6ddba5eb3812508c0bb42d4bc8e519fe.ppt
- Количество слайдов: 52

Overview Models in laboratory research The unique position of the mouse as a model Mouse genetics 101 Genetic engineering for hypothesis testing Genetic variation

Why Use Models? Allows for controlled experiments Environmental variables can be controlled Dosage or exposures can be controlled Experiments can be replicated


Phenotypes Genes Environment Chapel Hill Stochastic Processes
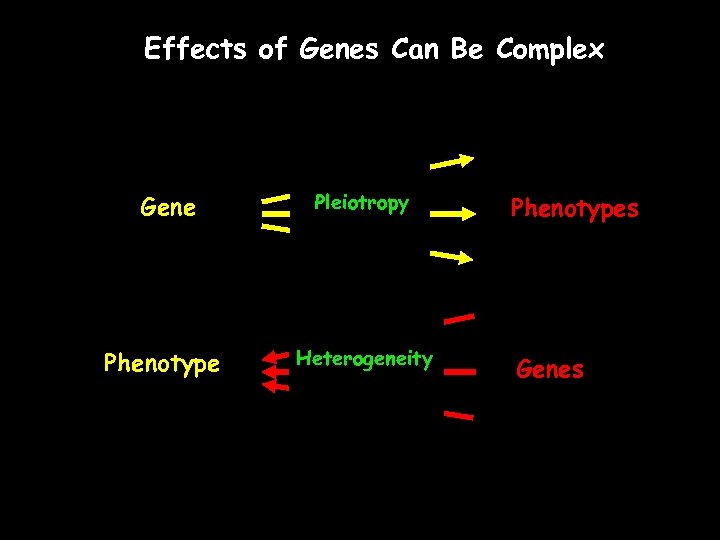
Effects of Genes Can Be Complex Gene Pleiotropy Phenotype Heterogeneity Phenotypes Genes
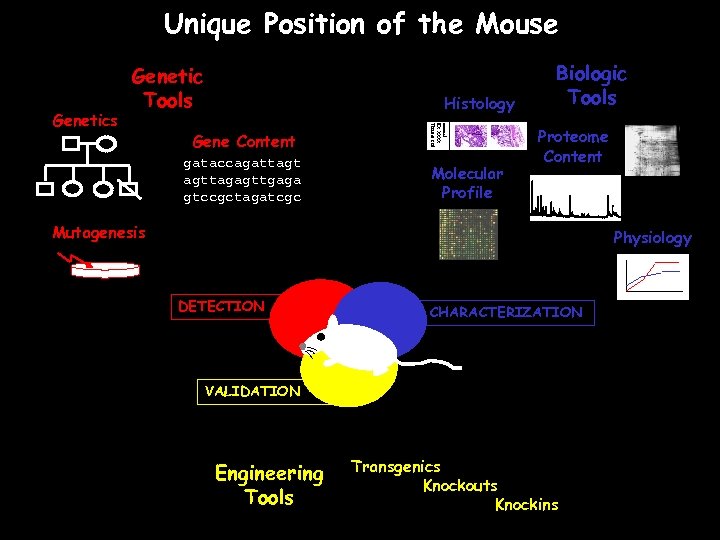
Unique Position of the Mouse Histology Gene Content gataccagattagt agttagagttgaga gtccgctagatcgc Strain: A/J ID: XXXX Tissue: col Genetics Genetic Tools Molecular Profile Biologic Tools Proteome Content Mutagenesis Physiology DETECTION CHARACTERIZATION VALIDATION Engineering Tools Transgenics Knockouts Knockins

Why Mice As an Experimental Organism? Hardy Requires little space Short life cycle Easily bred High fecundity Mammalian species Large amount of phenotypic variation Easy to genetically engineer
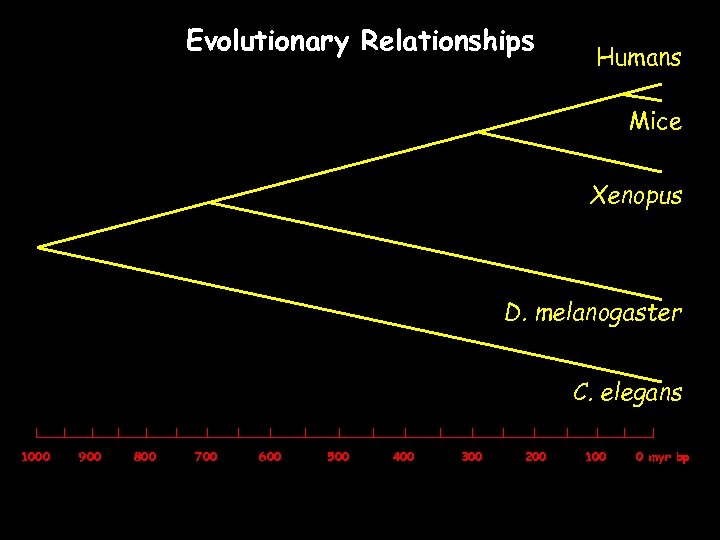
Evolutionary Relationships Humans Mice Xenopus D. melanogaster C. elegans 1000 900 800 700 600 500 400 300 200 100 0 myr bp
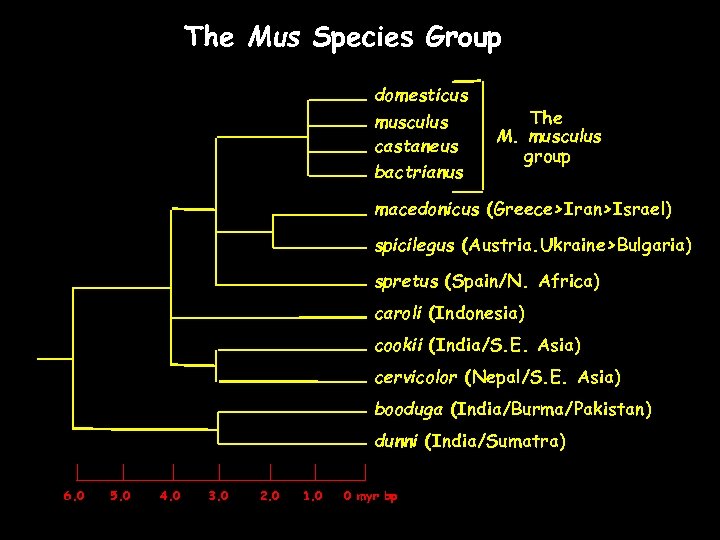
The Mus Species Group domesticus musculus castaneus bactrianus The M. musculus group macedonicus (Greece>Iran>Israel) spicilegus (Austria. Ukraine>Bulgaria) spretus (Spain/N. Africa) caroli (Indonesia) cookii (India/S. E. Asia) cervicolor (Nepal/S. E. Asia) booduga (India/Burma/Pakistan) dunni (India/Sumatra) 6. 0 5. 0 4. 0 3. 0 2. 0 1. 0 0 myr bp
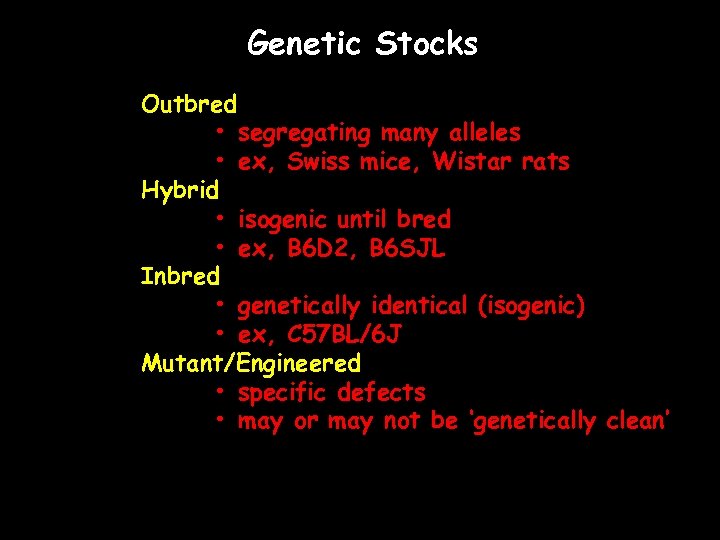
Genetic Stocks Outbred • segregating many alleles • ex, Swiss mice, Wistar rats Hybrid • isogenic until bred • ex, B 6 D 2, B 6 SJL Inbred • genetically identical (isogenic) • ex, C 57 BL/6 J Mutant/Engineered • specific defects • may or may not be ‘genetically clean’

Exercise A You have developed a compound that you think will help to prevent rejection of transplanted hearts, and you want to test this experimentally. The experiment will involve heart grafts between a donor and recipient mouse (whose own heart is not removed) with a control group and one treated with the compound. The following mouse strains are available: outbred ICR and CD 1 and inbred C 57 BL/6 J, A/J, and FVB/NJ. Which strains will you use as donor and recipient? Why?

Exercise B You also need to test the potential toxicity of the compound, and will want to do a long-term study with control and treated mice. You know it is not acutely toxic. Being a toxicologist, you reason that in this case since you wish to model humans who are genetically heterogeneous, you decide to use outbred genetically heterogeneous ICR mice, the strategy used by virtually all toxicologists. Do you decide to go with your initial intuition? Why?

Identify the ‘Experimental Unit’ The unit of randomization The experimental units must be capable of being assigned to different treatments Could be: a cage of animals a single animal an animal for a period of time a well in a tissue culture dish

The Problem With Genetic Heterogeneity Treated Control beagle mouse horse gerbil lion cat rabbit chicken crow frog hamster beaver dog toad

A Better Design Treated Control beagle mouse horse gerbil lion cat rabbit
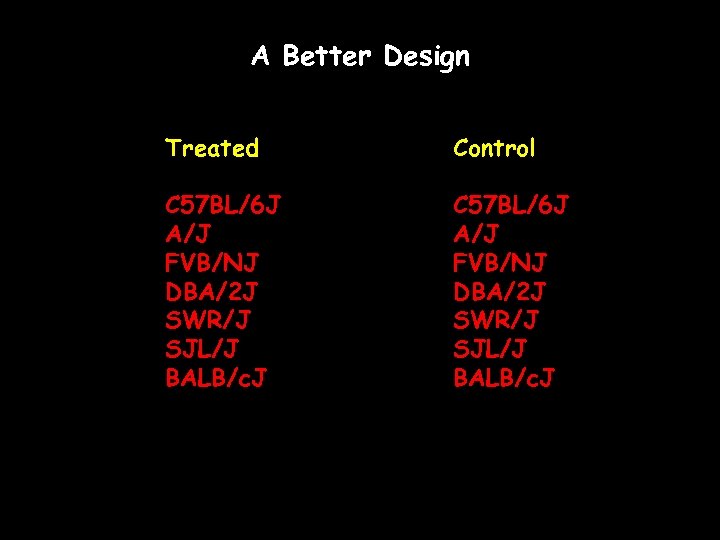
A Better Design Treated Control C 57 BL/6 J A/J FVB/NJ DBA/2 J SWR/J SJL/J BALB/c. J
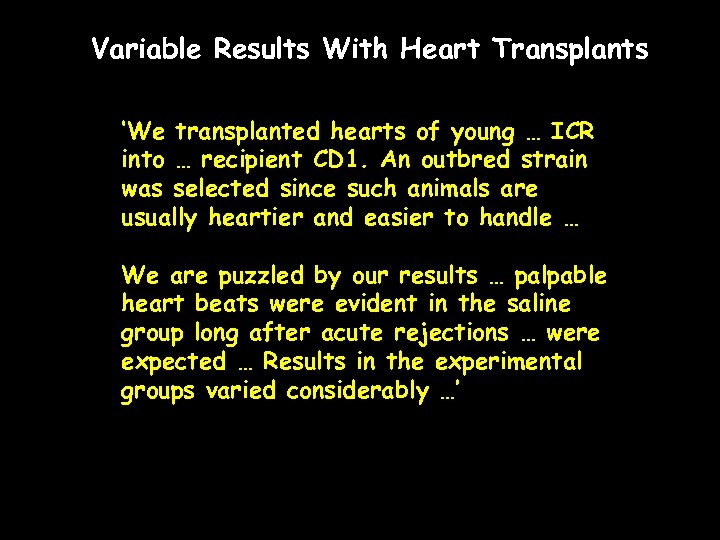
Variable Results With Heart Transplants ‘We transplanted hearts of young … ICR into … recipient CD 1. An outbred strain was selected since such animals are usually heartier and easier to handle … We are puzzled by our results … palpable heart beats were evident in the saline group long after acute rejections … were expected … Results in the experimental groups varied considerably …’
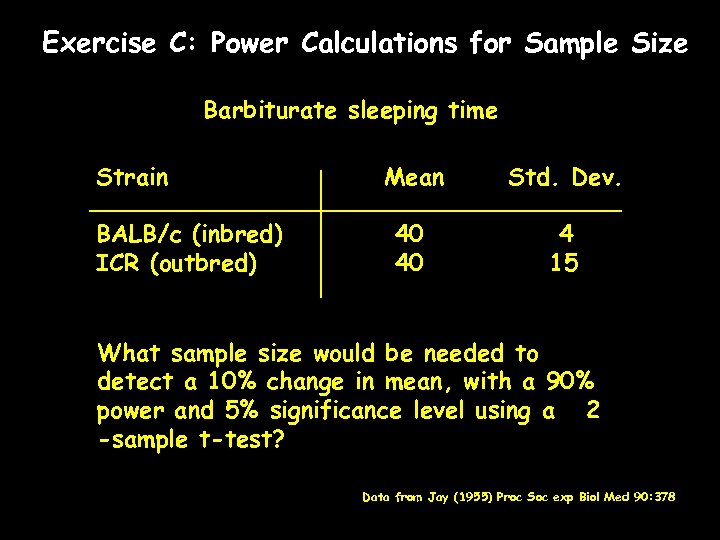
Exercise C: Power Calculations for Sample Size Barbiturate sleeping time Strain BALB/c (inbred) ICR (outbred) Mean Std. Dev. 40 40 4 15 What sample size would be needed to detect a 10% change in mean, with a 90% power and 5% significance level using a 2 -sample t-test? Data from Jay (1955) Proc Soc exp Biol Med 90: 378
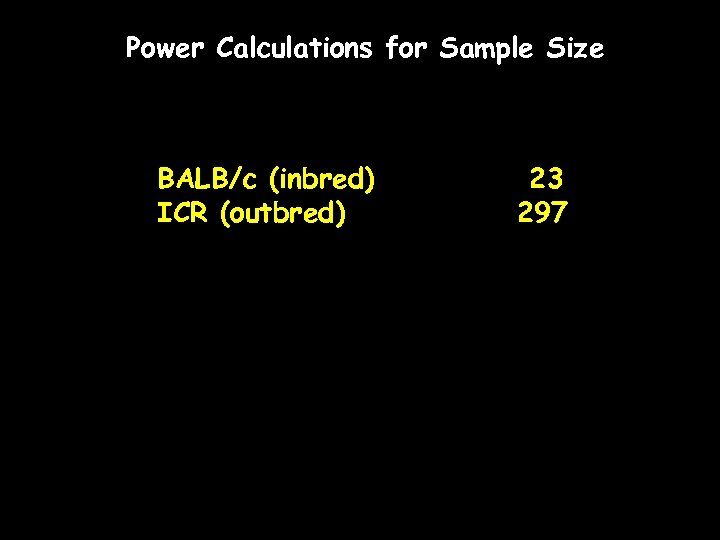
Power Calculations for Sample Size BALB/c (inbred) ICR (outbred) 23 297

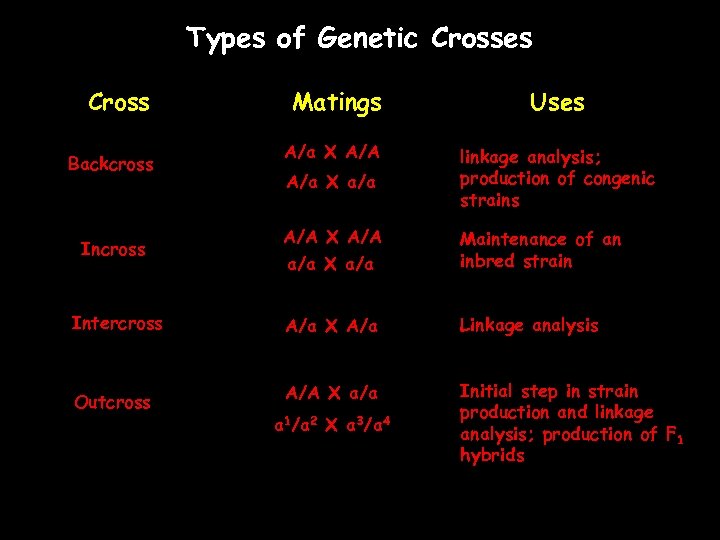
Types of Genetic Crosses Cross Backcross Matings Uses A/a X A/A linkage analysis; production of congenic strains A/a X a/a A/A X A/A a/a X a/a Maintenance of an inbred strain Intercross A/a X A/a Linkage analysis Outcross A/A X a/a Initial step in strain production and linkage analysis; production of F 1 hybrids Incross a 1/a 2 X a 3/a 4
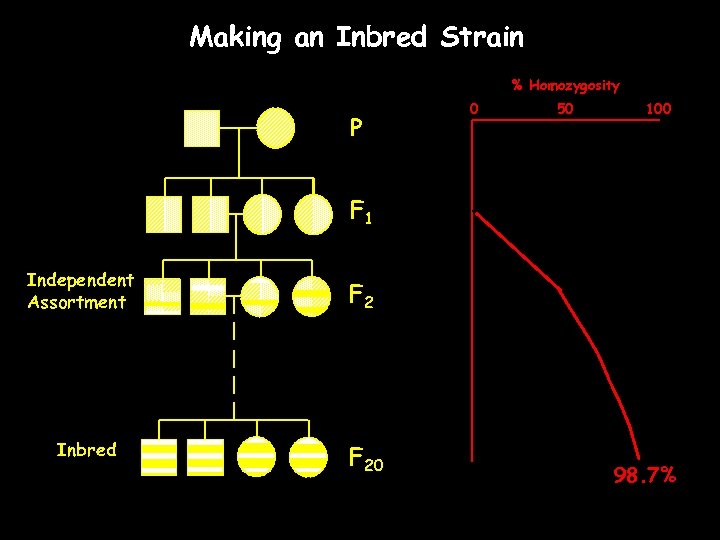
Making an Inbred Strain % Homozygosity P 0 50 100 F 1 Independent Assortment Inbred F 20 98. 7%

Inbred Strain 20 or more generation of brother x sister mating Isogenic Homozyogus Phenotypically uniform Long-term stability Unique strain characteristics International distribution Easily identifiable ‘immortal clone of genetically identical individuals’
Egfr. Wa 5/+ C 57 BL/6 J 129 S 1/Sv. Im. J BALB/c. J

‘The introduction of inbred strains into biology is probably comparable in importance with that of the analytical balance into chemistry’ Gruneberg (1952)

Outbred Stocks Widely used Characteristics not widely understood Advatages • cheap • easily available (no alternative for some species) • breed well • outbred like humans (? ? ? ) Disadvantages • unknown genetics (heterozygosity) • subject to genetic change (inbreeding, drift, selection) • lack of reliable background information • genotype not internationally distributed • not histocompatible • not easily identifiable
Outbred Mice CF 1 ICR CD 1 MF 1 Swiss-Webster B 6 D 2
Engineered Models Allows controlled experimental testing of • specific genes • specific environmental conditions or exposures Ideally suited to test specific hypothesis generated from human population studies or other laboratory findings
Engineered Models Transgenics • usually used to over-express genes • can be global or tissue-specific • can be temporally regulated Knockouts/knockins • usually used in inactivate genes • can be global or tissue-specific • can be temporally regulated • can introduce genes into a foreign locus • can make amino acid modifications
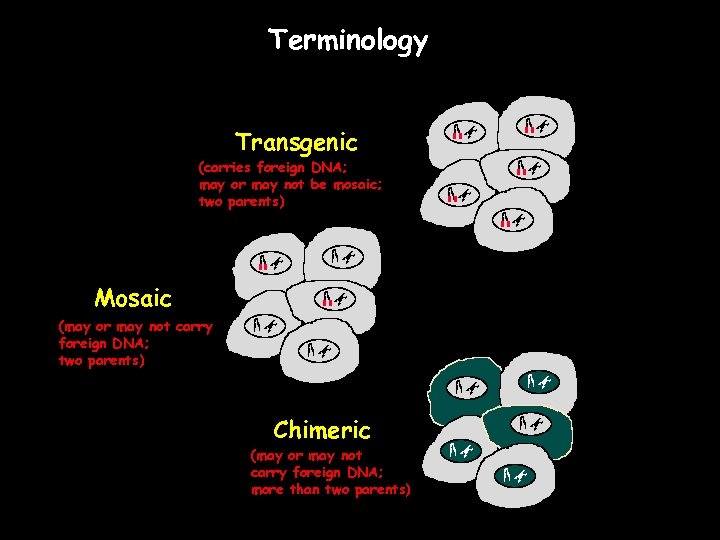
Terminology Transgenic (carries foreign DNA; may or may not be mosaic; two parents) Mosaic (may or may not carry foreign DNA; two parents) Chimeric (may or may not carry foreign DNA; more than two parents)
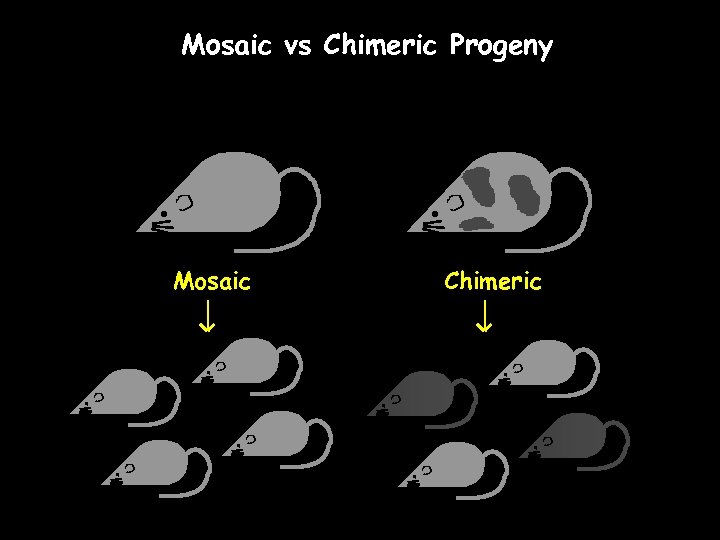
Mosaic vs Chimeric Progeny Mosaic Chimeric
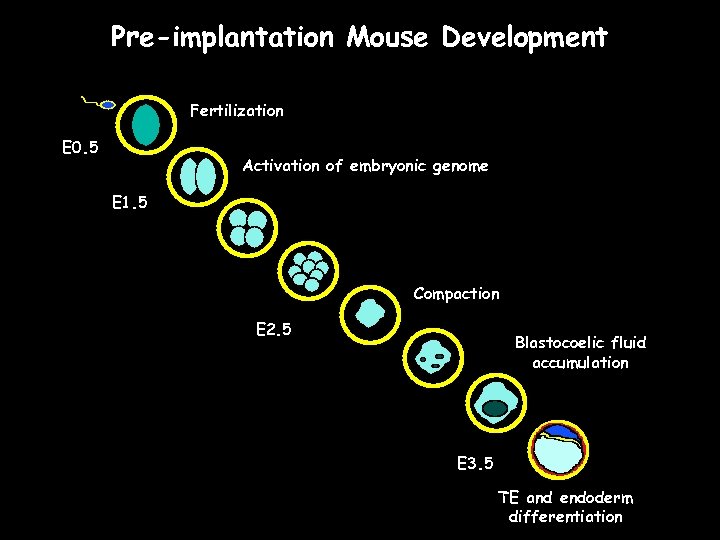
Pre-implantation Mouse Development Fertilization E 0. 5 Activation of embryonic genome E 1. 5 Compaction E 2. 5 Blastocoelic fluid accumulation E 3. 5 TE and endoderm differentiation
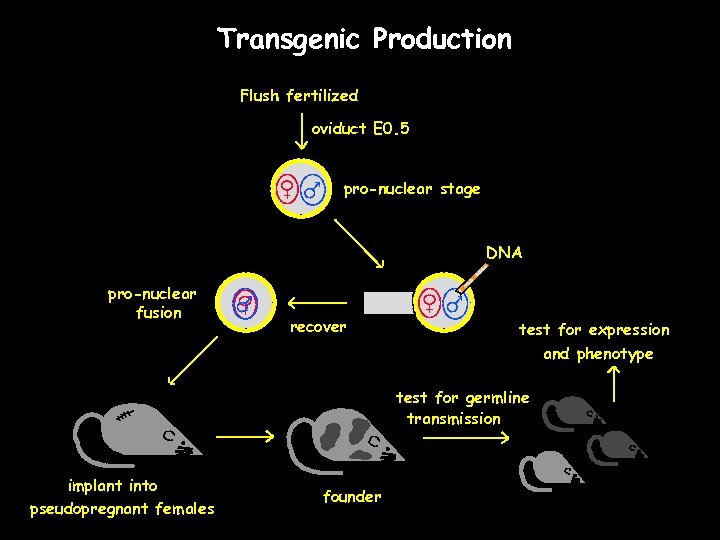
Transgenic Production Flush fertilized oviduct E 0. 5 pro-nuclear stage DNA pro-nuclear fusion recover test for expression and phenotype test for germline transmission implant into pseudopregnant females founder
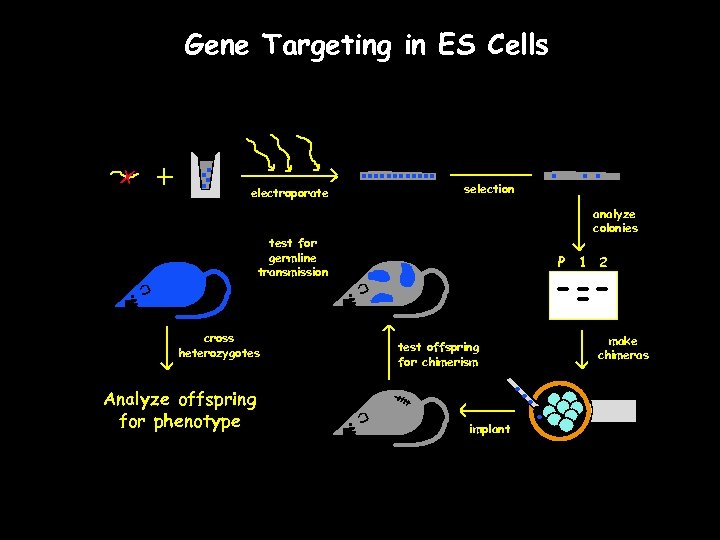
Gene Targeting in ES Cells electroporate selection analyze colonies test for germline transmission cross heterozygotes Analyze offspring for phenotype P 1 2 test offspring for chimerism implant make chimeras

Humanized Mice Gene knock-ins have been generated to introduce a variety of human genes that have relevance to toxicology: CYP, MHC, etc.

Humanized Mice Another approach to humanizing mice is tissue replacement via inter-species chimeras. This has been achieved for liver by using immunodeficient mice on a liver toxic transgenic background (urokinase-type plasminogen activator) by injecting human liver cells IV. Up to 90% of the mouse hepatocytes can be replaced by human hepatocytes.

Exercise D You obtain a mouse line from your collaborator that carries a knockout in PPAR-gamma. The collaborator made the mice by gene targeting in 129 strain derived ES cells. He made chimeras with the cells by blastocyst injection into C 57 BL/6 J embryos. After birth, he bred the chimeras to C 57 BL/6 J mice and then intercrossed heterozygous carriers to make the PPAR-gamma knockout homozygous line. You need wild-type controls for your experiment. What mice to you use for this? Why?
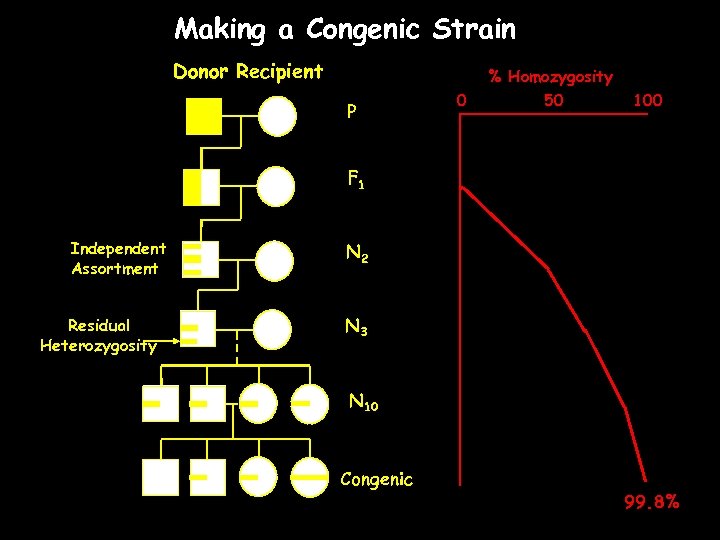
Making a Congenic Strain Donor Recipient P 0 % Homozygosity 50 100 F 1 Independent Assortment Residual Heterozygosity N 2 N 3 N 10 Congenic 99. 8%
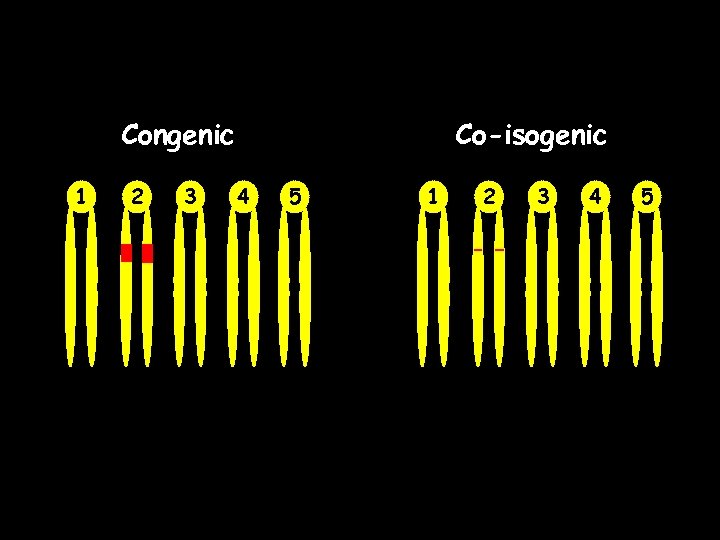
Congenic 1 2 3 Co-isogenic 4 5 1 2 3 4 5
Genetic Variation Significant extant genetic (and thus phenotypic) variation exists across mouse strains Can be used to identify a more ‘accurate’ model of specific human exposures or responses Phenotypic variation across inbred mouse strains is as great or greater than humans for virtually any trait
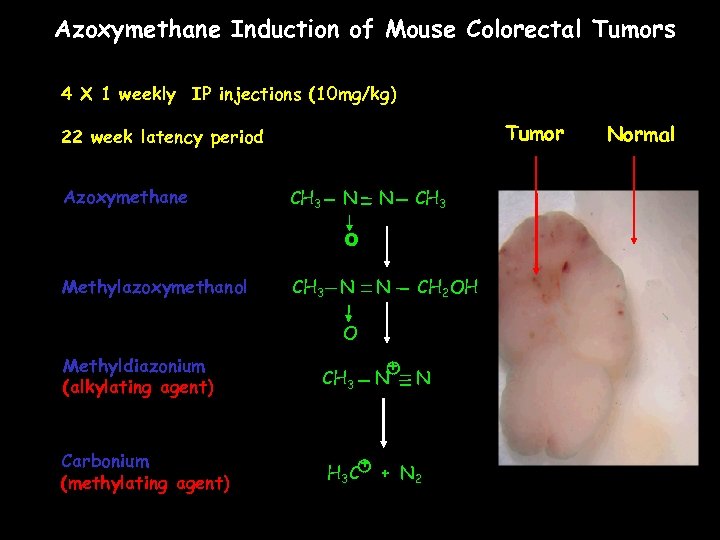
Azoxymethane Induction of Mouse Colorectal Tumors 4 X 1 weekly IP injections (10 mg/kg) Tumor 22 week latency period Azoxymethane CH 3 N N CH 3 N CH 2 OH O Methylazoxymethanol CH 3 N O Methyldiazonium (alkylating agent) Carbonium (methylating agent) + CH 3 C N + N 2 Normal
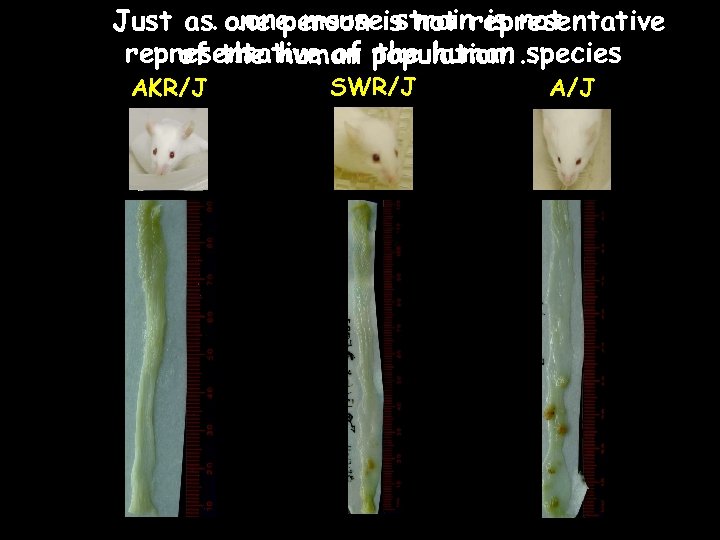
. . one mouse strain is not Just as one person is not representative of population. . of the human species AKR/J SWR/J A/J
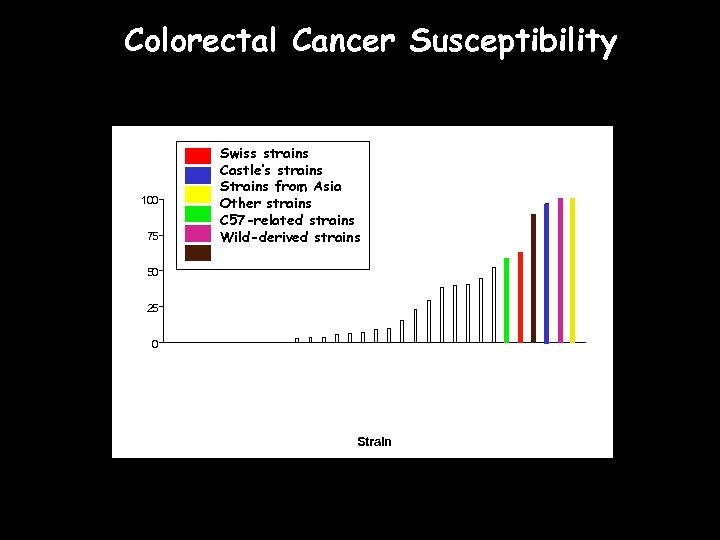
Colorectal Cancer Susceptibility 100 75 Swiss strains Castle’s strains Strains from Asia Other strains C 57 -related strains Wild-derived strains 50 25 0 Strain
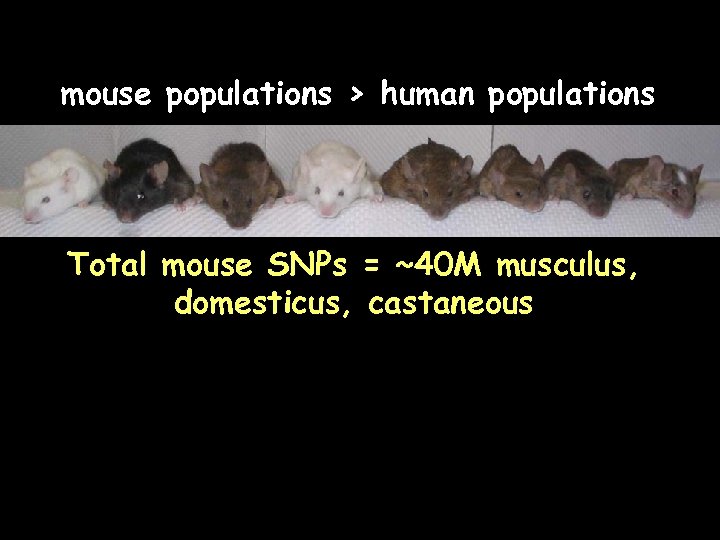
mouse populations > human populations Total mouse SNPs = ~40 M musculus, domesticus, castaneous
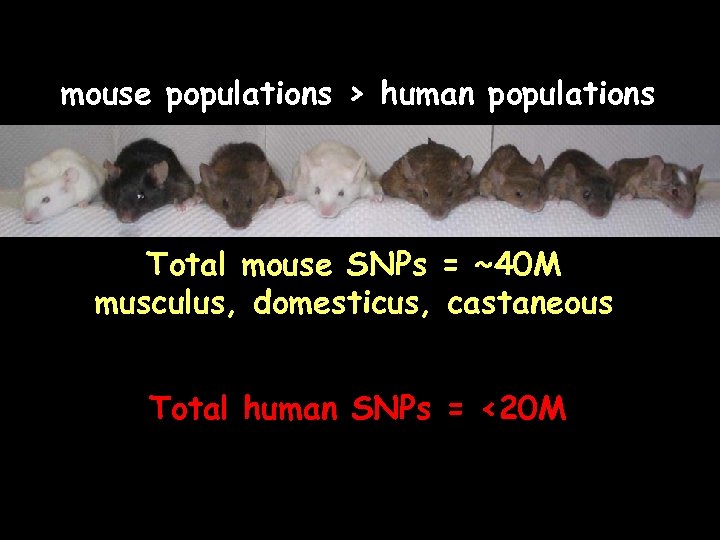
mouse populations > human populations Total mouse SNPs = ~40 M musculus, domesticus, castaneous Total human SNPs = <20 M
mouse populations > human populations Total mouse SNPs = ~65 M musculus, domesticus, castaneous + spretus Total human SNPs = <20 M
Why would human and mouse biology be similar? Evolutionary conservation!! - genome (gene content, arrangement and sequence) - structure (gross and molecular anatomy) - function (physiology and molecular circuits)
Why would human and mouse biology be similar? Evolutionary conservation!! - Phenotypic differences are due to a finite set of key genes - “Key” means nodes in regulatory networks
6ddba5eb3812508c0bb42d4bc8e519fe.ppt