98b29afa002c0bca98a437c5085c0414.ppt
- Количество слайдов: 56
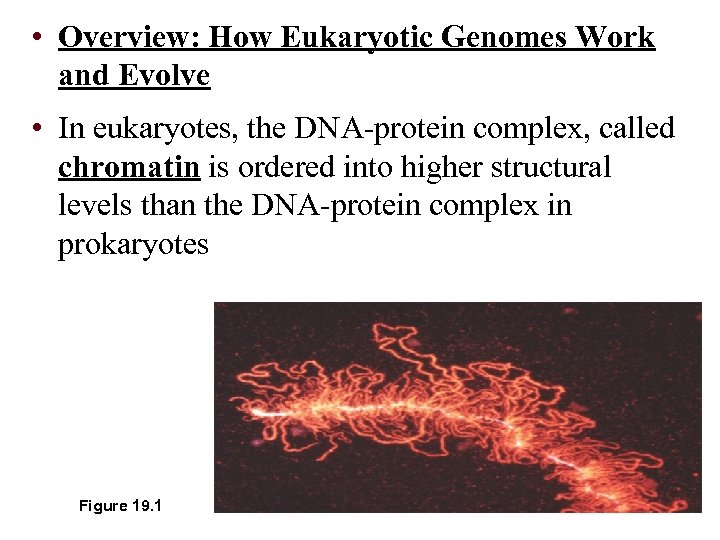 • Overview: How Eukaryotic Genomes Work and Evolve • In eukaryotes, the DNA-protein complex, called chromatin is ordered into higher structural levels than the DNA-protein complex in prokaryotes Figure 19. 1
• Overview: How Eukaryotic Genomes Work and Evolve • In eukaryotes, the DNA-protein complex, called chromatin is ordered into higher structural levels than the DNA-protein complex in prokaryotes Figure 19. 1
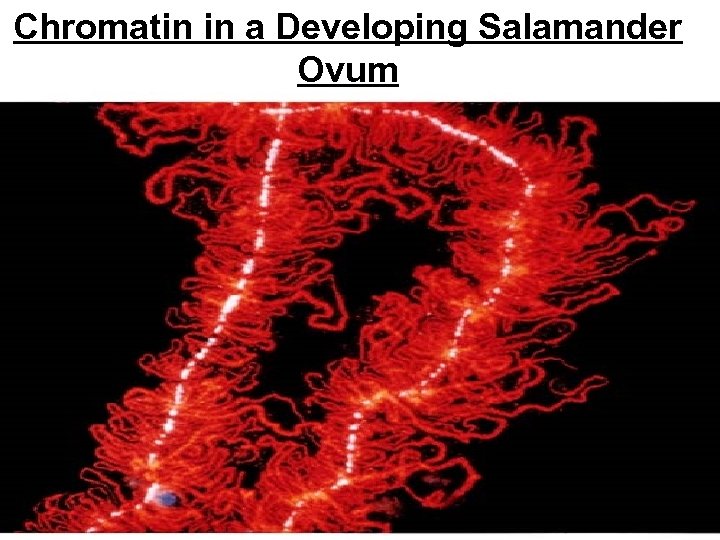 Chromatin in a Developing Salamander Ovum
Chromatin in a Developing Salamander Ovum
 • Both prokaryotes and eukaryotes • Must alter their patterns of gene expression in response to changes in environmental conditions
• Both prokaryotes and eukaryotes • Must alter their patterns of gene expression in response to changes in environmental conditions
 • Concept 19. 1: Chromatin structure is based on successive levels of DNA packing • Eukaryotic DNA • Is precisely combined with a large amount of protein • Eukaryotic chromosomes • Contain an enormous amount of DNA relative to their condensed length
• Concept 19. 1: Chromatin structure is based on successive levels of DNA packing • Eukaryotic DNA • Is precisely combined with a large amount of protein • Eukaryotic chromosomes • Contain an enormous amount of DNA relative to their condensed length
 Nucleosomes, or “Beads on a String” • Proteins called histones are responsible for the first level of DNA packing in chromatin • Bind tightly to DNA • The association of DNA and histones seems to remain intact throughout the cell cycle
Nucleosomes, or “Beads on a String” • Proteins called histones are responsible for the first level of DNA packing in chromatin • Bind tightly to DNA • The association of DNA and histones seems to remain intact throughout the cell cycle
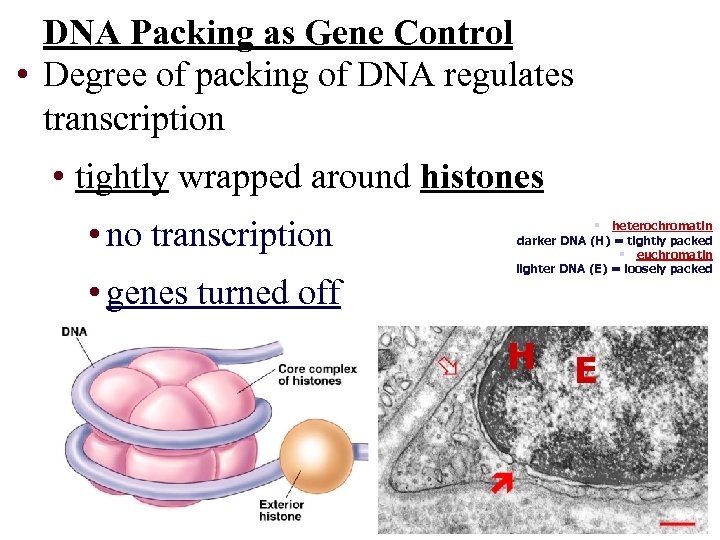 DNA Packing as Gene Control • Degree of packing of DNA regulates transcription • tightly wrapped around histones • no transcription • genes turned off § heterochromatin darker DNA (H) = tightly packed § euchromatin lighter DNA (E) = loosely packed H E
DNA Packing as Gene Control • Degree of packing of DNA regulates transcription • tightly wrapped around histones • no transcription • genes turned off § heterochromatin darker DNA (H) = tightly packed § euchromatin lighter DNA (E) = loosely packed H E
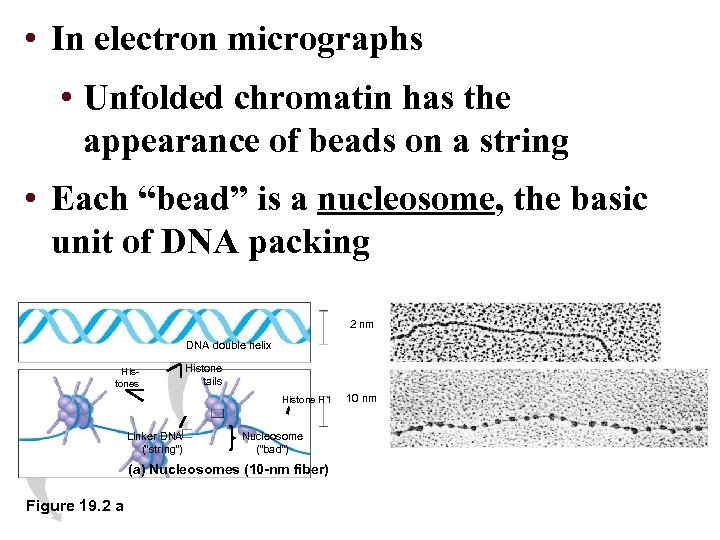 • In electron micrographs • Unfolded chromatin has the appearance of beads on a string • Each “bead” is a nucleosome, the basic unit of DNA packing 2 nm DNA double helix Histones Histone tails Histone H 1 Linker DNA (“string”) Nucleosome (“bad”) (a) Nucleosomes (10 -nm fiber) Figure 19. 2 a 10 nm
• In electron micrographs • Unfolded chromatin has the appearance of beads on a string • Each “bead” is a nucleosome, the basic unit of DNA packing 2 nm DNA double helix Histones Histone tails Histone H 1 Linker DNA (“string”) Nucleosome (“bad”) (a) Nucleosomes (10 -nm fiber) Figure 19. 2 a 10 nm
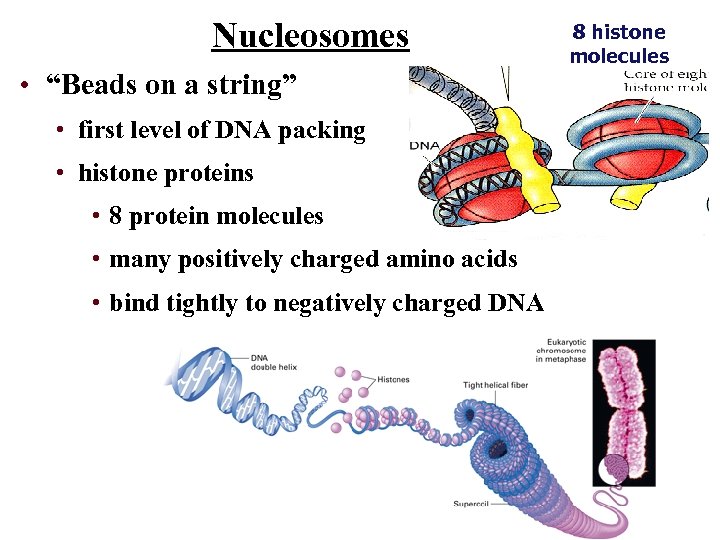 Nucleosomes • “Beads on a string” • first level of DNA packing • histone proteins • 8 protein molecules • many positively charged amino acids • bind tightly to negatively charged DNA 8 histone molecules
Nucleosomes • “Beads on a string” • first level of DNA packing • histone proteins • 8 protein molecules • many positively charged amino acids • bind tightly to negatively charged DNA 8 histone molecules
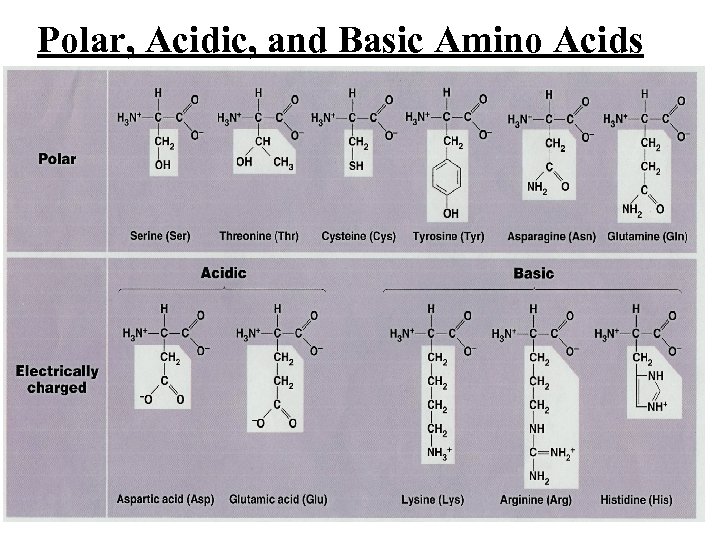 Polar, Acidic, and Basic Amino Acids
Polar, Acidic, and Basic Amino Acids
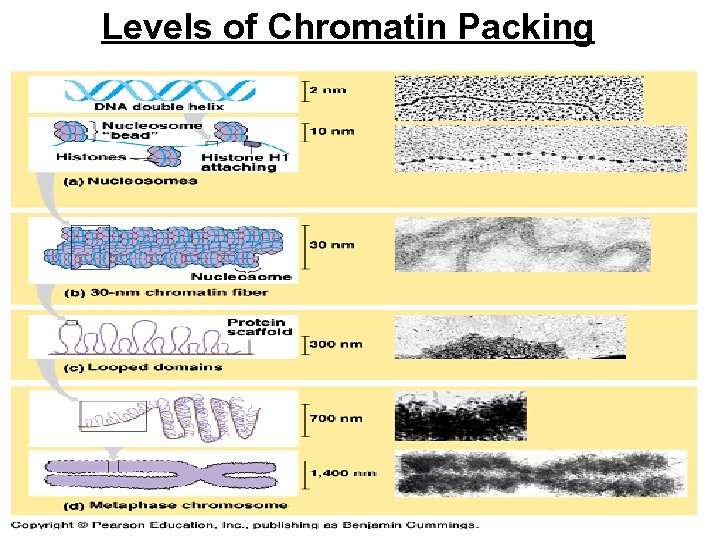 Levels of Chromatin Packing
Levels of Chromatin Packing
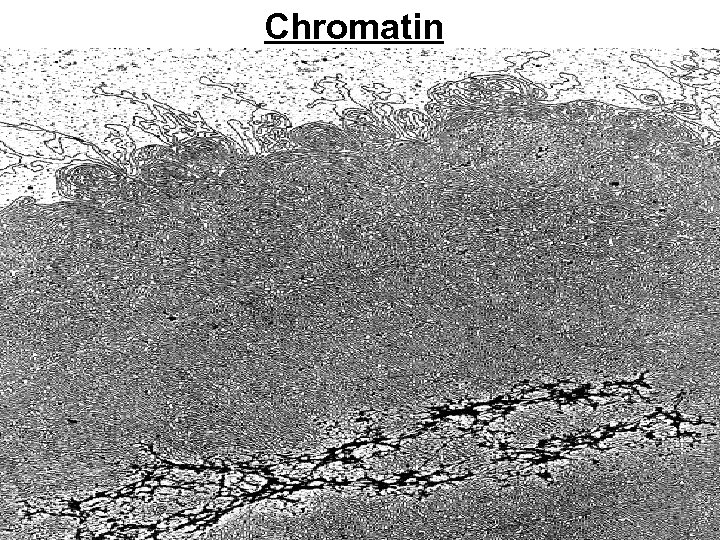 Chromatin
Chromatin
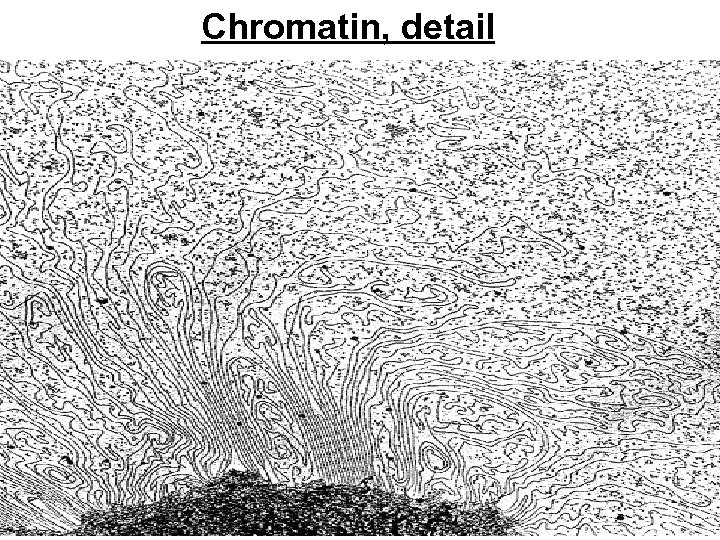 Chromatin, detail
Chromatin, detail
 • Concept 19. 2: Gene expression can be regulated at any stage, but the key step is transcription • All organisms must regulate which genes are expressed at any given time • During development of a multicellular organism cells undergo a process of specialization in form and function called cellular differentiation
• Concept 19. 2: Gene expression can be regulated at any stage, but the key step is transcription • All organisms must regulate which genes are expressed at any given time • During development of a multicellular organism cells undergo a process of specialization in form and function called cellular differentiation
 Differential Gene Expression • Each cell of a multicellular eukaryote expresses only a fraction of its genes • In each type of differentiated cell a unique subset of genes is expressed
Differential Gene Expression • Each cell of a multicellular eukaryote expresses only a fraction of its genes • In each type of differentiated cell a unique subset of genes is expressed
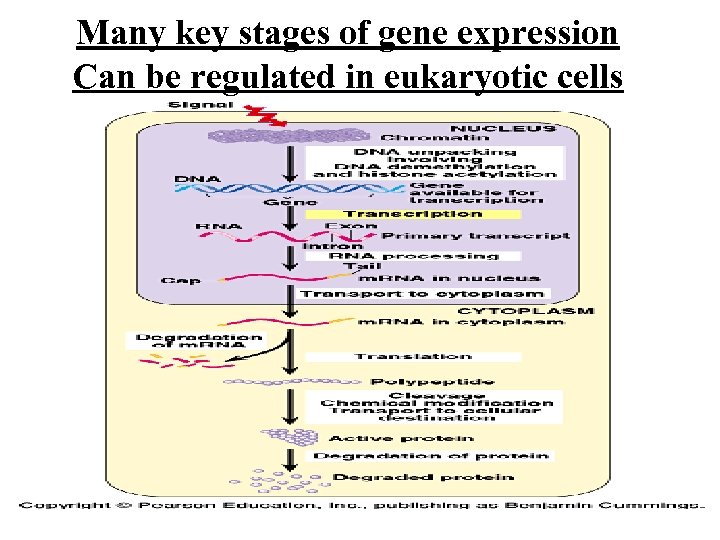 Many key stages of gene expression Can be regulated in eukaryotic cells
Many key stages of gene expression Can be regulated in eukaryotic cells
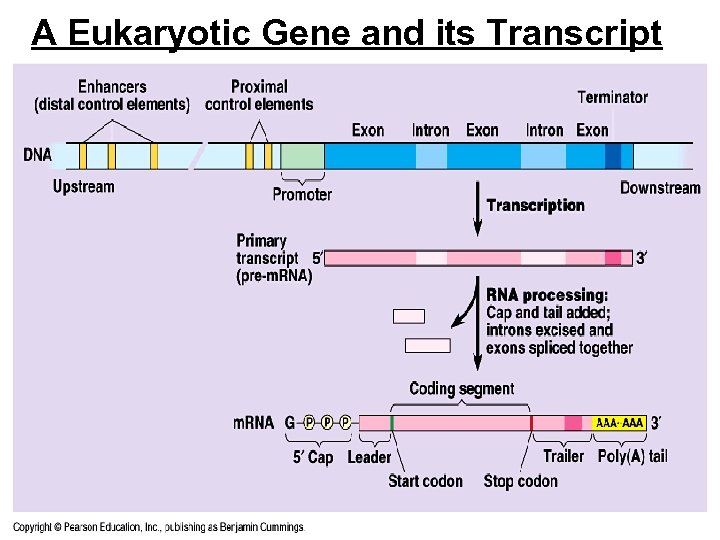 A Eukaryotic Gene and its Transcript
A Eukaryotic Gene and its Transcript
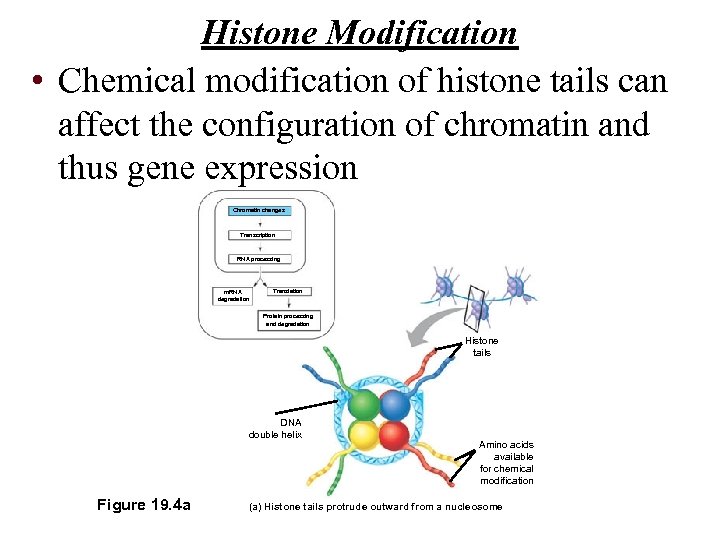 Histone Modification • Chemical modification of histone tails can affect the configuration of chromatin and thus gene expression Chromatin changes Transcription RNA processing m. RNA degradation Translation Protein processing and degradation Histone tails DNA double helix Figure 19. 4 a Amino acids available for chemical modification (a) Histone tails protrude outward from a nucleosome
Histone Modification • Chemical modification of histone tails can affect the configuration of chromatin and thus gene expression Chromatin changes Transcription RNA processing m. RNA degradation Translation Protein processing and degradation Histone tails DNA double helix Figure 19. 4 a Amino acids available for chemical modification (a) Histone tails protrude outward from a nucleosome
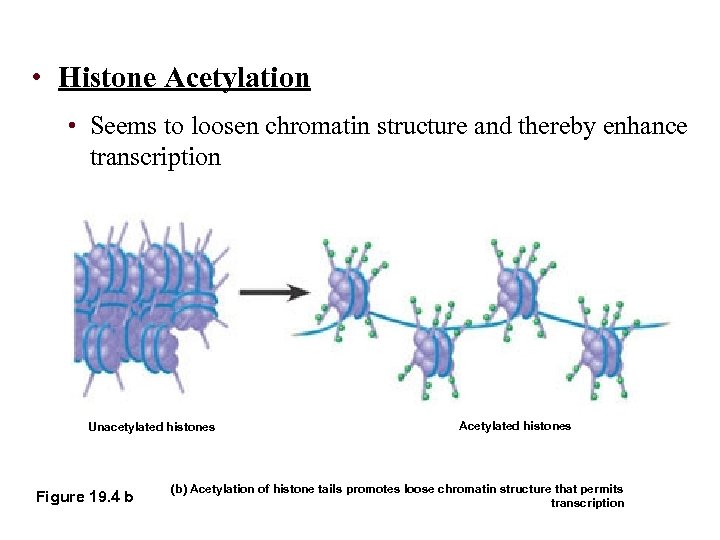 • Histone Acetylation • Seems to loosen chromatin structure and thereby enhance transcription Unacetylated histones Figure 19. 4 b Acetylated histones (b) Acetylation of histone tails promotes loose chromatin structure that permits transcription
• Histone Acetylation • Seems to loosen chromatin structure and thereby enhance transcription Unacetylated histones Figure 19. 4 b Acetylated histones (b) Acetylation of histone tails promotes loose chromatin structure that permits transcription
 DNA Methylation • Addition of methyl groups to certain bases in DNA is associated with reduced transcription in some species
DNA Methylation • Addition of methyl groups to certain bases in DNA is associated with reduced transcription in some species
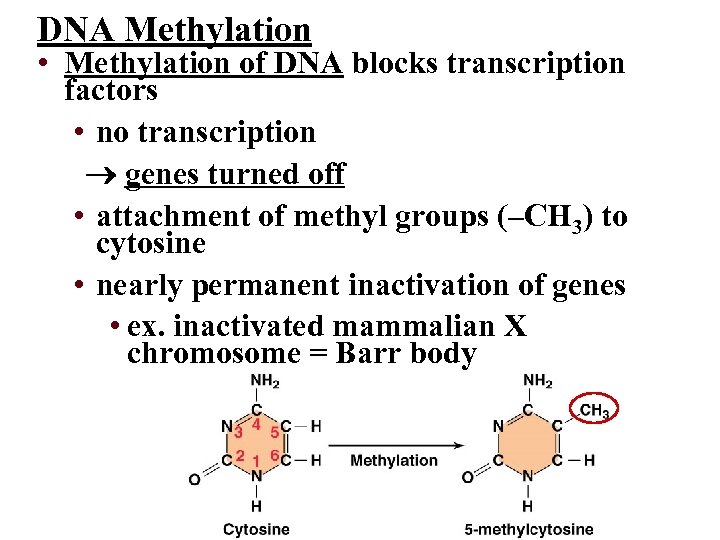 DNA Methylation • Methylation of DNA blocks transcription factors • no transcription genes turned off • attachment of methyl groups (–CH 3) to cytosine • nearly permanent inactivation of genes • ex. inactivated mammalian X chromosome = Barr body
DNA Methylation • Methylation of DNA blocks transcription factors • no transcription genes turned off • attachment of methyl groups (–CH 3) to cytosine • nearly permanent inactivation of genes • ex. inactivated mammalian X chromosome = Barr body
 • Epigenetic Inheritance is the inheritance of traits transmitted by mechanisms not directly involving the nucleotide sequence
• Epigenetic Inheritance is the inheritance of traits transmitted by mechanisms not directly involving the nucleotide sequence
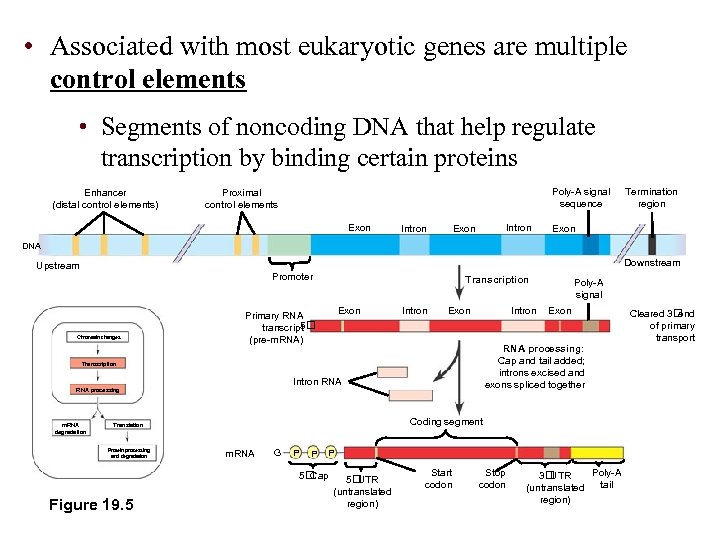 • Associated with most eukaryotic genes are multiple control elements • Segments of noncoding DNA that help regulate transcription by binding certain proteins Enhancer (distal control elements) Poly-A signal sequence Proximal control elements Exon Intron Exon Termination region Exon DNA Downstream Upstream Promoter Chromatin changes Transcription Exon Primary RNA 5 transcript (pre-m. RNA) Intron Exon Intron m. RNA degradation Intron RNA Coding segment Translation Protein processing and degradation m. RNA G P P P 5 Cap Figure 19. 5 Exon RNA processing: Cap and tail added; introns excised and exons spliced together Transcription RNA processing Poly-A signal 5 UTR (untranslated region) Start codon Stop codon Poly-A 3 UTR tail (untranslated region) Cleared 3 end of primary transport
• Associated with most eukaryotic genes are multiple control elements • Segments of noncoding DNA that help regulate transcription by binding certain proteins Enhancer (distal control elements) Poly-A signal sequence Proximal control elements Exon Intron Exon Termination region Exon DNA Downstream Upstream Promoter Chromatin changes Transcription Exon Primary RNA 5 transcript (pre-m. RNA) Intron Exon Intron m. RNA degradation Intron RNA Coding segment Translation Protein processing and degradation m. RNA G P P P 5 Cap Figure 19. 5 Exon RNA processing: Cap and tail added; introns excised and exons spliced together Transcription RNA processing Poly-A signal 5 UTR (untranslated region) Start codon Stop codon Poly-A 3 UTR tail (untranslated region) Cleared 3 end of primary transport
 • Proximal control elements are located close to the promoter • Distal control elements, groups of which are called enhancers may be far away from a gene or even in an intron
• Proximal control elements are located close to the promoter • Distal control elements, groups of which are called enhancers may be far away from a gene or even in an intron
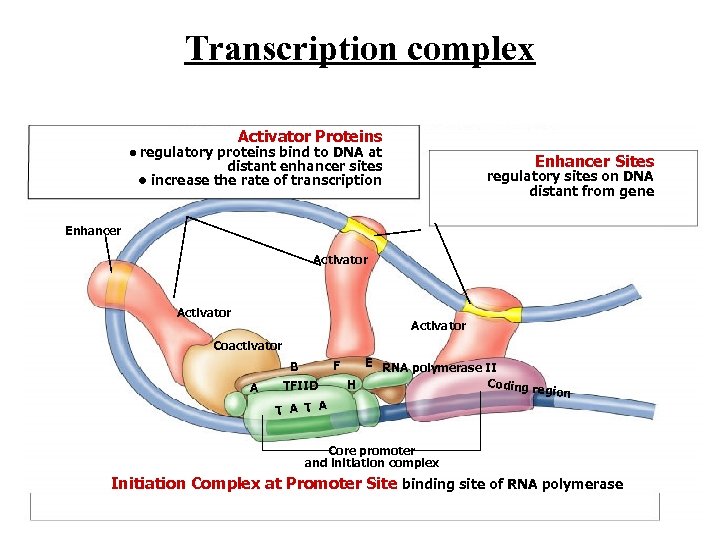 Transcription complex Activator Proteins • regulatory proteins bind to DNA at Enhancer Sites distant enhancer sites • increase the rate of transcription regulatory sites on DNA distant from gene Enhancer Activator Coactivator F B A TFIID T A E RNA polymerase II Coding r H egion Core promoter and initiation complex Initiation Complex at Promoter Site binding site of RNA polymerase
Transcription complex Activator Proteins • regulatory proteins bind to DNA at Enhancer Sites distant enhancer sites • increase the rate of transcription regulatory sites on DNA distant from gene Enhancer Activator Coactivator F B A TFIID T A E RNA polymerase II Coding r H egion Core promoter and initiation complex Initiation Complex at Promoter Site binding site of RNA polymerase
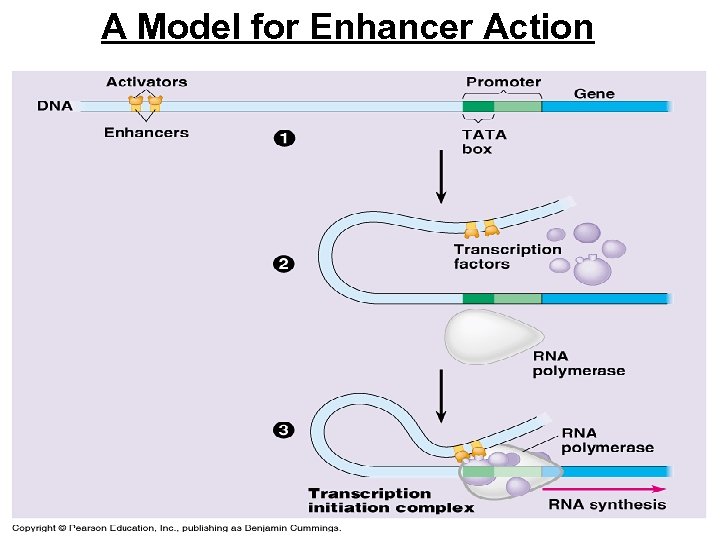 A Model for Enhancer Action
A Model for Enhancer Action
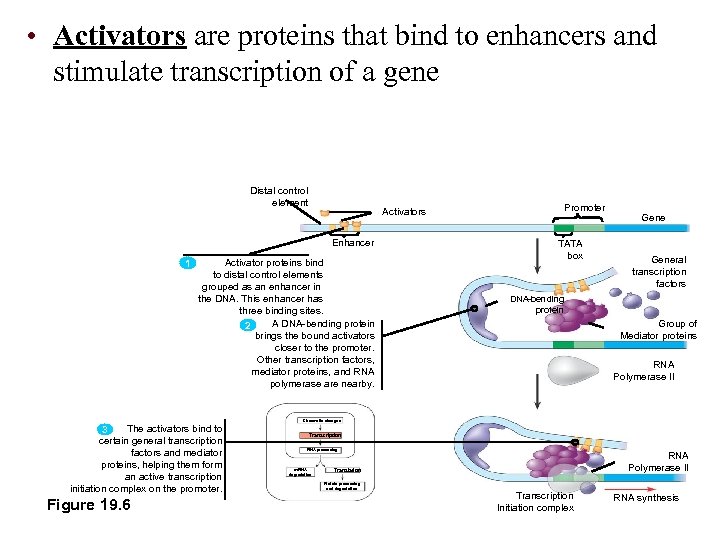 • Activators are proteins that bind to enhancers and stimulate transcription of a gene Distal control element Enhancer 1 Promoter Activators Activator proteins bind to distal control elements grouped as an enhancer in the DNA. This enhancer has three binding sites. A DNA-bending protein 2 brings the bound activators closer to the promoter. Other transcription factors, mediator proteins, and RNA polymerase are nearby. TATA box General transcription factors DNA-bending protein Group of Mediator proteins RNA Polymerase II Chromatin changes The activators bind to 3 certain general transcription factors and mediator proteins, helping them form an active transcription initiation complex on the promoter. Figure 19. 6 Transcription RNA processing m. RNA degradation RNA Polymerase II Translation Protein processing and degradation Transcription Initiation complex RNA synthesis
• Activators are proteins that bind to enhancers and stimulate transcription of a gene Distal control element Enhancer 1 Promoter Activators Activator proteins bind to distal control elements grouped as an enhancer in the DNA. This enhancer has three binding sites. A DNA-bending protein 2 brings the bound activators closer to the promoter. Other transcription factors, mediator proteins, and RNA polymerase are nearby. TATA box General transcription factors DNA-bending protein Group of Mediator proteins RNA Polymerase II Chromatin changes The activators bind to 3 certain general transcription factors and mediator proteins, helping them form an active transcription initiation complex on the promoter. Figure 19. 6 Transcription RNA processing m. RNA degradation RNA Polymerase II Translation Protein processing and degradation Transcription Initiation complex RNA synthesis
 • Some specific transcription factors function as repressors to inhibit expression of a particular gene • Some activators and repressors act indirectly by influencing chromatin structure
• Some specific transcription factors function as repressors to inhibit expression of a particular gene • Some activators and repressors act indirectly by influencing chromatin structure
 Post-Transcriptional Control • Alternative RNA splicing • variable processing of exons creates a family of proteins, depending on which RNA segments are treated as exons and which as introns
Post-Transcriptional Control • Alternative RNA splicing • variable processing of exons creates a family of proteins, depending on which RNA segments are treated as exons and which as introns
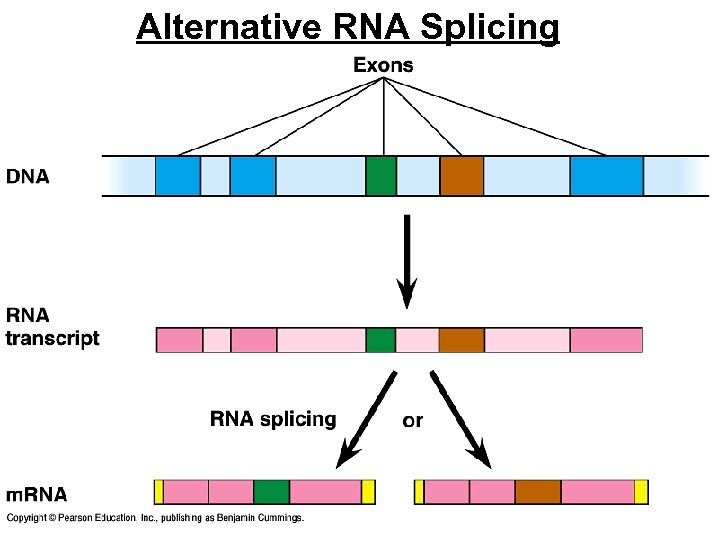 Alternative RNA Splicing
Alternative RNA Splicing
 Micro. RNAs (mi. RNAs) • small single-stranded RNA molecules that can bind to m. RNA • These can degrade m. RNA or block its translation • Inhibition of gene expression by RNA molecules = RNA INTERFERENCE (RNAi)
Micro. RNAs (mi. RNAs) • small single-stranded RNA molecules that can bind to m. RNA • These can degrade m. RNA or block its translation • Inhibition of gene expression by RNA molecules = RNA INTERFERENCE (RNAi)
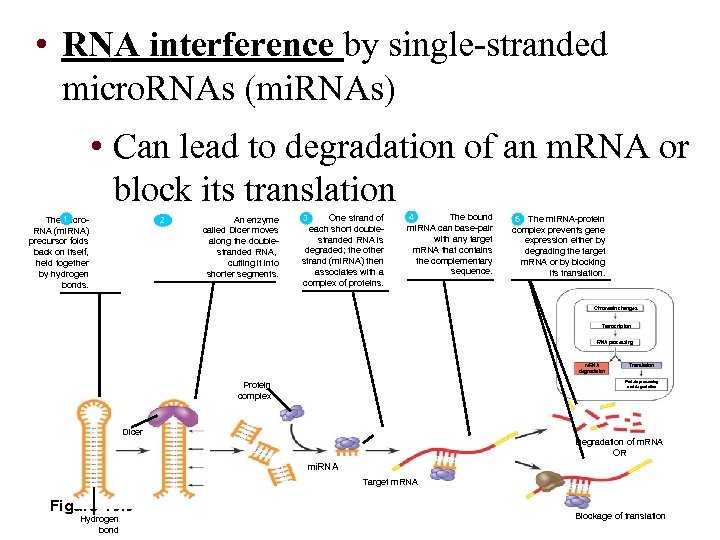 • RNA interference by single-stranded micro. RNAs (mi. RNAs) • Can lead to degradation of an m. RNA or block its translation The 1 micro. RNA (mi. RNA) precursor folds back on itself, held together by hydrogen bonds. 2 2 An enzyme called Dicer moves along the doublestranded RNA, cutting it into shorter segments. One strand of each short doublestranded RNA is degraded; the other strand (mi. RNA) then associates with a complex of proteins. 3 4 The bound mi. RNA can base-pair with any target m. RNA that contains the complementary sequence. 5 The mi. RNA-protein 5 complex prevents gene expression either by degrading the target m. RNA or by blocking its translation. Chromatin changes Transcription RNA processing m. RNA degradation Protein complex Translation Protein processing and degradation Dicer Degradation of m. RNA OR mi. RNA Target m. RNA Figure 19. 9 Hydrogen bond Blockage of translation
• RNA interference by single-stranded micro. RNAs (mi. RNAs) • Can lead to degradation of an m. RNA or block its translation The 1 micro. RNA (mi. RNA) precursor folds back on itself, held together by hydrogen bonds. 2 2 An enzyme called Dicer moves along the doublestranded RNA, cutting it into shorter segments. One strand of each short doublestranded RNA is degraded; the other strand (mi. RNA) then associates with a complex of proteins. 3 4 The bound mi. RNA can base-pair with any target m. RNA that contains the complementary sequence. 5 The mi. RNA-protein 5 complex prevents gene expression either by degrading the target m. RNA or by blocking its translation. Chromatin changes Transcription RNA processing m. RNA degradation Protein complex Translation Protein processing and degradation Dicer Degradation of m. RNA OR mi. RNA Target m. RNA Figure 19. 9 Hydrogen bond Blockage of translation
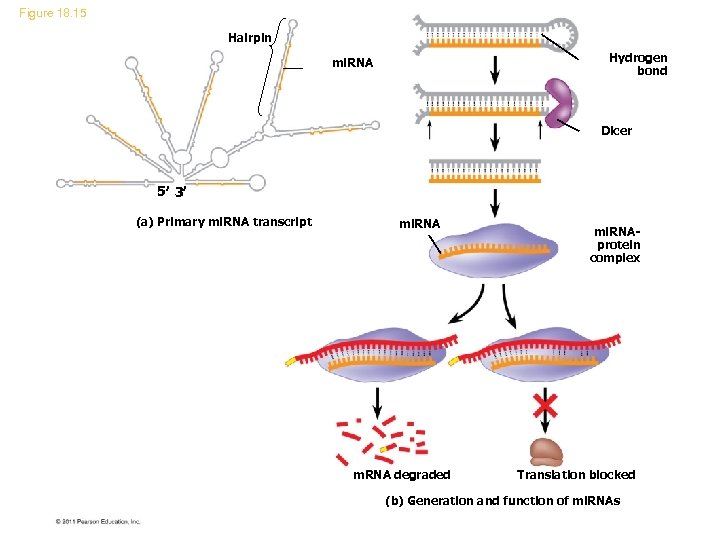 Figure 18. 15 Hairpin Hydrogen bond mi. RNA Dicer 5 3 (a) Primary mi. RNA transcript mi. RNA m. RNA degraded mi. RNAprotein complex Translation blocked (b) Generation and function of mi. RNAs
Figure 18. 15 Hairpin Hydrogen bond mi. RNA Dicer 5 3 (a) Primary mi. RNA transcript mi. RNA m. RNA degraded mi. RNAprotein complex Translation blocked (b) Generation and function of mi. RNAs
 Small Interfering RNAs (si. RNAs) • RNA interference (RNAi) is caused by si. RNAs • Ex: Yeast: si. RNA’s play a role in heterochromatin formation and can block large regions of the chromosome
Small Interfering RNAs (si. RNAs) • RNA interference (RNAi) is caused by si. RNAs • Ex: Yeast: si. RNA’s play a role in heterochromatin formation and can block large regions of the chromosome
 • The initiation of translation of selected m. RNAs can be blocked by regulatory proteins that bind to specific sequences or structures of the m. RNA • Alternatively, translation of all the m. RNAs in a cell may be regulated simultaneously
• The initiation of translation of selected m. RNAs can be blocked by regulatory proteins that bind to specific sequences or structures of the m. RNA • Alternatively, translation of all the m. RNAs in a cell may be regulated simultaneously
 • After translation various types of protein processing, including cleavage and the addition of chemical groups, are subject to control
• After translation various types of protein processing, including cleavage and the addition of chemical groups, are subject to control
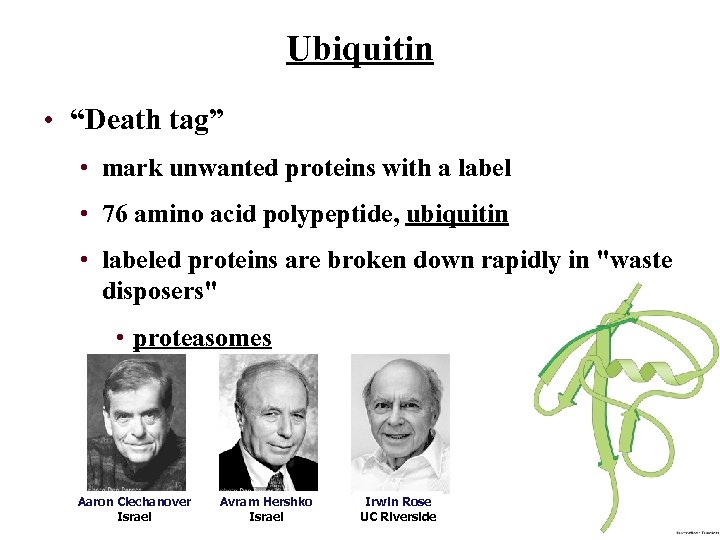 Ubiquitin • “Death tag” • mark unwanted proteins with a label • 76 amino acid polypeptide, ubiquitin • labeled proteins are broken down rapidly in "waste disposers" • proteasomes Aaron Ciechanover Israel Avram Hershko Israel Irwin Rose UC Riverside
Ubiquitin • “Death tag” • mark unwanted proteins with a label • 76 amino acid polypeptide, ubiquitin • labeled proteins are broken down rapidly in "waste disposers" • proteasomes Aaron Ciechanover Israel Avram Hershko Israel Irwin Rose UC Riverside
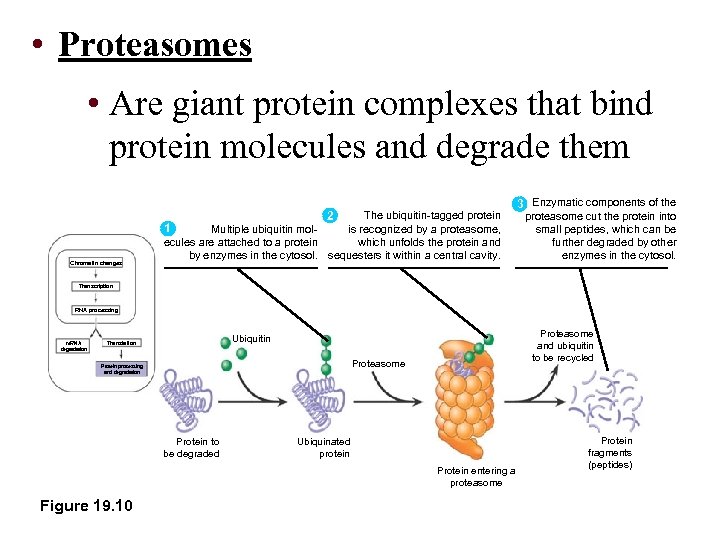 • Proteasomes • Are giant protein complexes that bind protein molecules and degrade them Chromatin changes The ubiquitin-tagged protein 2 1 is recognized by a proteasome, Multiple ubiquitin molwhich unfolds the protein and ecules are attached to a protein by enzymes in the cytosol. sequesters it within a central cavity. 3 Enzymatic components of the proteasome cut the protein into small peptides, which can be further degraded by other enzymes in the cytosol. Transcription RNA processing m. RNA degradation Proteasome and ubiquitin to be recycled Ubiquitin Translation Proteasome Protein processing and degradation Protein to be degraded Ubiquinated protein Protein entering a proteasome Figure 19. 10 Protein fragments (peptides)
• Proteasomes • Are giant protein complexes that bind protein molecules and degrade them Chromatin changes The ubiquitin-tagged protein 2 1 is recognized by a proteasome, Multiple ubiquitin molwhich unfolds the protein and ecules are attached to a protein by enzymes in the cytosol. sequesters it within a central cavity. 3 Enzymatic components of the proteasome cut the protein into small peptides, which can be further degraded by other enzymes in the cytosol. Transcription RNA processing m. RNA degradation Proteasome and ubiquitin to be recycled Ubiquitin Translation Proteasome Protein processing and degradation Protein to be degraded Ubiquinated protein Protein entering a proteasome Figure 19. 10 Protein fragments (peptides)
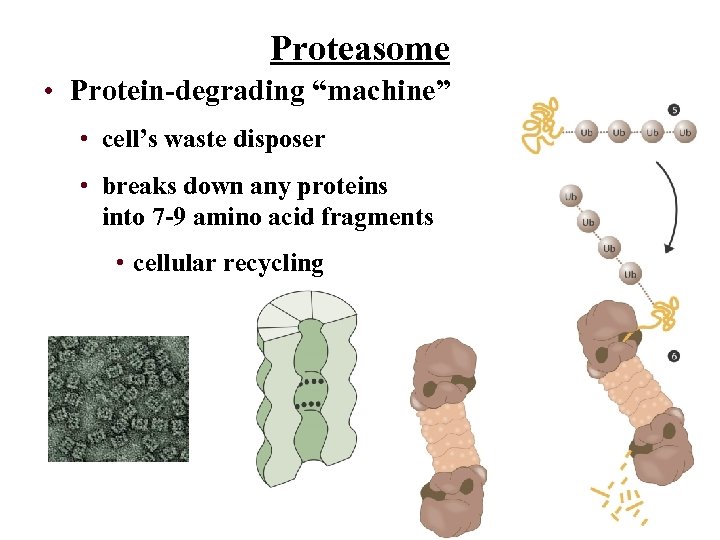 Proteasome • Protein-degrading “machine” • cell’s waste disposer • breaks down any proteins into 7 -9 amino acid fragments • cellular recycling
Proteasome • Protein-degrading “machine” • cell’s waste disposer • breaks down any proteins into 7 -9 amino acid fragments • cellular recycling
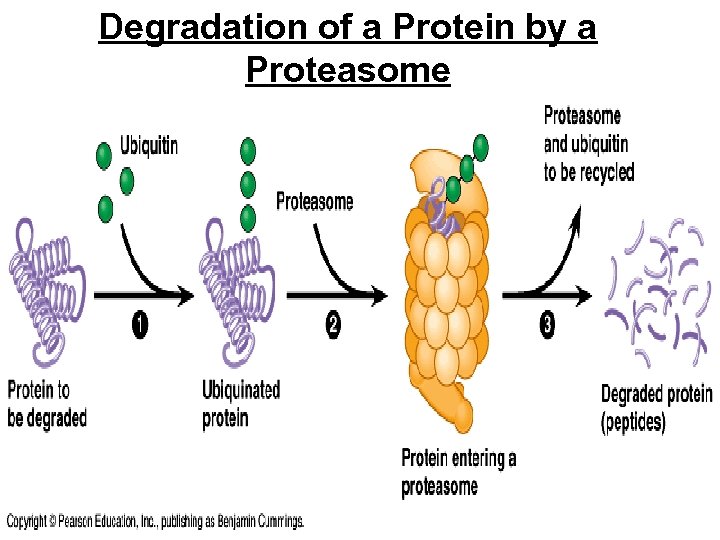 Degradation of a Protein by a Proteasome
Degradation of a Protein by a Proteasome
 • Concept 19. 4 Eukaryotic genomes can have many noncoding DNA sequences in addition to genes • The bulk of most eukaryotic genomes consists of noncoding DNA sequences, often described in the past as “junk DNA” • However, much evidence is accumulating that noncoding DNA plays important roles in the cell
• Concept 19. 4 Eukaryotic genomes can have many noncoding DNA sequences in addition to genes • The bulk of most eukaryotic genomes consists of noncoding DNA sequences, often described in the past as “junk DNA” • However, much evidence is accumulating that noncoding DNA plays important roles in the cell
 The Relationship Between Genomic Composition and Organismal Complexity • Compared with prokaryotic genomes, the genomes of eukaryotes • Generally are larger • Have longer genes • Contain a much greater amount of noncoding DNA both associated with genes and between genes
The Relationship Between Genomic Composition and Organismal Complexity • Compared with prokaryotic genomes, the genomes of eukaryotes • Generally are larger • Have longer genes • Contain a much greater amount of noncoding DNA both associated with genes and between genes
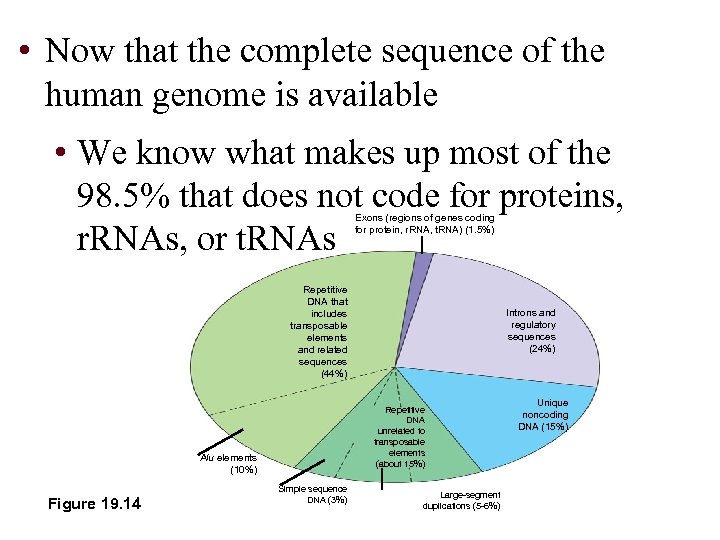 • Now that the complete sequence of the human genome is available • We know what makes up most of the 98. 5% that does not code for proteins, r. RNAs, or t. RNAs Exons (regions of genes coding for protein, r. RNA, t. RNA) (1. 5%) Repetitive DNA that includes transposable elements and related sequences (44%) Repetitive DNA unrelated to transposable elements (about 15%) Alu elements (10%) Figure 19. 14 Introns and regulatory sequences (24%) Simple sequence DNA (3%) Large-segment duplications (5 -6%) Unique noncoding DNA (15%)
• Now that the complete sequence of the human genome is available • We know what makes up most of the 98. 5% that does not code for proteins, r. RNAs, or t. RNAs Exons (regions of genes coding for protein, r. RNA, t. RNA) (1. 5%) Repetitive DNA that includes transposable elements and related sequences (44%) Repetitive DNA unrelated to transposable elements (about 15%) Alu elements (10%) Figure 19. 14 Introns and regulatory sequences (24%) Simple sequence DNA (3%) Large-segment duplications (5 -6%) Unique noncoding DNA (15%)
 • The first evidence for wandering DNA segments • Came from geneticist Barbara Mc. Clintock’s breeding experiments with Indian corn Figure 19. 15
• The first evidence for wandering DNA segments • Came from geneticist Barbara Mc. Clintock’s breeding experiments with Indian corn Figure 19. 15
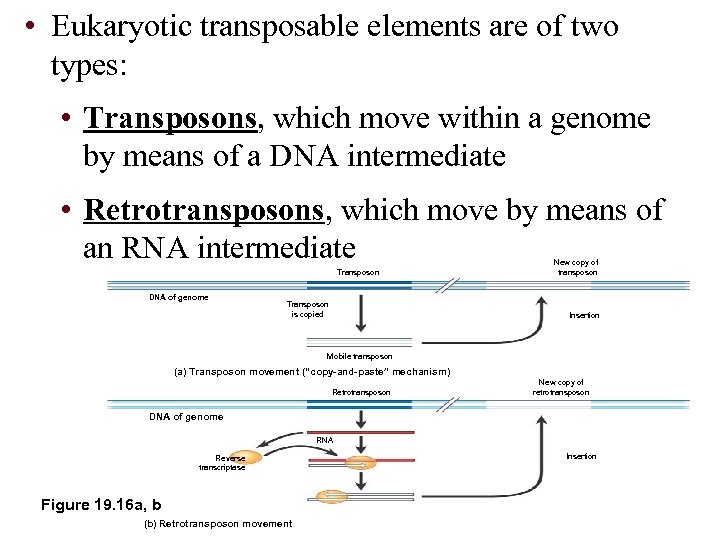 • Eukaryotic transposable elements are of two types: • Transposons, which move within a genome by means of a DNA intermediate • Retrotransposons, which move by means of an RNA intermediate Transposon DNA of genome Transposon is copied New copy of transposon Insertion Mobile transposon (a) Transposon movement (“copy-and-paste” mechanism) Retrotransposon New copy of retrotransposon DNA of genome RNA Reverse transcriptase Figure 19. 16 a, b (b) Retrotransposon movement Insertion
• Eukaryotic transposable elements are of two types: • Transposons, which move within a genome by means of a DNA intermediate • Retrotransposons, which move by means of an RNA intermediate Transposon DNA of genome Transposon is copied New copy of transposon Insertion Mobile transposon (a) Transposon movement (“copy-and-paste” mechanism) Retrotransposon New copy of retrotransposon DNA of genome RNA Reverse transcriptase Figure 19. 16 a, b (b) Retrotransposon movement Insertion
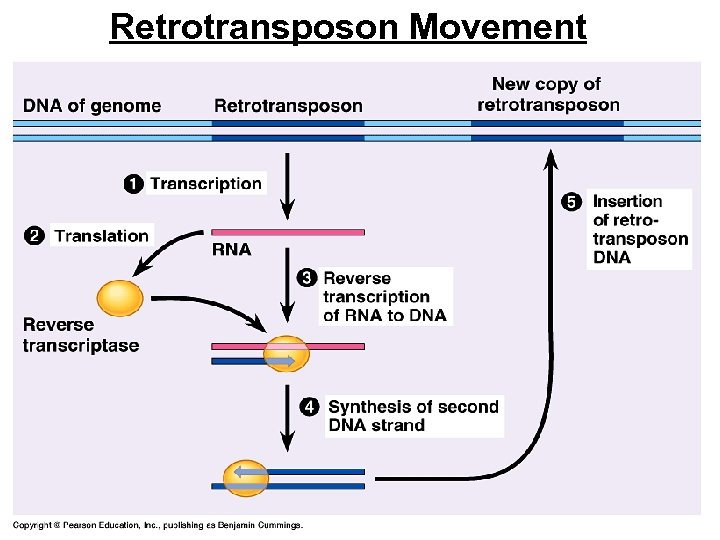 Retrotransposon Movement
Retrotransposon Movement
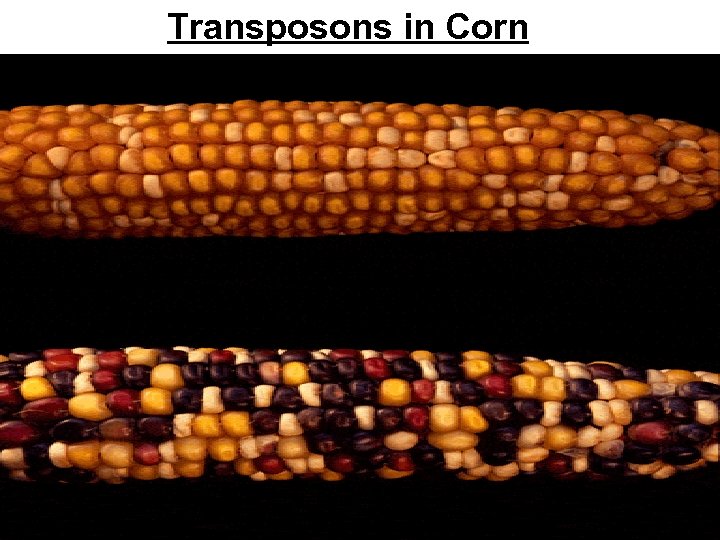 Transposons in Corn
Transposons in Corn
 Rearrangements of Parts of Genes: Exon Duplication and Exon Shuffling • A particular exon within a gene could be duplicated on one chromosome and deleted from the homologous chromosome
Rearrangements of Parts of Genes: Exon Duplication and Exon Shuffling • A particular exon within a gene could be duplicated on one chromosome and deleted from the homologous chromosome
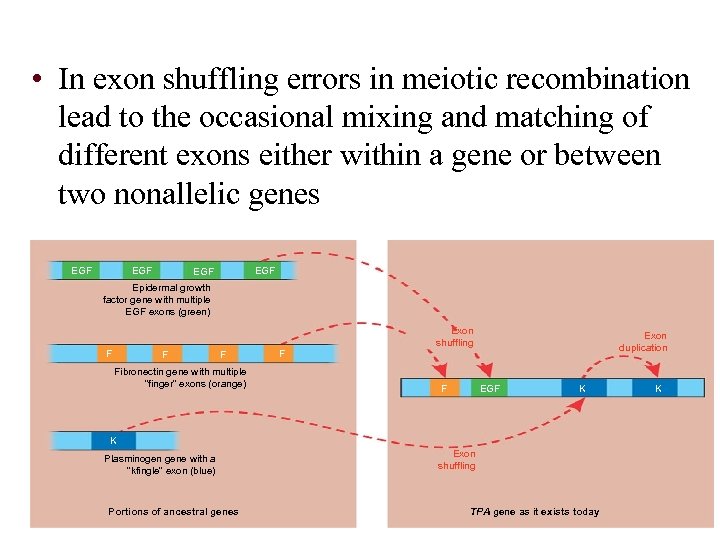 • In exon shuffling errors in meiotic recombination lead to the occasional mixing and matching of different exons either within a gene or between two nonallelic genes EGF EGF Epidermal growth factor gene with multiple EGF exons (green) F Fibronectin gene with multiple “finger” exons (orange) F Exon shuffling F Exon duplication EGF K K Plasminogen gene with a “kfingle” exon (blue) Figure 19. 20 Portions of ancestral genes Exon shuffling TPA gene as it exists today K
• In exon shuffling errors in meiotic recombination lead to the occasional mixing and matching of different exons either within a gene or between two nonallelic genes EGF EGF Epidermal growth factor gene with multiple EGF exons (green) F Fibronectin gene with multiple “finger” exons (orange) F Exon shuffling F Exon duplication EGF K K Plasminogen gene with a “kfingle” exon (blue) Figure 19. 20 Portions of ancestral genes Exon shuffling TPA gene as it exists today K
 How Transposable Elements Contribute to Genome Evolution • Movement of transposable elements or recombination between copies of the same element occasionally generates new sequence combinations that are beneficial to the organism • Some mechanisms can alter the functions of genes or their patterns of expression and regulation
How Transposable Elements Contribute to Genome Evolution • Movement of transposable elements or recombination between copies of the same element occasionally generates new sequence combinations that are beneficial to the organism • Some mechanisms can alter the functions of genes or their patterns of expression and regulation
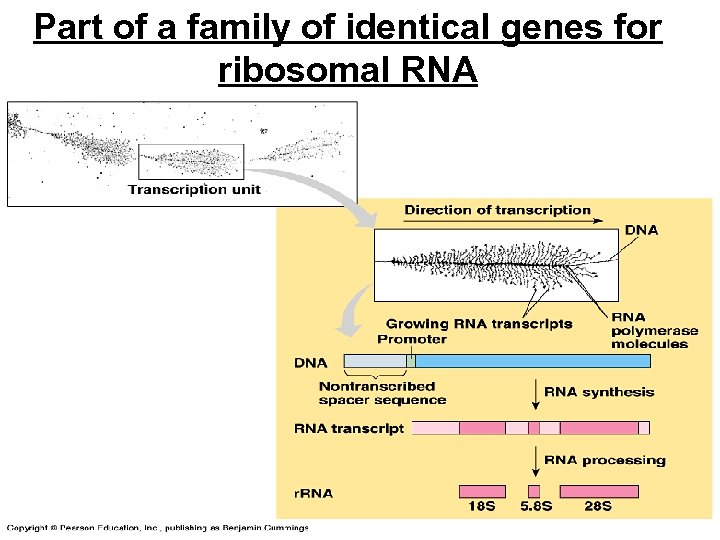 Part of a family of identical genes for ribosomal RNA
Part of a family of identical genes for ribosomal RNA
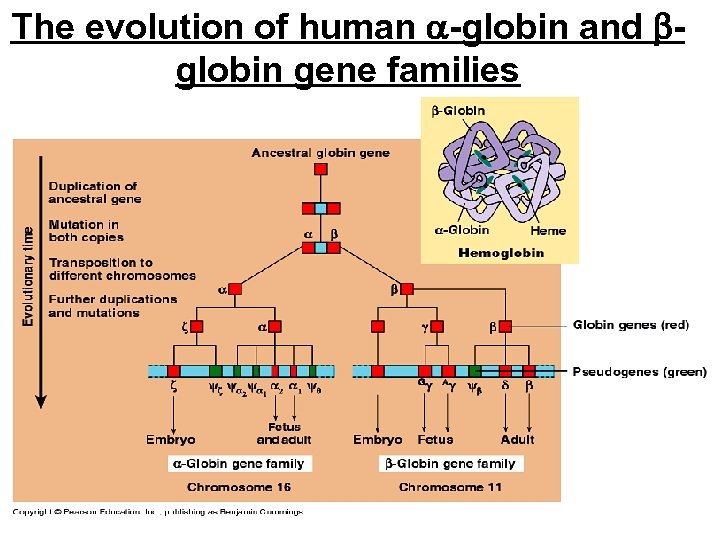 The evolution of human -globin and globin gene families
The evolution of human -globin and globin gene families
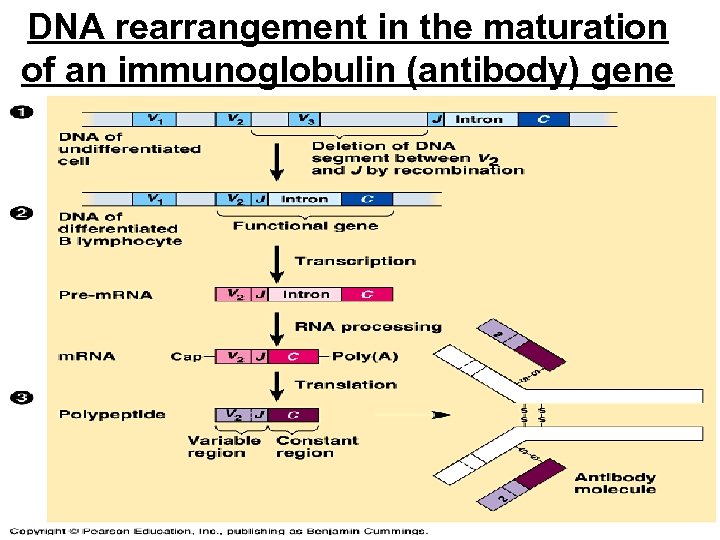 DNA rearrangement in the maturation of an immunoglobulin (antibody) gene
DNA rearrangement in the maturation of an immunoglobulin (antibody) gene
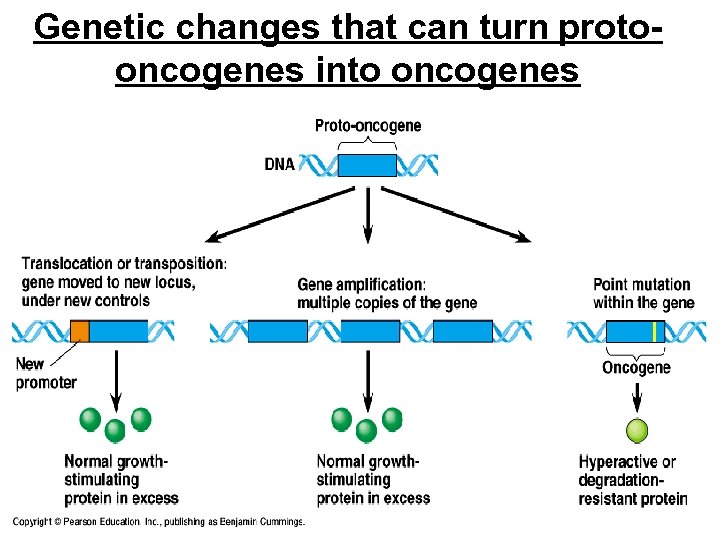 Genetic changes that can turn protooncogenes into oncogenes
Genetic changes that can turn protooncogenes into oncogenes
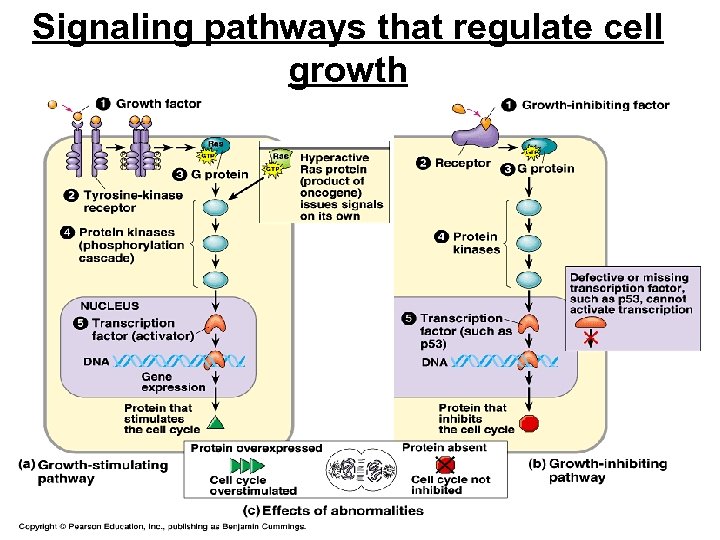 Signaling pathways that regulate cell growth
Signaling pathways that regulate cell growth
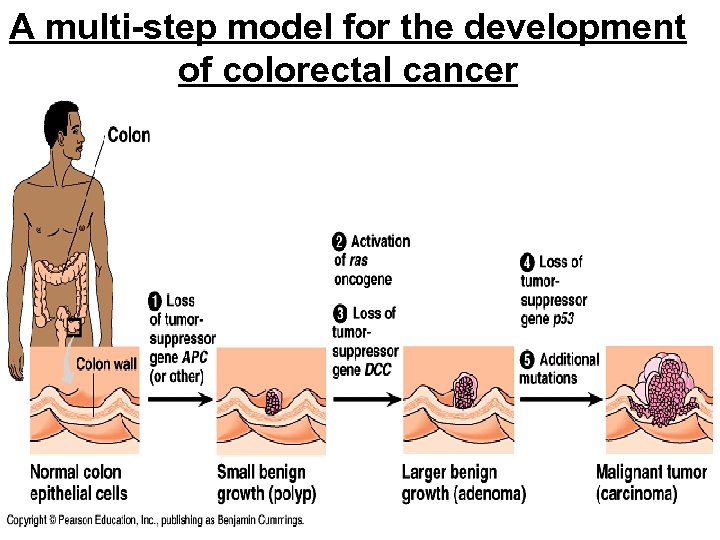 A multi-step model for the development of colorectal cancer
A multi-step model for the development of colorectal cancer


