4dfef6d281e2432c8756e5a2360f81fb.ppt
- Количество слайдов: 75
 OVERVIEW FOR EXAM 2
OVERVIEW FOR EXAM 2
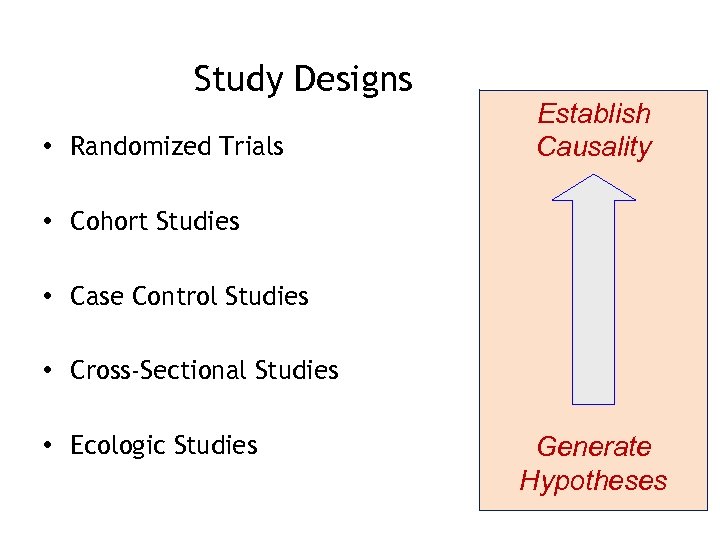 Study Designs • Randomized Trials Establish Causality • Cohort Studies • Case Control Studies • Cross-Sectional Studies • Ecologic Studies Generate Hypotheses
Study Designs • Randomized Trials Establish Causality • Cohort Studies • Case Control Studies • Cross-Sectional Studies • Ecologic Studies Generate Hypotheses
 Randomized Controlled Trials
Randomized Controlled Trials
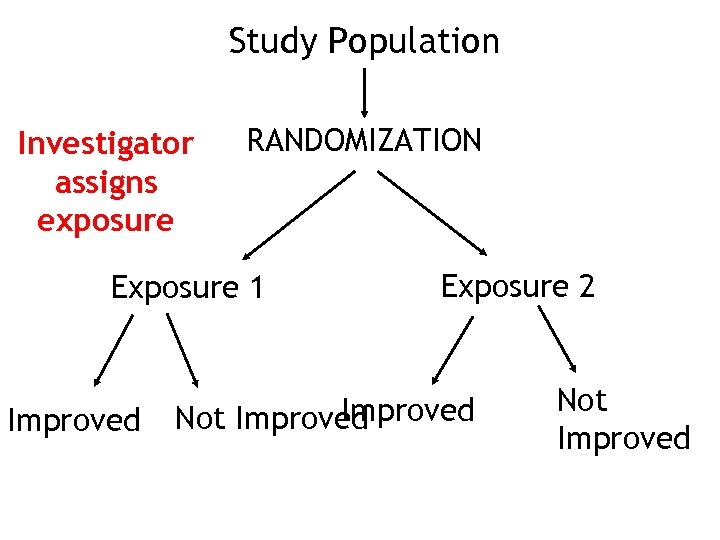 Study Population Investigator assigns exposure RANDOMIZATION Exposure 1 Improved Exposure 2 Improved Not Improved
Study Population Investigator assigns exposure RANDOMIZATION Exposure 1 Improved Exposure 2 Improved Not Improved
 Situations that favor the use of a RCT 1. Exposure of interest is a modifiable 2. Individuals are willing to relinquish control 3. Genuine uncertainties regarding the effects of the interventions 4. Effect of intervention on a rare outcome is of sufficient importance to justify a large study 5. Potential benefits must outweigh the risks
Situations that favor the use of a RCT 1. Exposure of interest is a modifiable 2. Individuals are willing to relinquish control 3. Genuine uncertainties regarding the effects of the interventions 4. Effect of intervention on a rare outcome is of sufficient importance to justify a large study 5. Potential benefits must outweigh the risks
 Typical comparison or control groups in RCTs 1) The Placebo (looks like intervention) 2) Alternative treatment 3) ‘Usual care’
Typical comparison or control groups in RCTs 1) The Placebo (looks like intervention) 2) Alternative treatment 3) ‘Usual care’
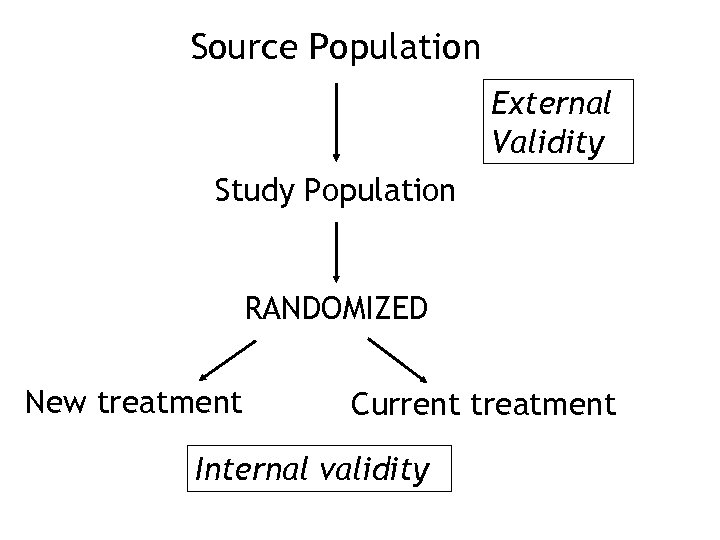 Source Population External Validity Study Population RANDOMIZED New treatment Current treatment Internal validity
Source Population External Validity Study Population RANDOMIZED New treatment Current treatment Internal validity
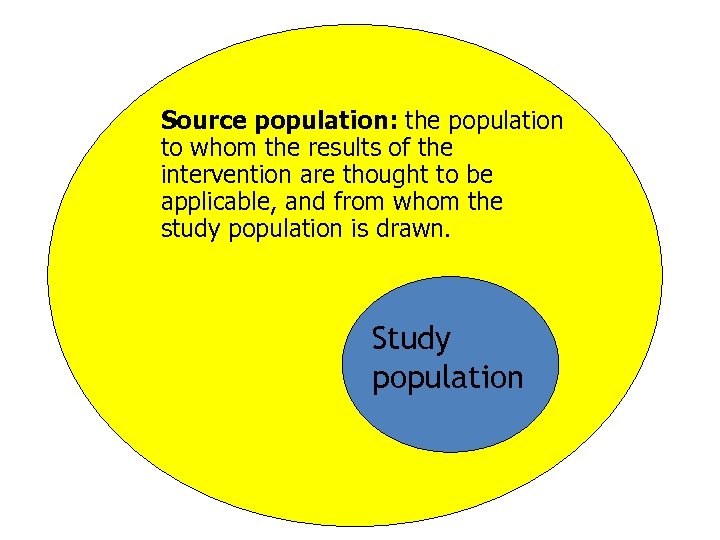 Source population: the population to whom the results of the intervention are thought to be applicable, and from whom the study population is drawn. Study population
Source population: the population to whom the results of the intervention are thought to be applicable, and from whom the study population is drawn. Study population
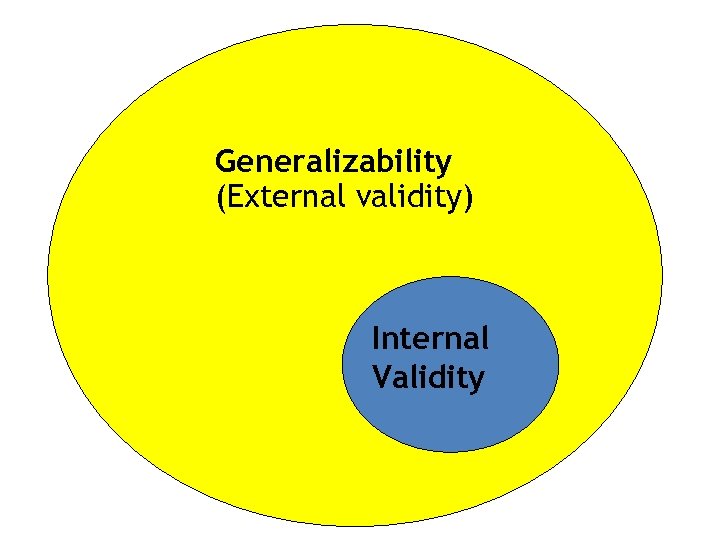 Generalizability (External validity) Internal Validity
Generalizability (External validity) Internal Validity
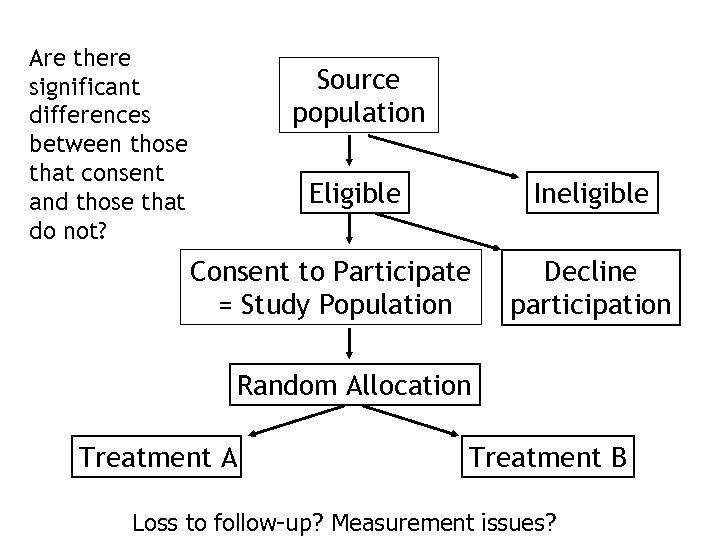 Are there significant differences between those that consent and those that do not? Source population Eligible Ineligible Consent to Participate = Study Population Decline participation Random Allocation Treatment A Treatment B Loss to follow-up? Measurement issues?
Are there significant differences between those that consent and those that do not? Source population Eligible Ineligible Consent to Participate = Study Population Decline participation Random Allocation Treatment A Treatment B Loss to follow-up? Measurement issues?
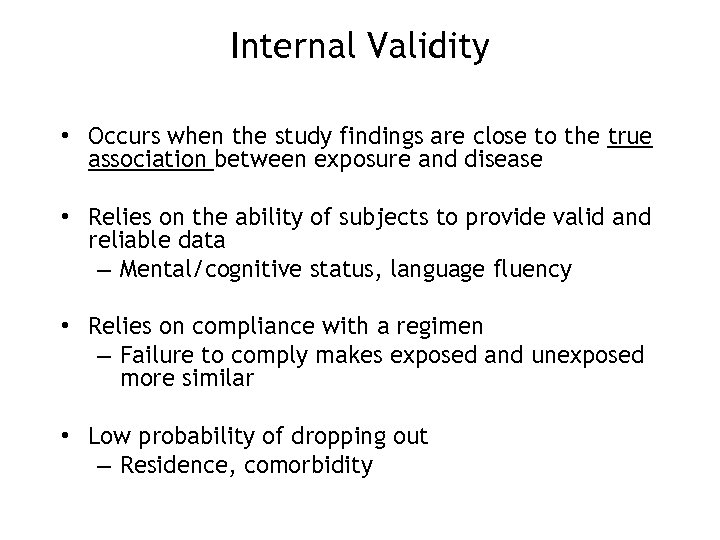 Internal Validity • Occurs when the study findings are close to the true association between exposure and disease • Relies on the ability of subjects to provide valid and reliable data – Mental/cognitive status, language fluency • Relies on compliance with a regimen – Failure to comply makes exposed and unexposed more similar • Low probability of dropping out – Residence, comorbidity
Internal Validity • Occurs when the study findings are close to the true association between exposure and disease • Relies on the ability of subjects to provide valid and reliable data – Mental/cognitive status, language fluency • Relies on compliance with a regimen – Failure to comply makes exposed and unexposed more similar • Low probability of dropping out – Residence, comorbidity
 External Validity • Occurs when the results from the study can be applied to the larger (source) population • Are there demographic differences between eligible and ineligible subgroups? • Difference between those that consent and do not consent? • Does your intervention mirror what will happen in the community or source population?
External Validity • Occurs when the results from the study can be applied to the larger (source) population • Are there demographic differences between eligible and ineligible subgroups? • Difference between those that consent and do not consent? • Does your intervention mirror what will happen in the community or source population?
 MASKING (aka blinding) • Observers and/or subjects are kept ignorant – Single blind or mask subjects – Double blind or mask observer and subject – Triple blind or mask observer, subject and analyst • Helps improve internal validity of the study
MASKING (aka blinding) • Observers and/or subjects are kept ignorant – Single blind or mask subjects – Double blind or mask observer and subject – Triple blind or mask observer, subject and analyst • Helps improve internal validity of the study
 How do we analyze the results of an RCT?
How do we analyze the results of an RCT?
 Measures of Association • Risk Ratio: Ratio of the Cumulative Incidence among exposed versus those not exposed • Rate Ratio: Ratio of the Incidence Rate in those exposed versus those not exposed.
Measures of Association • Risk Ratio: Ratio of the Cumulative Incidence among exposed versus those not exposed • Rate Ratio: Ratio of the Incidence Rate in those exposed versus those not exposed.
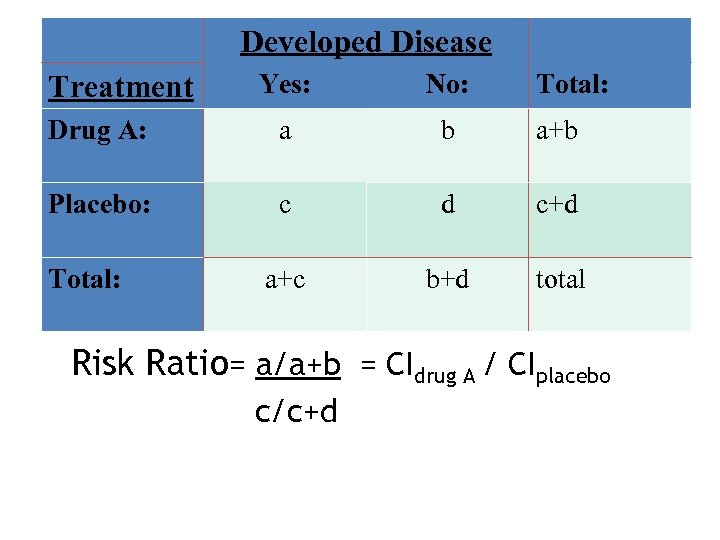 Developed Disease Yes: No: Drug A: a b a+b Placebo: c d c+d a+c b+d total Treatment Total: Risk Ratio= a/a+b = CIdrug A / CIplacebo c/c+d
Developed Disease Yes: No: Drug A: a b a+b Placebo: c d c+d a+c b+d total Treatment Total: Risk Ratio= a/a+b = CIdrug A / CIplacebo c/c+d
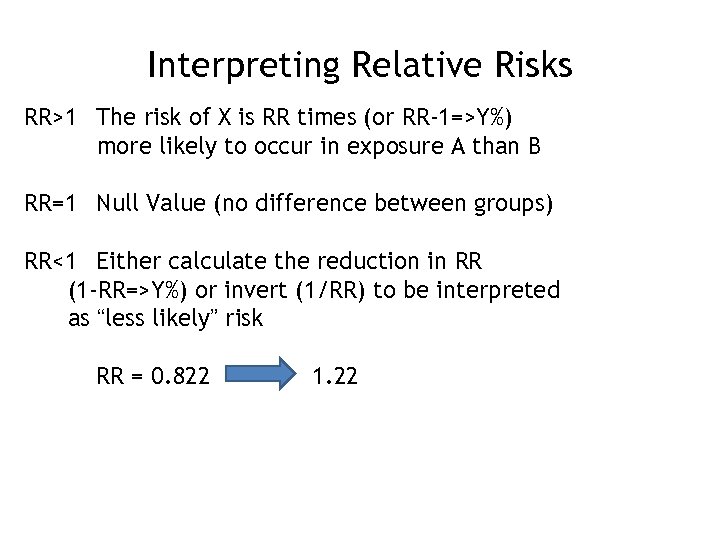 Interpreting Relative Risks RR>1 The risk of X is RR times (or RR-1=>Y%) more likely to occur in exposure A than B RR=1 Null Value (no difference between groups) RR<1 Either calculate the reduction in RR (1 -RR=>Y%) or invert (1/RR) to be interpreted as “less likely” risk RR = 0. 822 1. 22
Interpreting Relative Risks RR>1 The risk of X is RR times (or RR-1=>Y%) more likely to occur in exposure A than B RR=1 Null Value (no difference between groups) RR<1 Either calculate the reduction in RR (1 -RR=>Y%) or invert (1/RR) to be interpreted as “less likely” risk RR = 0. 822 1. 22
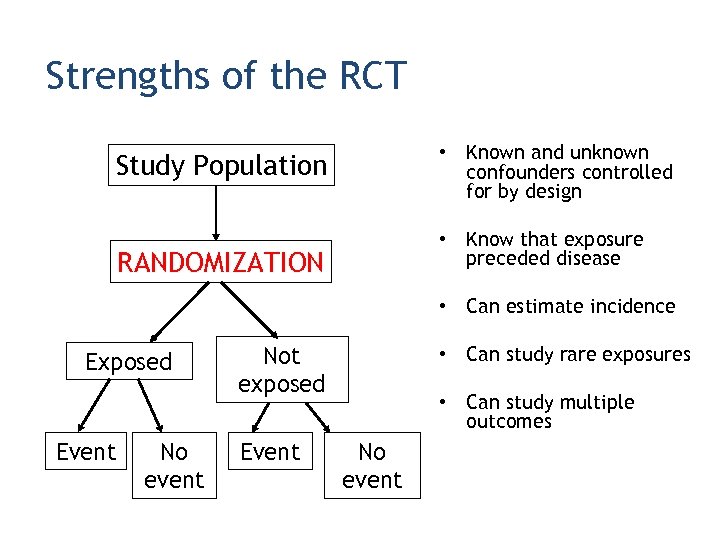 Strengths of the RCT • Known and unknown confounders controlled for by design Study Population • Know that exposure preceded disease RANDOMIZATION • Can estimate incidence Exposed Event No event Not exposed Event • Can study rare exposures • Can study multiple outcomes No event
Strengths of the RCT • Known and unknown confounders controlled for by design Study Population • Know that exposure preceded disease RANDOMIZATION • Can estimate incidence Exposed Event No event Not exposed Event • Can study rare exposures • Can study multiple outcomes No event
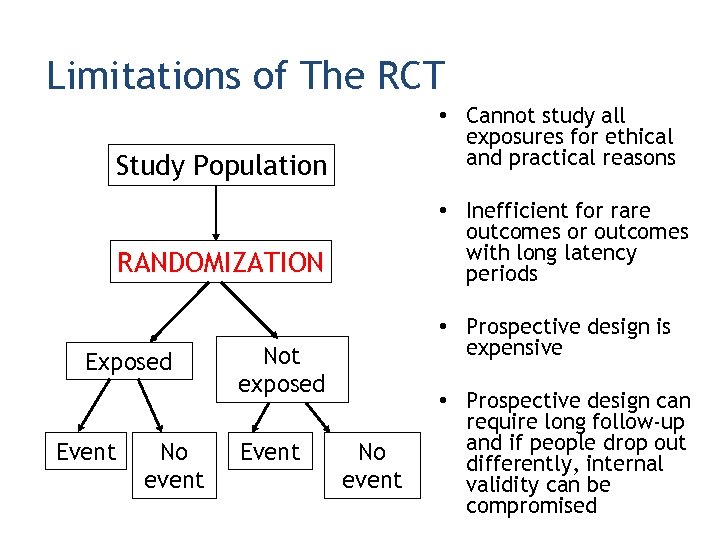 Limitations of The RCT • Cannot study all exposures for ethical and practical reasons Study Population • Inefficient for rare outcomes or outcomes with long latency periods RANDOMIZATION Exposed Event No event • Prospective design is expensive Not exposed Event No event • Prospective design can require long follow-up and if people drop out differently, internal validity can be compromised
Limitations of The RCT • Cannot study all exposures for ethical and practical reasons Study Population • Inefficient for rare outcomes or outcomes with long latency periods RANDOMIZATION Exposed Event No event • Prospective design is expensive Not exposed Event No event • Prospective design can require long follow-up and if people drop out differently, internal validity can be compromised
 Cohort Studies
Cohort Studies
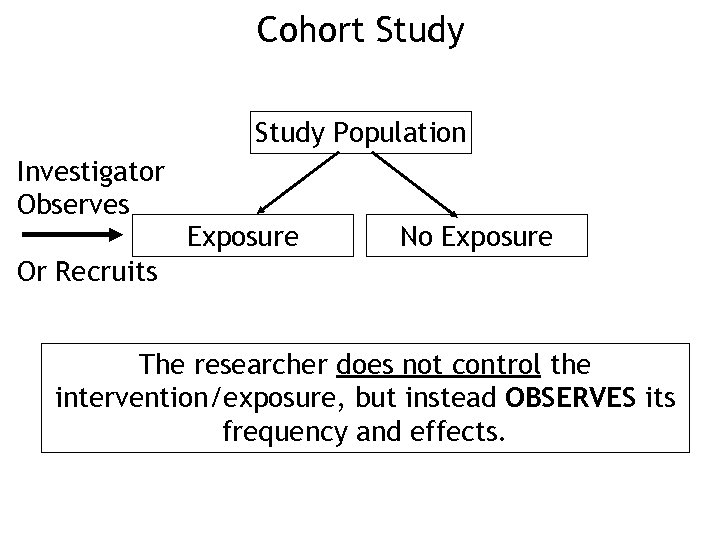 Cohort Study Population Investigator Observes Exposure No Exposure Or Recruits The researcher does not control the intervention/exposure, but instead OBSERVES its frequency and effects.
Cohort Study Population Investigator Observes Exposure No Exposure Or Recruits The researcher does not control the intervention/exposure, but instead OBSERVES its frequency and effects.
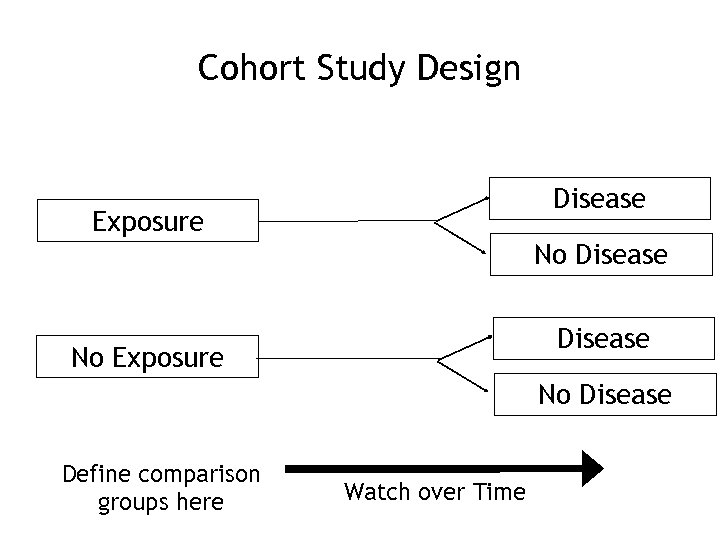 Cohort Study Design Disease Exposure No Disease No Exposure No Disease Define comparison groups here Watch over Time
Cohort Study Design Disease Exposure No Disease No Exposure No Disease Define comparison groups here Watch over Time
 Measuring Exposure • Clear • Objective • Measurable – Smoked 100 cigarettes over the past month – Influenza vaccine in the last month
Measuring Exposure • Clear • Objective • Measurable – Smoked 100 cigarettes over the past month – Influenza vaccine in the last month
 Measuring Outcome • Clear • Objective • Measurable – Physician diagnosis in medical record – Specified ICD codes in hospital discharge data – Cause of death on death certificate
Measuring Outcome • Clear • Objective • Measurable – Physician diagnosis in medical record – Specified ICD codes in hospital discharge data – Cause of death on death certificate
 Strengths of Cohort Studies 1. Temporality (exposure before disease) 2. Efficient for rare or unusual exposures 3. Assess multiple outcomes from a single exposure 4. Can estimate incidence
Strengths of Cohort Studies 1. Temporality (exposure before disease) 2. Efficient for rare or unusual exposures 3. Assess multiple outcomes from a single exposure 4. Can estimate incidence
 Weaknesses of Cohort Studies 1. Expensive 2. Inefficient for studying rare diseases 3. Not good for diseases that take a long time to develop (e. g. long latency) 4. People can change their exposure classification (unlike an RCT) 5. Differential loss-to-follow-up between exposure groups can bias associations
Weaknesses of Cohort Studies 1. Expensive 2. Inefficient for studying rare diseases 3. Not good for diseases that take a long time to develop (e. g. long latency) 4. People can change their exposure classification (unlike an RCT) 5. Differential loss-to-follow-up between exposure groups can bias associations
 Two Main Types of Cohort Studies • Prospective • Retrospective
Two Main Types of Cohort Studies • Prospective • Retrospective
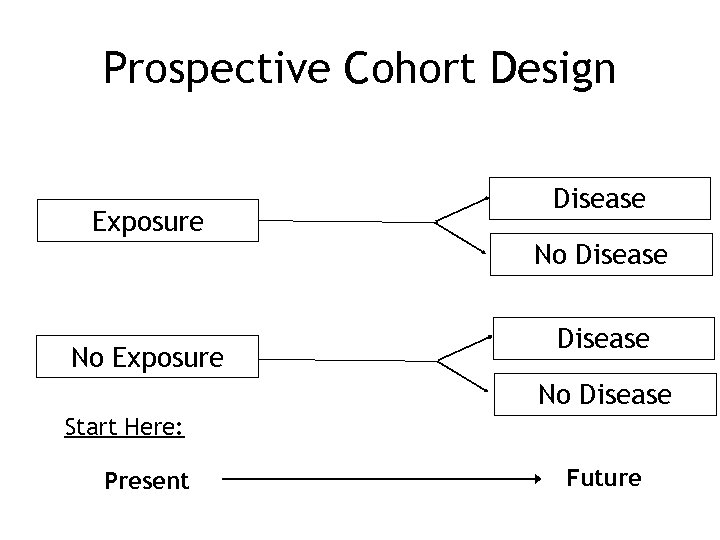 Prospective Cohort Design Exposure Disease No Disease Start Here: Present Future
Prospective Cohort Design Exposure Disease No Disease Start Here: Present Future
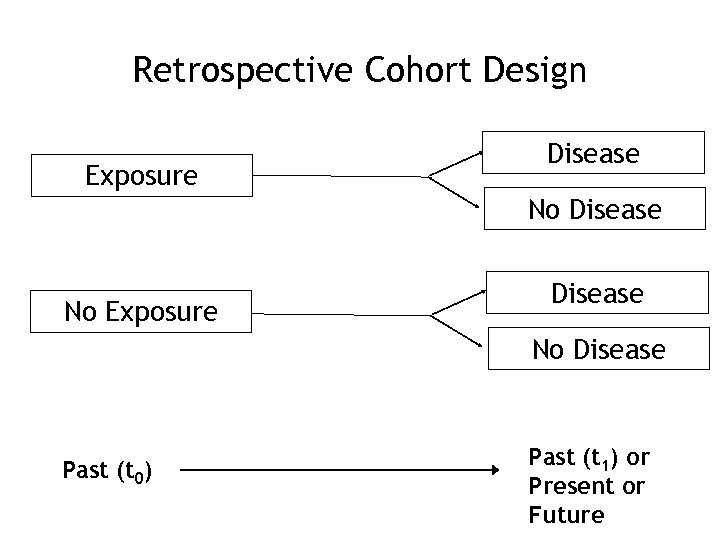 Retrospective Cohort Design Exposure Disease No Disease Past (t 0) Past (t 1) or Present or Future
Retrospective Cohort Design Exposure Disease No Disease Past (t 0) Past (t 1) or Present or Future
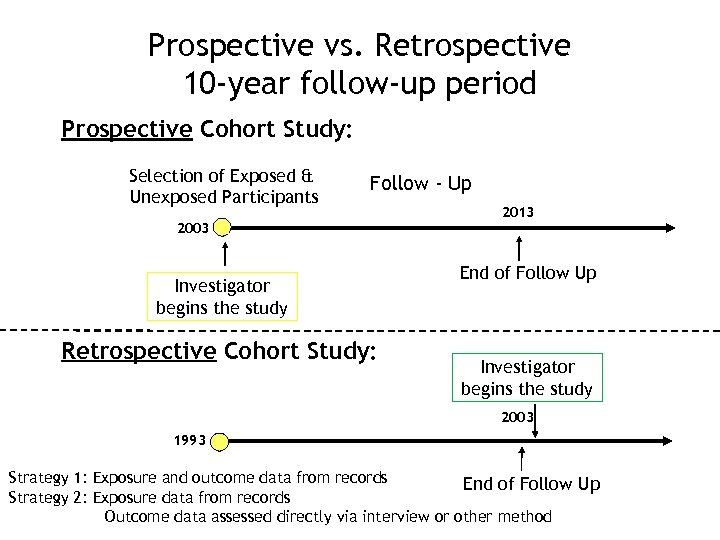 Prospective vs. Retrospective 10 -year follow-up period Prospective Cohort Study: Selection of Exposed & Unexposed Participants Follow - Up 2003 Investigator begins the study Retrospective Cohort Study: 2013 End of Follow Up Investigator begins the study 2003 1993 Strategy 1: Exposure and outcome data from records End of Follow Up Strategy 2: Exposure data from records Outcome data assessed directly via interview or other method
Prospective vs. Retrospective 10 -year follow-up period Prospective Cohort Study: Selection of Exposed & Unexposed Participants Follow - Up 2003 Investigator begins the study Retrospective Cohort Study: 2013 End of Follow Up Investigator begins the study 2003 1993 Strategy 1: Exposure and outcome data from records End of Follow Up Strategy 2: Exposure data from records Outcome data assessed directly via interview or other method
 Prospective Cohort Studies • Exposure at initiation of study • Outcome that may occur in future • Followed over time
Prospective Cohort Studies • Exposure at initiation of study • Outcome that may occur in future • Followed over time
 Retrospective Cohort Studies • Exposure recorded in past – Employment records – Med records – Typically not as good as in the prospective study • Disease incidence (or mortality) assessed from past, present, or future – Great for long latency diseases • Example: Followed over 10 year conceptually but can be actually assessed in one year (e. g. through record review)
Retrospective Cohort Studies • Exposure recorded in past – Employment records – Med records – Typically not as good as in the prospective study • Disease incidence (or mortality) assessed from past, present, or future – Great for long latency diseases • Example: Followed over 10 year conceptually but can be actually assessed in one year (e. g. through record review)
 Advantages of Retrospective Cohort Design • Timely and temporal • Less expensive than Prospective cohort • Good for diseases of long latency
Advantages of Retrospective Cohort Design • Timely and temporal • Less expensive than Prospective cohort • Good for diseases of long latency
 Disadvantages of Retrospective Cohort Design • Reliance on available information • Quality? • Ascertainment biases in outcomes – Exposure is known
Disadvantages of Retrospective Cohort Design • Reliance on available information • Quality? • Ascertainment biases in outcomes – Exposure is known
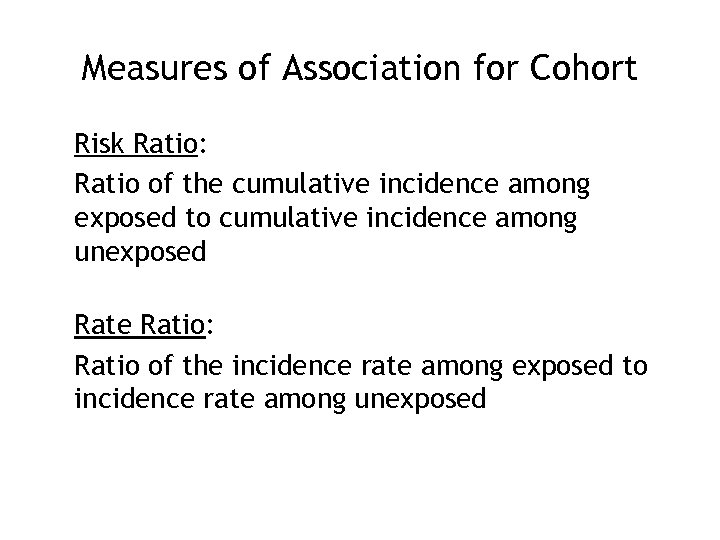 Measures of Association for Cohort Risk Ratio: Ratio of the cumulative incidence among exposed to cumulative incidence among unexposed Rate Ratio: Ratio of the incidence rate among exposed to incidence rate among unexposed
Measures of Association for Cohort Risk Ratio: Ratio of the cumulative incidence among exposed to cumulative incidence among unexposed Rate Ratio: Ratio of the incidence rate among exposed to incidence rate among unexposed
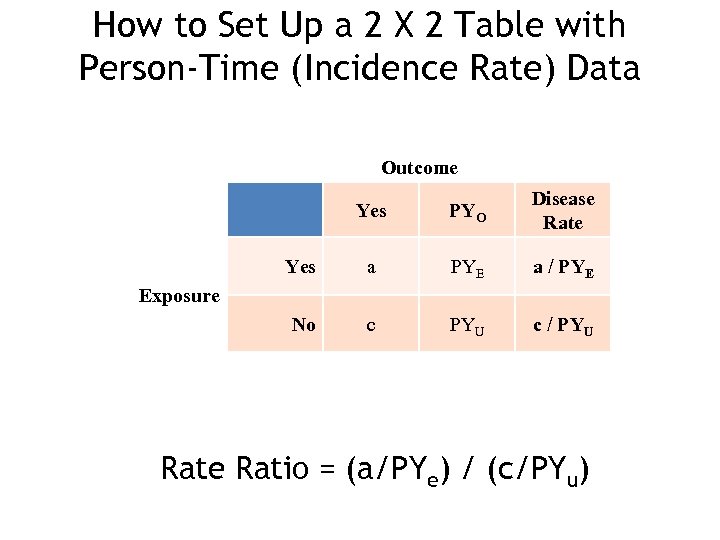 How to Set Up a 2 X 2 Table with Person-Time (Incidence Rate) Data Outcome Yes PYO Disease Rate Yes a PYE a / PYE No c PYU c / PYU Exposure Ratio = (a/PYe) / (c/PYu)
How to Set Up a 2 X 2 Table with Person-Time (Incidence Rate) Data Outcome Yes PYO Disease Rate Yes a PYE a / PYE No c PYU c / PYU Exposure Ratio = (a/PYe) / (c/PYu)
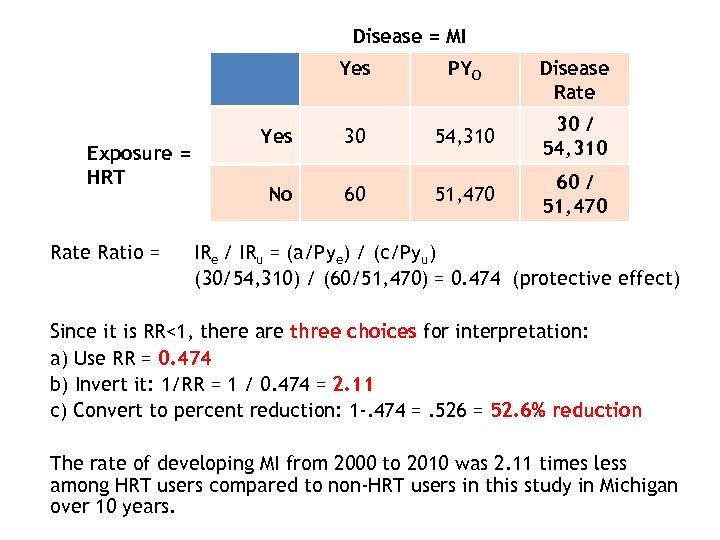 Disease = MI Yes Exposure = HRT Rate Ratio = PYO Disease Rate Yes 30 54, 310 30 / 54, 310 No 60 51, 470 60 / 51, 470 IRe / IRu = (a/Pye) / (c/Pyu) (30/54, 310) / (60/51, 470) = 0. 474 (protective effect) Since it is RR<1, there are three choices for interpretation: a) Use RR = 0. 474 b) Invert it: 1/RR = 1 / 0. 474 = 2. 11 c) Convert to percent reduction: 1 -. 474 =. 526 = 52. 6% reduction The rate of developing MI from 2000 to 2010 was 2. 11 times less among HRT users compared to non-HRT users in this study in Michigan over 10 years.
Disease = MI Yes Exposure = HRT Rate Ratio = PYO Disease Rate Yes 30 54, 310 30 / 54, 310 No 60 51, 470 60 / 51, 470 IRe / IRu = (a/Pye) / (c/Pyu) (30/54, 310) / (60/51, 470) = 0. 474 (protective effect) Since it is RR<1, there are three choices for interpretation: a) Use RR = 0. 474 b) Invert it: 1/RR = 1 / 0. 474 = 2. 11 c) Convert to percent reduction: 1 -. 474 =. 526 = 52. 6% reduction The rate of developing MI from 2000 to 2010 was 2. 11 times less among HRT users compared to non-HRT users in this study in Michigan over 10 years.
 Case Control Studies
Case Control Studies
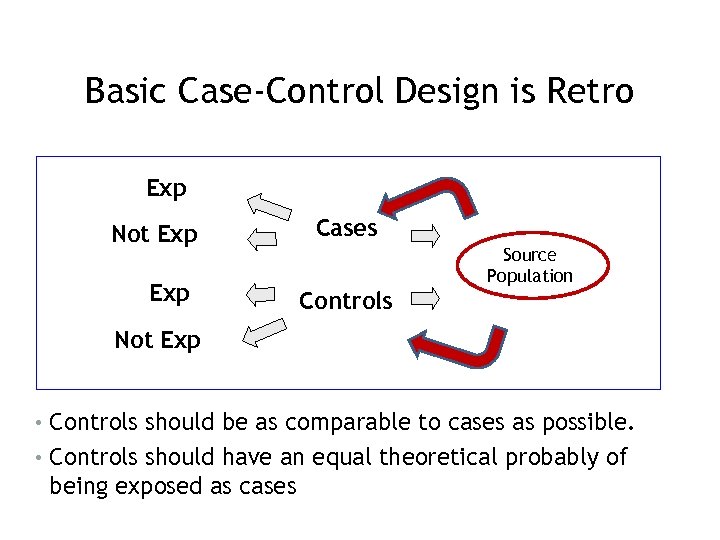 Basic Case-Control Design is Retro Exp Not Exp Cases Controls Source Population Not Exp • Controls should be as comparable to cases as possible. • Controls should have an equal theoretical probably of being exposed as cases
Basic Case-Control Design is Retro Exp Not Exp Cases Controls Source Population Not Exp • Controls should be as comparable to cases as possible. • Controls should have an equal theoretical probably of being exposed as cases
 When to Conduct a Case-Control Study? • When the disease or outcome is rare – Ex: Studying risk factors for birth defects • When little is known about the disease – Ex. Early studies of AIDS
When to Conduct a Case-Control Study? • When the disease or outcome is rare – Ex: Studying risk factors for birth defects • When little is known about the disease – Ex. Early studies of AIDS
 Selection of Cases • Decide on a case definition • Decide whether PREVALENT or INCIDENT cases • Where will you get cases? – Think about who you want to generalize the results to in the future.
Selection of Cases • Decide on a case definition • Decide whether PREVALENT or INCIDENT cases • Where will you get cases? – Think about who you want to generalize the results to in the future.
 Selection of Controls • Controls should be as comparable to cases as possible. – Age, sex, ethnicity, geography, income, education • Controls should have an equal theoretical probability of being exposed as cases • Controls have to have the ability to be cases – General population set of controls used for prostate cancer case-control study?
Selection of Controls • Controls should be as comparable to cases as possible. – Age, sex, ethnicity, geography, income, education • Controls should have an equal theoretical probability of being exposed as cases • Controls have to have the ability to be cases – General population set of controls used for prostate cancer case-control study?
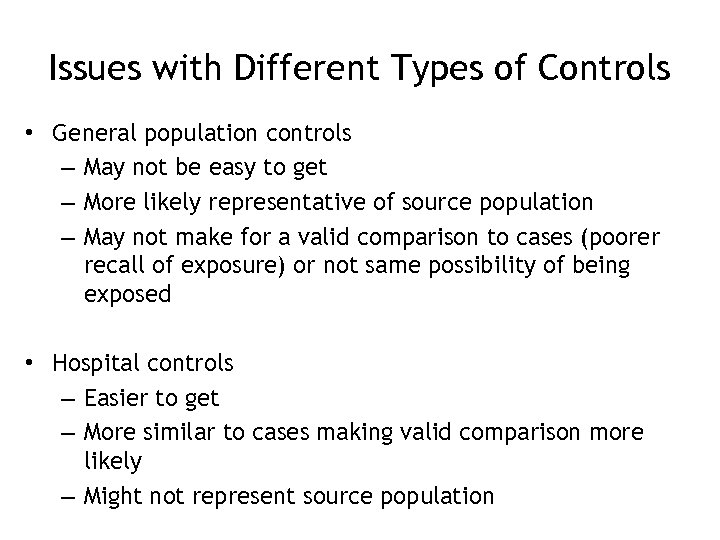 Issues with Different Types of Controls • General population controls – May not be easy to get – More likely representative of source population – May not make for a valid comparison to cases (poorer recall of exposure) or not same possibility of being exposed • Hospital controls – Easier to get – More similar to cases making valid comparison more likely – Might not represent source population
Issues with Different Types of Controls • General population controls – May not be easy to get – More likely representative of source population – May not make for a valid comparison to cases (poorer recall of exposure) or not same possibility of being exposed • Hospital controls – Easier to get – More similar to cases making valid comparison more likely – Might not represent source population
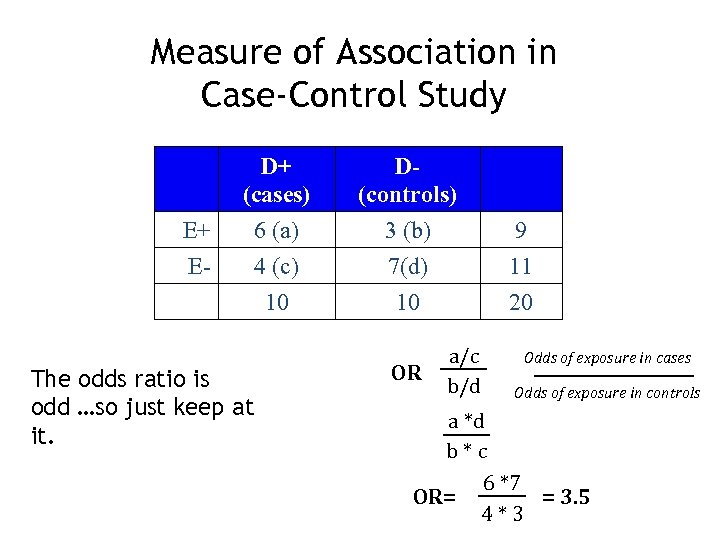 Measure of Association in Case-Control Study E+ E- D+ (cases) 6 (a) 4 (c) 10 The odds ratio is odd …so just keep at it. D(controls) 3 (b) 7(d) 10 OR a/c b/d 9 11 20 Odds of exposure in cases Odds of exposure in controls a *d b*c 6 *7 OR= = 3. 5 4*3
Measure of Association in Case-Control Study E+ E- D+ (cases) 6 (a) 4 (c) 10 The odds ratio is odd …so just keep at it. D(controls) 3 (b) 7(d) 10 OR a/c b/d 9 11 20 Odds of exposure in cases Odds of exposure in controls a *d b*c 6 *7 OR= = 3. 5 4*3
 Advantages of Case-Control • Relatively easy to conduct • The best design for rare diseases • An important foundational step to evaluate association to motivate cohort study
Advantages of Case-Control • Relatively easy to conduct • The best design for rare diseases • An important foundational step to evaluate association to motivate cohort study
 Disadvantages of Case-Control • Usually can’t estimate even basic population measures of disease or exposure frequency • usually no prevalence or incidence data • Typically, can’t separate forward causation from reverse causation • Increased breastfeeding associated with growth decline in toddlers (actually the toddlers that were sick with diarrheal diseases breastfeed more)
Disadvantages of Case-Control • Usually can’t estimate even basic population measures of disease or exposure frequency • usually no prevalence or incidence data • Typically, can’t separate forward causation from reverse causation • Increased breastfeeding associated with growth decline in toddlers (actually the toddlers that were sick with diarrheal diseases breastfeed more)
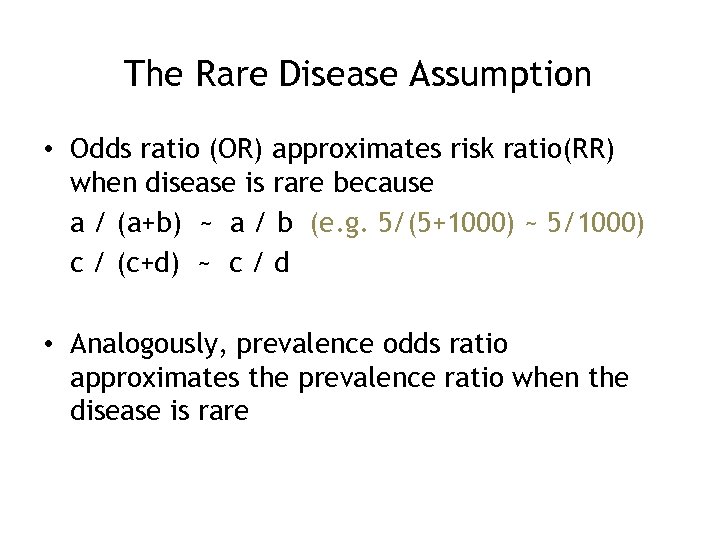 The Rare Disease Assumption • Odds ratio (OR) approximates risk ratio(RR) when disease is rare because a / (a+b) ~ a / b (e. g. 5/(5+1000) ~ 5/1000) c / (c+d) ~ c / d • Analogously, prevalence odds ratio approximates the prevalence ratio when the disease is rare
The Rare Disease Assumption • Odds ratio (OR) approximates risk ratio(RR) when disease is rare because a / (a+b) ~ a / b (e. g. 5/(5+1000) ~ 5/1000) c / (c+d) ~ c / d • Analogously, prevalence odds ratio approximates the prevalence ratio when the disease is rare
 Cross-sectional and Ecological Study Design
Cross-sectional and Ecological Study Design
 Cross Sectional Studies: • Simultaneously assess disease and exposure in an individual • Occurs a single point of time (no follow-up) • A study of prevalences and their interrelationship • Measures of Association: – Prevalence odds ratio – Prevalence ratio
Cross Sectional Studies: • Simultaneously assess disease and exposure in an individual • Occurs a single point of time (no follow-up) • A study of prevalences and their interrelationship • Measures of Association: – Prevalence odds ratio – Prevalence ratio
 Advantages of Cross Sectional Studies • Faster and less expensive than cohort • Often done to get information to apply to the population at large (source population) • Get good estimates of prevalence of many exposures and outcomes simultaneously
Advantages of Cross Sectional Studies • Faster and less expensive than cohort • Often done to get information to apply to the population at large (source population) • Get good estimates of prevalence of many exposures and outcomes simultaneously
 Disadvantages of Cross Sectional Studies: • Temporality most often not known (but sometimes is known) • Can’t capture the change in risk factors or disease over time • Not good for rare diseases • Not good for rare exposures
Disadvantages of Cross Sectional Studies: • Temporality most often not known (but sometimes is known) • Can’t capture the change in risk factors or disease over time • Not good for rare diseases • Not good for rare exposures
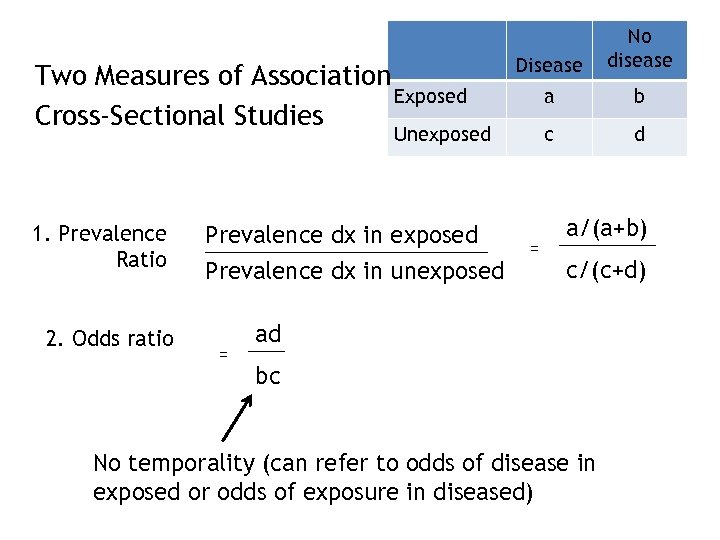 Two Measures of Association Exposed Cross-Sectional Studies Disease No disease a b c d Unexposed 1. Prevalence Ratio 2. Odds ratio Prevalence dx in exposed Prevalence dx in unexposed = = a/(a+b) c/(c+d) ad bc No temporality (can refer to odds of disease in exposed or odds of exposure in diseased)
Two Measures of Association Exposed Cross-Sectional Studies Disease No disease a b c d Unexposed 1. Prevalence Ratio 2. Odds ratio Prevalence dx in exposed Prevalence dx in unexposed = = a/(a+b) c/(c+d) ad bc No temporality (can refer to odds of disease in exposed or odds of exposure in diseased)
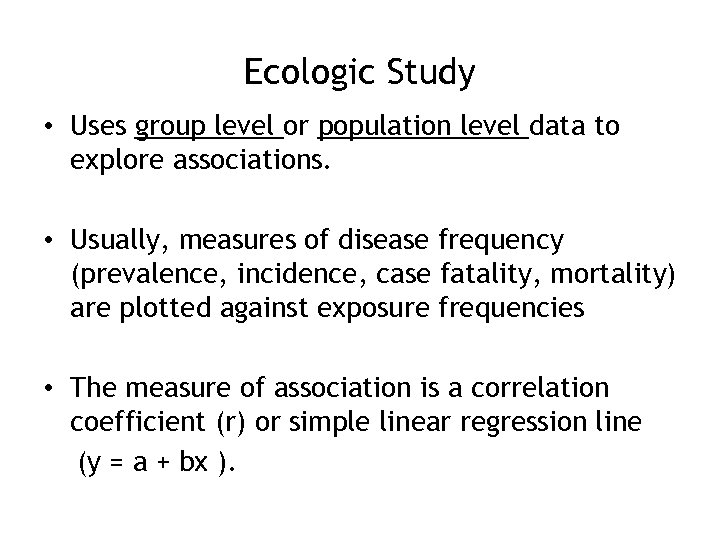 Ecologic Study • Uses group level or population level data to explore associations. • Usually, measures of disease frequency (prevalence, incidence, case fatality, mortality) are plotted against exposure frequencies • The measure of association is a correlation coefficient (r) or simple linear regression line (y = a + bx ).
Ecologic Study • Uses group level or population level data to explore associations. • Usually, measures of disease frequency (prevalence, incidence, case fatality, mortality) are plotted against exposure frequencies • The measure of association is a correlation coefficient (r) or simple linear regression line (y = a + bx ).
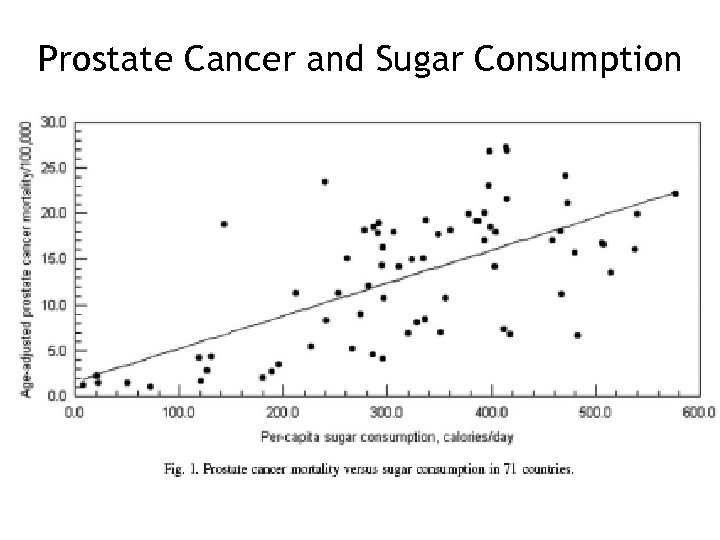 Prostate Cancer and Sugar Consumption
Prostate Cancer and Sugar Consumption
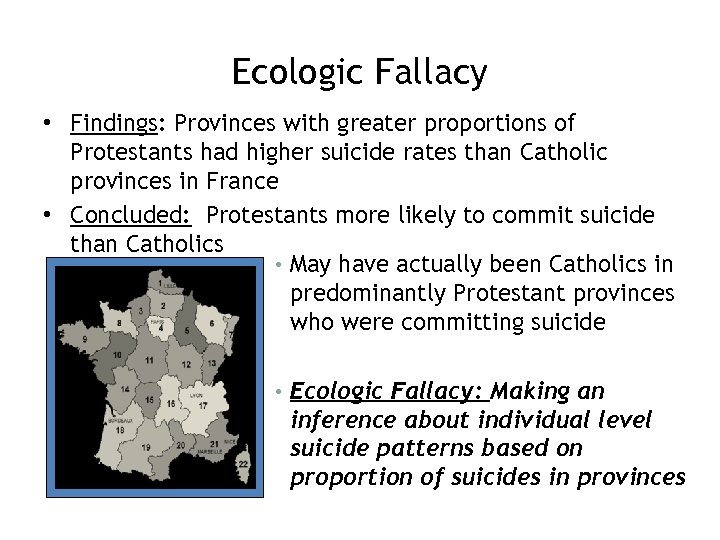 Ecologic Fallacy • Findings: Provinces with greater proportions of Protestants had higher suicide rates than Catholic provinces in France • Concluded: Protestants more likely to commit suicide than Catholics • May have actually been Catholics in predominantly Protestant provinces who were committing suicide • Ecologic Fallacy: Making an inference about individual level suicide patterns based on proportion of suicides in provinces
Ecologic Fallacy • Findings: Provinces with greater proportions of Protestants had higher suicide rates than Catholic provinces in France • Concluded: Protestants more likely to commit suicide than Catholics • May have actually been Catholics in predominantly Protestant provinces who were committing suicide • Ecologic Fallacy: Making an inference about individual level suicide patterns based on proportion of suicides in provinces
 A few questions to consider Where can incidence be estimated? What designs are good for rare exposures? What designs are good for rare outcomes? Is randomization to ensure external validity or internal validity? • What designs can you estimate RR? • What designs do you need to use an OR? • What is the difference between an exposure OR and a disease OR? • •
A few questions to consider Where can incidence be estimated? What designs are good for rare exposures? What designs are good for rare outcomes? Is randomization to ensure external validity or internal validity? • What designs can you estimate RR? • What designs do you need to use an OR? • What is the difference between an exposure OR and a disease OR? • •
 Other Risk Estimates
Other Risk Estimates
 How do we estimate public health effect of an exposure? Attributable Risk Estimates of Effect • Attributable Risk (AR) • Attributable Risk Percent (AR%) • Population Attributable Risk (PAR) • Population Attributable Risk Percent (PAR%) These statistics address the question: • How much of the disease that occurs can be attributed to a certain exposure?
How do we estimate public health effect of an exposure? Attributable Risk Estimates of Effect • Attributable Risk (AR) • Attributable Risk Percent (AR%) • Population Attributable Risk (PAR) • Population Attributable Risk Percent (PAR%) These statistics address the question: • How much of the disease that occurs can be attributed to a certain exposure?
 • AR and PAR tell us: how many cases of disease could be eliminated if we completely eliminate the exposure • AR% and PAR% tell us: what percent of cases could be eliminated if we completely eliminate the exposure
• AR and PAR tell us: how many cases of disease could be eliminated if we completely eliminate the exposure • AR% and PAR% tell us: what percent of cases could be eliminated if we completely eliminate the exposure
 Measures of Attribution and Effect • Attributable Risk (AR) is a Risk Difference (RD) – It estimates the excess risk of disease in those exposed compared with those non-exposed. – AR = Incidence Exposed – Incidence Not Exposed • Group of Interest: Exposed • Quantifies the risk of disease in the “exposed” group attributable to the exposure
Measures of Attribution and Effect • Attributable Risk (AR) is a Risk Difference (RD) – It estimates the excess risk of disease in those exposed compared with those non-exposed. – AR = Incidence Exposed – Incidence Not Exposed • Group of Interest: Exposed • Quantifies the risk of disease in the “exposed” group attributable to the exposure
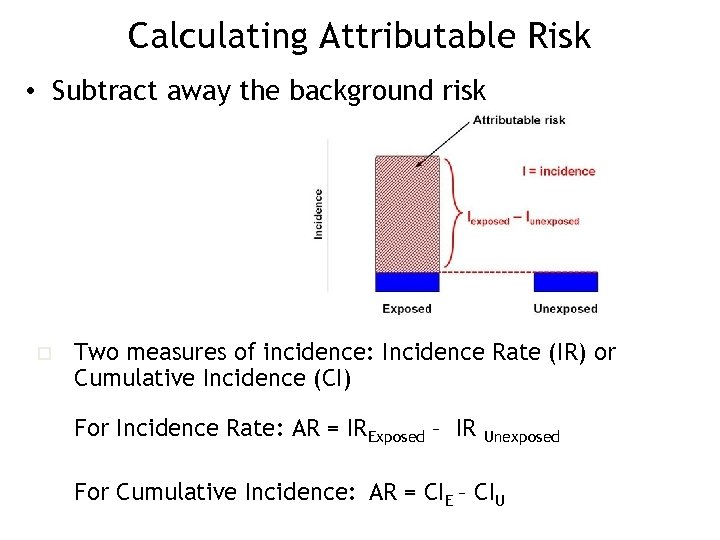 Calculating Attributable Risk • Subtract away the background risk o Two measures of incidence: Incidence Rate (IR) or Cumulative Incidence (CI) For Incidence Rate: AR = IRExposed – IR Unexposed For Cumulative Incidence: AR = CIE – CIU
Calculating Attributable Risk • Subtract away the background risk o Two measures of incidence: Incidence Rate (IR) or Cumulative Incidence (CI) For Incidence Rate: AR = IRExposed – IR Unexposed For Cumulative Incidence: AR = CIE – CIU
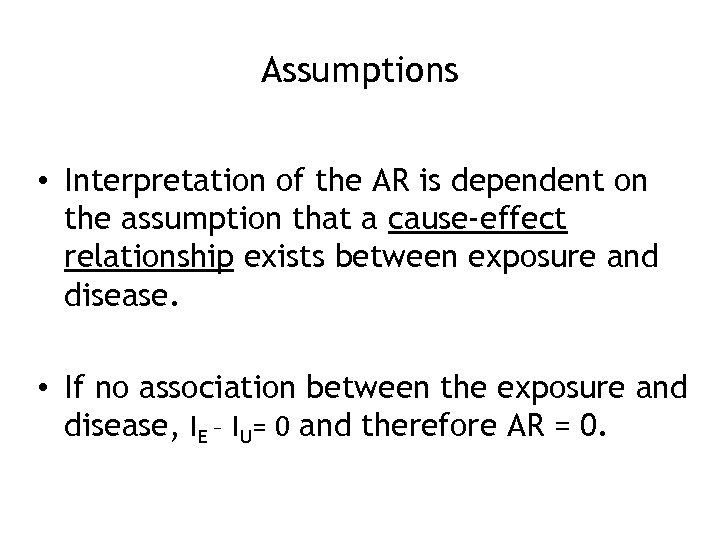 Assumptions • Interpretation of the AR is dependent on the assumption that a cause-effect relationship exists between exposure and disease. • If no association between the exposure and disease, IE – IU= 0 and therefore AR = 0.
Assumptions • Interpretation of the AR is dependent on the assumption that a cause-effect relationship exists between exposure and disease. • If no association between the exposure and disease, IE – IU= 0 and therefore AR = 0.
 How does AR compare to other measures of association? • The RR is a measure of the strength of the association and the possibility of a causal relationship. • The AR indicates the potential for prevention, if the exposure could be eliminated.
How does AR compare to other measures of association? • The RR is a measure of the strength of the association and the possibility of a causal relationship. • The AR indicates the potential for prevention, if the exposure could be eliminated.
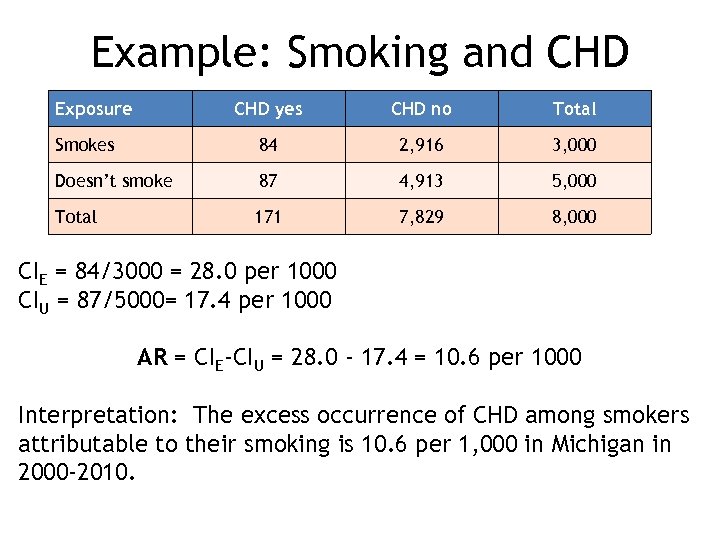 Example: Smoking and CHD Exposure CHD yes CHD no Total Smokes 84 2, 916 3, 000 Doesn’t smoke 87 4, 913 5, 000 Total 171 7, 829 8, 000 CIE = 84/3000 = 28. 0 per 1000 CIU = 87/5000= 17. 4 per 1000 AR = CIE-CIU = 28. 0 - 17. 4 = 10. 6 per 1000 Interpretation: The excess occurrence of CHD among smokers attributable to their smoking is 10. 6 per 1, 000 in Michigan in 2000 -2010.
Example: Smoking and CHD Exposure CHD yes CHD no Total Smokes 84 2, 916 3, 000 Doesn’t smoke 87 4, 913 5, 000 Total 171 7, 829 8, 000 CIE = 84/3000 = 28. 0 per 1000 CIU = 87/5000= 17. 4 per 1000 AR = CIE-CIU = 28. 0 - 17. 4 = 10. 6 per 1000 Interpretation: The excess occurrence of CHD among smokers attributable to their smoking is 10. 6 per 1, 000 in Michigan in 2000 -2010.
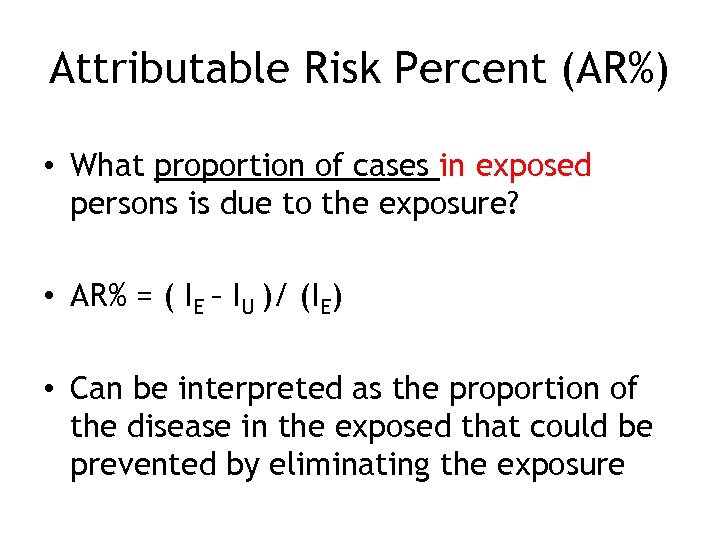 Attributable Risk Percent (AR%) • What proportion of cases in exposed persons is due to the exposure? • AR% = ( IE – IU )/ (IE) • Can be interpreted as the proportion of the disease in the exposed that could be prevented by eliminating the exposure
Attributable Risk Percent (AR%) • What proportion of cases in exposed persons is due to the exposure? • AR% = ( IE – IU )/ (IE) • Can be interpreted as the proportion of the disease in the exposed that could be prevented by eliminating the exposure
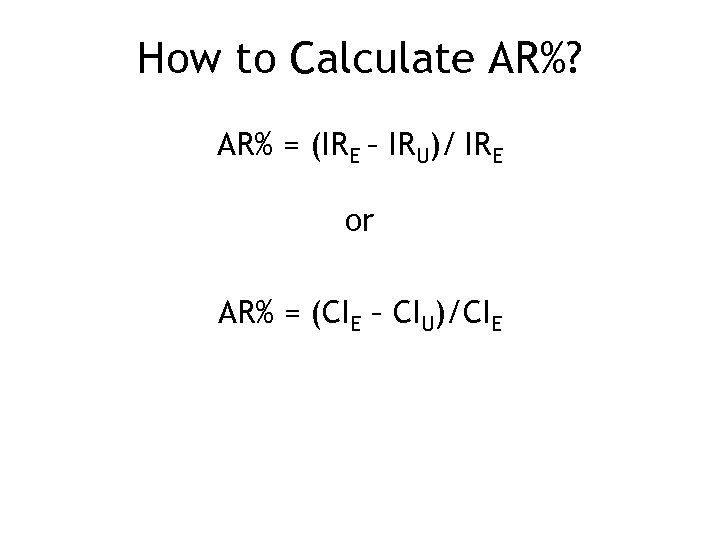 How to Calculate AR%? AR% = (IRE – IRU)/ IRE or AR% = (CIE – CIU)/CIE
How to Calculate AR%? AR% = (IRE – IRU)/ IRE or AR% = (CIE – CIU)/CIE
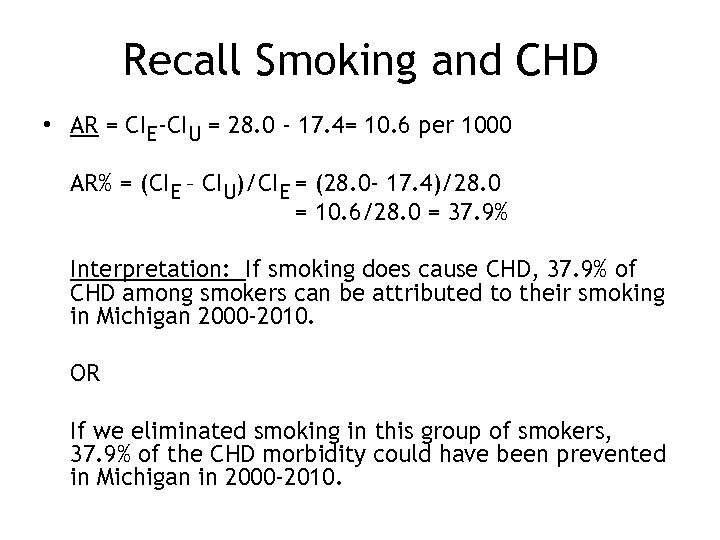 Recall Smoking and CHD • AR = CIE-CIU = 28. 0 - 17. 4= 10. 6 per 1000 AR% = (CIE – CIU)/CIE = (28. 0 - 17. 4)/28. 0 = 10. 6/28. 0 = 37. 9% Interpretation: If smoking does cause CHD, 37. 9% of CHD among smokers can be attributed to their smoking in Michigan 2000 -2010. OR If we eliminated smoking in this group of smokers, 37. 9% of the CHD morbidity could have been prevented in Michigan in 2000 -2010.
Recall Smoking and CHD • AR = CIE-CIU = 28. 0 - 17. 4= 10. 6 per 1000 AR% = (CIE – CIU)/CIE = (28. 0 - 17. 4)/28. 0 = 10. 6/28. 0 = 37. 9% Interpretation: If smoking does cause CHD, 37. 9% of CHD among smokers can be attributed to their smoking in Michigan 2000 -2010. OR If we eliminated smoking in this group of smokers, 37. 9% of the CHD morbidity could have been prevented in Michigan in 2000 -2010.
 Population Attributable Risk (PAR) • The PAR estimates the excess rate of disease in the “total study population” of exposed and nonexposed that is attributable to the exposure.
Population Attributable Risk (PAR) • The PAR estimates the excess rate of disease in the “total study population” of exposed and nonexposed that is attributable to the exposure.
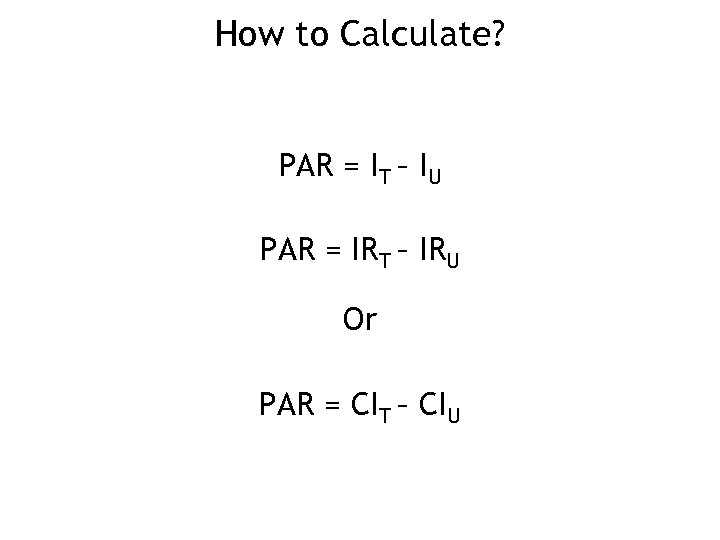 How to Calculate? PAR = IT – IU PAR = IRT – IRU Or PAR = CIT – CIU
How to Calculate? PAR = IT – IU PAR = IRT – IRU Or PAR = CIT – CIU
 PAR alternative calculation Alternatively, the PAR can be calculated as PAR = (AR)*Pe Where AR is the attributable risk and Pe is the proportion of exposed people in the population.
PAR alternative calculation Alternatively, the PAR can be calculated as PAR = (AR)*Pe Where AR is the attributable risk and Pe is the proportion of exposed people in the population.
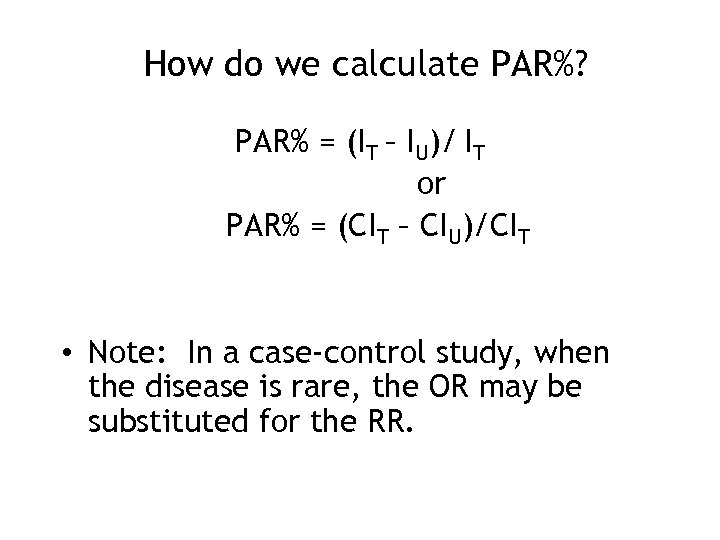 How do we calculate PAR%? PAR% = (IT – IU)/ IT or PAR% = (CIT – CIU)/CIT • Note: In a case-control study, when the disease is rare, the OR may be substituted for the RR.
How do we calculate PAR%? PAR% = (IT – IU)/ IT or PAR% = (CIT – CIU)/CIT • Note: In a case-control study, when the disease is rare, the OR may be substituted for the RR.
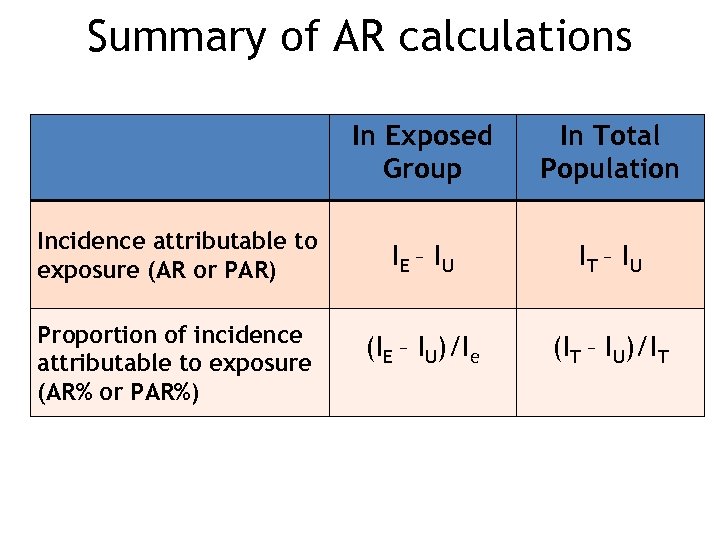 Summary of AR calculations In Exposed Group Incidence attributable to exposure (AR or PAR) Proportion of incidence attributable to exposure (AR% or PAR%) In Total Population IE – I U IT – I U (IE – IU)/Ie (IT – IU)/IT
Summary of AR calculations In Exposed Group Incidence attributable to exposure (AR or PAR) Proportion of incidence attributable to exposure (AR% or PAR%) In Total Population IE – I U IT – I U (IE – IU)/Ie (IT – IU)/IT
 A few AR – PAR questions • Can you use the odds ratio from a casecontrol study in an AR calculation? • Is the PAR larger than the AR? Why? • If Michigan and Ohio have the same cumulative incidence of disease in exposed and unexposed, will they have the same AR?
A few AR – PAR questions • Can you use the odds ratio from a casecontrol study in an AR calculation? • Is the PAR larger than the AR? Why? • If Michigan and Ohio have the same cumulative incidence of disease in exposed and unexposed, will they have the same AR?
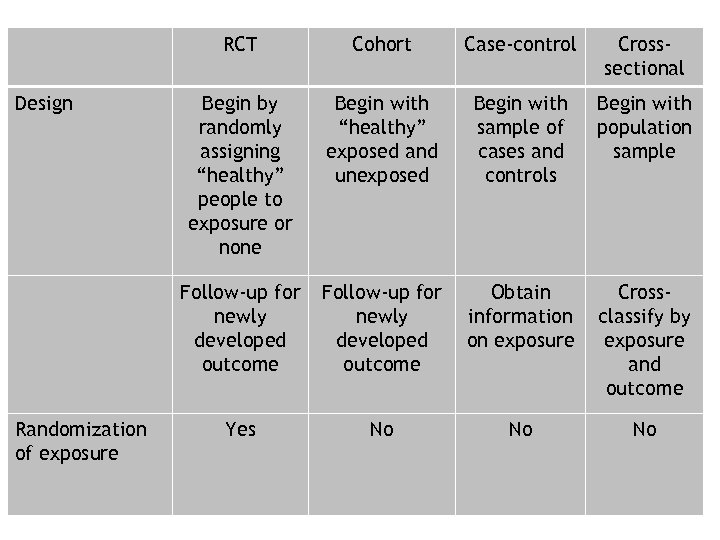 RCT Randomization of exposure Case-control Crosssectional Begin by randomly assigning “healthy” people to exposure or none Begin with “healthy” exposed and unexposed Begin with sample of cases and controls Begin with population sample Follow-up for newly developed outcome Design Cohort Follow-up for newly developed outcome Obtain information on exposure Crossclassify by exposure and outcome Yes No No No
RCT Randomization of exposure Case-control Crosssectional Begin by randomly assigning “healthy” people to exposure or none Begin with “healthy” exposed and unexposed Begin with sample of cases and controls Begin with population sample Follow-up for newly developed outcome Design Cohort Follow-up for newly developed outcome Obtain information on exposure Crossclassify by exposure and outcome Yes No No No
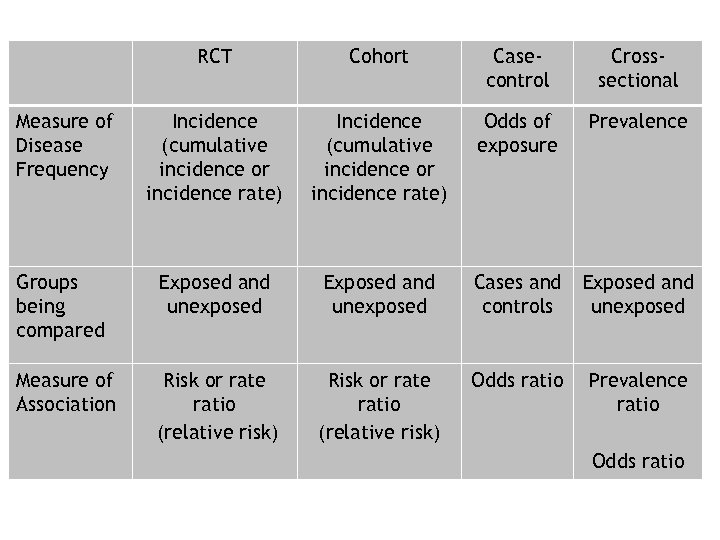 RCT Cohort Casecontrol Crosssectional Measure of Disease Frequency Incidence (cumulative incidence or incidence rate) Odds of exposure Prevalence Groups being compared Exposed and unexposed Cases and controls Exposed and unexposed Measure of Association Risk or rate ratio (relative risk) Odds ratio Prevalence ratio Odds ratio
RCT Cohort Casecontrol Crosssectional Measure of Disease Frequency Incidence (cumulative incidence or incidence rate) Odds of exposure Prevalence Groups being compared Exposed and unexposed Cases and controls Exposed and unexposed Measure of Association Risk or rate ratio (relative risk) Odds ratio Prevalence ratio Odds ratio


