highest Finding Levels FY 99 L 1 L 2 L 3 No Finding FY 99 (total) 1. 6 23. 5 16. 2 58. 5 FY 99 (I) (Q 1 -Q 3) FY 99 (F) (Q 4) 0. 9 18. 3 61. 7 3. 6 36. 8 10. 1 49. 2
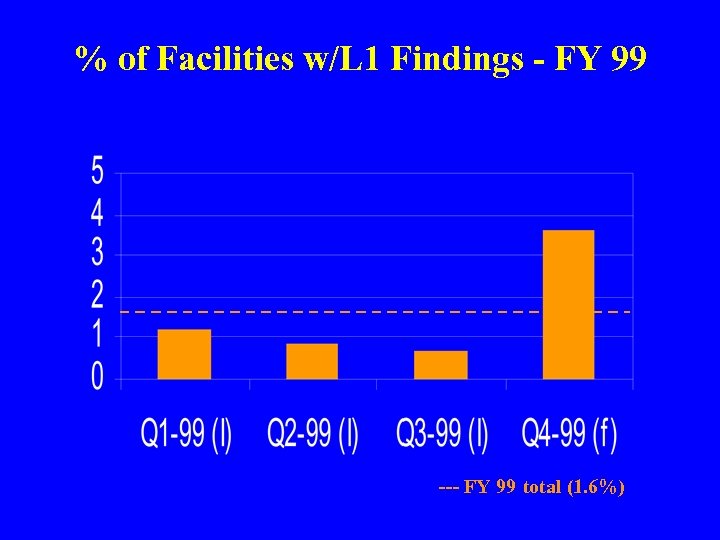 % of Facilities w/L 1 Findings - FY 99 --- FY 99 total (1. 6%)
% of Facilities w/L 1 Findings - FY 99 --- FY 99 total (1. 6%)
 Inspection Findings Under the Final Regulations • Level Changes & Consequences • L 1 & L 2 Findings ~ Two Year Results • Future Trend
Inspection Findings Under the Final Regulations • Level Changes & Consequences • L 1 & L 2 Findings ~ Two Year Results • Future Trend
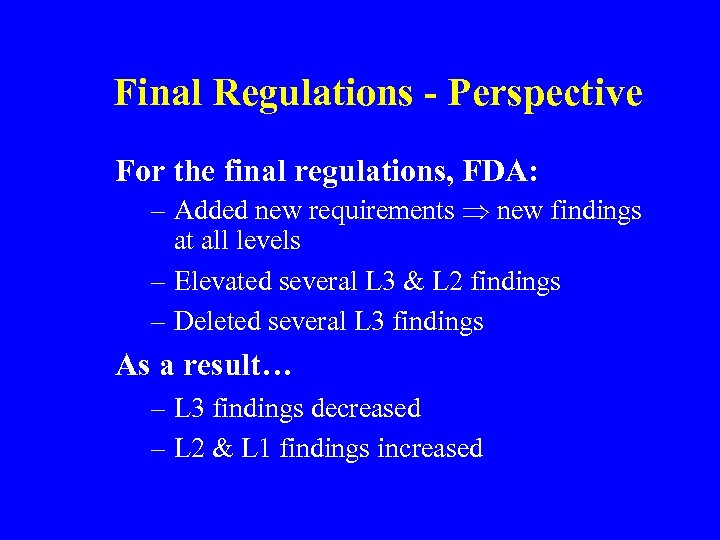 Final Regulations - Perspective For the final regulations, FDA: – Added new requirements new findings at all levels – Elevated several L 3 & L 2 findings – Deleted several L 3 findings As a result… – L 3 findings decreased – L 2 & L 1 findings increased
Final Regulations - Perspective For the final regulations, FDA: – Added new requirements new findings at all levels – Elevated several L 3 & L 2 findings – Deleted several L 3 findings As a result… – L 3 findings decreased – L 2 & L 1 findings increased
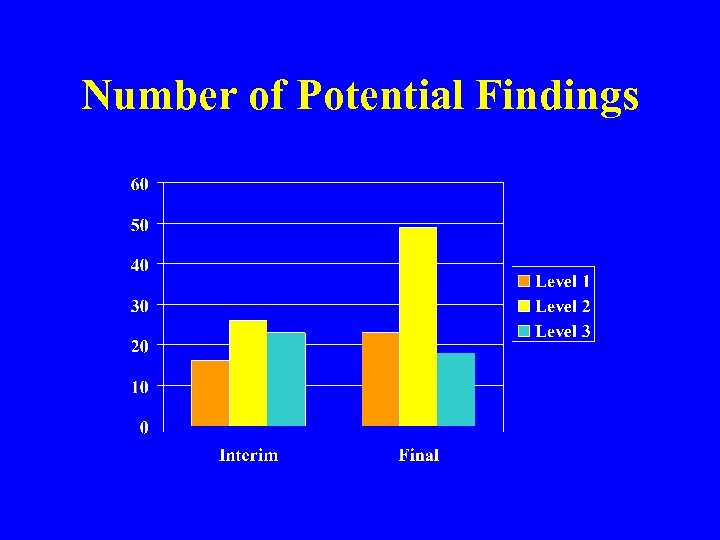 Number of Potential Findings
Number of Potential Findings
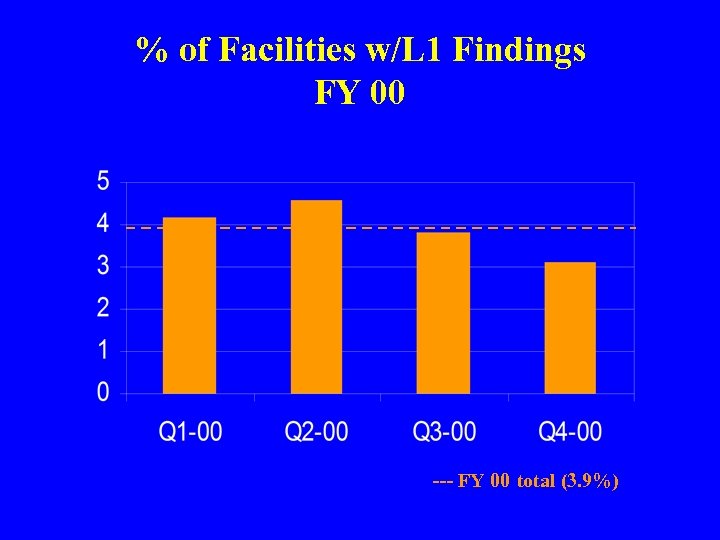 % of Facilities w/L 1 Findings FY 00 --- FY 00 total (3. 9%)
% of Facilities w/L 1 Findings FY 00 --- FY 00 total (3. 9%)
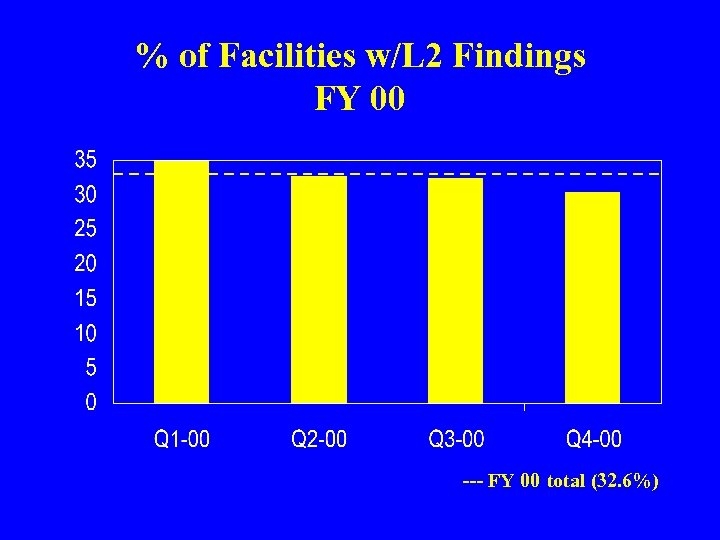 % of Facilities w/L 2 Findings FY 00 --- FY 00 total (32. 6%)
% of Facilities w/L 2 Findings FY 00 --- FY 00 total (32. 6%)
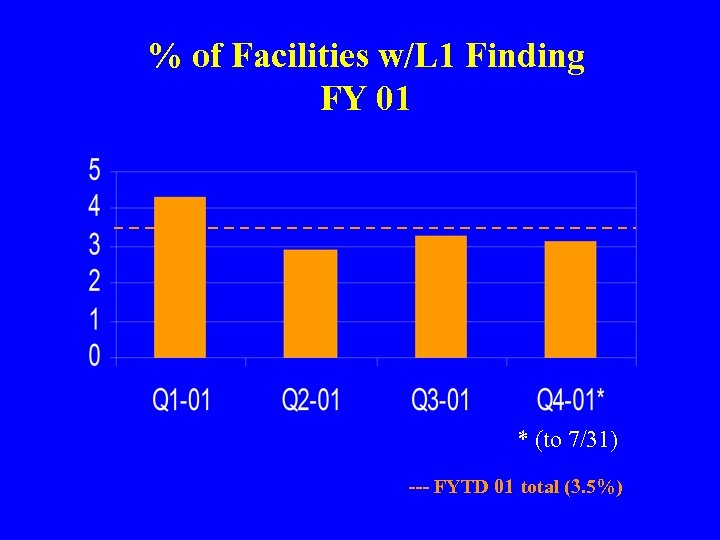 % of Facilities w/L 1 Finding FY 01 * (to 7/31) --- FYTD 01 total (3. 5%)
% of Facilities w/L 1 Finding FY 01 * (to 7/31) --- FYTD 01 total (3. 5%)
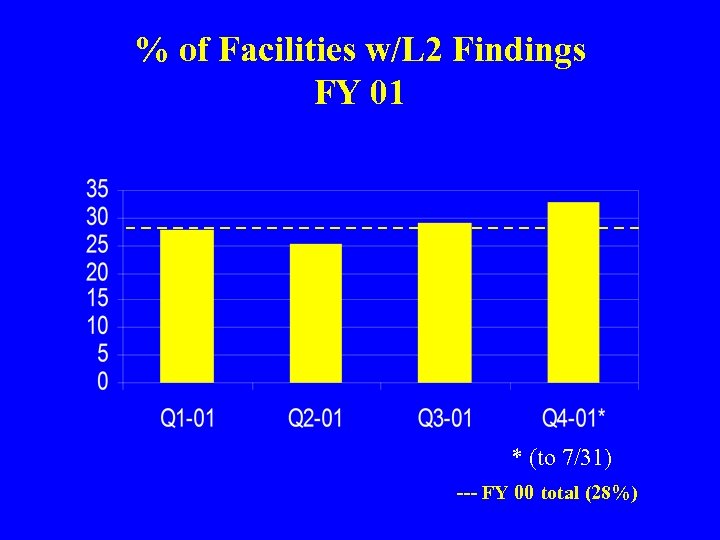 % of Facilities w/L 2 Findings FY 01 * (to 7/31) --- FY 00 total (28%)
% of Facilities w/L 2 Findings FY 01 * (to 7/31) --- FY 00 total (28%)
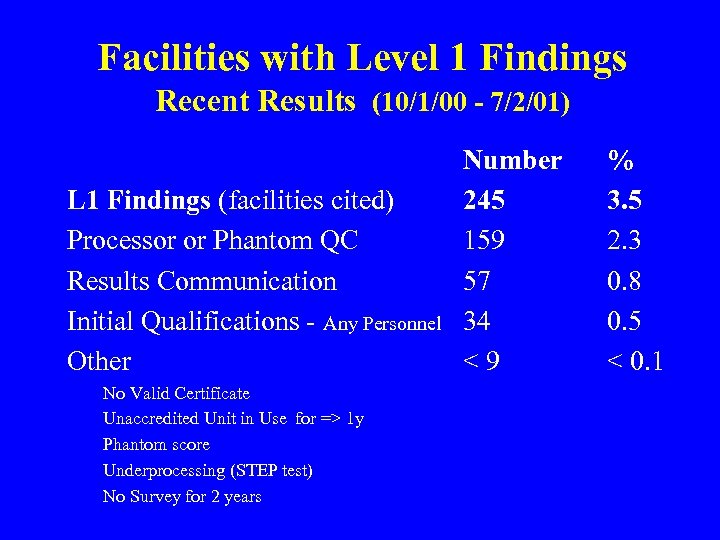 Facilities with Level 1 Findings Recent Results (10/1/00 - 7/2/01) L 1 Findings (facilities cited) Processor or Phantom QC Results Communication Initial Qualifications - Any Personnel Other No Valid Certificate Unaccredited Unit in Use for => 1 y Phantom score Underprocessing (STEP test) No Survey for 2 years Number 245 159 57 34 <9 % 3. 5 2. 3 0. 8 0. 5 < 0. 1
Facilities with Level 1 Findings Recent Results (10/1/00 - 7/2/01) L 1 Findings (facilities cited) Processor or Phantom QC Results Communication Initial Qualifications - Any Personnel Other No Valid Certificate Unaccredited Unit in Use for => 1 y Phantom score Underprocessing (STEP test) No Survey for 2 years Number 245 159 57 34 <9 % 3. 5 2. 3 0. 8 0. 5 < 0. 1
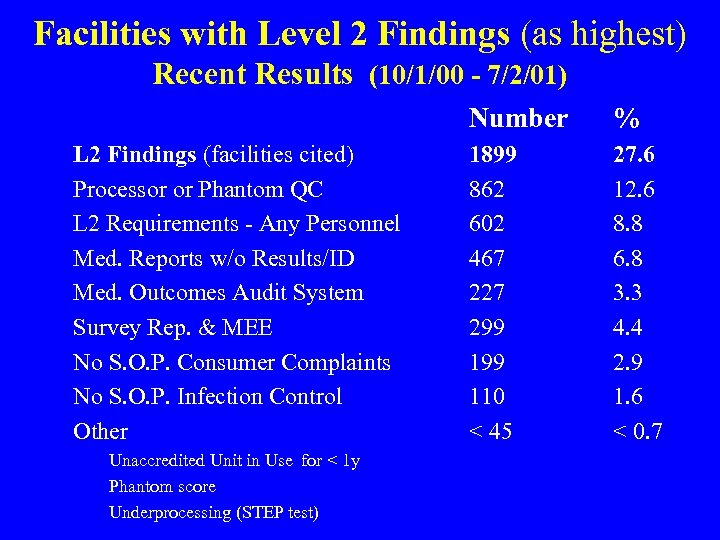 Facilities with Level 2 Findings (as highest) Recent Results (10/1/00 - 7/2/01) Number L 2 Findings (facilities cited) Processor or Phantom QC L 2 Requirements - Any Personnel Med. Reports w/o Results/ID Med. Outcomes Audit System Survey Rep. & MEE No S. O. P. Consumer Complaints No S. O. P. Infection Control Other Unaccredited Unit in Use for < 1 y Phantom score Underprocessing (STEP test) % 1899 862 602 467 227 299 110 < 45 27. 6 12. 6 8. 8 6. 8 3. 3 4. 4 2. 9 1. 6 < 0. 7
Facilities with Level 2 Findings (as highest) Recent Results (10/1/00 - 7/2/01) Number L 2 Findings (facilities cited) Processor or Phantom QC L 2 Requirements - Any Personnel Med. Reports w/o Results/ID Med. Outcomes Audit System Survey Rep. & MEE No S. O. P. Consumer Complaints No S. O. P. Infection Control Other Unaccredited Unit in Use for < 1 y Phantom score Underprocessing (STEP test) % 1899 862 602 467 227 299 110 < 45 27. 6 12. 6 8. 8 6. 8 3. 3 4. 4 2. 9 1. 6 < 0. 7
 FUTURE TREND Based on experience to date, we believe that in the foreseeable future (~ 1 year) … • % Facilities with L 1 citations < 2. 5 • % Facilities with L 2 citations < 25. 0 • % Facilities with L 3 citations < 10. 0
FUTURE TREND Based on experience to date, we believe that in the foreseeable future (~ 1 year) … • % Facilities with L 1 citations < 2. 5 • % Facilities with L 2 citations < 25. 0 • % Facilities with L 3 citations < 10. 0
 PROGRAMS UNDERWAY • Demonstration Program – Scheduled to start May 2002 – About 300 eligible facilities (14 states) in the pool – ~ 150 will be inspected once in 2 years • New Mammographic Modality – FFDM – Currently only GE’s Senographe 2000 D (since 6/00) others expected in the near future – Small number of facilities & units – 8 hours training in FFDM are the only citations implemented to date
PROGRAMS UNDERWAY • Demonstration Program – Scheduled to start May 2002 – About 300 eligible facilities (14 states) in the pool – ~ 150 will be inspected once in 2 years • New Mammographic Modality – FFDM – Currently only GE’s Senographe 2000 D (since 6/00) others expected in the near future – Small number of facilities & units – 8 hours training in FFDM are the only citations implemented to date
 . . . for more Information on MQSA • MQSA Internet home page: http: //www. fda. gov/cdrh/mammography • MQSA facility hotline: 1 -800 -838 -7715 • (DSMA) Facts on Demand: 1 -800 -899 -0381
. . . for more Information on MQSA • MQSA Internet home page: http: //www. fda. gov/cdrh/mammography • MQSA facility hotline: 1 -800 -838 -7715 • (DSMA) Facts on Demand: 1 -800 -899 -0381


