cc2bca34afd56b9d39c0ad5da29a62c2.ppt
- Количество слайдов: 62

Osteoanabolic Therapy for Osteoporosis John P. Bilezikian, MD Professor of Medicine College of Physicians and Surgeons Columbia University New York, NY USA Skeletal Endocrinology Brescia, Italy 18 September 2015
THE HOLY GRAIL?

WHY PARATHYROID HORMONE AS AN OSTEOANABOLIC THERAPY WHEN…. . “Parathyroid hormone is bad for bones”
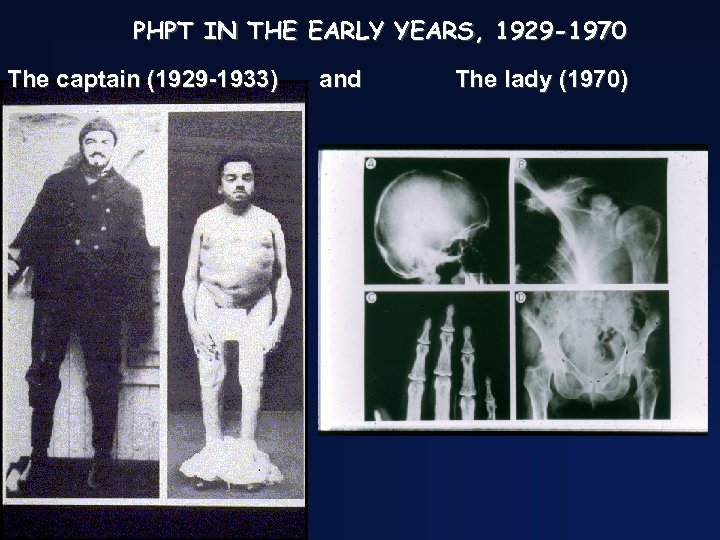
PHPT IN THE EARLY YEARS, 1929 -1970 The captain (1929 -1933) and The lady (1970)
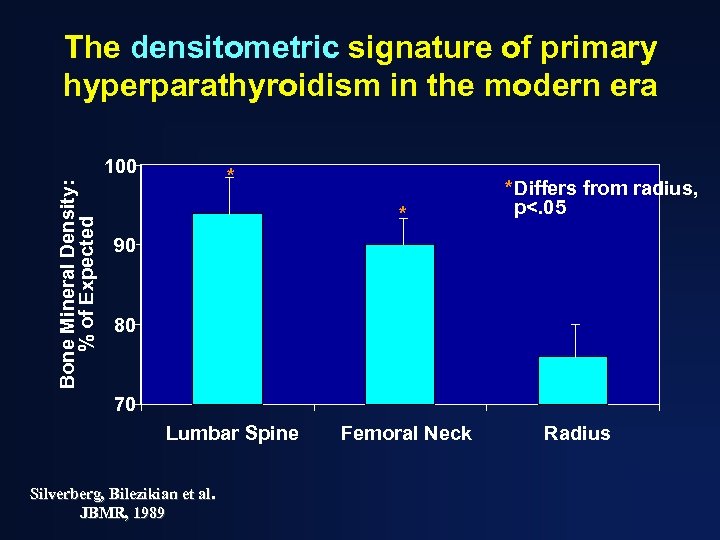
The densitometric signature of primary hyperparathyroidism in the modern era Bone Mineral Density: % of Expected 100 * * *Differs from radius, p<. 05 90 80 70 Lumbar Spine Silverberg, Bilezikian et al. JBMR, 1989 Femoral Neck Radius
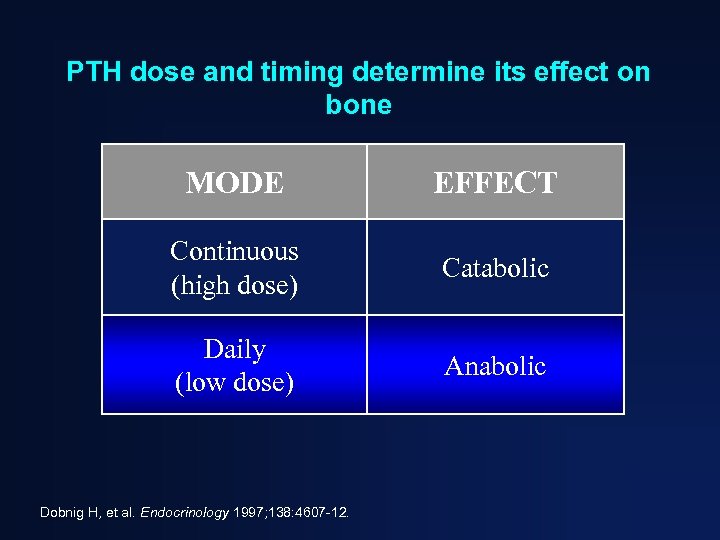
PTH dose and timing determine its effect on bone MODE EFFECT Continuous (high dose) Catabolic Daily (low dose) Anabolic Dobnig H, et al. Endocrinology 1997; 138: 4607 -12.
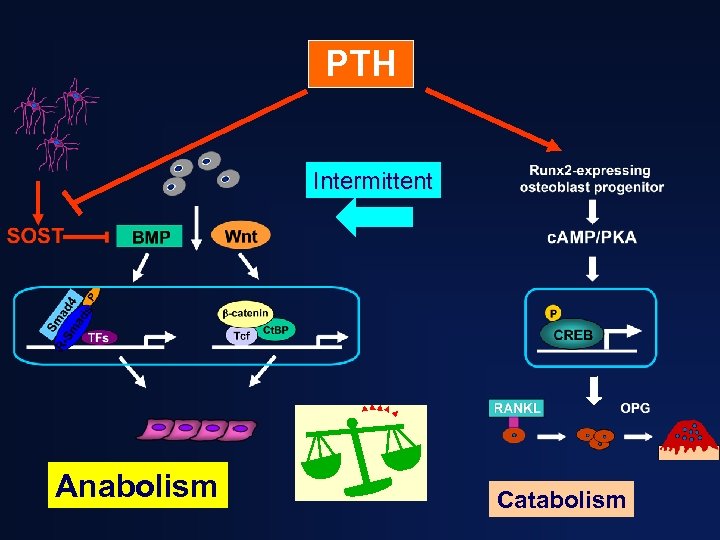
PTH Intermittent Anabolism Catabolism

Three keys to the anabolic potential of PTH • Low dose • Intermittent administration • Pulsatility with rapid “on” and “off” kinetics Under these conditions…. .
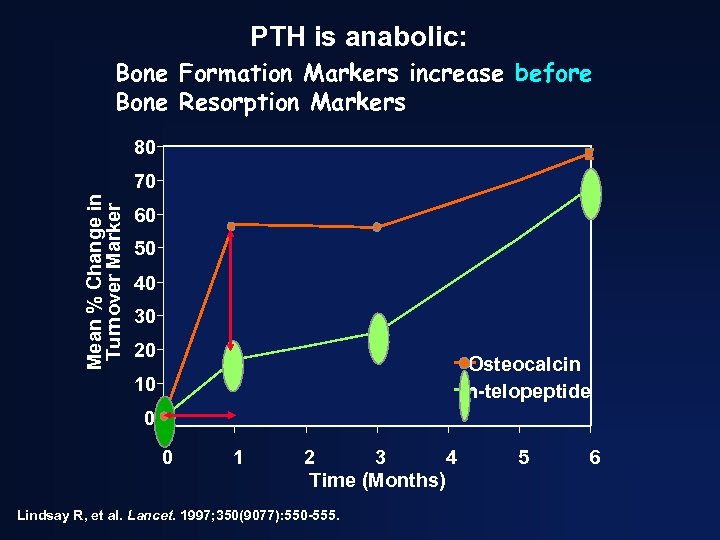
PTH is anabolic: Bone Formation Markers increase before Bone Resorption Markers 80 Mean % Change in Turnover Marker 70 60 50 40 30 20 Osteocalcin n-telopeptide 10 0 0 1 2 3 4 Time (Months) Lindsay R, et al. Lancet. 1997; 350(9077): 550 -555. 5 6
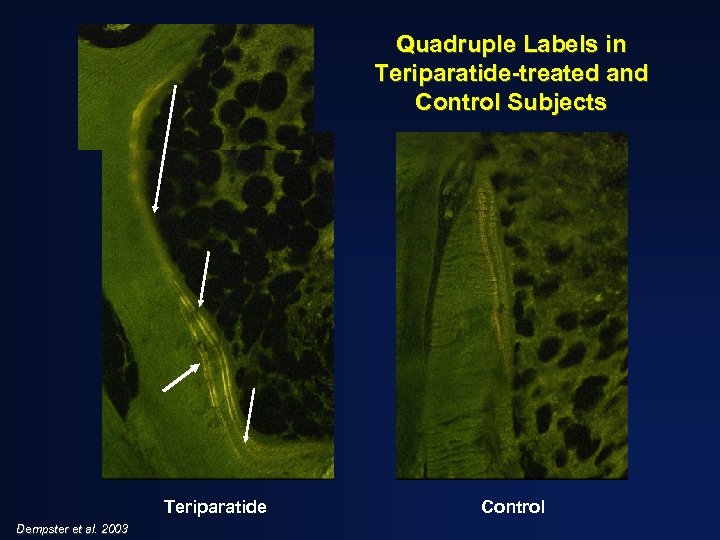
Quadruple Labels in Teriparatide-treated and Control Subjects Teriparatide Dempster et al. 2003 Control
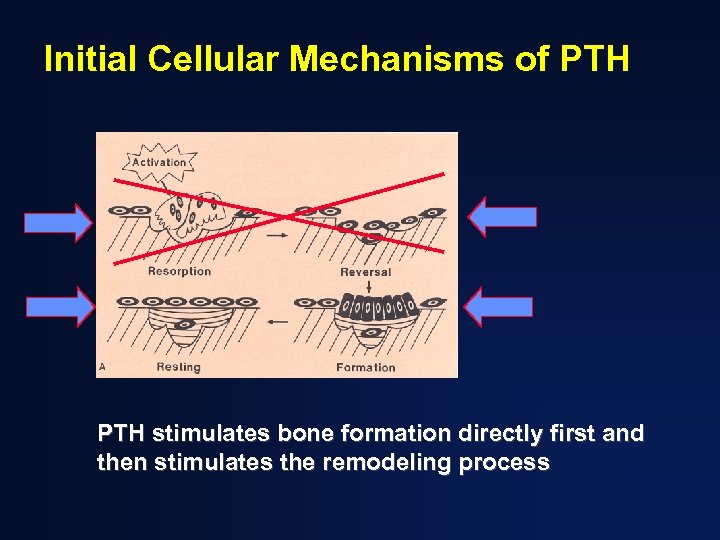
Initial Cellular Mechanisms of PTH stimulates bone formation directly first and then stimulates the remodeling process
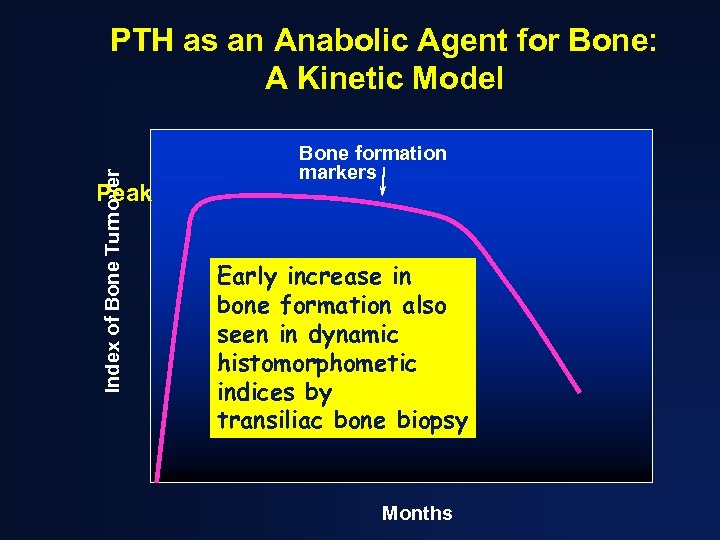
Index of Bone Turnover PTH as an Anabolic Agent for Bone: A Kinetic Model Peak Bone formation markers Early increase in bone formation also seen in dynamic histomorphometic indices by transiliac bone biopsy Months
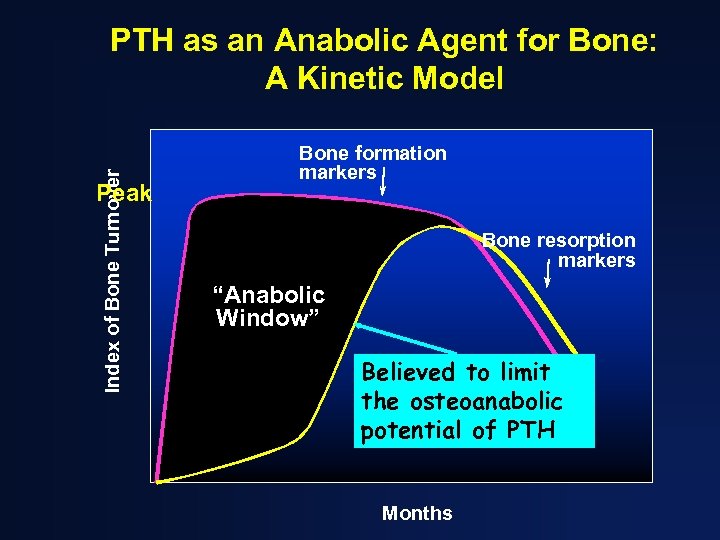
Index of Bone Turnover PTH as an Anabolic Agent for Bone: A Kinetic Model Peak Bone formation markers Bone resorption markers “Anabolic Window” Believed to limit the osteoanabolic potential of PTH Months
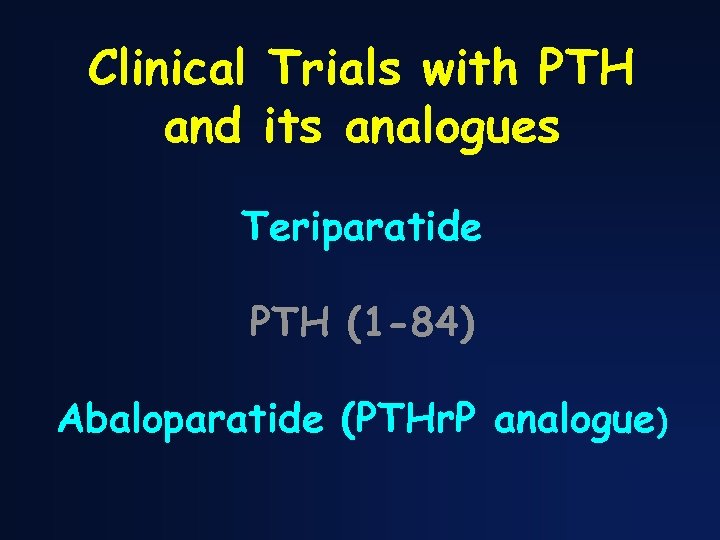
Clinical Trials with PTH and its analogues Teriparatide PTH (1 -84) Abaloparatide (PTHr. P analogue)
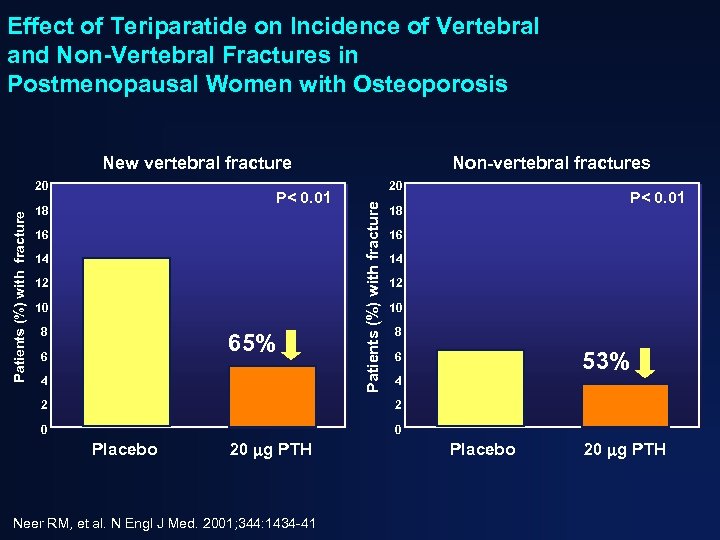
Effect of Teriparatide on Incidence of Vertebral and Non-Vertebral Fractures in Postmenopausal Women with Osteoporosis New vertebral fracture P< 0. 01 18 16 14 12 10 8 65% 6 4 20 Patients (%) with fracture 20 Non-vertebral fractures P< 0. 01 18 16 14 12 10 8 53% 6 4 2 2 0 0 Placebo 20 g PTH Neer RM, et al. N Engl J Med. 2001; 344: 1434 -41 Placebo 20 g PTH
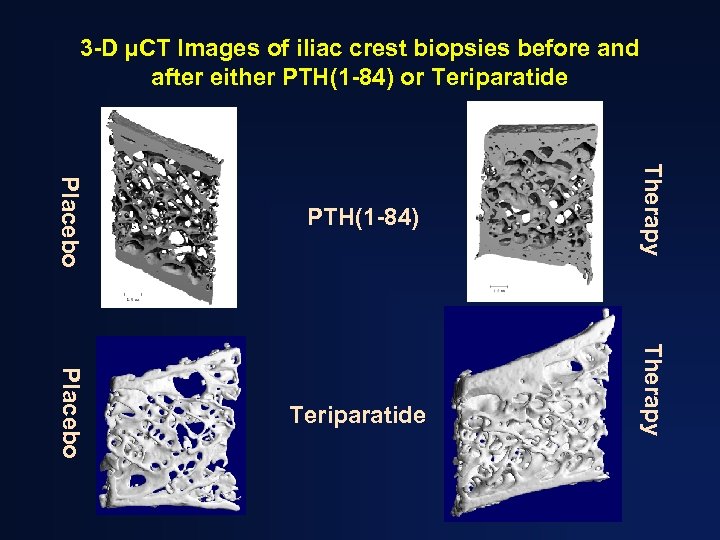
3 -D µCT Images of iliac crest biopsies before and after either PTH(1 -84) or Teriparatide Therapy Placebo PTH(1 -84)

Despite efficacy of teriparatide as a therapy for osteoporosis, there are “issues” • Animal toxicity data (osteosarcoma), still a concern to some prescribers and patients

Update on Osteosarcoma (Cipriani, Irani, and Bilezikian, J Bone Miner Res, 2012) • 3 -5 cases of osteosarcoma have been reported in patients who have received teriparatide since 2002 (Harper et al. JBMR, 2007) • Epidemiological considerations – >2. 0 million patients have been treated with teriparatide and PTH(1 -84) – The background incidence of osteosarcoma in adults 1/250, 000 – The cases reported are fewer than what would be expected on coincidental, epidemiological grounds

Update on Osteosarcoma (Andrews et al, J Bone Miner Res, 2012) • 15 -year FDA-mandated surveillance “Osteosarcoma Surveillance Study” • The report covers the first 7 years • 1448 cases of osteosarcoma identified (62% of all US cases over this period) • 549 patients or proxies interviewed (representative of the entire cohort) • No history of teriparatide use in any patient

Safety of PTH • No oncogenic signals after 12 years of human use, worldwide • The likelihood that osteosarcoma is a human toxicity when used in the way it is being used would appear to be remote. • Surveillance (and the black box) continues
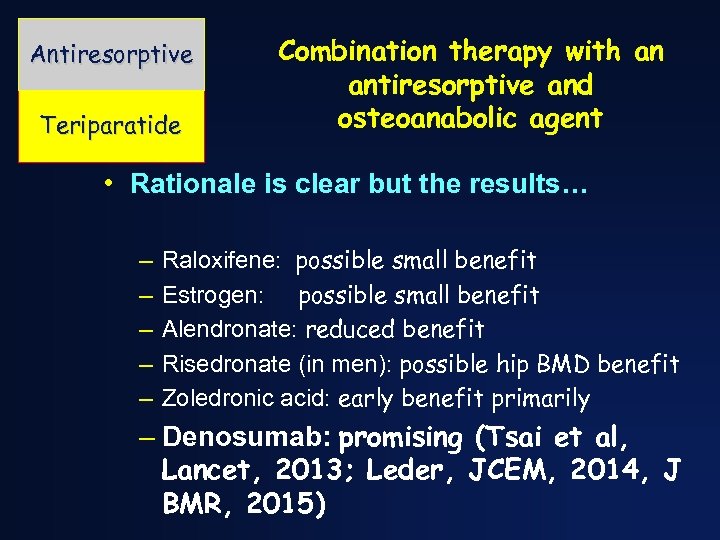
Antiresorptive Teriparatide Combination therapy with an antiresorptive and osteoanabolic agent • Rationale is clear but the results… – – – Raloxifene: possible small benefit Estrogen: possible small benefit Alendronate: reduced benefit Risedronate (in men): possible hip BMD benefit Zoledronic acid: early benefit primarily – Denosumab: promising (Tsai et al, Lancet, 2013; Leder, JCEM, 2014, J BMR, 2015)
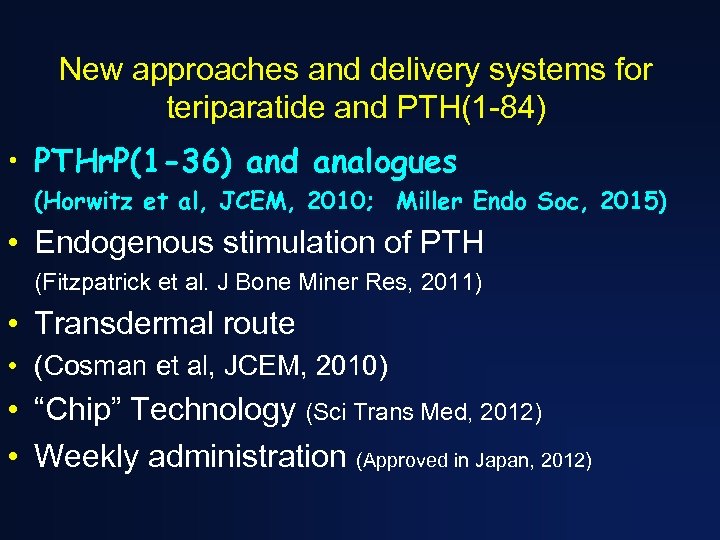
New approaches and delivery systems for teriparatide and PTH(1 -84) • PTHr. P(1 -36) and analogues (Horwitz et al, JCEM, 2010; Miller Endo Soc, 2015) • Endogenous stimulation of PTH (Fitzpatrick et al. J Bone Miner Res, 2011) • Transdermal route • (Cosman et al, JCEM, 2010) • “Chip” Technology (Sci Trans Med, 2012) • Weekly administration (Approved in Japan, 2012)
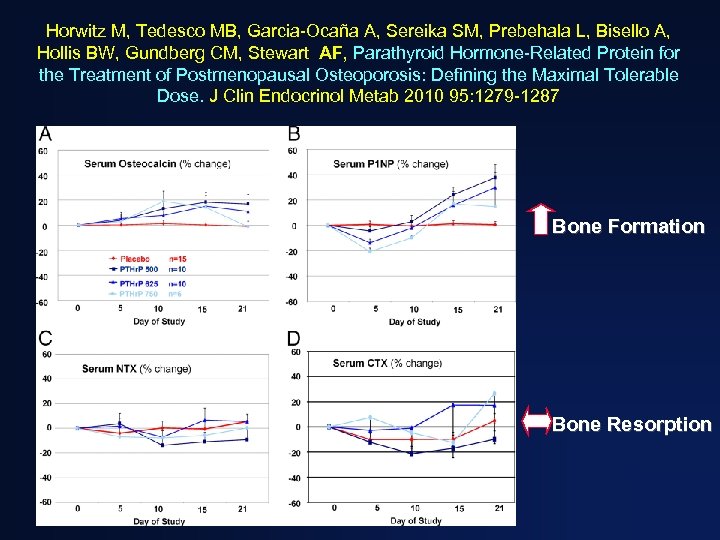
Horwitz M, Tedesco MB, Garcia-Ocaña A, Sereika SM, Prebehala L, Bisello A, Hollis BW, Gundberg CM, Stewart AF, Parathyroid Hormone-Related Protein for the Treatment of Postmenopausal Osteoporosis: Defining the Maximal Tolerable Dose. J Clin Endocrinol Metab 2010 95: 1279 -1287 Bone Formation Bone Resorption
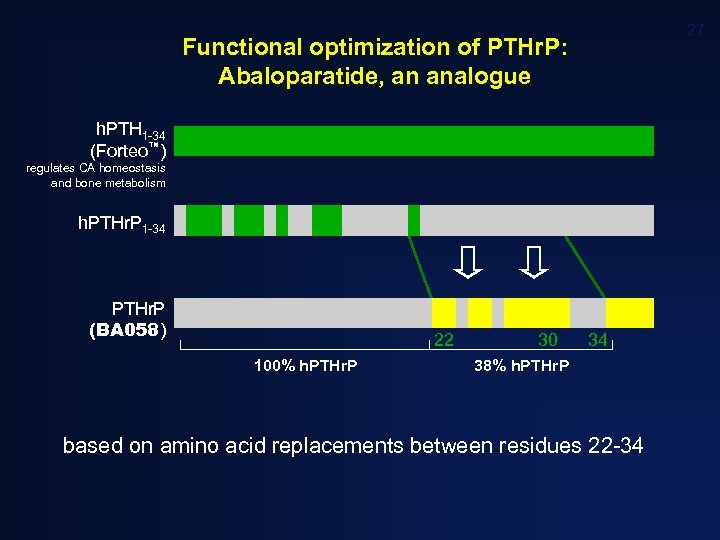
27 Functional optimization of PTHr. P: Abaloparatide, an analogue h. PTH 1 -34 (Forteo™) regulates CA homeostasis and bone metabolism h. PTHr. P 1 -34 PTHr. P (BA 058) 22 100% h. PTHr. P 30 34 38% h. PTHr. P based on amino acid replacements between residues 22 -34
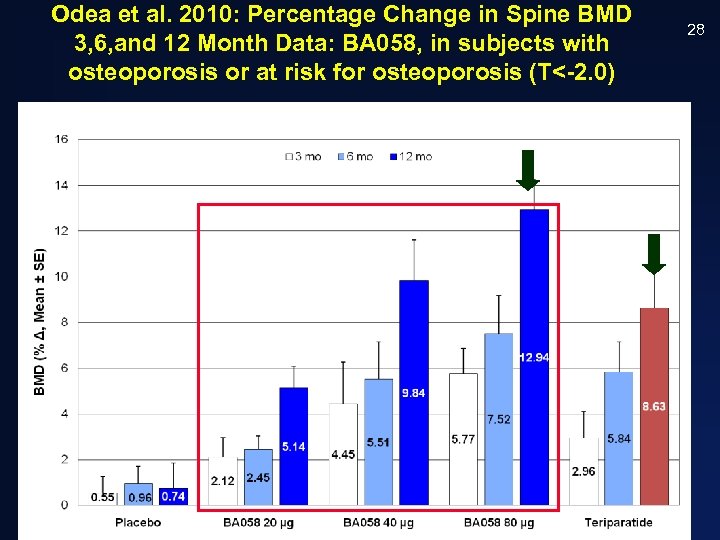
Odea et al. 2010: Percentage Change in Spine BMD 3, 6, and 12 Month Data: BA 058, in subjects with osteoporosis or at risk for osteoporosis (T<-2. 0) CONFIDENTIAL 28
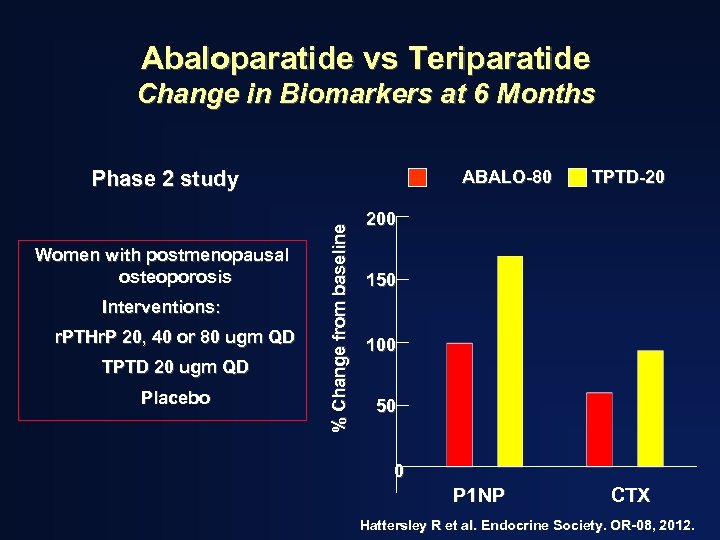
Abaloparatide vs Teriparatide Change in Biomarkers at 6 Months Phase 2 study Interventions: r. PTHr. P 20, 40 or 80 ugm QD TPTD 20 ugm QD Placebo % Change from baseline Women with postmenopausal osteoporosis ABALO-80 TPTD-20 200 150 100 50 0 P 1 NP CTX Hattersley R et al. Endocrine Society. OR-08, 2012.
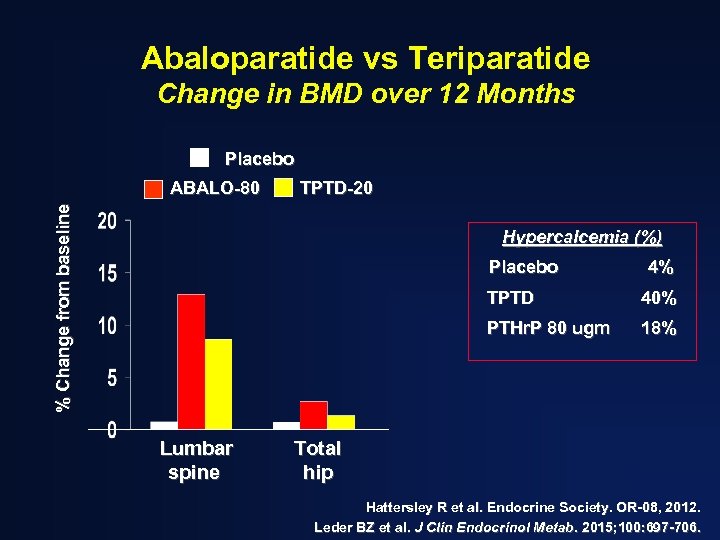
Abaloparatide vs Teriparatide Change in BMD over 12 Months Placebo TPTD-20 % Change from baseline ABALO-80 Hypercalcemia (%) Placebo 4% TPTD PTHr. P 80 ugm Lumbar spine 40% 18% Total hip Hattersley R et al. Endocrine Society. OR-08, 2012. Leder BZ et al. J Clin Endocrinol Metab. 2015; 100: 697 -706.

Update on BA 058 (Abaloparatide) • International Phase 3 trial ended, September, 2014 • Results made available, December, 2014 • Presented by Miller et al, Endocrine Society, March, 2015

Phase 3 Trial Design of Abaloparatide Clinical Trial Randomization N = 2463 Placebo Abaloparatide 80 mcg Daily SC Teriparatide 20 mcg Daily SC Months Miller et al, 2015 6 12 18
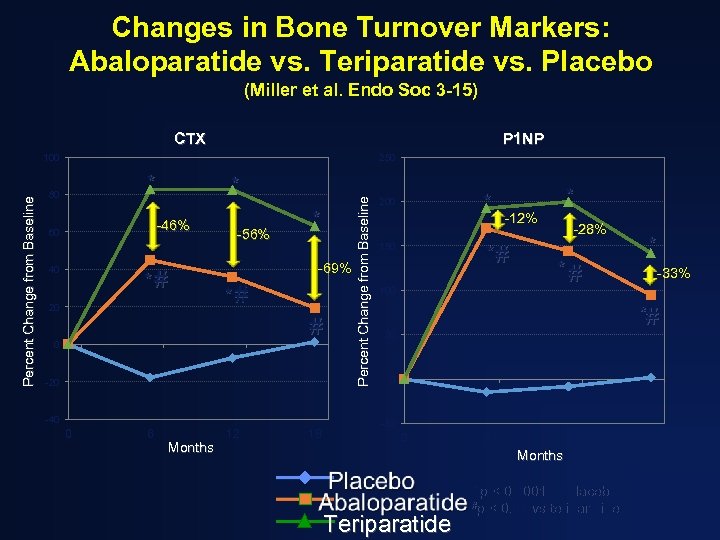
Changes in Bone Turnover Markers: Abaloparatide vs. Teriparatide vs. Placebo (Miller et al. Endo Soc 3 -15) CTX P 1 NP 250 * 80 * -46% 60 40 *# 20 -56% * -69% *# # 0 -20 -40 0 6 Months 12 18 Percent Change from Baseline 100 * 200 * -12% *# 150 100 -28% *# * -33% *# 50 0 -50 0 6 12 Months Teriparatide *p < 0. 0001 vs placebo #p < 0. 01 vs teriparatide 18
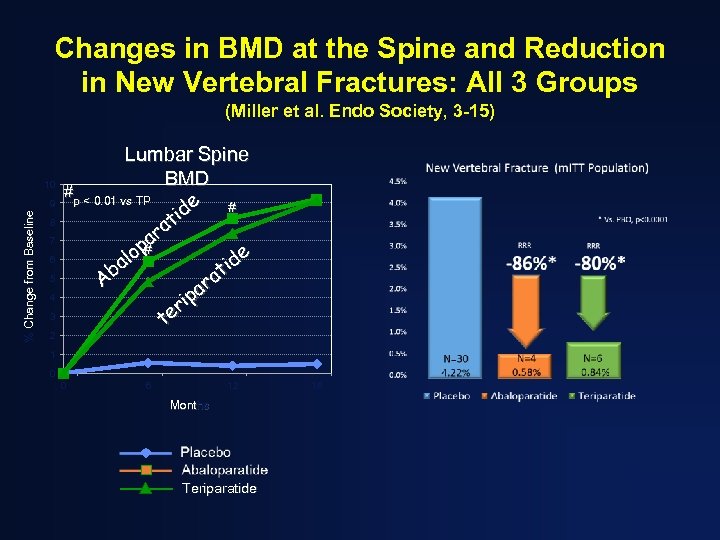
Changes in BMD at the Spine and Reduction in New Vertebral Fractures: All 3 Groups (Miller et al. Endo Society, 3 -15) 10 % Change from Baseline 9 8 7 6 5 4 3 2 Lumbar Spine BMD #p < 0. 01 vs TP e # id t ra pa e lo # a id t Ab ra a ip r te 1 0 0 6 12 Months Teriparatide 18
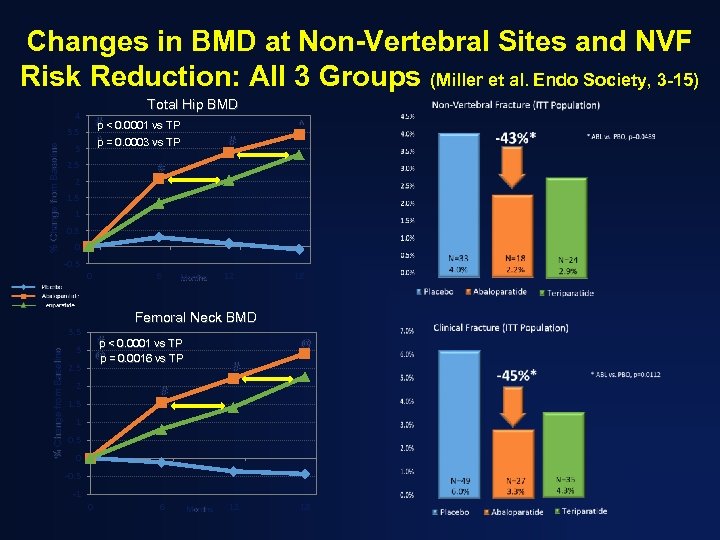
Changes in BMD at Non-Vertebral Sites and NVF Risk Reduction: All 3 Groups (Miller et al. Endo Society, 3 -15) Total Hip BMD 4 # < 0. 0001 vs TP p ^ = 0. 0003 vs TP p % Change from Baseline 3. 5 3 2. 5 ^ # # 2 1. 5 1 0. 5 0 -0. 5 0 Months 12 18 Femoral Neck BMD 3. 5 % Change from Baseline 6 # < 0. 0001 vs TP p 3 @ @ = 0. 0016 vs TP p 2. 5 2 # # 1. 5 1 0. 5 0 -0. 5 -1 0 6 Months 12 18
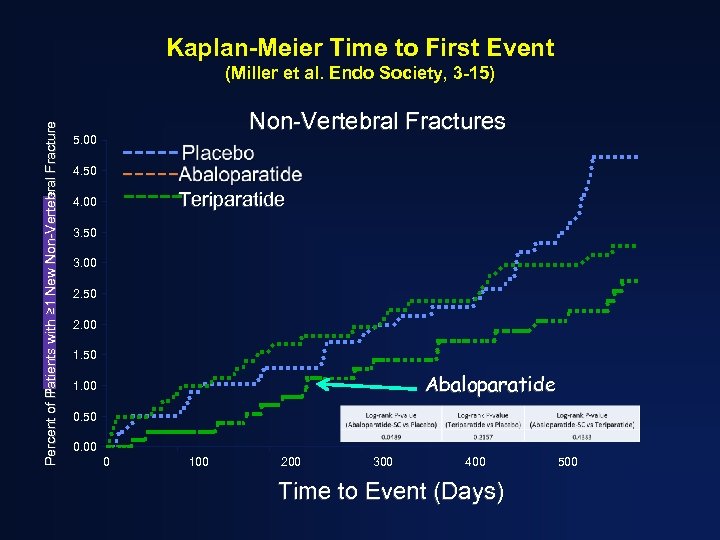
Kaplan-Meier Time to First Event Percent of Patients with ≥ 1 New Non-Vertebral Fracture Percent of Patients with Fracture (Miller et al. Endo Society, 3 -15) Non-Vertebral Fractures 5. 00 4. 50 Teriparatide 4. 00 3. 50 3. 00 2. 50 2. 00 1. 50 Abaloparatide 1. 00 0. 50 0. 00 0 100 200 300 400 Time to Event (Days) 500
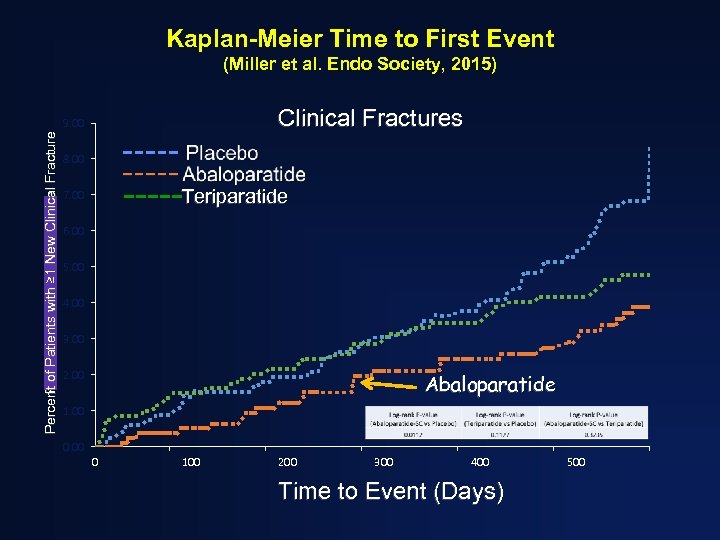
Kaplan-Meier Time to First Event (Miller et al. Endo Society, 2015) Clinical Fractures Percent of Patients with ≥ 1 New Clinical Fracture Percent of Patients with Fracture 9. 00 8. 00 Teriparatide 7. 00 6. 00 5. 00 4. 00 3. 00 2. 00 Abaloparatide 1. 00 0 100 200 300 400 Time to Event (Days) 500
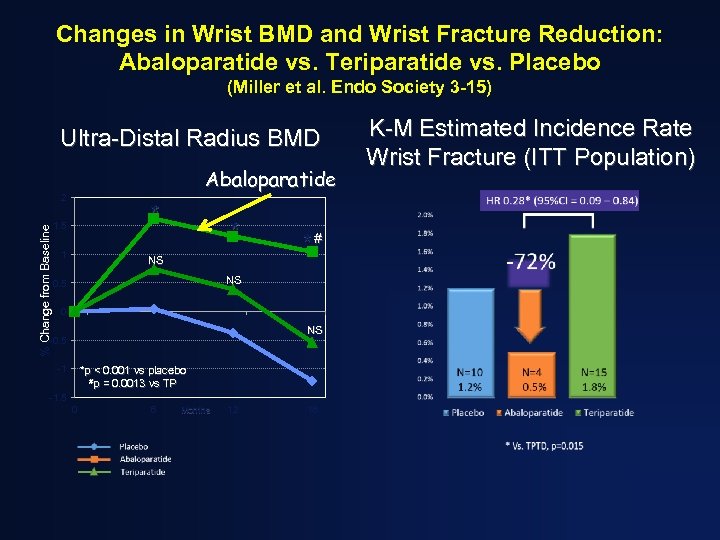
Changes in Wrist BMD and Wrist Fracture Reduction: Abaloparatide vs. Teriparatide vs. Placebo (Miller et al. Endo Society 3 -15) Ultra-Distal Radius BMD Abaloparatide % Change from Baseline 2 * 1. 5 1 * NS *# NS 0. 5 0 NS -0. 5 -1 *p < 0. 001 vs placebo #p = 0. 0013 vs TP -1. 5 0 6 Months 12 18 K-M Estimated Incidence Rate Wrist Fracture (ITT Population)
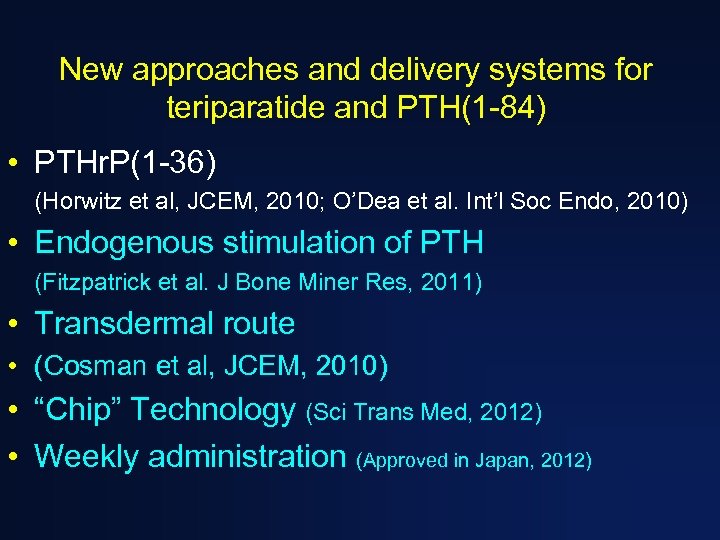
New approaches and delivery systems for teriparatide and PTH(1 -84) • PTHr. P(1 -36) (Horwitz et al, JCEM, 2010; O’Dea et al. Int’l Soc Endo, 2010) • Endogenous stimulation of PTH (Fitzpatrick et al. J Bone Miner Res, 2011) • Transdermal route • (Cosman et al, JCEM, 2010) • “Chip” Technology (Sci Trans Med, 2012) • Weekly administration (Approved in Japan, 2012)

Other Potential Applications • • Historical notes Review of therapeutic efficacy in Osteoporosis New PTHs and delivery systems Applications of PTH to specific conditions – – GIO (to be covered in another lecture) Accelerate Fracture healing Atypical Fractures Hypoparathyroidism (to be covered in another lecture)

PTH to accelerate fracture healing • In animals, fracture healing is enhanced (Ellegard M et al. CTI, 2010) • In human subjects – with Colles’ Fracture, 20 ug, but not 40 ug (the primary endpoint), reduced the median time to radiographic healing (Aspenberg et al. J Bone Miner Res, 2010) – With pelvic fracture, PTH(1 -84) reduced the time to radiographic healing (Peichl et al. J Bone Joint Surg 2011) – Case Reports: numerous – Reviews: (J Orthop Trauma , 2013, Borges, Freitas and Bilezikian, Arq Bras Endocinol Metab, 2013, Campbell et al. expert Opin Biol Ther, 2015). – Personal Experience: Lecoultre J et al. (Rev Med Suisee, 2015)
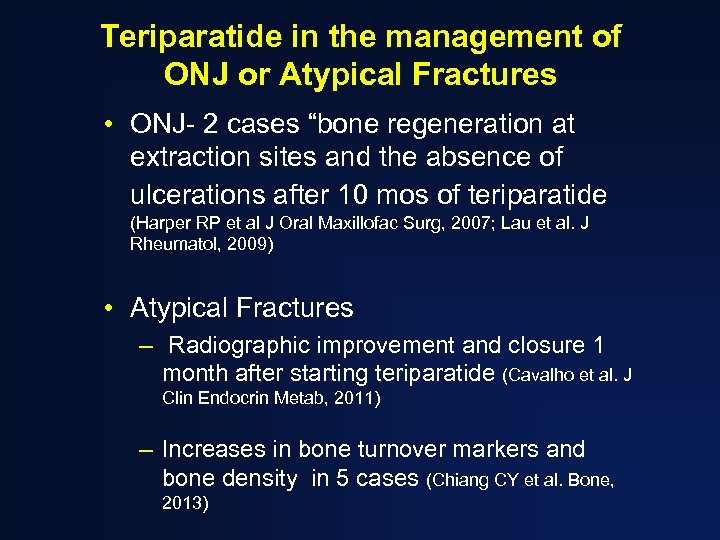
Teriparatide in the management of ONJ or Atypical Fractures • ONJ- 2 cases “bone regeneration at extraction sites and the absence of ulcerations after 10 mos of teriparatide (Harper RP et al J Oral Maxillofac Surg, 2007; Lau et al. J Rheumatol, 2009) • Atypical Fractures – Radiographic improvement and closure 1 month after starting teriparatide (Cavalho et al. J Clin Endocrin Metab, 2011) – Increases in bone turnover markers and bone density in 5 cases (Chiang CY et al. Bone, 2013)

Osteoanabolic therapy with PTH and PTHr. P peptides…. • Provides a therapeutic mechanism by which bone gain and reduced fracture incidence is associated with microstructural improvements…… • A most worthy goal!
THE FUTURE OF OSTEOANABOLIC THERAPY

Clues to a new therapeutic approach: Sclerosteosis & van Buchem’s Disease • increased bone mass throughout skeleton. • very low fracture risk • due to absence of sclerostin - an inhibitor of Wnt signaling and bone formation Janssens and Van Hul. Hum Mol Genet. 2002; 11: 2385 -93.

Heterozygous carriers of Sclerosteosis and van Buchem’s disease do not appear to have complications as seen in the homozygous subjects • Higher bone density • Increased markers of bone formation • Levels of sclerostin are intermediate • No long-term or progressive sequellae
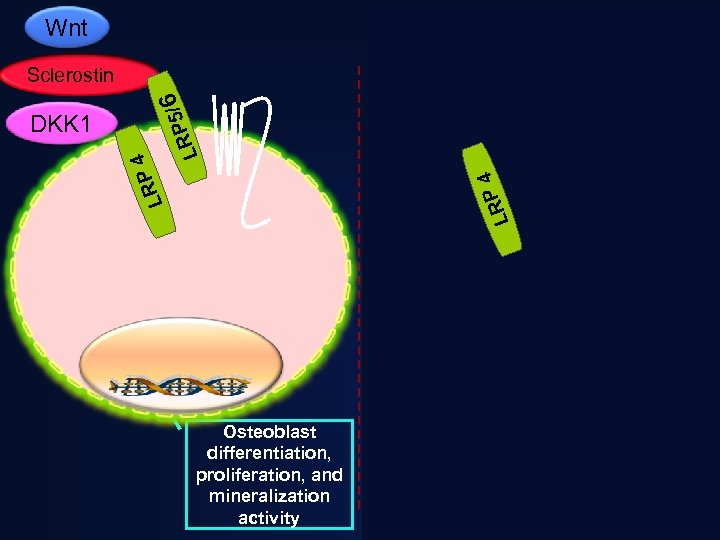
Wnt Sclerostin APC / RP 5 L 6 DS H Frat 1 4 LRP AXIN DKK 1 APC LRP 4 DKK 1 6 P 5/ LR Sclerostin GSK 3β βcatenin P DS H AXIN Proteosomal Degradation βcatenin Osteoblast differentiation, proliferation, and mineralization activity Osteoblast: Reduced activity
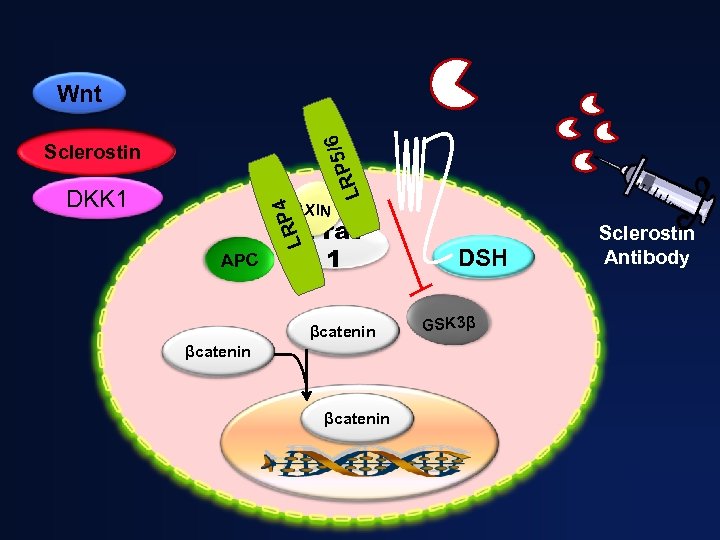
/6 Wnt RP 5 L Sclerostin AXIN LRP 4 DKK 1 APC Frat 1 βcatenin DSH GSK 3β Sclerostin Antibody
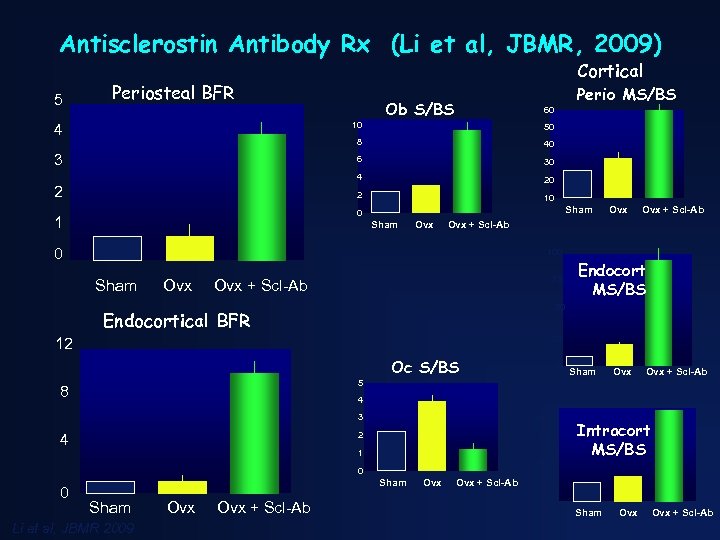
Antisclerostin Antibody Rx (Li et al, JBMR, 2009) 5 Cortical Periosteal BFR Perio MS/BS Ob S/BS 60 4 10 50 8 40 3 6 30 4 20 2 10 2 Sham 0 1 Sham Ovx Ovx + Scl-Ab 0 100 Sham Ovx 75 Ovx + Scl-Ab Endocort MS/BS 50 Endocortical BFR 25 12 Oc S/BS 0 Sham Ovx + Scl-Ab 5 8 4 3 2 4 40 30 1 20 Intracort MS/BS 0 0 Sham Li et al, JBMR 2009 Ovx Ovx + Scl-Ab 10 0 Sham Ovx + Scl-Ab
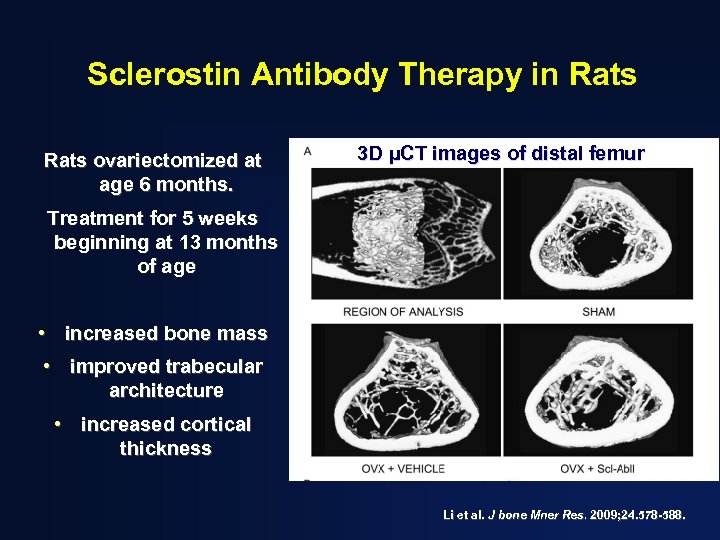
Sclerostin Antibody Therapy in Rats ovariectomized at age 6 months. 3 D µCT images of distal femur Treatment for 5 weeks beginning at 13 months of age • increased bone mass • improved trabecular architecture • increased cortical thickness Li et al. J bone Mner Res. 2009; 24. 578 -588.
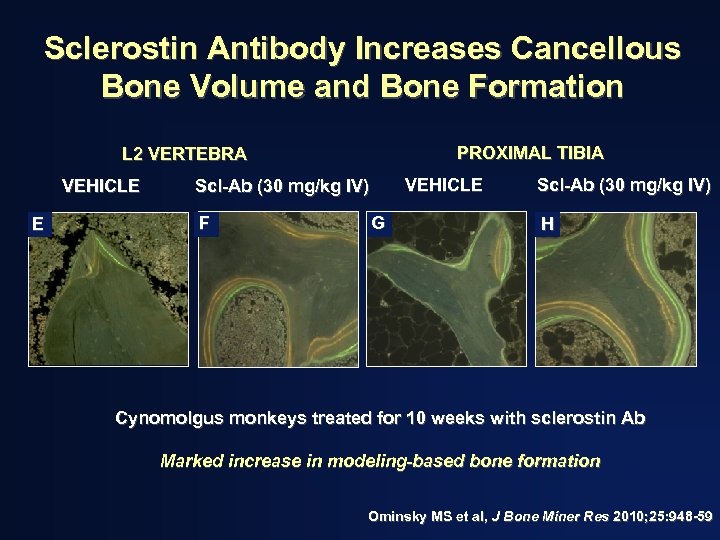
Sclerostin Antibody Increases Cancellous Bone Volume and Bone Formation PROXIMAL TIBIA L 2 VERTEBRA VEHICLE E VEHICLE Scl-Ab (30 mg/kg IV) F G Scl-Ab (30 mg/kg IV) H Cynomolgus monkeys treated for 10 weeks with sclerostin Ab Marked increase in modeling-based bone formation Ominsky MS et al, J Bone Miner Res 2010; 25: 948 -59
CLINICAL TRIALS AND MECHANISMS OF THERAPEUTICS: ANTISCLEROSTIN ANTIBODY Human Studies • Romosozumab (osteoporosis) • Blosozumab (osteoporosis) • BPS 804 (adult onset adult-onset hypophosphatasia- MO 27)

Phase 2 CLINICAL TRIAL: Romosozumab in Postmenopausal Women with Low Bone Density. Mc. Clung et al. N Eng J Med January, 2014, – 419 Postmenopausal women, 55 -85 yrs old – BMD: T < -2. 0 and > -3. 5 • Mean T-scores: LS: -2. 29; TH: -1. 53; FN: -1. 93 – 6 sc dosing regimens monthly (70, 140, 210 mg) or q 3 mos (140, 210 mg); PLB – Comparators: open label- ALN (70 mg/weekly; TPTD 20 ug daily)
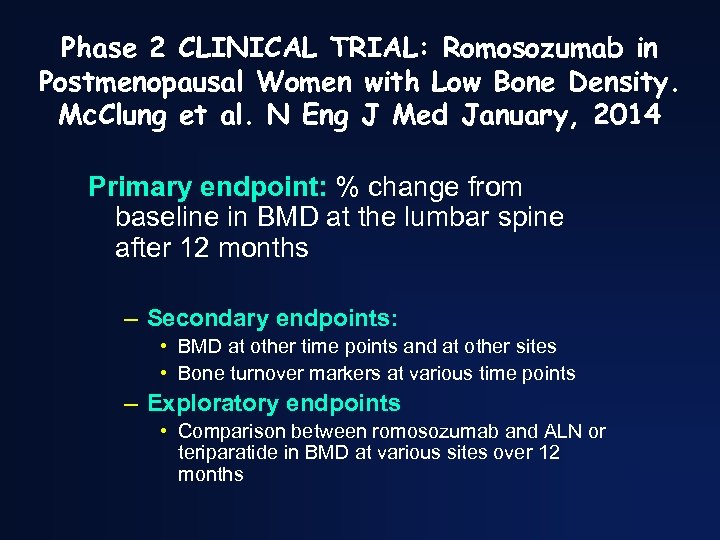
Phase 2 CLINICAL TRIAL: Romosozumab in Postmenopausal Women with Low Bone Density. Mc. Clung et al. N Eng J Med January, 2014 Primary endpoint: % change from baseline in BMD at the lumbar spine after 12 months – Secondary endpoints: • BMD at other time points and at other sites • Bone turnover markers at various time points – Exploratory endpoints • Comparison between romosozumab and ALN or teriparatide in BMD at various sites over 12 months
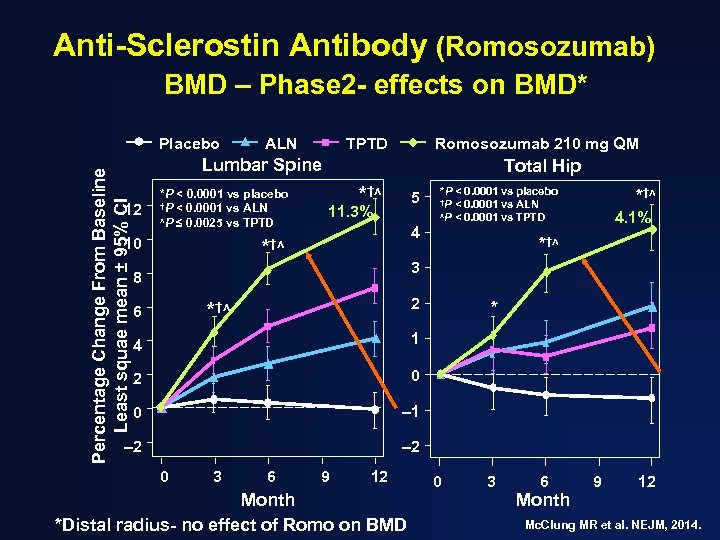
Anti-Sclerostin Antibody (Romosozumab) BMD – Phase 2 - effects on BMD* Percentage Change From Baseline Least squae mean ± 95% CI Placebo ALN TPTD Romosozumab 210 mg QM Lumbar Spine 12 *P < 0. 0001 vs placebo †P < 0. 0001 vs ALN ʌP ≤ 0. 0025 vs TPTD 10 Total Hip *†ʌ 5 11. 3% *P < 0. 0001 vs placebo †P < 0. 0001 vs ALN ʌP < 0. 0001 vs TPTD 4 *†ʌ 4. 1% *†ʌ 3 8 2 *†ʌ 6 4 1 2 0 0 – 1 – 2 * – 2 0 3 6 9 12 Month *Distal radius- no effect of Romo on BMD 0 3 6 Month 9 12 Mc. Clung MR et al. NEJM, 2014.
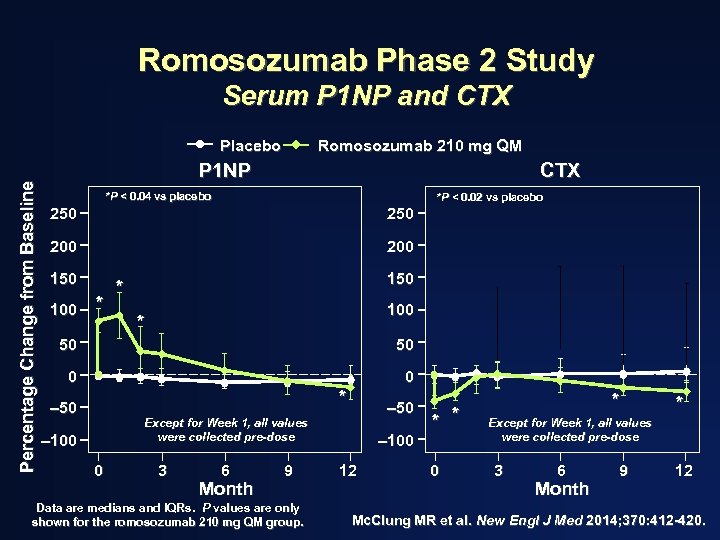
Romosozumab Phase 2 Study Serum P 1 NP and CTX Percentage Change from Baseline Placebo Romosozumab 210 mg QM P 1 NP CTX *P < 0. 04 vs placebo *P < 0. 02 vs placebo 250 200 150 100 * 150 * 100 * 50 50 0 0 * – 50 Except for Week 1, all values were collected pre-dose – 100 0 3 6 Month 9 Data are medians and IQRs. P values are only shown for the romosozumab 210 mg QM group. – 100 12 * * 0 * * Except for Week 1, all values were collected pre-dose 3 6 Month 9 12 Mc. Clung MR et al. New Engl J Med 2014; 370: 412 -420.
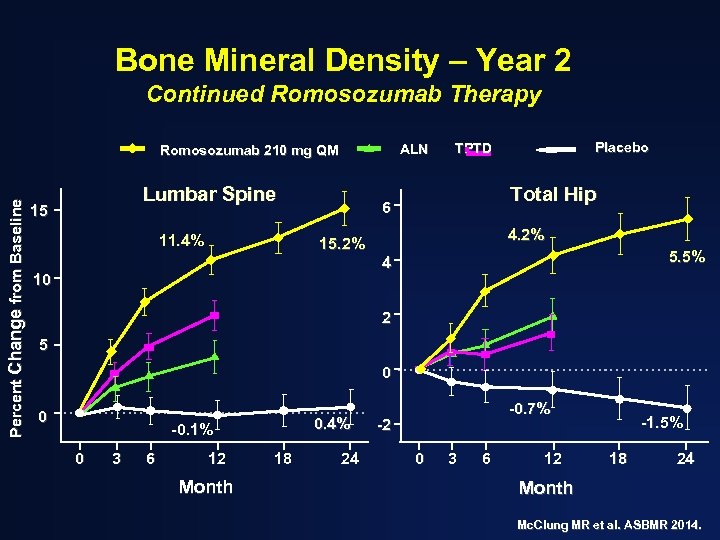
Bone Mineral Density – Year 2 Continued Romosozumab Therapy ALN Percent Change from Baseline Romosozumab 210 mg QM Lumbar Spine 15 11. 4% Placebo TPTD Total Hip 6 4. 2% 15. 2% 5. 5% 4 10 2 5 0 0 0. 4% -0. 1% 0 3 6 12 Month 18 24 -0. 7% -2 0 3 6 12 -1. 5% 18 24 Month Mc. Clung MR et al. ASBMR 2014.

Effects of Sclerostin Inhibition with Romosozumab in human subjects Phase I and II studies: • Early, marked but transient increase in markers of bone formation • Modest, persistent reduction in bone resorption • Substantial increase in BMD • Phase III studies are underway

CLINICAL TRIALS AND MECHANISMS OF THERAPEUTICS: ANTISCLEROSTIN ANTIBODY • Romosozumab (osteoporosis) • Blosozumab (osteoporosis)
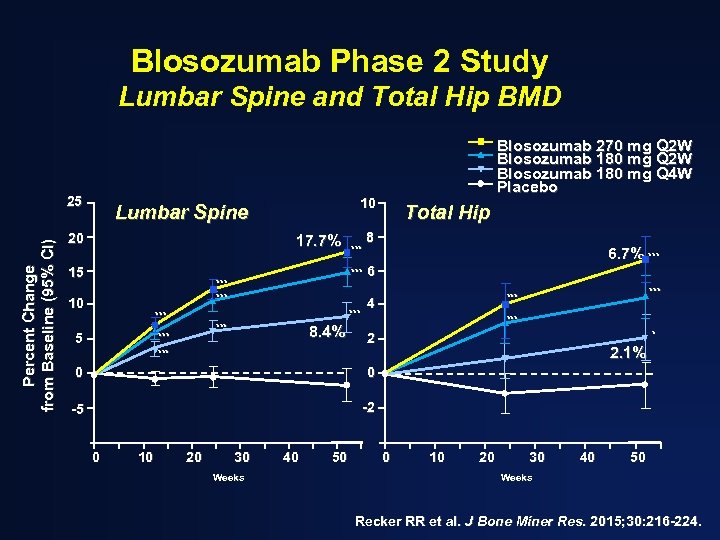
Blosozumab Phase 2 Study Lumbar Spine and Total Hip BMD Percent Change from Baseline (95% CI) 25 10 Lumbar Spine 20 17. 7% 15 *** *** *** 10 *** Blosozumab 270 mg Q 2 W Blosozumab 180 mg Q 4 W Placebo 8. 4% Total Hip 8 6. 7% *** 6 *** 4 *** * 2 2. 1% *** 0 0 -5 -2 0 10 20 30 Weeks 40 50 0 10 20 30 40 50 Weeks Recker RR et al. J Bone Miner Res. 2015; 30: 216 -224.
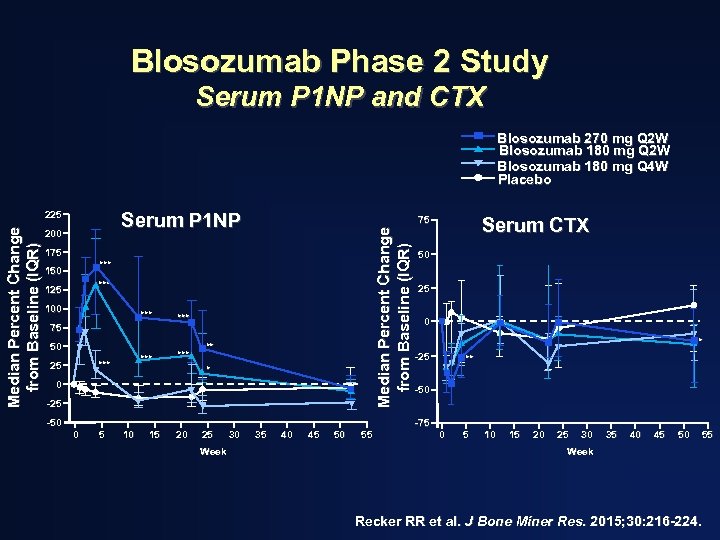
Blosozumab Phase 2 Study Serum P 1 NP and CTX Blosozumab 270 mg Q 2 W Blosozumab 180 mg Q 4 W Placebo Serum P 1 NP 200 175 *** 150 *** 125 100 *** *** 75 50 *** 25 ** * 0 -25 -50 0 5 10 15 20 25 Week 30 Serum CTX 75 Median Percent Change from Baseline (IQR) 225 35 40 45 50 55 50 25 0 * -25 ** -50 -75 0 5 10 15 20 25 30 35 40 45 50 55 Week Recker RR et al. J Bone Miner Res. 2015; 30: 216 -224.

CLINICAL TRIALS AND MECHANISMS OF THERAPEUTICS: ANTISCLEROSTIN ANTIBODY • Blosozumab (osteoporosis) – Further develop halted on April 29, 2015 because the company could not develop a subcutaneous formulation that was not associated with local skin reactions.

Osteoanabolic Therapy for Osteoporosis: the present and the future Teriparatide Abaloparatide Romosozumab

Summary Osteoanabolic therapy has the potential to restore skeletal microstructure and uniquely transform osteoporotic bone towards normal.

THANK YOU!
cc2bca34afd56b9d39c0ad5da29a62c2.ppt