f3a3b2499b49b12a7e72124f2cea0998.ppt
- Количество слайдов: 57

Overview of Bioterrorism Agents Nicole Balmer M. D. 08/25/2006

Lab Response Network § The Laboratory Response Network (LRN) was established by the Department of Health and Human Services, Centers for Disease Control and Prevention (CDC). § Operational August 1999. § Includes labs in all 50 states, UK, Canada, Australia and vet labs.

Lab Response Network § National Labs: Have unique resources to handle highly infectious agents and confirm diagnosis § Reference Labs: Allows conclusive enough results to allow emergency response § Sentinel Labs: “rule out and refer” § Denver Health; must meet certain standards

CDC Category A Agents § § § Category A Variola major: Smallpox Bacillus anthracis: Anthrax Yersinia pestis: Plague Clostridium botulinum (botulinum toxins): Botulism § Francisella tularensis: Tularemia § Filoviruses and Arenaviruses (e. g. , Ebola virus, Lassa virus): Viral hemorrhagic fevers
CDC Categories B & C § § § Category B Coxiella burnetii: Q fever Brucella spp. : Brucellosis Burkholderia mallei: Glanders Burkholderia pseudomallei: Melioidosis Alphaviruses (VEE, EEE, WEEa): Encephalitis Rickettsia prowazekii: Typhus fever Toxins (e. g. , Ricin, Staphylococcal enterotoxin B): Toxic syndromes Chlamydia psittaci: Psittacosis Food safety threats (e. g. , Salmonella spp. , Escherichia coli O 157: H 7) Water safety threats (e. g. , Vibrio cholerae, Cryptosporidium parvum) § Category C § Emerging threat agents (e. g. , Nipah virus, hantavirus)

Biosafety Levels § BSL-1: Microorganisms that are not known to cause disease in healthy humans § BSL-2: Agents of moderate risk to personnel and the environment § BSL-3: Agents which may cause serious or potentially lethal diseases as a result of exposure by the inhalation route § BSL-4: Dangerous and exotic agents that pose a high individual risk of aerosol-transmitted laboratory infections and life-threatening disease
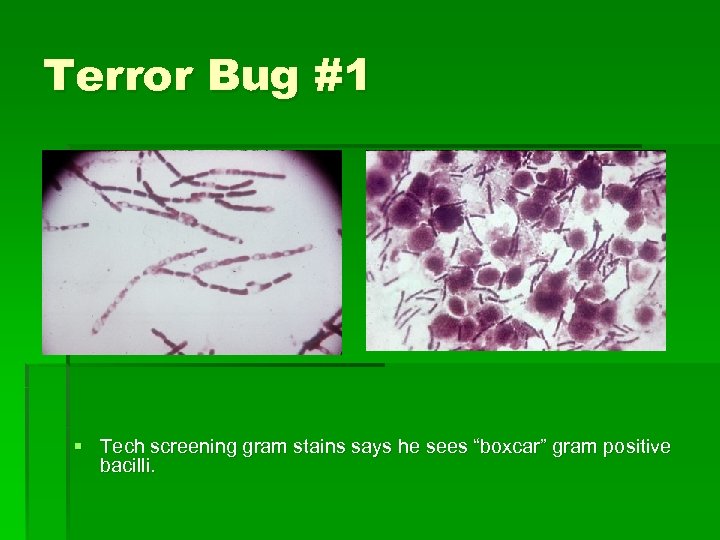
Terror Bug #1 § Tech screening gram stains says he sees “boxcar” gram positive bacilli.
Terror Bug # 1 First decision: “Boxcar” Gram positive rods Aerobic Anaerobic
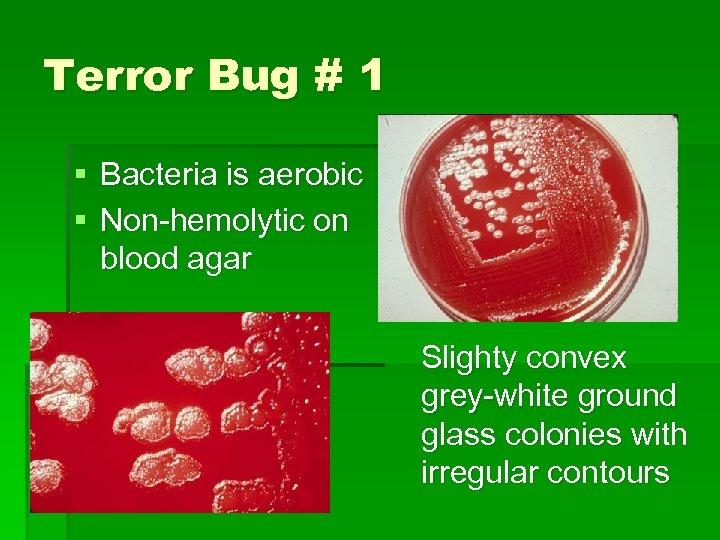
Terror Bug # 1 § Bacteria is aerobic § Non-hemolytic on blood agar Slighty convex grey-white ground glass colonies with irregular contours

Terror Bug # 1 § Also known as “Medusa Head” colonies

Motility test § Organism in question in Non-motile

Bacillus anthracis

Bacillus anthracis § Large gram positive bacilli in short chains (boxcars) which look encapsulated. § Non-hemolytic § Medusa head colonies § India Ink stain: + capsule (optional) § Non-motile § Notify FBI, state public health lab and state public health department
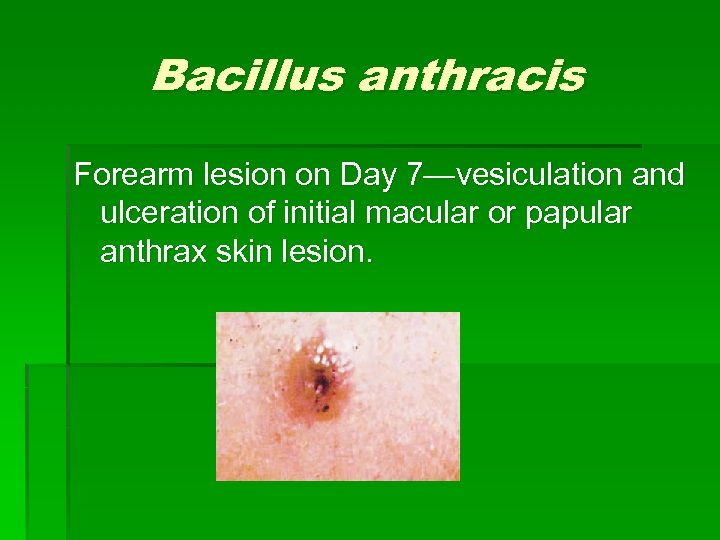
Bacillus anthracis Forearm lesion on Day 7—vesiculation and ulceration of initial macular or papular anthrax skin lesion.
Other Bacillus species § Other Bacillus
Terror Bug # 1 First decision: “Boxcar” Gram positive rods Aerobic Anaerobic

Clostridium § Anaerobic spore forming gram positive bacilli

Clostridium botulinum § § § Food-borne botulism Wound botulism Infant botulism Adult intestinal colonization Injection-related Inhalational

Clostridium botulism § Lethal foodborne intoxication with toxin types A, B, E, or F; shorter incubation period --->poorer prognosis § phage-mediated, systemic-acting A-B neurotoxin (botulinum toxin = botulin) released at cell lysis § Mode of Action -- one of most extremely potent neurotoxins known § Lethal dose to humans, less than 1 mcg.

Clostridium botulism § A-B toxin ingested, binds specific receptors on peripheral cholinergic nerve endings (neuromuscular junctions) where it blocks release of presynaptic acetylcholine (excitatory neurotransmitter) blocking muscle stimulation and resulting in flaccid paralysis § Early: nausea, vomiting, weakness, lassitude (lack of energy), dizziness, constipation § Later: double vision, difficulty in swallowing and speaking § Final: death due to respiratory paralysis

Clostridium botulism § Acceptable specimens: § § Enema fluid Nasal swab Serum Stool § DH lab (and other sentinel labs) will only accept suspected bioterrorism specimens to send to Reference lab § Any manipulation of specimen= BSL-3 § All work areas must be disinfected

Clostridium botulism § Lab Identification § Microscopic detection or Cx (culture) are often unsuccessful (few organisms and slow growing) § Toxin detected and typed in lab via toxicity and antitoxin neutralization tests in mice or by ELISA

Clostridium botulism § Colonies commonly show some spreading and have an irregular edge. On egg yolk medium, they usually exhibit surface iridescence when examined by oblique light. This luster zone, often referred to as a pearly layer, usually extends beyond and follows the irregular contour of the colony.

Clostridium botulism § Laboratory confirmation of toxin presence is via a mouse bioassay, and identification of the toxin type is performed by a mouse toxin neutralization test. § New methods of detection: In vitro methods of detection, including polymerase chain reactionbased detection of clostridial genes and ELISA identification of toxin, but these methods are not widely available outside of research institutions.

Use of Clostridium botulism § Japanese in World War II carried out human experiments on prisoners in Manchuria. § World War II, the British secretly used a botulism-impregnated grenade in the assassination of a German Gestapo officer. § The United States studied botulinum toxin as a military bioweapon until President Nixon signed the Biological and Toxin Weapons Convention in 1972, § Iraq and the Soviet Union stockpiled neurotoxin, with Iraq admitting to weaponizing thousands of liters of toxin in warheads after the 1991 Gulf War. § An attempt at terrorist use of Clostridium toxin in the early 1990 s by the Japanese Aum Shinryko cult against American military targets was unsuccessful.

Clostridium botulism § These were jars of contaminated Jalapeño peppers involved in an outbreak of botulism in Pontiac, Michigan, April, 1977.
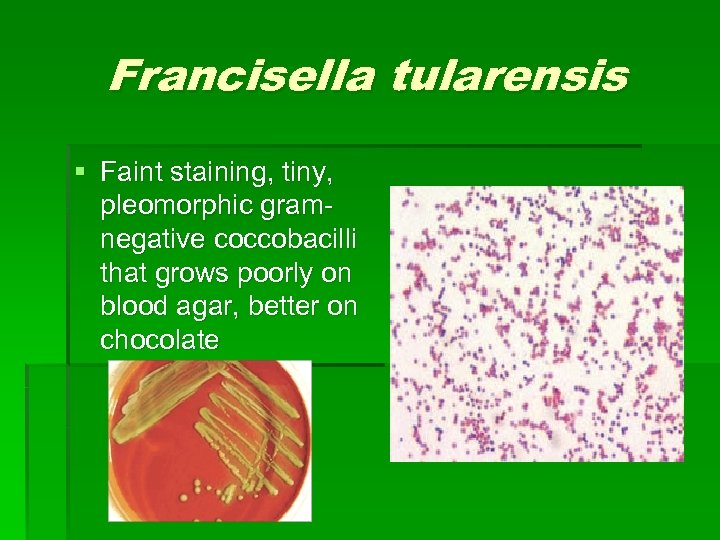
Francisella tularensis § Faint staining, tiny, pleomorphic gramnegative coccobacilli that grows poorly on blood agar, better on chocolate
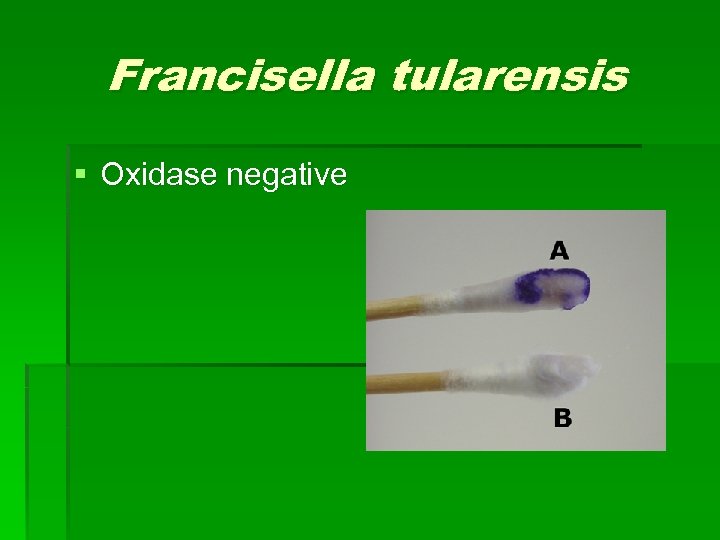
Francisella tularensis § Oxidase negative

Francisella tularensis § Catalase weakly positive
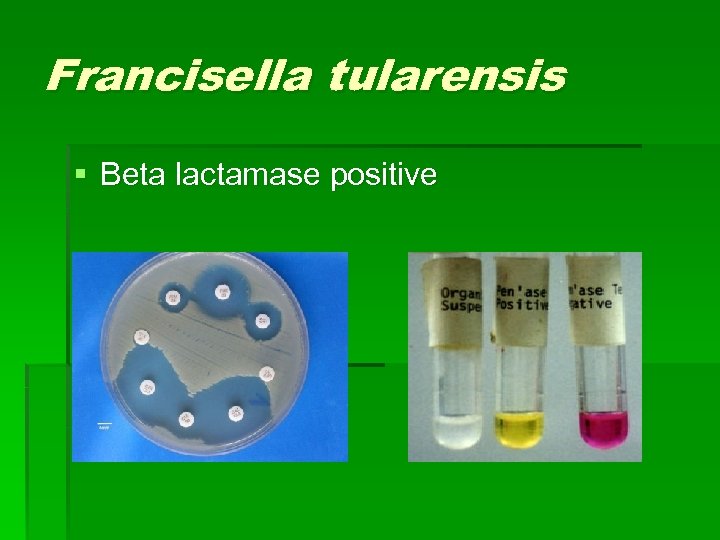
Francisella tularensis § Beta lactamase positive

Francisella tularensis § Satellite test negative

Francisella tularensis § Urease negative
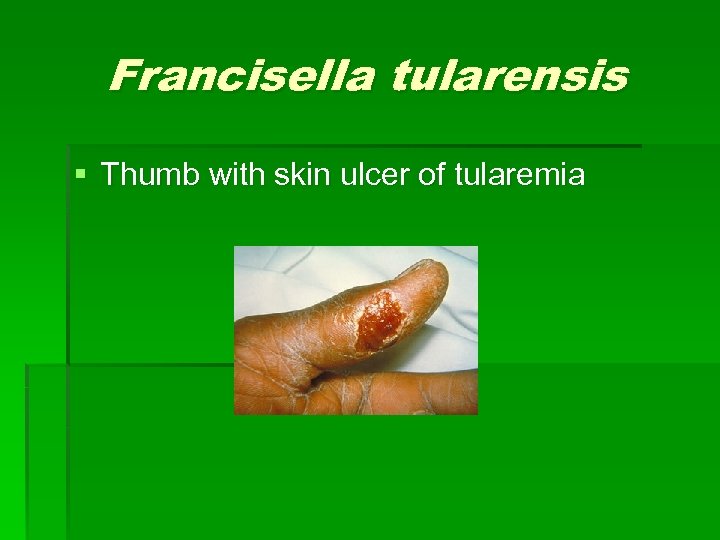
Francisella tularensis § Thumb with skin ulcer of tularemia

Francisella tularensis § Usually misidentified using commercial ID systems such as Microscan. § Usually ID’d as H. influinzae (satellite positive) or Actinobacillus spp. (beta lactamase negative)
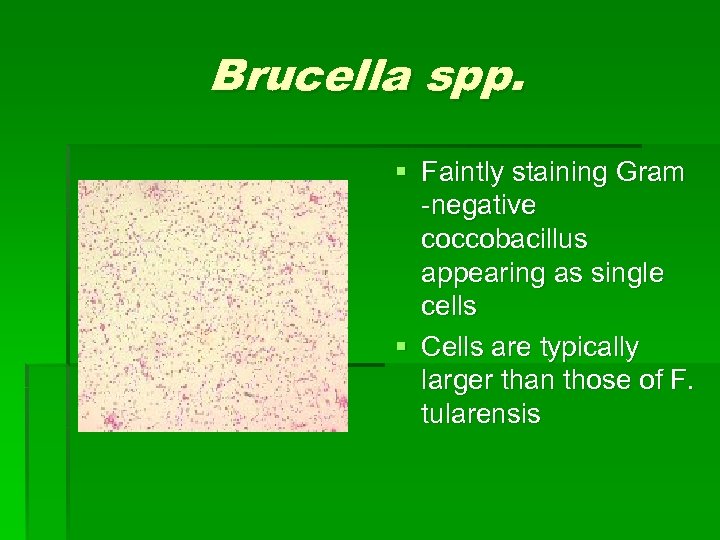
Brucella spp. § Faintly staining Gram -negative coccobacillus appearing as single cells § Cells are typically larger than those of F. tularensis

Brucella spp. § § § Colony Characteristics Usually no visible or pinpoint at 24 hrs. Grows slowly on most standard lab media including sheep blood, chocolate, and TSA. Grows on Martin-Lewis and Thayer-Martin agars. After 48 hrs. appears translucent, pinpoint and smooth Non-hemolytic on sheep blood agar Some strains can grow on Mac. Conkey agar
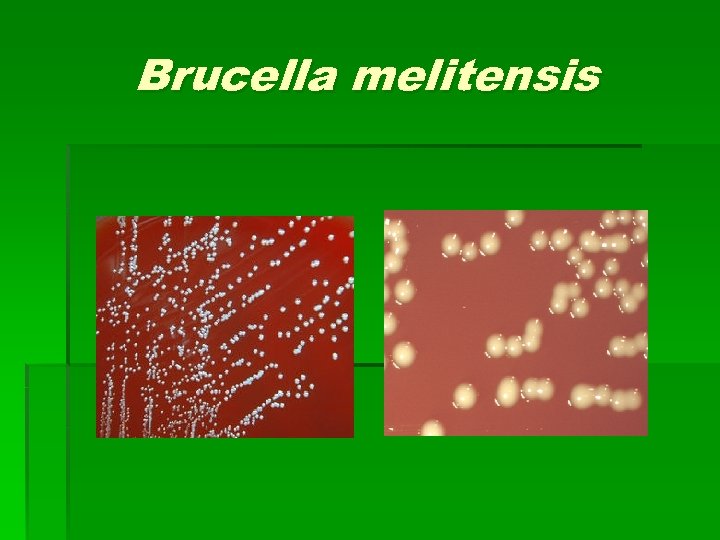
Brucella melitensis
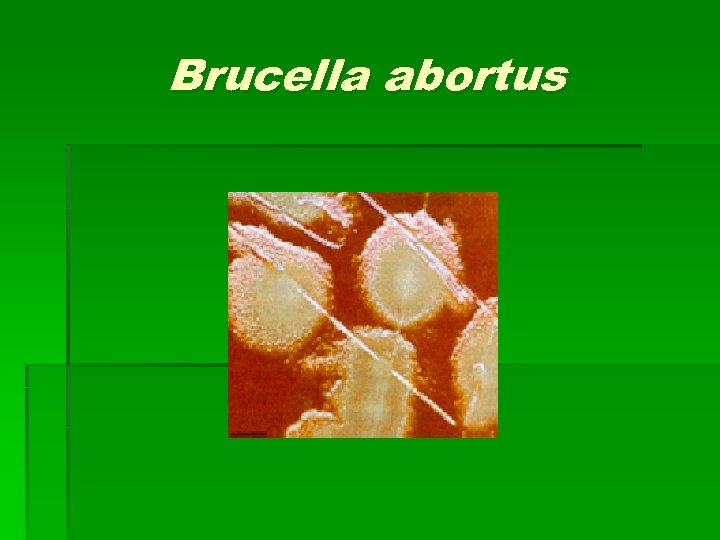
Brucella abortus

Brucella spp. § § § Non-Motile Catalase Positive Oxidase Positive (B. canis is variable) § Urease Positive (Strong, some with 5 min to 2 hrs)
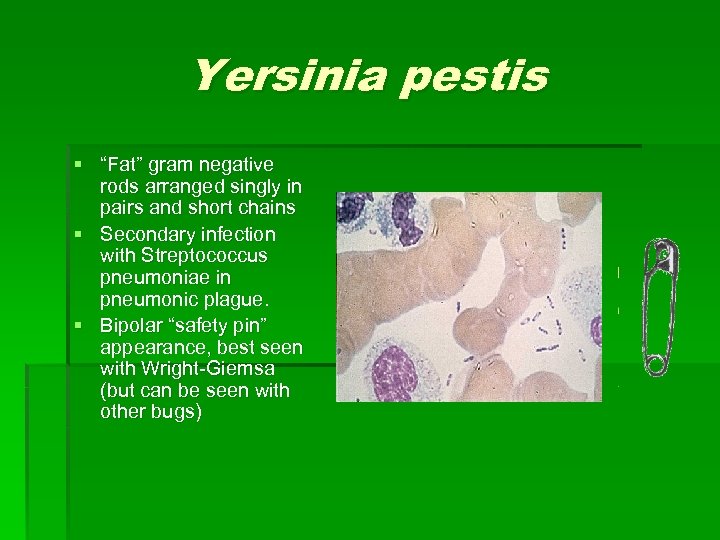
Yersinia pestis § “Fat” gram negative rods arranged singly in pairs and short chains § Secondary infection with Streptococcus pneumoniae in pneumonic plague. § Bipolar “safety pin” appearance, best seen with Wright-Giemsa (but can be seen with other bugs)

Yersinia pestis § Gray to white, light yellow, opaque, pinpoint, nonhemolytic nonlactose fermenting colonies. § With age, cultures have fried egg appearance § Broth tube: 24 hrs. – “stalactite” growth § 48 hrs. “cotton fluff”
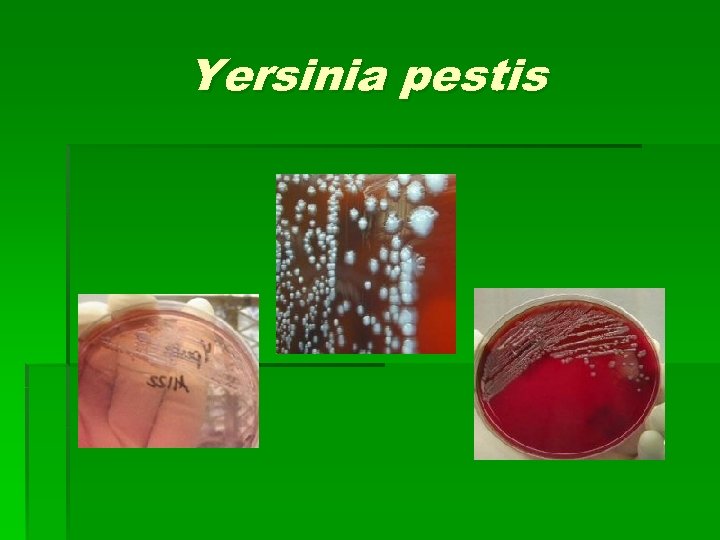
Yersinia pestis

Yersinia pestis § § § § Oxidase negative Catalase positive Urea negative Indole negative K/A Nonmotile at 37 C Grows better at 28 C

Indole § Ability of a bacteria to breakdown the amino acid trytophan
Yersinia pestis
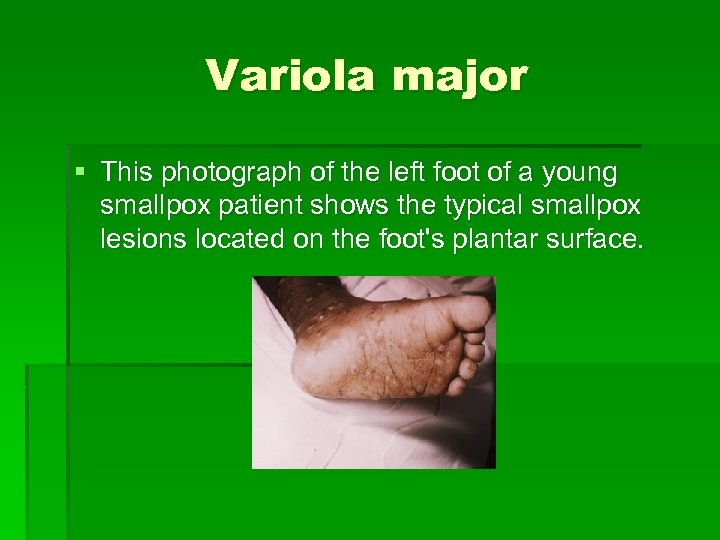
Variola major § This photograph of the left foot of a young smallpox patient shows the typical smallpox lesions located on the foot's plantar surface.

Smallpox § Smallpox infection can be rapidly confirmed in the laboratory by electron microscopic examination of vesicular or pustular liquid or scabs. § Definitive laboratory identification and characterization of the virus involves growth of the virus in the cell culture or on chorioallantoic egg membrane and characterization of strains by use of biologic assays, including the polymerase chain reaction (PCR), restriction fragment-length polymorphism analysis (RFLP) and ELISA. Confirmation using these methods can be accomplished in a few hours.

Smallpox § BSL-4 § Pharyngeal swab, scab matter, nasal swab, serum Virus may survive in scabs from patient for several weeks. (Note: Virons in scabs may remain viable for years, but their being bound in fibrin probably reduces their practical danger).

Hemorrhagic Fever Viruses § The two viruses considered to be the greatest bioterrorism threats are Ebola and Marburg, the two members of the filovirus family

Hemorrhagic Fever Viruses § This was the local Red Cross team in Kikwit, Zaire, during the Ebola VHF outbreak in 1995. This team went to the homes in the area to bring patients with suspected Ebola viral hemorrhagic fever (VHF) to the Kikwit hospital, as well as to remove corpses.

Hemorrhagic Fever Viruses § BSL-4 § Antigen-Capture ELISA, RT-PCR (most useful clinically) § Ig. M by Antibody-Capture ELISA, Viral isolation § Acute and convalescent Ig. G serologies in survivors (only helpful retrospectively)

References § 1. http: //www. bt. cdc. gov/Agentlist. asp § 2. Gaido, L. Denver Health Microbiology Procedure Manual-Bioterrorism Agents, 2004. § 3. Arnon SS et al. Botulinum toxin as a biological weapon: Medical and Public Health Management. JAMA. 2001; 285(8): 1059 -70. § 4. Dennis DT et al. Tularemia as a biological weapon: Medical and Public Health Management. JAMA. 2001; 285(21): 2763 -73

References § 5. Ingelsby TV et al. Plague as a biological weapon: Medical and Public Health Management. JAMA. 200 o; 283(17): 2281 -90.

Dr. Elmer Koneman’s contribution (August 26, 2006): I have read through Nicole Balmer's Power Point presentation on Bioterrorism agents posted on the Web. A couple of years ago I worked with Jim Beebe, Head of the Colo Dept. Health Micro Lab, in publishing a bioterrorism CD. Dr. Balmer leads one through the in-laboratory identification of Francisella tularensis, including the use of an automated system. Dr. Beebe and I, in creating the algorithm for the identification of this highly contagious microbe, indicated that any slow-growing isolate, growing poorly if at all on blood agar but with tiny colonies on chocolate agar, that appear as poorly staining tiny gram negative cocco-bacilli on gram stain, that are cytochrome oxidase-negative and are non-motile in a direct mount preparation should be sent immediately to the state laboratory with no attempt to make an in-laboratory identification. We essentially have established a similar algorithm for the direct preliminary exam for Brucella, leading to immediate referral to the state lab of any suspicious isolate, again to prevent any chance for a laboratory acquired infection resulting from a full in-house work up. I would like to bring this precaution to your attention. I believe regulations mitigate that the agents of bioterrorism should be definitively worked up only in a Level 3 microbiology laboratory. This may not be true for all the agents, but I know it is for F. tularensis and Brucella species. The chances are high that laboratory-acquired infections may occur from handling these isolates and work up in Level-2 laboratories or below elevates this possibility. Most hospital or even university labs don't meet this criteria; therefore, any time F. tularensis or Brucella species is suspected, the State Lab should be consulted and the cultures forwarded under their guidelines. Next are two slides onto which I transcribed the presumptive identification algorithms of F. tularensis and Brucella sp. These were taken from the CD: "The Bacterial Agents of Bioterrorism", authored by James Beebe, Elmer Koneman, and Christie Grueser, with the copyright being held by CACMLE. The full disk is available from CACMLE, and is their self-study product is #96 A 05 CD. You might consider acquiring this disk for additional information and future reference (call 303 321 1734 for details). Thank you. Elmer W. Koneman, M. D.
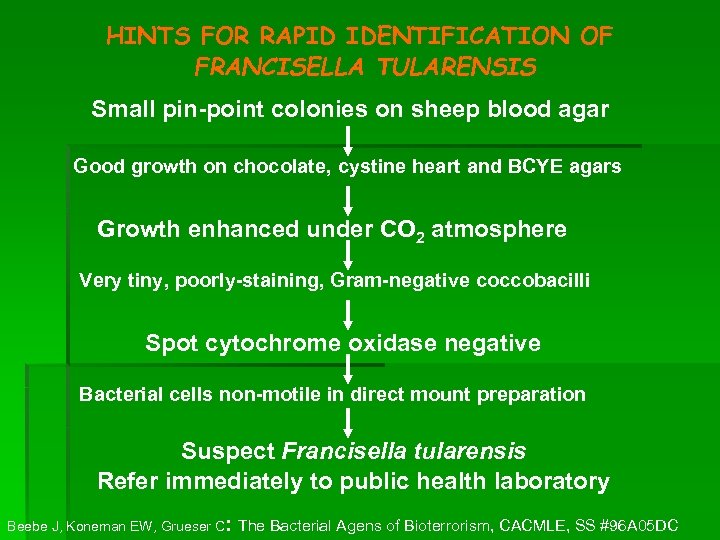
HINTS FOR RAPID IDENTIFICATION OF FRANCISELLA TULARENSIS Small pin-point colonies on sheep blood agar Good growth on chocolate, cystine heart and BCYE agars Growth enhanced under CO 2 atmosphere Very tiny, poorly-staining, Gram-negative coccobacilli Spot cytochrome oxidase negative Bacterial cells non-motile in direct mount preparation Suspect Francisella tularensis Refer immediately to public health laboratory : Beebe J, Koneman EW, Grueser C The Bacterial Agens of Bioterrorism, CACMLE, SS #96 A 05 DC
HINTS FOR RAPID IDENTIFICATION OF BRUCELLA SPECIES Slow growth of tiny, gray-white, shiny colonies on BA Very pale-staining gram-negative coccobacilli on Gram stain Spot cytochrome oxidase positive Rapid hydrolysis of Urea: Positive reaction in 1 -4 Hours on Christensen’s urea agar Suspect BRUCELLA SPECIES Consult Public Health Laboratory Immediately Beebe J, Koneman EW, Grueser C: The Bacterial Agens of Bioterrorism, CACMLE, SS#96 A 05 DC
f3a3b2499b49b12a7e72124f2cea0998.ppt