2597f4f0e82fd091cbbf38ba3e2d47d1.ppt
- Количество слайдов: 78
 Orientation to data collection processes and inaugural quarterly discussion of latest data on ICAP programs MER April 25 2007 ICAP quarterly data meeting
Orientation to data collection processes and inaugural quarterly discussion of latest data on ICAP programs MER April 25 2007 ICAP quarterly data meeting
 Objectives • Review and discuss the process of data collection, cleaning, analysis and dissemination in NY and in-country • Share latest data on ICAP supported programs across 14 countries – Discuss how data are or can be used for program improvement • Discuss format of future ICAP quarterly data meetings
Objectives • Review and discuss the process of data collection, cleaning, analysis and dissemination in NY and in-country • Share latest data on ICAP supported programs across 14 countries – Discuss how data are or can be used for program improvement • Discuss format of future ICAP quarterly data meetings
 Outline • Unified Reporting System (URS) • Site Census • Program and Facility Characteristics Tracking System (P-Fa. CTS) • Care and treatment data • PMTCT(+) data • TB/HIV data • Patient-level data • Open discussion
Outline • Unified Reporting System (URS) • Site Census • Program and Facility Characteristics Tracking System (P-Fa. CTS) • Care and treatment data • PMTCT(+) data • TB/HIV data • Patient-level data • Open discussion
 ICAP/CU-supported countries
ICAP/CU-supported countries
 ICAP supports an estimated 694 programmatic activities at 260 facilities in 14 countries (2. 7 activities per site) • Need to capture standard donor and ICAP indicators on the scale and quality of ICAP-supported programs on a quarterly basis. – Care & treatment: 166 indicators (203 of 222 sites reporting as of 4/07) – PMTCT+: 37 indicators (105/115 sites reporting as of 04/07) – TB/HIV: 22 indicators (not yet started, expected from 141 sites) – Testing & counseling, infant diagnosis, adherence support in the works • Programs are expanding, diversity of programming is expanding – Need for more indicators? !
ICAP supports an estimated 694 programmatic activities at 260 facilities in 14 countries (2. 7 activities per site) • Need to capture standard donor and ICAP indicators on the scale and quality of ICAP-supported programs on a quarterly basis. – Care & treatment: 166 indicators (203 of 222 sites reporting as of 4/07) – PMTCT+: 37 indicators (105/115 sites reporting as of 04/07) – TB/HIV: 22 indicators (not yet started, expected from 141 sites) – Testing & counseling, infant diagnosis, adherence support in the works • Programs are expanding, diversity of programming is expanding – Need for more indicators? !
 Unified Reporting System (URS) What: Central reporting system that utilizes a web-based application for capturing and disseminating data on multiple ICAPsupported activities and programs Purpose: To streamline, standardize, and increase the efficiency and utilization of routinely collected data by any ICAP staff member
Unified Reporting System (URS) What: Central reporting system that utilizes a web-based application for capturing and disseminating data on multiple ICAPsupported activities and programs Purpose: To streamline, standardize, and increase the efficiency and utilization of routinely collected data by any ICAP staff member
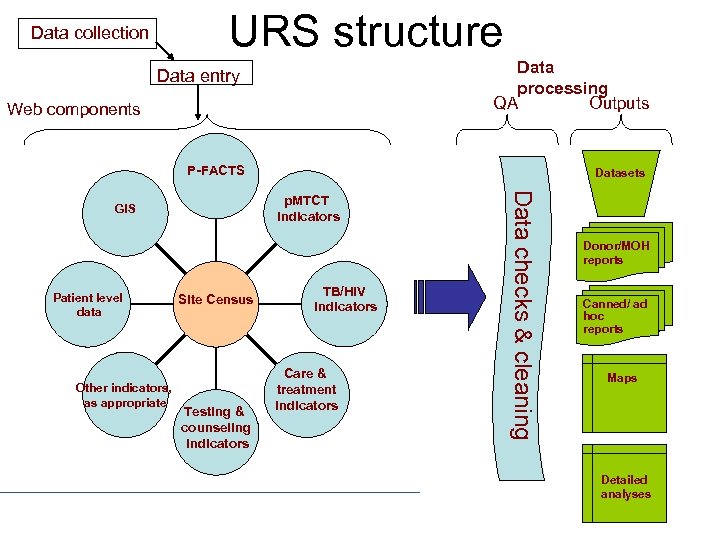 URS structure Data collection Data processing QA Outputs Data entry Web components P-FACTS Other indicators, as appropriate Site Census Testing & counseling indicators TB/HIV indicators Care & treatment indicators CIEISIN Data checks & cleaning p. MTCT indicators GIS Patient level data Datasets Donor/MOH reports Canned/ ad hoc reports Maps Detailed analyses
URS structure Data collection Data processing QA Outputs Data entry Web components P-FACTS Other indicators, as appropriate Site Census Testing & counseling indicators TB/HIV indicators Care & treatment indicators CIEISIN Data checks & cleaning p. MTCT indicators GIS Patient level data Datasets Donor/MOH reports Canned/ ad hoc reports Maps Detailed analyses
 Site Census module What: Real-time inventory of all planned, current, and closed ICAP sites. Supported activities, funding source(s) and their targets are also captured Purpose: To have one up to date master list of sites, activities and targets that all ICAP staff can refer to for planning and evaluation
Site Census module What: Real-time inventory of all planned, current, and closed ICAP sites. Supported activities, funding source(s) and their targets are also captured Purpose: To have one up to date master list of sites, activities and targets that all ICAP staff can refer to for planning and evaluation
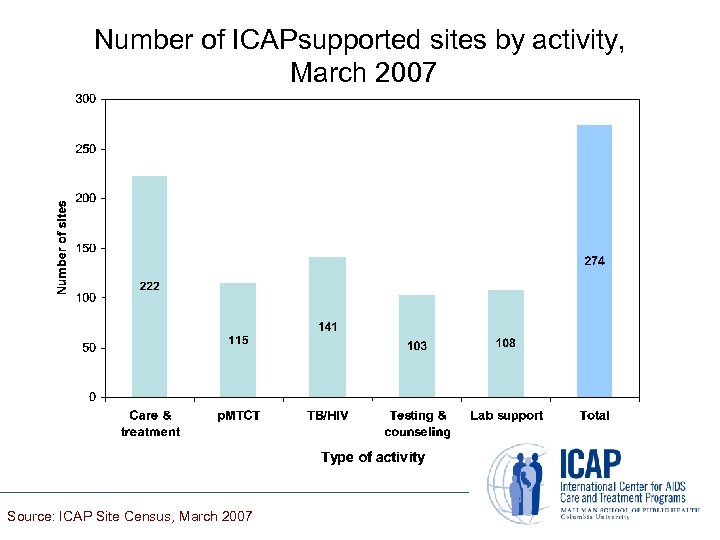 Number of ICAPsupported sites by activity, March 2007 Source: ICAP Site Census, March 2007
Number of ICAPsupported sites by activity, March 2007 Source: ICAP Site Census, March 2007
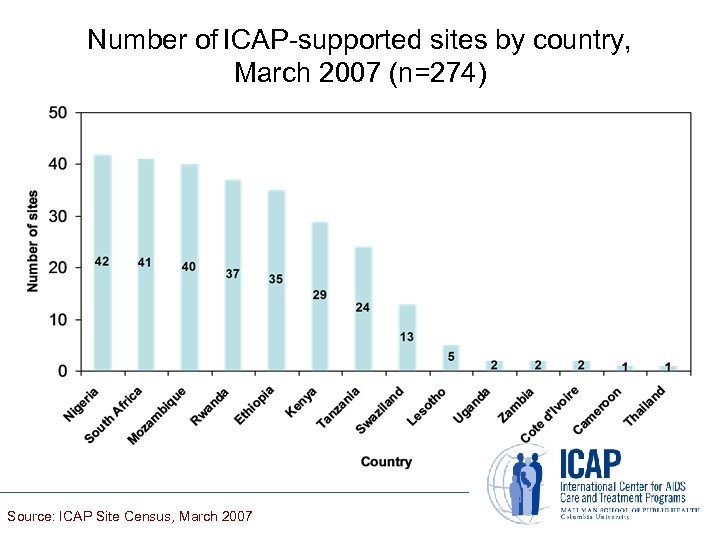 Number of ICAP-supported sites by country, March 2007 (n=274) Source: ICAP Site Census, March 2007
Number of ICAP-supported sites by country, March 2007 (n=274) Source: ICAP Site Census, March 2007
 Program and Facility Characteristics Tracking System (P-Fa. CTS) module What: Collects program and facility information on ICAP-supported care and treatment programs semi-annually Purpose: To describe the scope, diversity, and comprehensiveness of ICAP-supported care and treatment programs, and evaluate multi-level factors that influence program performance and patient-level outcomes
Program and Facility Characteristics Tracking System (P-Fa. CTS) module What: Collects program and facility information on ICAP-supported care and treatment programs semi-annually Purpose: To describe the scope, diversity, and comprehensiveness of ICAP-supported care and treatment programs, and evaluate multi-level factors that influence program performance and patient-level outcomes
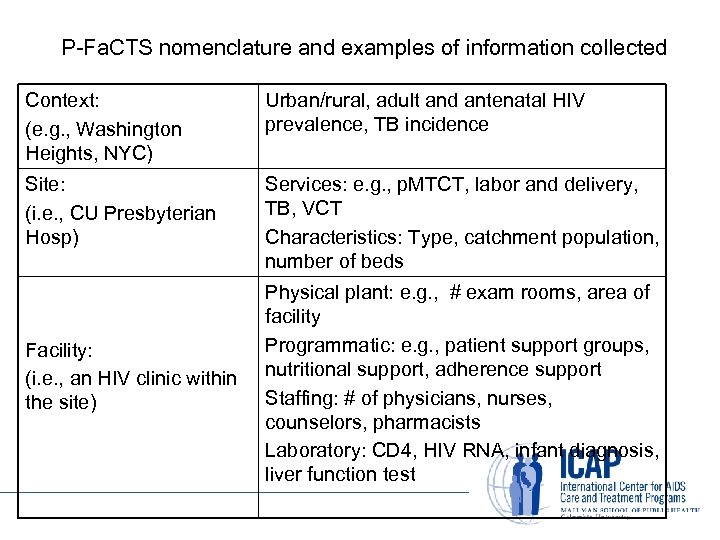 P-Fa. CTS nomenclature and examples of information collected Context: (e. g. , Washington Heights, NYC) Urban/rural, adult and antenatal HIV prevalence, TB incidence Site: (i. e. , CU Presbyterian Hosp) Services: e. g. , p. MTCT, labor and delivery, TB, VCT Characteristics: Type, catchment population, number of beds Facility: (i. e. , an HIV clinic within the site) Physical plant: e. g. , # exam rooms, area of facility Programmatic: e. g. , patient support groups, nutritional support, adherence support Staffing: # of physicians, nurses, counselors, pharmacists Laboratory: CD 4, HIV RNA, infant diagnosis, liver function test
P-Fa. CTS nomenclature and examples of information collected Context: (e. g. , Washington Heights, NYC) Urban/rural, adult and antenatal HIV prevalence, TB incidence Site: (i. e. , CU Presbyterian Hosp) Services: e. g. , p. MTCT, labor and delivery, TB, VCT Characteristics: Type, catchment population, number of beds Facility: (i. e. , an HIV clinic within the site) Physical plant: e. g. , # exam rooms, area of facility Programmatic: e. g. , patient support groups, nutritional support, adherence support Staffing: # of physicians, nurses, counselors, pharmacists Laboratory: CD 4, HIV RNA, infant diagnosis, liver function test
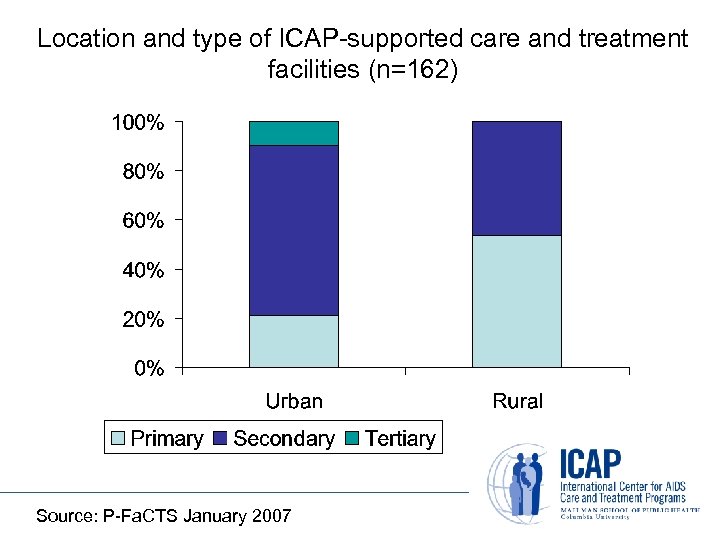 Location and type of ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
Location and type of ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
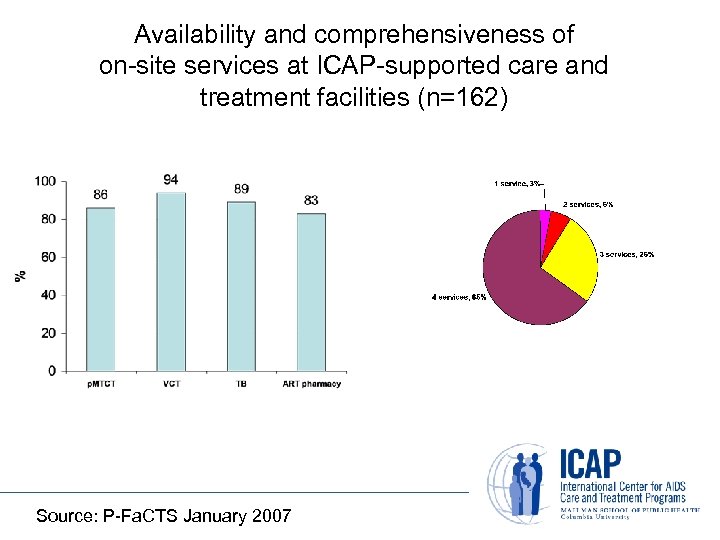 Availability and comprehensiveness of on-site services at ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
Availability and comprehensiveness of on-site services at ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
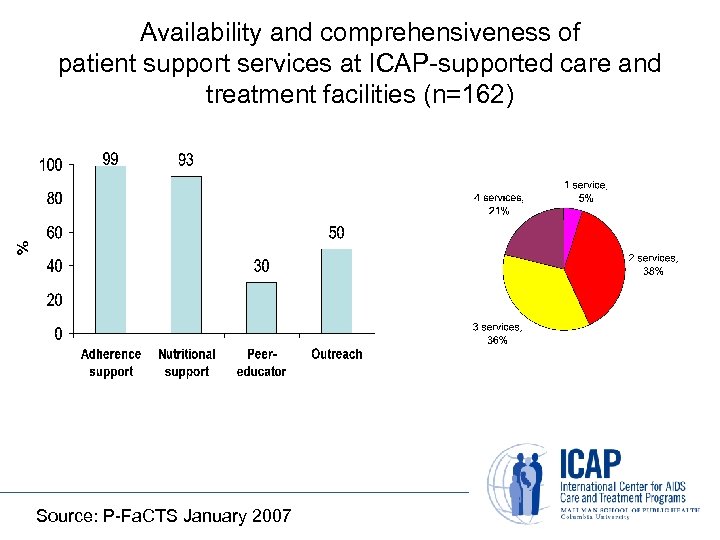 Availability and comprehensiveness of patient support services at ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
Availability and comprehensiveness of patient support services at ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
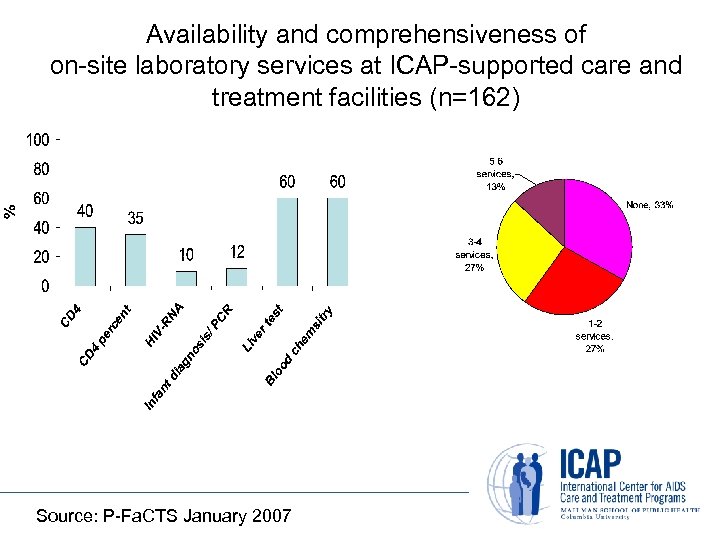 Availability and comprehensiveness of on-site laboratory services at ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
Availability and comprehensiveness of on-site laboratory services at ICAP-supported care and treatment facilities (n=162) Source: P-Fa. CTS January 2007
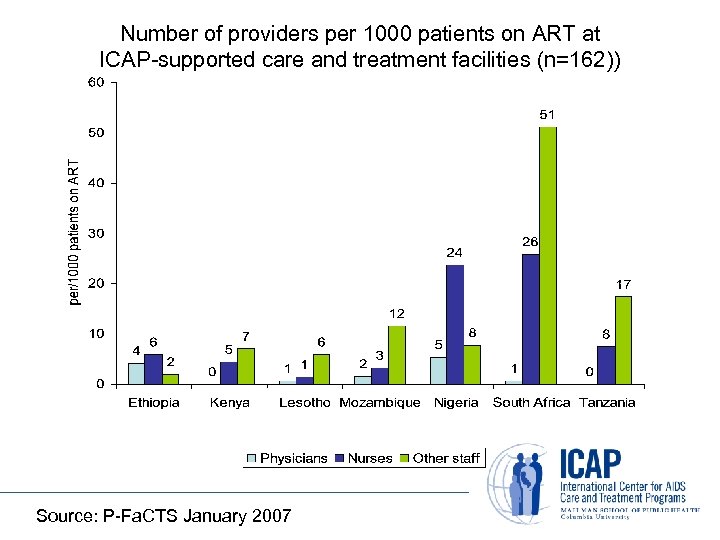 Number of providers per 1000 patients on ART at ICAP-supported care and treatment facilities (n=162)) Source: P-Fa. CTS January 2007
Number of providers per 1000 patients on ART at ICAP-supported care and treatment facilities (n=162)) Source: P-Fa. CTS January 2007
 P-Fa. CTS summary • Care and treatment programs vary in comprehensiveness of services offered • Activities and components of ICAPsupported care and treatment programs are dynamic, therefore necessary to conduct routine surveys • PFa. CTS data can be used in conjunction with program data for program planning and evaluation
P-Fa. CTS summary • Care and treatment programs vary in comprehensiveness of services offered • Activities and components of ICAPsupported care and treatment programs are dynamic, therefore necessary to conduct routine surveys • PFa. CTS data can be used in conjunction with program data for program planning and evaluation
 Care and treatment
Care and treatment
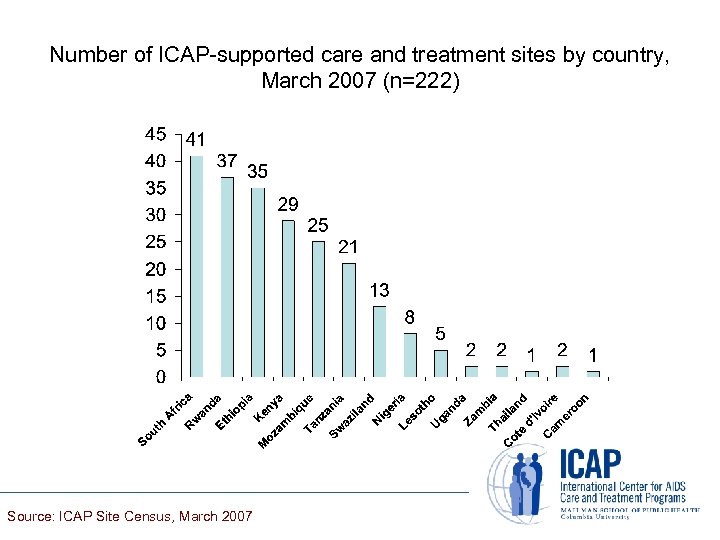 Number of ICAP-supported care and treatment sites by country, March 2007 (n=222) Source: ICAP Site Census, March 2007
Number of ICAP-supported care and treatment sites by country, March 2007 (n=222) Source: ICAP Site Census, March 2007
 Care and treatment aggregate indicators received on quarterly basis by facility (currently 209/222 in 10 countries) • Pre-ART and ART care enrollment by age, sex and pregnancy status • CD 4 count for ART patients (baseline, 6 and 12 months) • ART regimens by age • ART discontinuation and reasons • Number of trainings on ART and palliative care
Care and treatment aggregate indicators received on quarterly basis by facility (currently 209/222 in 10 countries) • Pre-ART and ART care enrollment by age, sex and pregnancy status • CD 4 count for ART patients (baseline, 6 and 12 months) • ART regimens by age • ART discontinuation and reasons • Number of trainings on ART and palliative care
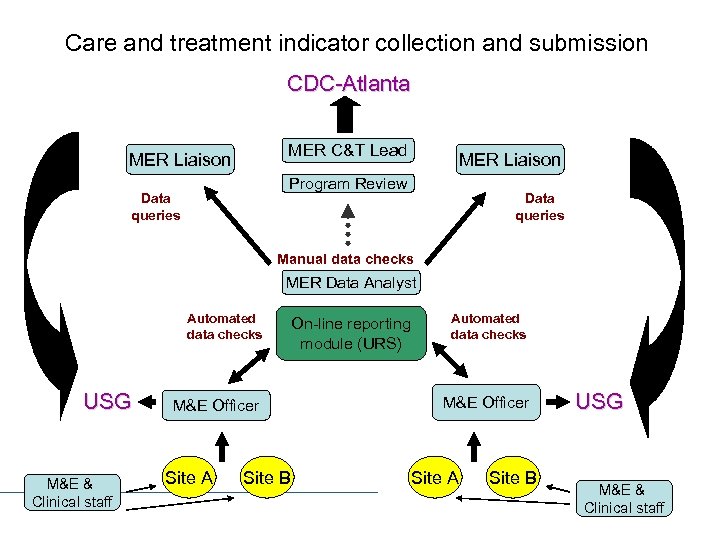 Care and treatment indicator collection and submission CDC-Atlanta MER C&T Lead MER Liaison Program Review Data queries Manual data checks MER Data Analyst Automated data checks USG M&E & Clinical staff M&E Officer Site A Site B On-line reporting module (URS) Automated data checks M&E Officer Site A Site B USG M&E & Clinical staff
Care and treatment indicator collection and submission CDC-Atlanta MER C&T Lead MER Liaison Program Review Data queries Manual data checks MER Data Analyst Automated data checks USG M&E & Clinical staff M&E Officer Site A Site B On-line reporting module (URS) Automated data checks M&E Officer Site A Site B USG M&E & Clinical staff
 Uses of care and treatment data • • • In-country USG reporting Track 1. 0 reporting (Atlanta) Dissemination of data for program evaluation and planning – Summary tables and graphs • By country and time period – Slide set for presentations • Important trends – In-depth analyses • Abstracts from Implementer and IAS meeting
Uses of care and treatment data • • • In-country USG reporting Track 1. 0 reporting (Atlanta) Dissemination of data for program evaluation and planning – Summary tables and graphs • By country and time period – Slide set for presentations • Important trends – In-depth analyses • Abstracts from Implementer and IAS meeting
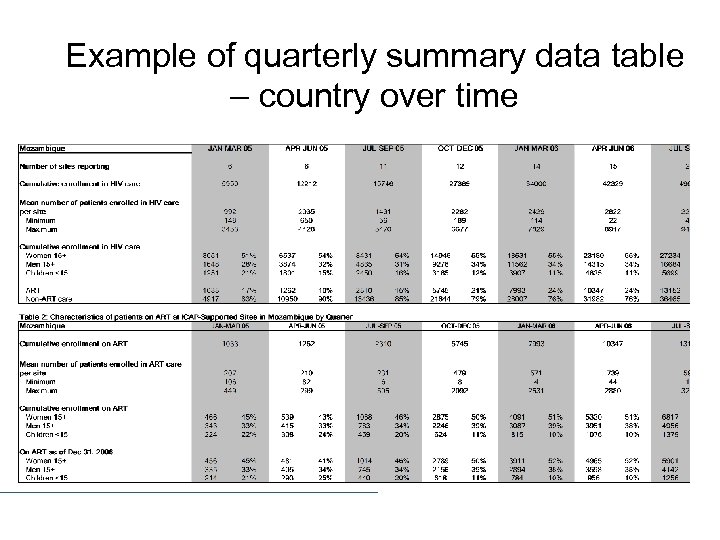 Example of quarterly summary data table – country over time
Example of quarterly summary data table – country over time
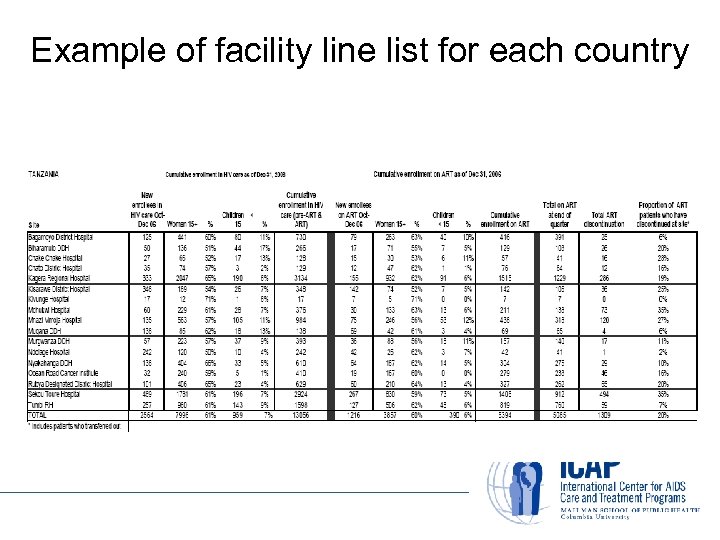 Example of facility line list for each country
Example of facility line list for each country
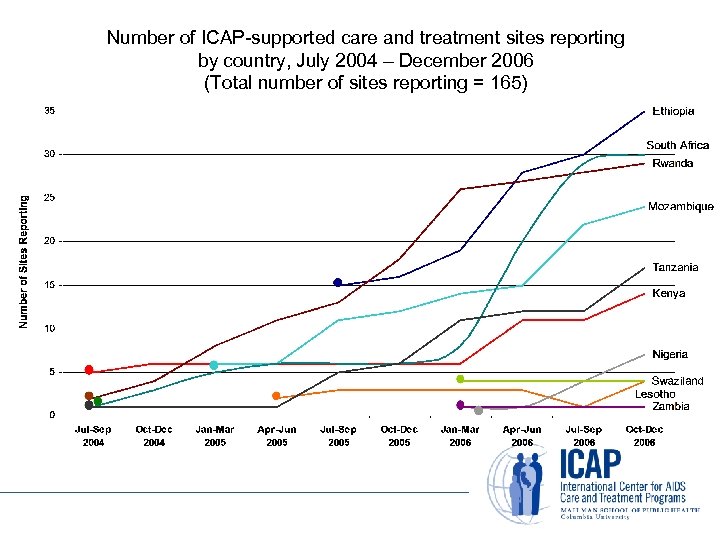 Number of ICAP-supported care and treatment sites reporting by country, July 2004 – December 2006 (Total number of sites reporting = 165)
Number of ICAP-supported care and treatment sites reporting by country, July 2004 – December 2006 (Total number of sites reporting = 165)
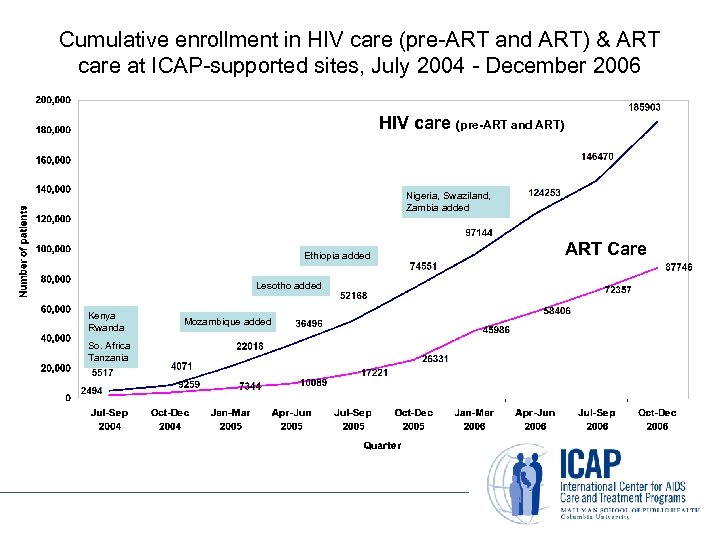 Cumulative enrollment in HIV care (pre-ART and ART) & ART care at ICAP-supported sites, July 2004 - December 2006 HIV care (pre-ART and ART) Nigeria, Swaziland, Zambia added Ethiopia added Lesotho added Kenya Rwanda So. Africa Tanzania Mozambique added ART Care
Cumulative enrollment in HIV care (pre-ART and ART) & ART care at ICAP-supported sites, July 2004 - December 2006 HIV care (pre-ART and ART) Nigeria, Swaziland, Zambia added Ethiopia added Lesotho added Kenya Rwanda So. Africa Tanzania Mozambique added ART Care
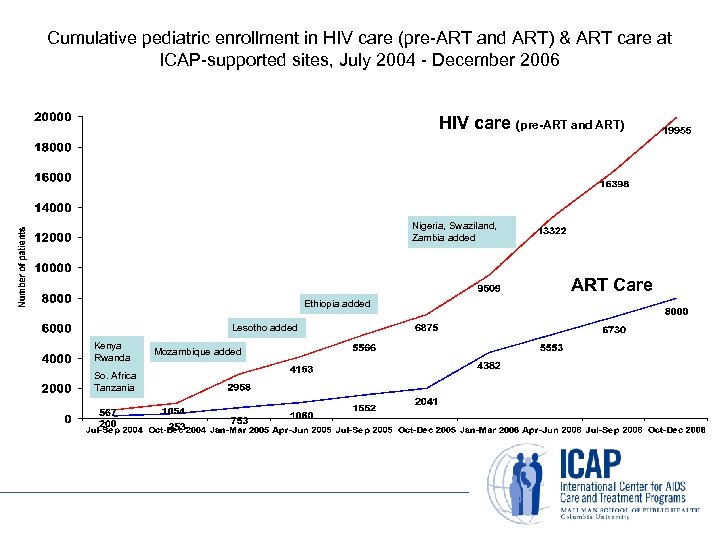 Cumulative pediatric enrollment in HIV care (pre-ART and ART) & ART care at ICAP-supported sites, July 2004 - December 2006 HIV care (pre-ART and ART) Nigeria, Swaziland, Zambia added ART Care Ethiopia added Lesotho added Kenya Rwanda So. Africa Tanzania Mozambique added
Cumulative pediatric enrollment in HIV care (pre-ART and ART) & ART care at ICAP-supported sites, July 2004 - December 2006 HIV care (pre-ART and ART) Nigeria, Swaziland, Zambia added ART Care Ethiopia added Lesotho added Kenya Rwanda So. Africa Tanzania Mozambique added
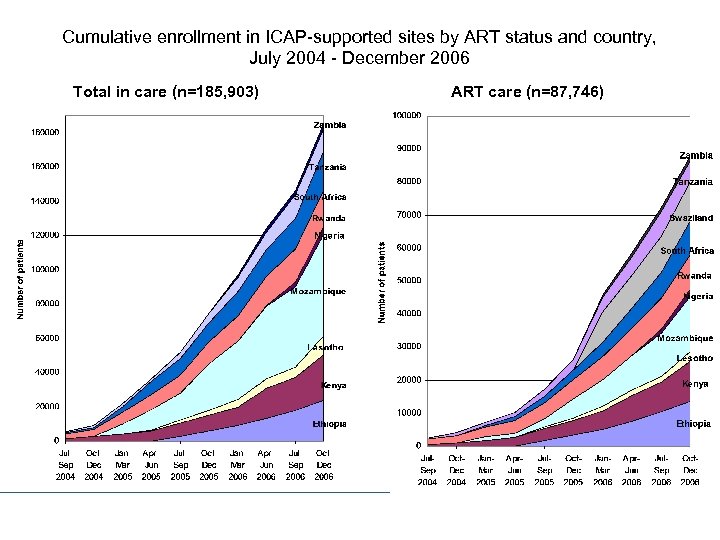 Cumulative enrollment in ICAP-supported sites by ART status and country, July 2004 - December 2006 Total in care (n=185, 903) ART care (n=87, 746)
Cumulative enrollment in ICAP-supported sites by ART status and country, July 2004 - December 2006 Total in care (n=185, 903) ART care (n=87, 746)
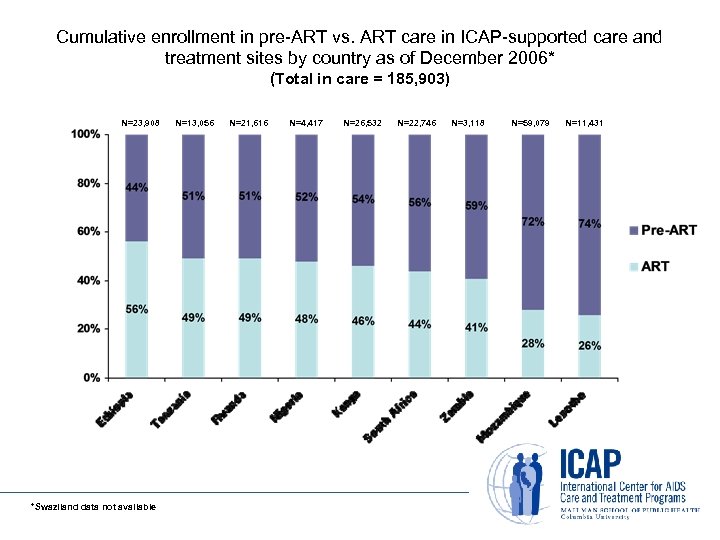 Cumulative enrollment in pre-ART vs. ART care in ICAP-supported care and treatment sites by country as of December 2006* (Total in care = 185, 903) N=23, 908 *Swaziland data not available N=13, 056 N=21, 616 N=4, 417 N=26, 532 N=22, 746 N=3, 118 N=59, 079 N=11, 431
Cumulative enrollment in pre-ART vs. ART care in ICAP-supported care and treatment sites by country as of December 2006* (Total in care = 185, 903) N=23, 908 *Swaziland data not available N=13, 056 N=21, 616 N=4, 417 N=26, 532 N=22, 746 N=3, 118 N=59, 079 N=11, 431
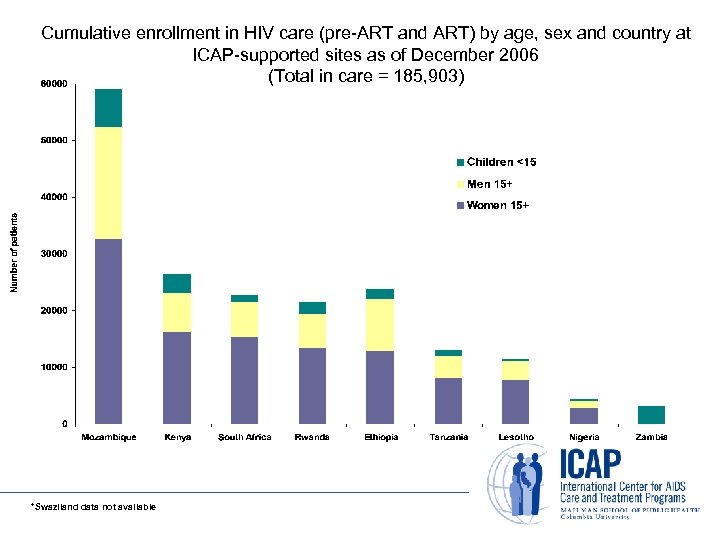 Cumulative enrollment in HIV care (pre-ART and ART) by age, sex and country at ICAP-supported sites as of December 2006 (Total in care = 185, 903) *Swaziland data not available
Cumulative enrollment in HIV care (pre-ART and ART) by age, sex and country at ICAP-supported sites as of December 2006 (Total in care = 185, 903) *Swaziland data not available
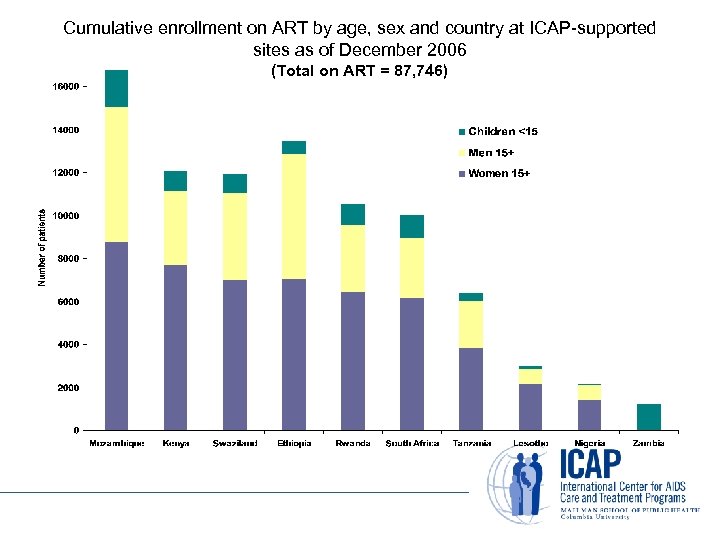 Cumulative enrollment on ART by age, sex and country at ICAP-supported sites as of December 2006 (Total on ART = 87, 746)
Cumulative enrollment on ART by age, sex and country at ICAP-supported sites as of December 2006 (Total on ART = 87, 746)
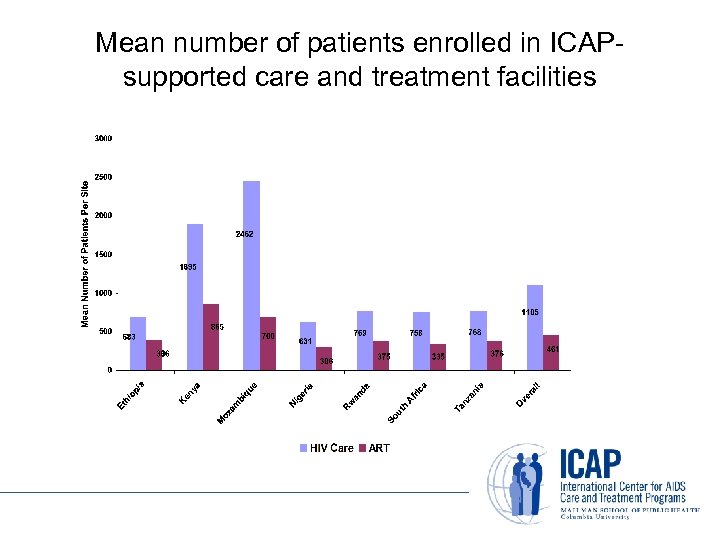 Mean number of patients enrolled in ICAPsupported care and treatment facilities
Mean number of patients enrolled in ICAPsupported care and treatment facilities
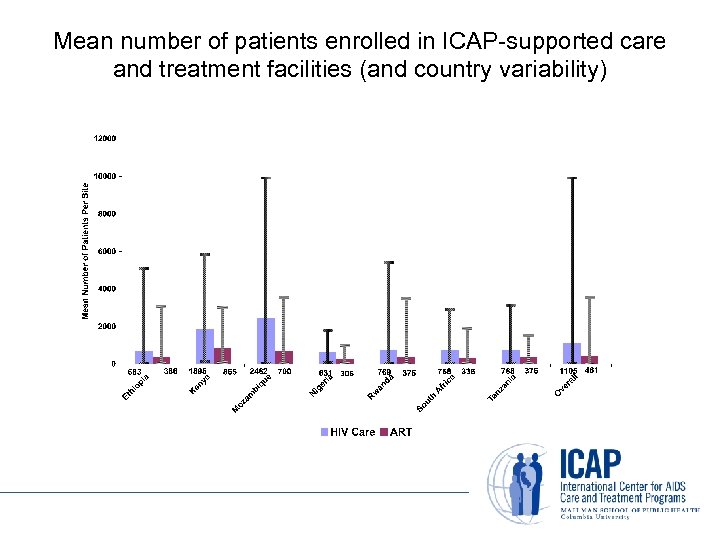 Mean number of patients enrolled in ICAP-supported care and treatment facilities (and country variability)
Mean number of patients enrolled in ICAP-supported care and treatment facilities (and country variability)
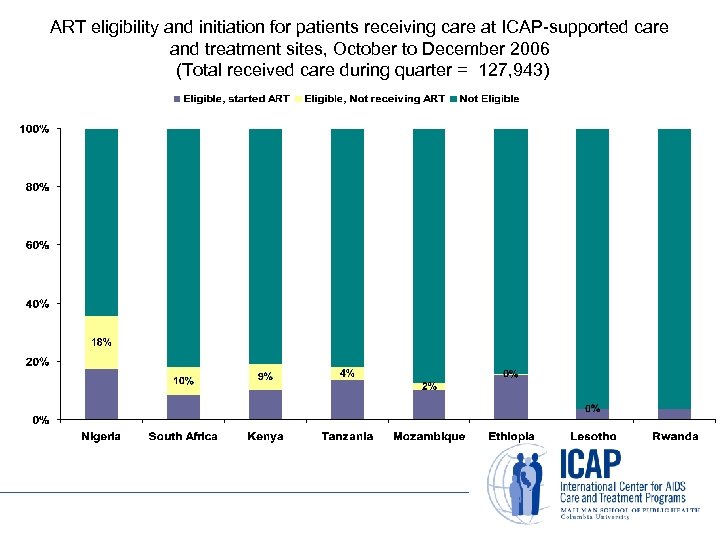 ART eligibility and initiation for patients receiving care at ICAP-supported care and treatment sites, October to December 2006 (Total received care during quarter = 127, 943)
ART eligibility and initiation for patients receiving care at ICAP-supported care and treatment sites, October to December 2006 (Total received care during quarter = 127, 943)
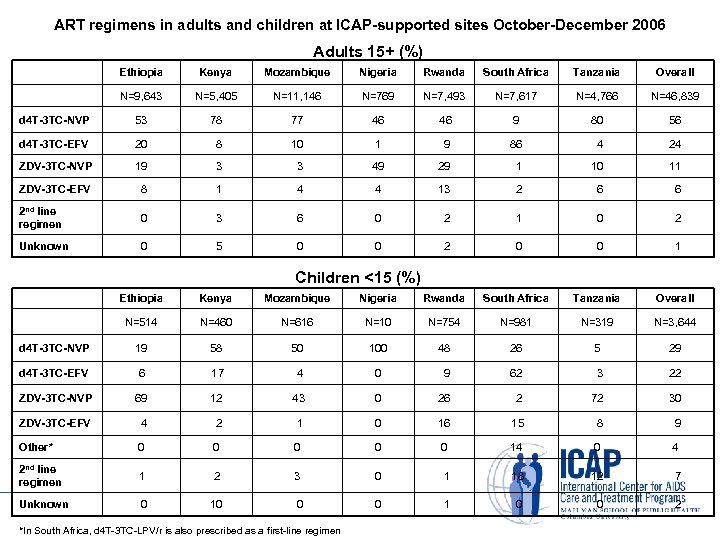 ART regimens in adults and children at ICAP-supported sites October-December 2006 Adults 15+ (%) Ethiopia Kenya Mozambique Nigeria Rwanda South Africa Tanzania Overall N=9, 643 N=5, 405 N=11, 146 N=769 N=7, 493 N=7, 617 N=4, 766 N=46, 839 d 4 T-3 TC-NVP 53 78 77 46 9 80 56 d 4 T-3 TC-EFV 20 8 10 1 9 86 4 24 ZDV-3 TC-NVP 19 3 3 49 29 1 10 11 ZDV-3 TC-EFV 8 1 4 4 13 2 6 6 2 nd line regimen 0 3 6 0 2 1 0 2 Unknown 0 5 0 0 2 0 0 1 Children <15 (%) Ethiopia Kenya Mozambique Nigeria Rwanda South Africa Tanzania Overall N=514 N=460 N=616 N=10 N=754 N=981 N=319 N=3, 644 d 4 T-3 TC-NVP 19 58 50 100 48 26 5 29 d 4 T-3 TC-EFV 6 17 4 0 9 62 3 22 ZDV-3 TC-NVP 69 12 43 0 26 2 72 30 ZDV-3 TC-EFV 4 2 1 0 16 15 8 9 Other* 0 0 0 14 0 4 2 nd line regimen 1 2 3 0 1 18 12 7 Unknown 0 10 0 0 1 0 0 2 *In South Africa, d 4 T-3 TC-LPV/r is also prescribed as a first-line regimen
ART regimens in adults and children at ICAP-supported sites October-December 2006 Adults 15+ (%) Ethiopia Kenya Mozambique Nigeria Rwanda South Africa Tanzania Overall N=9, 643 N=5, 405 N=11, 146 N=769 N=7, 493 N=7, 617 N=4, 766 N=46, 839 d 4 T-3 TC-NVP 53 78 77 46 9 80 56 d 4 T-3 TC-EFV 20 8 10 1 9 86 4 24 ZDV-3 TC-NVP 19 3 3 49 29 1 10 11 ZDV-3 TC-EFV 8 1 4 4 13 2 6 6 2 nd line regimen 0 3 6 0 2 1 0 2 Unknown 0 5 0 0 2 0 0 1 Children <15 (%) Ethiopia Kenya Mozambique Nigeria Rwanda South Africa Tanzania Overall N=514 N=460 N=616 N=10 N=754 N=981 N=319 N=3, 644 d 4 T-3 TC-NVP 19 58 50 100 48 26 5 29 d 4 T-3 TC-EFV 6 17 4 0 9 62 3 22 ZDV-3 TC-NVP 69 12 43 0 26 2 72 30 ZDV-3 TC-EFV 4 2 1 0 16 15 8 9 Other* 0 0 0 14 0 4 2 nd line regimen 1 2 3 0 1 18 12 7 Unknown 0 10 0 0 1 0 0 2 *In South Africa, d 4 T-3 TC-LPV/r is also prescribed as a first-line regimen
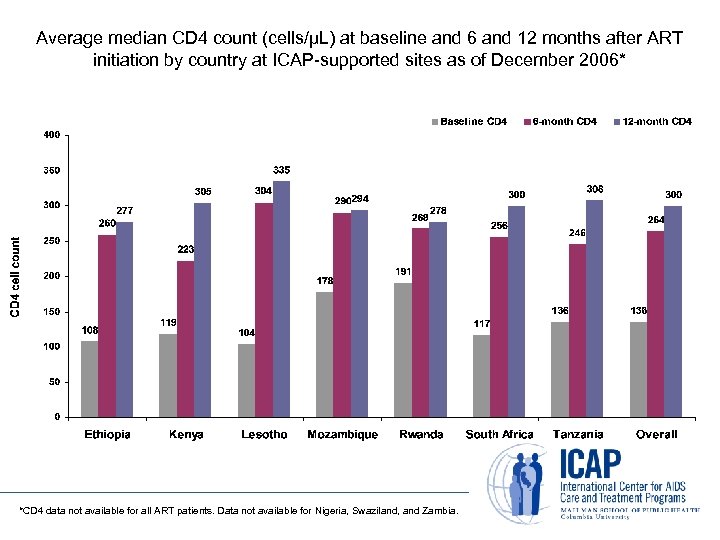 Average median CD 4 count (cells/µL) at baseline and 6 and 12 months after ART initiation by country at ICAP-supported sites as of December 2006* *CD 4 data not available for all ART patients. Data not available for Nigeria, Swaziland, and Zambia.
Average median CD 4 count (cells/µL) at baseline and 6 and 12 months after ART initiation by country at ICAP-supported sites as of December 2006* *CD 4 data not available for all ART patients. Data not available for Nigeria, Swaziland, and Zambia.
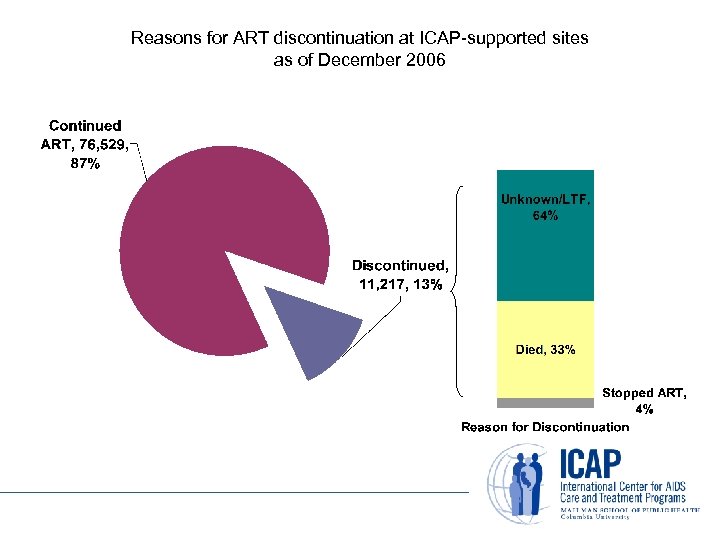 Reasons for ART discontinuation at ICAP-supported sites as of December 2006
Reasons for ART discontinuation at ICAP-supported sites as of December 2006
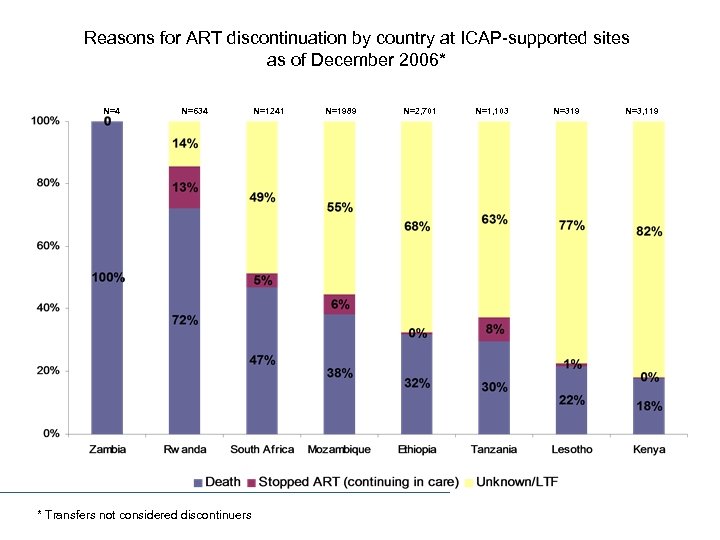 Reasons for ART discontinuation by country at ICAP-supported sites as of December 2006* N=4 N=634 * Transfers not considered discontinuers N=1241 N=1989 N=2, 701 N=1, 103 N=319 N=3, 119
Reasons for ART discontinuation by country at ICAP-supported sites as of December 2006* N=4 N=634 * Transfers not considered discontinuers N=1241 N=1989 N=2, 701 N=1, 103 N=319 N=3, 119
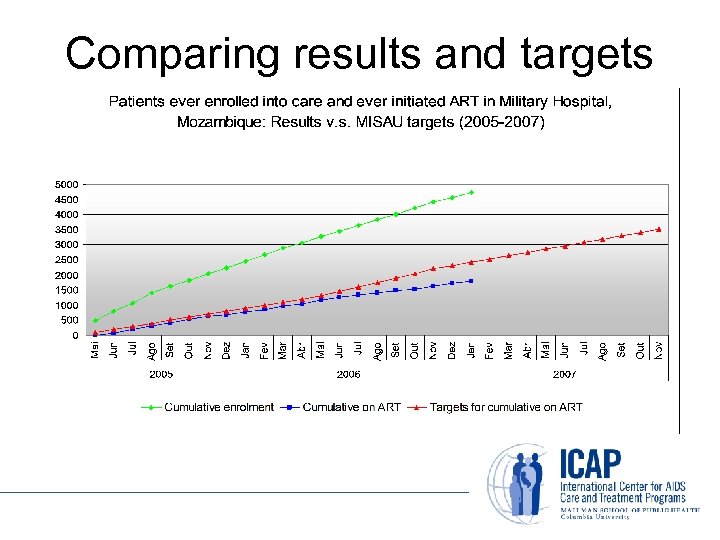 Comparing results and targets
Comparing results and targets
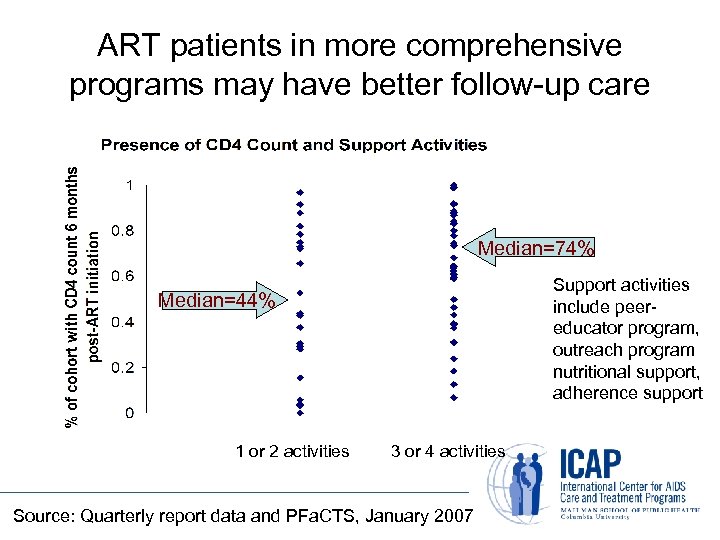 ART patients in more comprehensive programs may have better follow-up care Median=74% Support activities include peereducator program, outreach program nutritional support, adherence support Median=44% 1 or 2 activities 3 or 4 activities Source: Quarterly report data and PFa. CTS, January 2007
ART patients in more comprehensive programs may have better follow-up care Median=74% Support activities include peereducator program, outreach program nutritional support, adherence support Median=44% 1 or 2 activities 3 or 4 activities Source: Quarterly report data and PFa. CTS, January 2007
 PMTCT/PMTCT-plus
PMTCT/PMTCT-plus
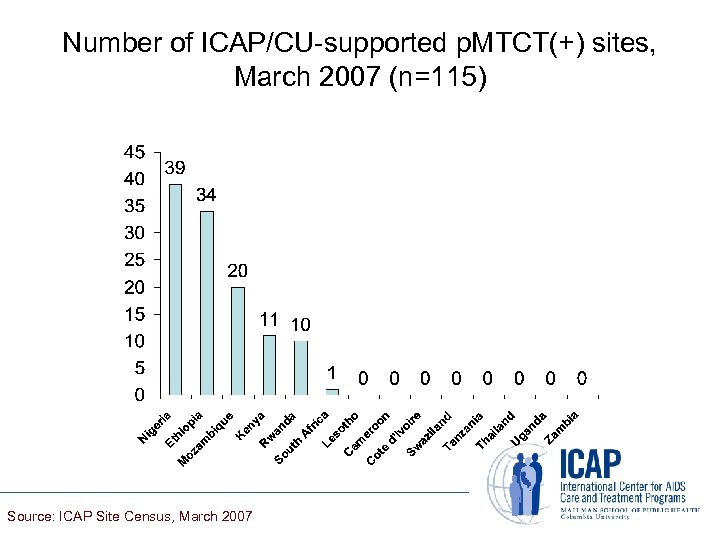 Number of ICAP/CU-supported p. MTCT(+) sites, March 2007 (n=115) Source: ICAP Site Census, March 2007
Number of ICAP/CU-supported p. MTCT(+) sites, March 2007 (n=115) Source: ICAP Site Census, March 2007
 PMTCT(+) Currently: 115 facilities in 5 countries • Developed list of 39 indicators (counseling and testing in ANC, prophylaxis to mother and infant, maternity, follow-up of exposed infant) • 95 of 115 sites in 5 countries reported 4 key indicators (Oct-Dec 2006) – Next round will include all 39 indicators • Expect close collaboration and communication btwn M&E and PMTCT program staff
PMTCT(+) Currently: 115 facilities in 5 countries • Developed list of 39 indicators (counseling and testing in ANC, prophylaxis to mother and infant, maternity, follow-up of exposed infant) • 95 of 115 sites in 5 countries reported 4 key indicators (Oct-Dec 2006) – Next round will include all 39 indicators • Expect close collaboration and communication btwn M&E and PMTCT program staff

 ICAP model • Family focused care • PMTCT is the entry point for HIV-infected women to access care and ART if eligible • ANC testing is the first step of the process – But need to go beyond that to ensure that: • women are accessing care and treatment and; • HIV-exposed infants receive follow-up
ICAP model • Family focused care • PMTCT is the entry point for HIV-infected women to access care and ART if eligible • ANC testing is the first step of the process – But need to go beyond that to ensure that: • women are accessing care and treatment and; • HIV-exposed infants receive follow-up
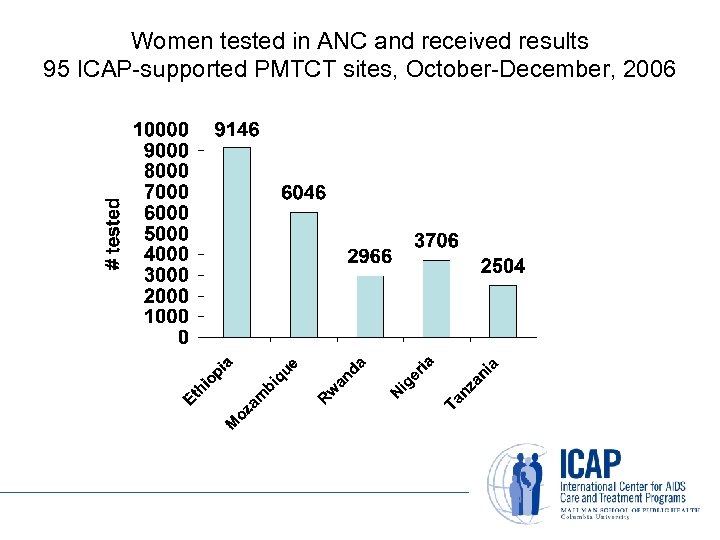 Women tested in ANC and received results 95 ICAP-supported PMTCT sites, October-December, 2006
Women tested in ANC and received results 95 ICAP-supported PMTCT sites, October-December, 2006
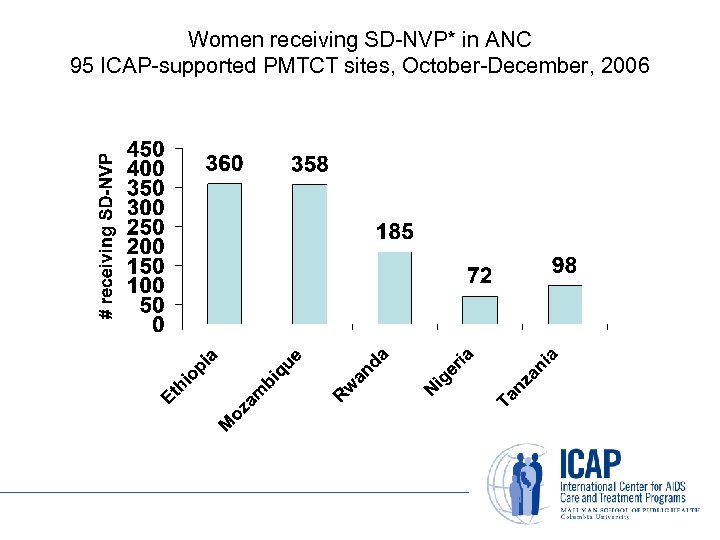 Women receiving SD-NVP* in ANC 95 ICAP-supported PMTCT sites, October-December, 2006
Women receiving SD-NVP* in ANC 95 ICAP-supported PMTCT sites, October-December, 2006
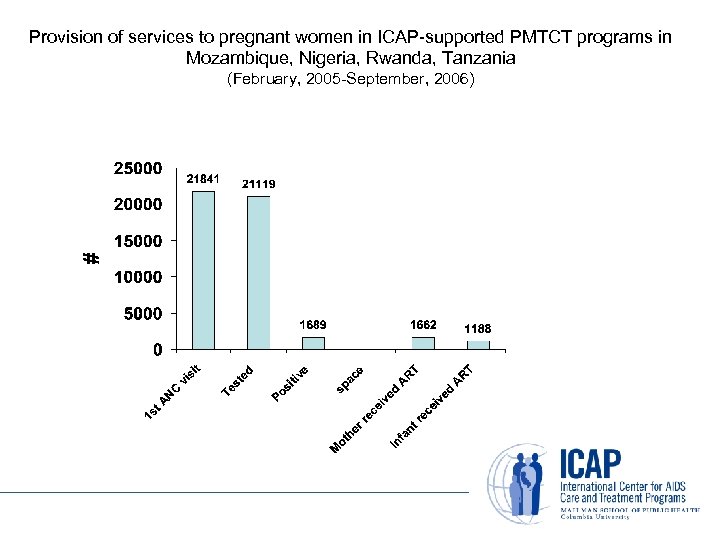 Provision of services to pregnant women in ICAP-supported PMTCT programs in Mozambique, Nigeria, Rwanda, Tanzania (February, 2005 -September, 2006)
Provision of services to pregnant women in ICAP-supported PMTCT programs in Mozambique, Nigeria, Rwanda, Tanzania (February, 2005 -September, 2006)
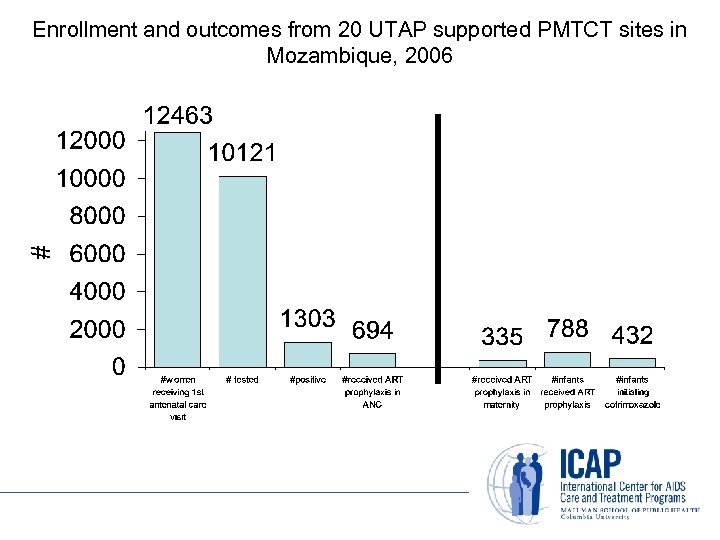 Enrollment and outcomes from 20 UTAP supported PMTCT sites in Mozambique, 2006
Enrollment and outcomes from 20 UTAP supported PMTCT sites in Mozambique, 2006
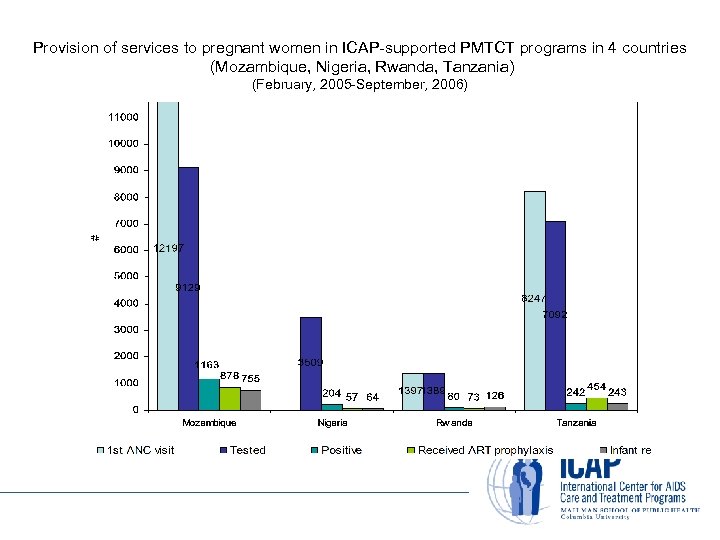 Provision of services to pregnant women in ICAP-supported PMTCT programs in 4 countries (Mozambique, Nigeria, Rwanda, Tanzania) (February, 2005 -September, 2006)
Provision of services to pregnant women in ICAP-supported PMTCT programs in 4 countries (Mozambique, Nigeria, Rwanda, Tanzania) (February, 2005 -September, 2006)
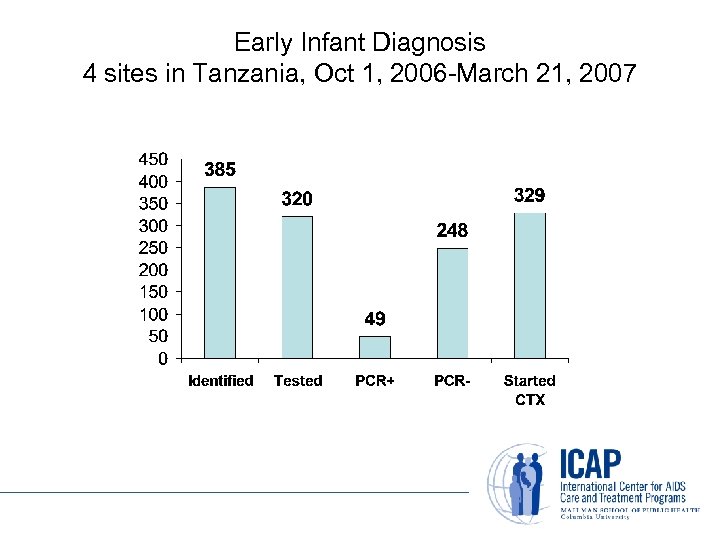 Early Infant Diagnosis 4 sites in Tanzania, Oct 1, 2006 -March 21, 2007
Early Infant Diagnosis 4 sites in Tanzania, Oct 1, 2006 -March 21, 2007
 Limitations of aggregate data • Cannot evaluate whether pregnant women are enrolled in care and treatment when eligible • Difficult to measure efficacy of PMTCT program without linking mother’s PMTCT and infant prophylaxis and infant outcome
Limitations of aggregate data • Cannot evaluate whether pregnant women are enrolled in care and treatment when eligible • Difficult to measure efficacy of PMTCT program without linking mother’s PMTCT and infant prophylaxis and infant outcome
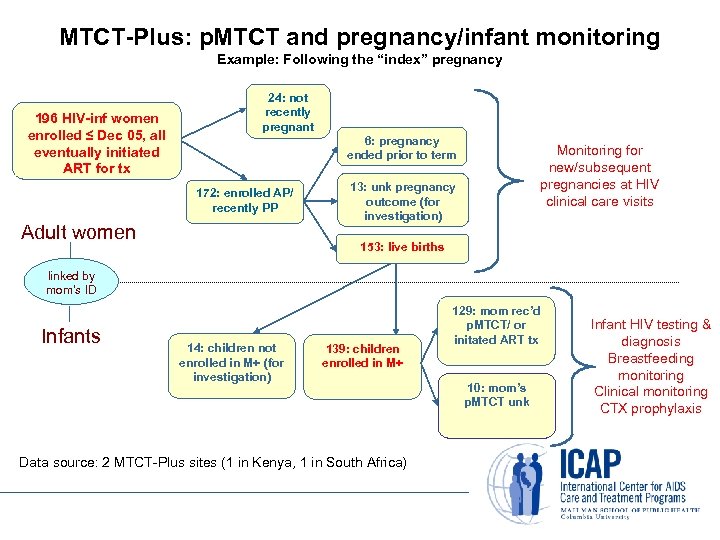 MTCT-Plus: p. MTCT and pregnancy/infant monitoring Example: Following the “index” pregnancy 196 HIV-inf women enrolled ≤ Dec 05, all eventually initiated ART for tx 24: not recently pregnant 6: pregnancy ended prior to term 172: enrolled AP/ recently PP Adult women Monitoring for new/subsequent pregnancies at HIV clinical care visits 13: unk pregnancy outcome (for investigation) 153: live births linked by mom’s ID Infants 14: children not enrolled in M+ (for investigation) 139: children enrolled in M+ Data source: 2 MTCT-Plus sites (1 in Kenya, 1 in South Africa) 129: mom rec’d p. MTCT/ or initated ART tx 10: mom’s p. MTCT unk Infant HIV testing & diagnosis Breastfeeding monitoring Clinical monitoring CTX prophylaxis
MTCT-Plus: p. MTCT and pregnancy/infant monitoring Example: Following the “index” pregnancy 196 HIV-inf women enrolled ≤ Dec 05, all eventually initiated ART for tx 24: not recently pregnant 6: pregnancy ended prior to term 172: enrolled AP/ recently PP Adult women Monitoring for new/subsequent pregnancies at HIV clinical care visits 13: unk pregnancy outcome (for investigation) 153: live births linked by mom’s ID Infants 14: children not enrolled in M+ (for investigation) 139: children enrolled in M+ Data source: 2 MTCT-Plus sites (1 in Kenya, 1 in South Africa) 129: mom rec’d p. MTCT/ or initated ART tx 10: mom’s p. MTCT unk Infant HIV testing & diagnosis Breastfeeding monitoring Clinical monitoring CTX prophylaxis
 Targeted Evaluations • Addresses “Why” • Helps identify barriers and facilitating factors • Provides details
Targeted Evaluations • Addresses “Why” • Helps identify barriers and facilitating factors • Provides details
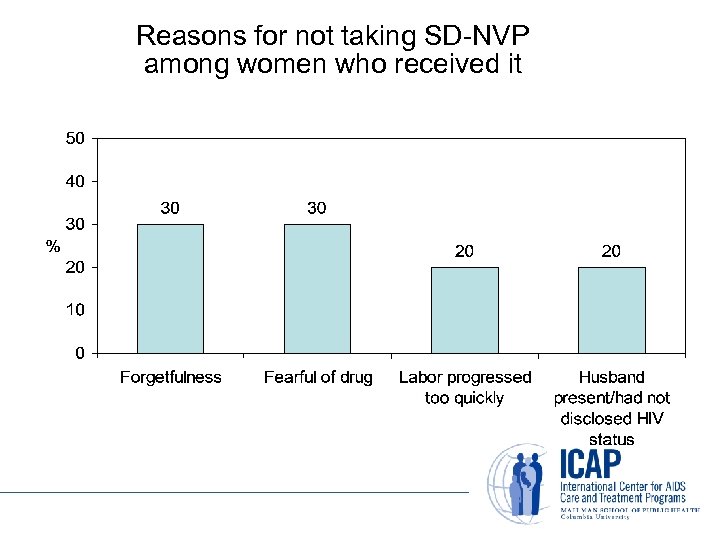 Reasons for not taking SD-NVP among women who received it
Reasons for not taking SD-NVP among women who received it
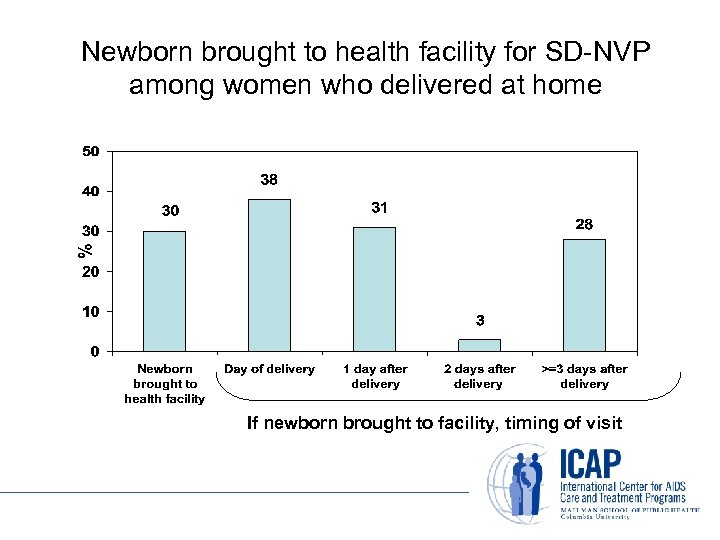 Newborn brought to health facility for SD-NVP among women who delivered at home If newborn brought to facility, timing of visit
Newborn brought to health facility for SD-NVP among women who delivered at home If newborn brought to facility, timing of visit
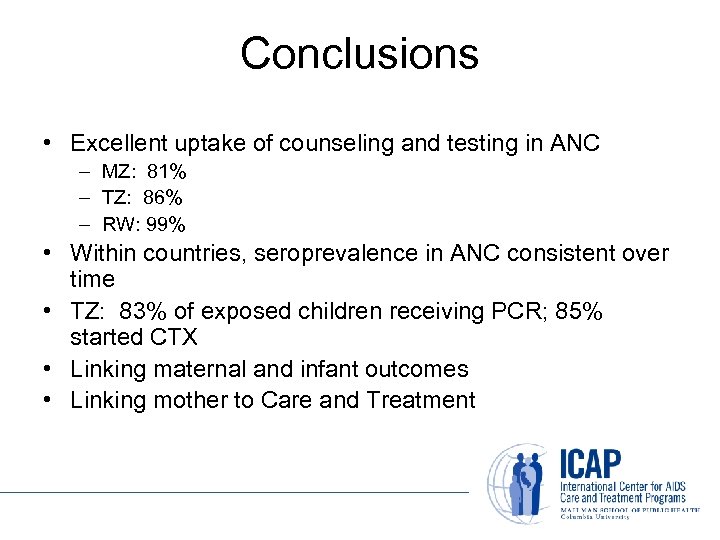 Conclusions • Excellent uptake of counseling and testing in ANC – MZ: 81% – TZ: 86% – RW: 99% • Within countries, seroprevalence in ANC consistent over time • TZ: 83% of exposed children receiving PCR; 85% started CTX • Linking maternal and infant outcomes • Linking mother to Care and Treatment
Conclusions • Excellent uptake of counseling and testing in ANC – MZ: 81% – TZ: 86% – RW: 99% • Within countries, seroprevalence in ANC consistent over time • TZ: 83% of exposed children receiving PCR; 85% started CTX • Linking maternal and infant outcomes • Linking mother to Care and Treatment
 TB/HIV
TB/HIV
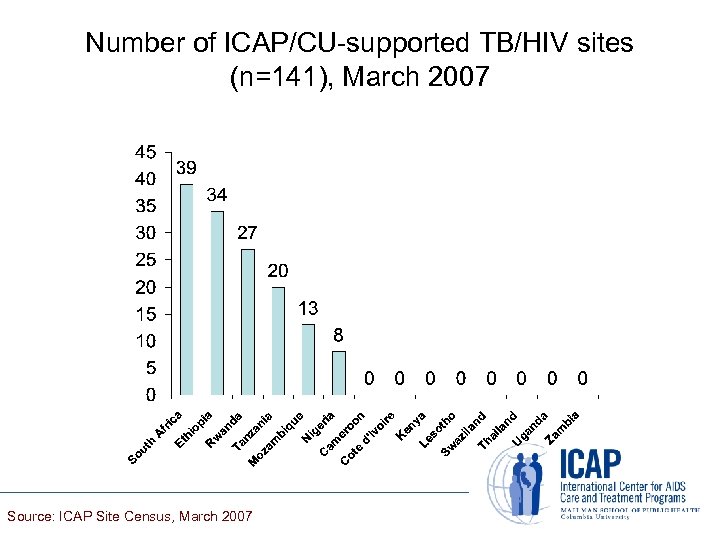 Number of ICAP/CU-supported TB/HIV sites (n=141), March 2007 Source: ICAP Site Census, March 2007
Number of ICAP/CU-supported TB/HIV sites (n=141), March 2007 Source: ICAP Site Census, March 2007
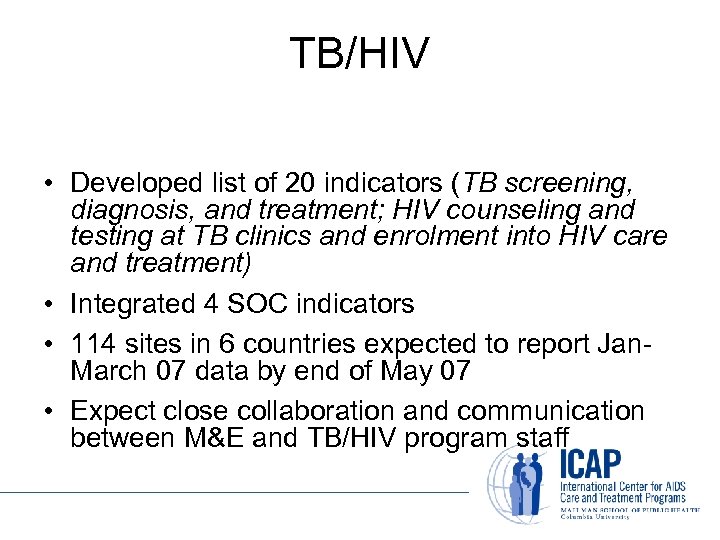 TB/HIV • Developed list of 20 indicators (TB screening, diagnosis, and treatment; HIV counseling and testing at TB clinics and enrolment into HIV care and treatment) • Integrated 4 SOC indicators • 114 sites in 6 countries expected to report Jan. March 07 data by end of May 07 • Expect close collaboration and communication between M&E and TB/HIV program staff
TB/HIV • Developed list of 20 indicators (TB screening, diagnosis, and treatment; HIV counseling and testing at TB clinics and enrolment into HIV care and treatment) • Integrated 4 SOC indicators • 114 sites in 6 countries expected to report Jan. March 07 data by end of May 07 • Expect close collaboration and communication between M&E and TB/HIV program staff
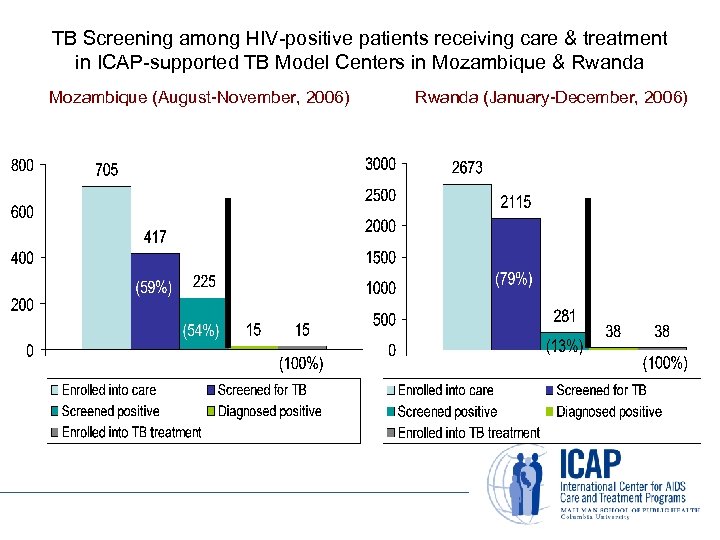 TB Screening among HIV-positive patients receiving care & treatment in ICAP-supported TB Model Centers in Mozambique & Rwanda Mozambique (August-November, 2006) Rwanda (January-December, 2006)
TB Screening among HIV-positive patients receiving care & treatment in ICAP-supported TB Model Centers in Mozambique & Rwanda Mozambique (August-November, 2006) Rwanda (January-December, 2006)
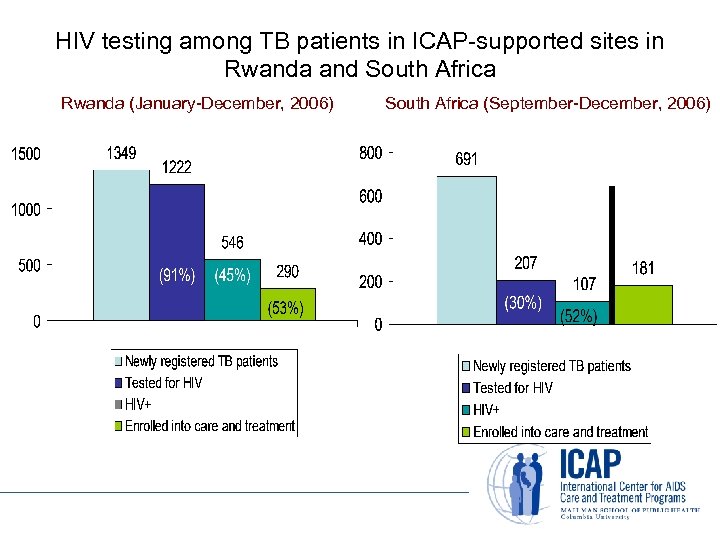 HIV testing among TB patients in ICAP-supported sites in Rwanda and South Africa Rwanda (January-December, 2006) South Africa (September-December, 2006)
HIV testing among TB patients in ICAP-supported sites in Rwanda and South Africa Rwanda (January-December, 2006) South Africa (September-December, 2006)
 Conclusions • Good implementation of TB screening in Rwanda and Mozambique (SOC target is over 90%) • HIV counseling and testing at TB clinics is efficient way to identify patients • Hard to capture linkages data TB clinic HIV clinic; HIV clinic TB clinic
Conclusions • Good implementation of TB screening in Rwanda and Mozambique (SOC target is over 90%) • HIV counseling and testing at TB clinics is efficient way to identify patients • Hard to capture linkages data TB clinic HIV clinic; HIV clinic TB clinic
 Patient-level data on care and treatment
Patient-level data on care and treatment
 What is meant by patient-level data? • Information collected for each patient at every clinic visit: * Clinical data * Laboratory results * Medication * Patient disposition/status
What is meant by patient-level data? • Information collected for each patient at every clinic visit: * Clinical data * Laboratory results * Medication * Patient disposition/status
 Purpose of collecting patient-level data with electronic databases • Consistent with MOH priorities • Improve quality of care (e. g. tracked missed visits, identify patients who have missed visits) • Automate routine reporting (e. g. reduce burden of MOH and other routine reporting) • Compare patient outcomes across sites to evaluate models of care
Purpose of collecting patient-level data with electronic databases • Consistent with MOH priorities • Improve quality of care (e. g. tracked missed visits, identify patients who have missed visits) • Automate routine reporting (e. g. reduce burden of MOH and other routine reporting) • Compare patient outcomes across sites to evaluate models of care

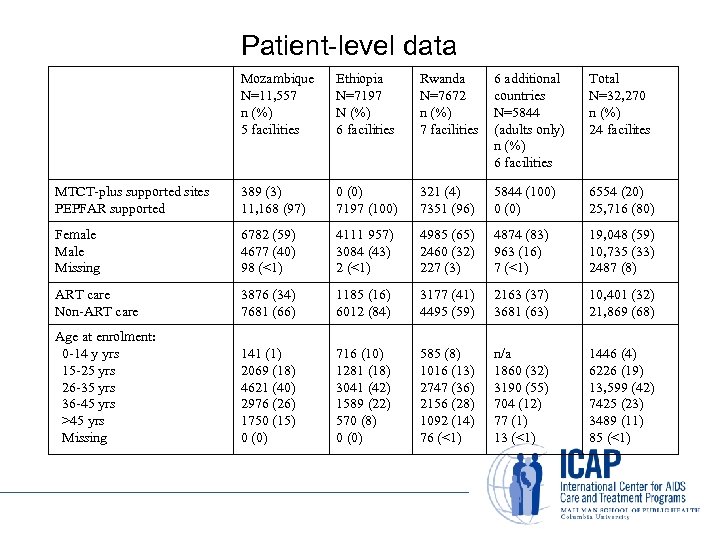 Patient-level data Mozambique N=11, 557 n (%) 5 facilities Ethiopia N=7197 N (%) 6 facilities Rwanda N=7672 n (%) 7 facilities 6 additional countries N=5844 (adults only) n (%) 6 facilities Total N=32, 270 n (%) 24 facilites MTCT-plus supported sites PEPFAR supported 389 (3) 11, 168 (97) 0 (0) 7197 (100) 321 (4) 7351 (96) 5844 (100) 0 (0) 6554 (20) 25, 716 (80) Female Missing 6782 (59) 4677 (40) 98 (<1) 4111 957) 3084 (43) 2 (<1) 4985 (65) 2460 (32) 227 (3) 4874 (83) 963 (16) 7 (<1) 19, 048 (59) 10, 735 (33) 2487 (8) ART care Non-ART care 3876 (34) 7681 (66) 1185 (16) 6012 (84) 3177 (41) 4495 (59) 2163 (37) 3681 (63) 10, 401 (32) 21, 869 (68) Age at enrolment: 0 -14 y yrs 15 -25 yrs 26 -35 yrs 36 -45 yrs >45 yrs Missing 141 (1) 2069 (18) 4621 (40) 2976 (26) 1750 (15) 0 (0) 716 (10) 1281 (18) 3041 (42) 1589 (22) 570 (8) 0 (0) 585 (8) 1016 (13) 2747 (36) 2156 (28) 1092 (14) 76 (<1) n/a 1860 (32) 3190 (55) 704 (12) 77 (1) 13 (<1) 1446 (4) 6226 (19) 13, 599 (42) 7425 (23) 3489 (11) 85 (<1)
Patient-level data Mozambique N=11, 557 n (%) 5 facilities Ethiopia N=7197 N (%) 6 facilities Rwanda N=7672 n (%) 7 facilities 6 additional countries N=5844 (adults only) n (%) 6 facilities Total N=32, 270 n (%) 24 facilites MTCT-plus supported sites PEPFAR supported 389 (3) 11, 168 (97) 0 (0) 7197 (100) 321 (4) 7351 (96) 5844 (100) 0 (0) 6554 (20) 25, 716 (80) Female Missing 6782 (59) 4677 (40) 98 (<1) 4111 957) 3084 (43) 2 (<1) 4985 (65) 2460 (32) 227 (3) 4874 (83) 963 (16) 7 (<1) 19, 048 (59) 10, 735 (33) 2487 (8) ART care Non-ART care 3876 (34) 7681 (66) 1185 (16) 6012 (84) 3177 (41) 4495 (59) 2163 (37) 3681 (63) 10, 401 (32) 21, 869 (68) Age at enrolment: 0 -14 y yrs 15 -25 yrs 26 -35 yrs 36 -45 yrs >45 yrs Missing 141 (1) 2069 (18) 4621 (40) 2976 (26) 1750 (15) 0 (0) 716 (10) 1281 (18) 3041 (42) 1589 (22) 570 (8) 0 (0) 585 (8) 1016 (13) 2747 (36) 2156 (28) 1092 (14) 76 (<1) n/a 1860 (32) 3190 (55) 704 (12) 77 (1) 13 (<1) 1446 (4) 6226 (19) 13, 599 (42) 7425 (23) 3489 (11) 85 (<1)
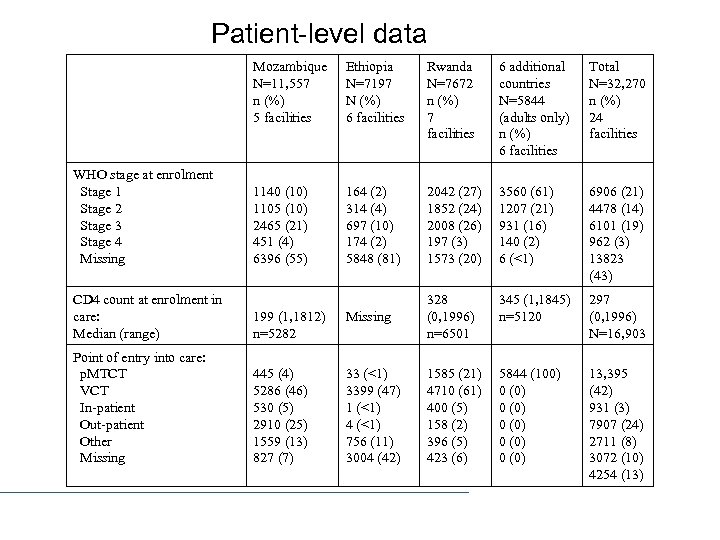 Patient-level data Mozambique N=11, 557 n (%) 5 facilities WHO stage at enrolment Stage 1 Stage 2 Stage 3 Stage 4 Missing Ethiopia N=7197 N (%) 6 facilities Rwanda N=7672 n (%) 7 facilities 6 additional countries N=5844 (adults only) n (%) 6 facilities Total N=32, 270 n (%) 24 facilities 1140 (10) 1105 (10) 2465 (21) 451 (4) 6396 (55) 164 (2) 314 (4) 697 (10) 174 (2) 5848 (81) 2042 (27) 1852 (24) 2008 (26) 197 (3) 1573 (20) 3560 (61) 1207 (21) 931 (16) 140 (2) 6 (<1) 6906 (21) 4478 (14) 6101 (19) 962 (3) 13823 (43) 345 (1, 1845) n=5120 297 (0, 1996) N=16, 903 5844 (100) 0 (0) 0 (0) 13, 395 (42) 931 (3) 7907 (24) 2711 (8) 3072 (10) 4254 (13) CD 4 count at enrolment in care: Median (range) 199 (1, 1812) n=5282 Missing 328 (0, 1996) n=6501 Point of entry into care: p. MTCT VCT In-patient Out-patient Other Missing 445 (4) 5286 (46) 530 (5) 2910 (25) 1559 (13) 827 (7) 33 (<1) 3399 (47) 1 (<1) 4 (<1) 756 (11) 3004 (42) 1585 (21) 4710 (61) 400 (5) 158 (2) 396 (5) 423 (6)
Patient-level data Mozambique N=11, 557 n (%) 5 facilities WHO stage at enrolment Stage 1 Stage 2 Stage 3 Stage 4 Missing Ethiopia N=7197 N (%) 6 facilities Rwanda N=7672 n (%) 7 facilities 6 additional countries N=5844 (adults only) n (%) 6 facilities Total N=32, 270 n (%) 24 facilities 1140 (10) 1105 (10) 2465 (21) 451 (4) 6396 (55) 164 (2) 314 (4) 697 (10) 174 (2) 5848 (81) 2042 (27) 1852 (24) 2008 (26) 197 (3) 1573 (20) 3560 (61) 1207 (21) 931 (16) 140 (2) 6 (<1) 6906 (21) 4478 (14) 6101 (19) 962 (3) 13823 (43) 345 (1, 1845) n=5120 297 (0, 1996) N=16, 903 5844 (100) 0 (0) 0 (0) 13, 395 (42) 931 (3) 7907 (24) 2711 (8) 3072 (10) 4254 (13) CD 4 count at enrolment in care: Median (range) 199 (1, 1812) n=5282 Missing 328 (0, 1996) n=6501 Point of entry into care: p. MTCT VCT In-patient Out-patient Other Missing 445 (4) 5286 (46) 530 (5) 2910 (25) 1559 (13) 827 (7) 33 (<1) 3399 (47) 1 (<1) 4 (<1) 756 (11) 3004 (42) 1585 (21) 4710 (61) 400 (5) 158 (2) 396 (5) 423 (6)
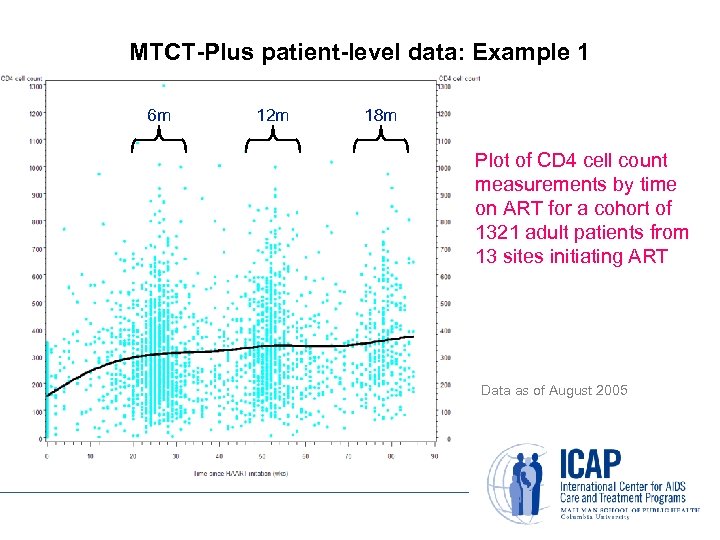 MTCT-Plus patient-level data: Example 1 6 m 12 m 18 m Plot of CD 4 cell count measurements by time on ART for a cohort of 1321 adult patients from 13 sites initiating ART Data as of August 2005
MTCT-Plus patient-level data: Example 1 6 m 12 m 18 m Plot of CD 4 cell count measurements by time on ART for a cohort of 1321 adult patients from 13 sites initiating ART Data as of August 2005
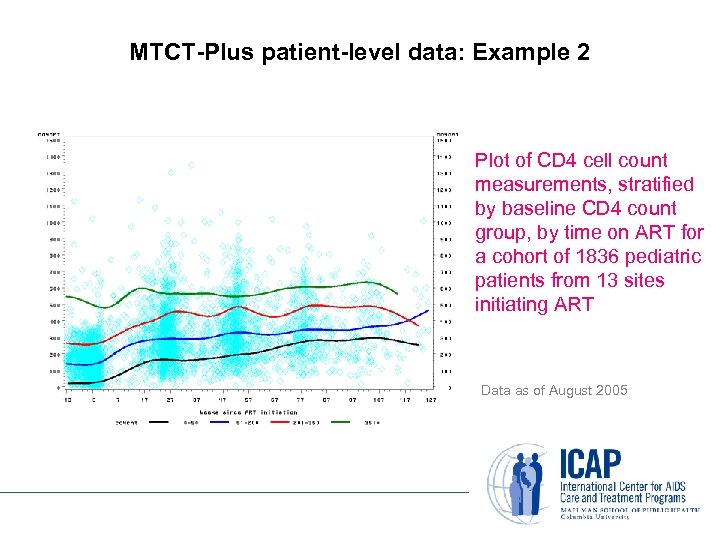 MTCT-Plus patient-level data: Example 2 Plot of CD 4 cell count measurements, stratified by baseline CD 4 count group, by time on ART for a cohort of 1836 pediatric patients from 13 sites initiating ART Data as of August 2005
MTCT-Plus patient-level data: Example 2 Plot of CD 4 cell count measurements, stratified by baseline CD 4 count group, by time on ART for a cohort of 1836 pediatric patients from 13 sites initiating ART Data as of August 2005
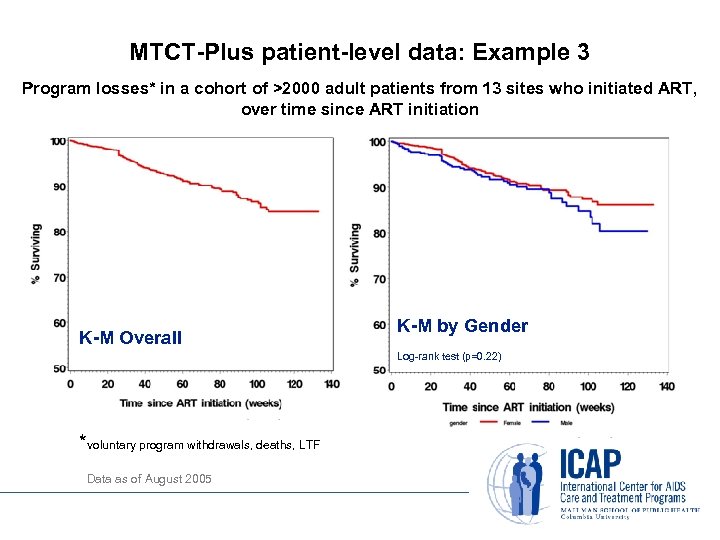 MTCT-Plus patient-level data: Example 3 Program losses* in a cohort of >2000 adult patients from 13 sites who initiated ART, over time since ART initiation K-M Overall K-M by Gender *voluntary program withdrawals, deaths, LTF Data as of August 2005 K-M by Gender Log-rank test (p=0. 22)
MTCT-Plus patient-level data: Example 3 Program losses* in a cohort of >2000 adult patients from 13 sites who initiated ART, over time since ART initiation K-M Overall K-M by Gender *voluntary program withdrawals, deaths, LTF Data as of August 2005 K-M by Gender Log-rank test (p=0. 22)
 Future patient-level data analyses • Measure outcomes (e. g. survival, change in CD 4 count) of patients in more ICAPsupported care and treatment programs • Identify facility and program-level characteristics that independently influence patient outcomes
Future patient-level data analyses • Measure outcomes (e. g. survival, change in CD 4 count) of patients in more ICAPsupported care and treatment programs • Identify facility and program-level characteristics that independently influence patient outcomes
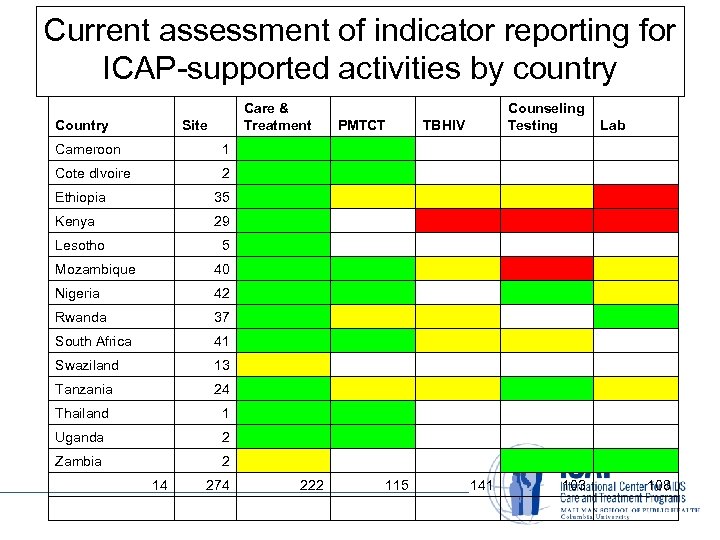 Current assessment of indicator reporting for ICAP-supported activities by country Care & Treatment Site PMTCT TBHIV Counseling Testing Lab Cameroon 1 Cote d. Ivoire 2 Ethiopia 35 Kenya 29 5 Mozambique 40 Nigeria 42 Rwanda 37 South Africa 41 Swaziland 13 Tanzania 24 Thailand 1 Uganda 2 Zambia 2 Lesotho 14 274 222 115 141 103 108
Current assessment of indicator reporting for ICAP-supported activities by country Care & Treatment Site PMTCT TBHIV Counseling Testing Lab Cameroon 1 Cote d. Ivoire 2 Ethiopia 35 Kenya 29 5 Mozambique 40 Nigeria 42 Rwanda 37 South Africa 41 Swaziland 13 Tanzania 24 Thailand 1 Uganda 2 Zambia 2 Lesotho 14 274 222 115 141 103 108
 Key messages and recommendations
Key messages and recommendations
 Discussion • Implications for country work plans • Ideas for future format for quarterly data meeting • How better we can feedback data to country offices? – Currently indicator tables, graphs, line listings – Dataset of indicators for local analysis – PFa. CTS summary tables, linelistings, and datasets • Future: – Online canned and ad hoc reports from URS – Combine info from all URS modules – Online mapping capabilities from URS – Country profiles (annual, semiannual)? – In-country quarterly data reviews? – Other formats/fora?
Discussion • Implications for country work plans • Ideas for future format for quarterly data meeting • How better we can feedback data to country offices? – Currently indicator tables, graphs, line listings – Dataset of indicators for local analysis – PFa. CTS summary tables, linelistings, and datasets • Future: – Online canned and ad hoc reports from URS – Combine info from all URS modules – Online mapping capabilities from URS – Country profiles (annual, semiannual)? – In-country quarterly data reviews? – Other formats/fora?
 Where to find MER outputs • P/MER Public • Eventually on web
Where to find MER outputs • P/MER Public • Eventually on web


