a4c660c752a38fb5220b3231158031ef.ppt
- Количество слайдов: 23
 Organisms in Space Greg Leonard and Darren Hughes Mains Associates 1
Organisms in Space Greg Leonard and Darren Hughes Mains Associates 1
 History - Dogs in Space A Russian stray dog named Laika was the first biological specimen to orbit the Earth. She flew aboard the Sputnik 2 spacecraft launched on November 3, 1959. Laika became an international celebrity, with several countries, from Romania to Mongolia, celebrating the event with commemorative stamps. 2
History - Dogs in Space A Russian stray dog named Laika was the first biological specimen to orbit the Earth. She flew aboard the Sputnik 2 spacecraft launched on November 3, 1959. Laika became an international celebrity, with several countries, from Romania to Mongolia, celebrating the event with commemorative stamps. 2
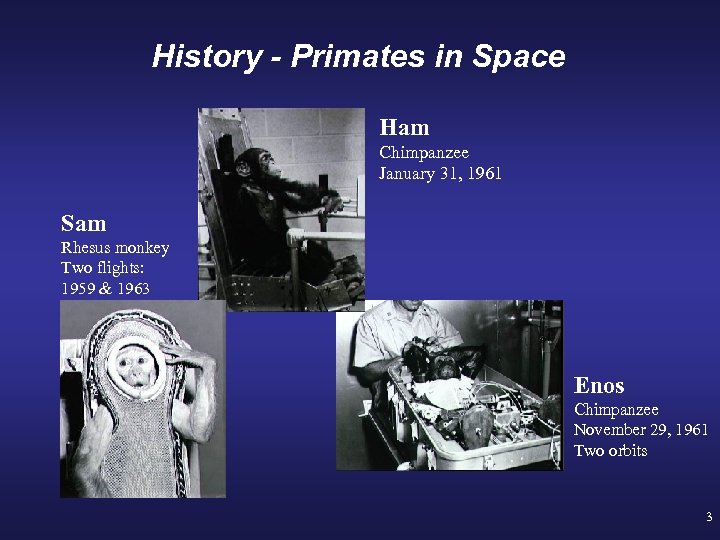 History - Primates in Space Ham Chimpanzee January 31, 1961 Sam Rhesus monkey Two flights: 1959 & 1963 Enos Chimpanzee November 29, 1961 Two orbits 3
History - Primates in Space Ham Chimpanzee January 31, 1961 Sam Rhesus monkey Two flights: 1959 & 1963 Enos Chimpanzee November 29, 1961 Two orbits 3
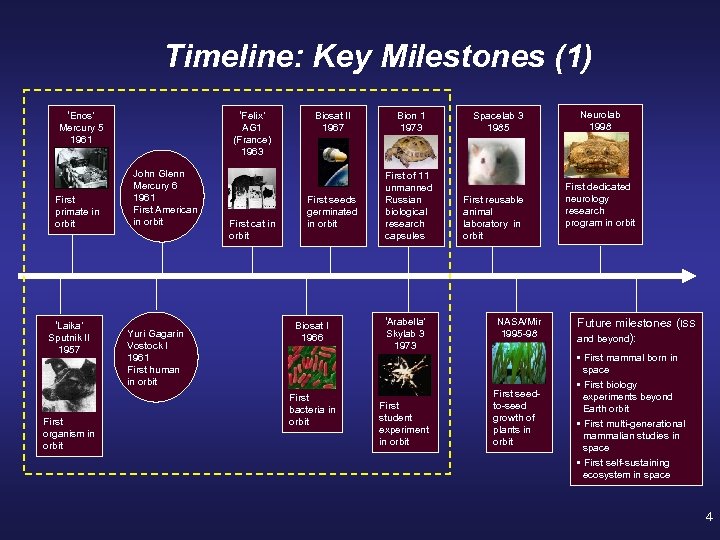 Timeline: Key Milestones (1) ‘Enos’ Mercury 5 1961 First primate in orbit ‘Laika’ Sputnik II 1957 First organism in orbit ‘Felix’ AG 1 (France) 1963 John Glenn Mercury 6 1961 First American in orbit Yuri Gagarin Vostock I 1961 First human in orbit First cat in orbit Biosat II 1967 First seeds germinated in orbit Biosat I 1966 First bacteria in orbit Bion 1 1973 First of 11 unmanned Russian biological research capsules ‘Arabella’ Skylab 3 1973 First student experiment in orbit Spacelab 3 1985 First reusable animal laboratory in orbit NASA/Mir 1995 -98 First seedto-seed growth of plants in orbit Neurolab 1998 First dedicated neurology research program in orbit Future milestones (ISS and beyond): • First mammal born in space • First biology experiments beyond Earth orbit • First multi-generational mammalian studies in space • First self-sustaining ecosystem in space 4
Timeline: Key Milestones (1) ‘Enos’ Mercury 5 1961 First primate in orbit ‘Laika’ Sputnik II 1957 First organism in orbit ‘Felix’ AG 1 (France) 1963 John Glenn Mercury 6 1961 First American in orbit Yuri Gagarin Vostock I 1961 First human in orbit First cat in orbit Biosat II 1967 First seeds germinated in orbit Biosat I 1966 First bacteria in orbit Bion 1 1973 First of 11 unmanned Russian biological research capsules ‘Arabella’ Skylab 3 1973 First student experiment in orbit Spacelab 3 1985 First reusable animal laboratory in orbit NASA/Mir 1995 -98 First seedto-seed growth of plants in orbit Neurolab 1998 First dedicated neurology research program in orbit Future milestones (ISS and beyond): • First mammal born in space • First biology experiments beyond Earth orbit • First multi-generational mammalian studies in space • First self-sustaining ecosystem in space 4
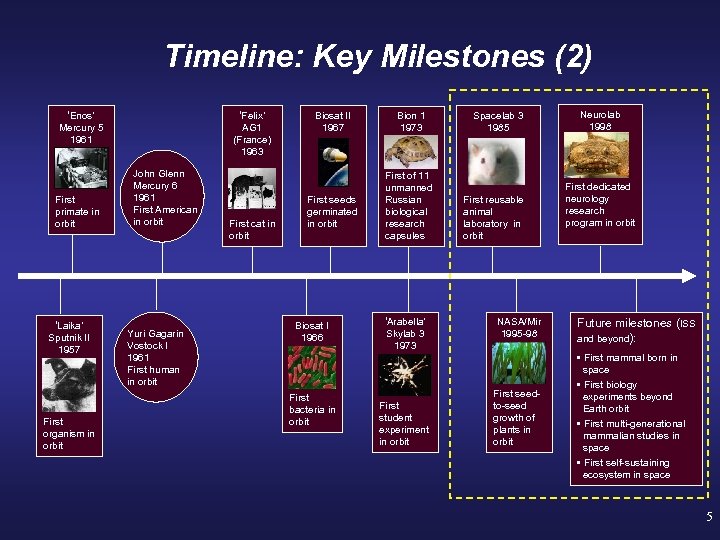 Timeline: Key Milestones (2) ‘Enos’ Mercury 5 1961 First primate in orbit ‘Laika’ Sputnik II 1957 First organism in orbit ‘Felix’ AG 1 (France) 1963 John Glenn Mercury 6 1961 First American in orbit Yuri Gagarin Vostock I 1961 First human in orbit First cat in orbit Biosat II 1967 First seeds germinated in orbit Biosat I 1966 First bacteria in orbit Bion 1 1973 First of 11 unmanned Russian biological research capsules ‘Arabella’ Skylab 3 1973 First student experiment in orbit Spacelab 3 1985 First reusable animal laboratory in orbit NASA/Mir 1995 -98 First seedto-seed growth of plants in orbit Neurolab 1998 First dedicated neurology research program in orbit Future milestones (ISS and beyond): • First mammal born in space • First biology experiments beyond Earth orbit • First multi-generational mammalian studies in space • First self-sustaining ecosystem in space 5
Timeline: Key Milestones (2) ‘Enos’ Mercury 5 1961 First primate in orbit ‘Laika’ Sputnik II 1957 First organism in orbit ‘Felix’ AG 1 (France) 1963 John Glenn Mercury 6 1961 First American in orbit Yuri Gagarin Vostock I 1961 First human in orbit First cat in orbit Biosat II 1967 First seeds germinated in orbit Biosat I 1966 First bacteria in orbit Bion 1 1973 First of 11 unmanned Russian biological research capsules ‘Arabella’ Skylab 3 1973 First student experiment in orbit Spacelab 3 1985 First reusable animal laboratory in orbit NASA/Mir 1995 -98 First seedto-seed growth of plants in orbit Neurolab 1998 First dedicated neurology research program in orbit Future milestones (ISS and beyond): • First mammal born in space • First biology experiments beyond Earth orbit • First multi-generational mammalian studies in space • First self-sustaining ecosystem in space 5
 Some Organisms Studied in Space Bacteria Aeromonas proteolytica Bacillus mycoides Bacillus subtilis Bacillus thuringiensis Burkholderia cepacia Chaetomium globosum Deinococcus radiodurans Escherichia coli Nematospiroides dubius Rhodotorula rubra Salmonella typhimurium Trichophyton terrestre Invertebrates Acheta domesticus (Cricket) Araneus diadematus (Spider) Biomphalaria glabrata (Snail) Caenorhabditis elegans (Nematode) Cynops pyrrhogaster (Newt) Drosophila melanogaster (Fruit fly) Habrobracon juglandis (Wasp) Manduca sexta (Tobacco hornworm) Pelomyxa carolinensis (Amoeba) Pothetria dispar (Gypsy moth) Tribolium confusum (Beetle) Trigonoscelis gigas (Beetle) Plants Aesculus hippocastanum L. (Horse chestnut) Arabidopsis thaliana (Thale cress) Avena sativa (Oat) Brassica rapa (Field mustard) Capsicum annuum (Ornamental pepper) Ceratodon (Moss) Ceratopteris (Fern) Ceratophyllum demersum (Hornweed) Cucumis sativus (Cucumber) Dactylis glomerata L. (Orchard grass) Daucus carota (Carrot) Digitalis lanata (Foxglove) Digitalis purpurea L. (Foxglove) Elodea (Waterweed) Flammulina velutipes, Agaricales (Fungus) Glycine max (Soybean) Haplopappus gracilis (Haplopappus) Helianthus annuus L. (Sunflower) Hemerocallis (Daylily) Lepidium sativum (Garden cress) Linum usitatissimum (Flax) Lycoperscion esculentum (Tomato) Neurospora crassa (Fungus) Nicotiana tabacum (Tobacco) Oryza sativa (Rice) Physarum polycephalum (Slime mold) Pseudotsuga menziesii (Douglas fir) Pseudotsuga taeda (Loblolly pine) Saccharomyces cerevisiae (Yeast) Tradescantia (Spiderwort) Triticum aestivum (Wheat) Triticum vulgare (Wheat) Vigna radiata (Mung bean) Zea mays (Corn) Vertebrates Canis familiaris (Dog) Felix maniculata (Cat) Homo sapiens (Human) Macaca mulatta (Rhesus monkey) Macaca nemestrina (Pigtail macaque monkey) Mus musculus (Mouse) Oryctolagus cuniculus (Rabbit) Pan troglodytes (Chimpanzee) Perognathus longimembris (Pocket mouse) Rattus norvegicus (Rat) Saimiri sciureus (Squirrel monkey) Testudo horsfieldi Gray (Tortoise) Birds Coturnix coturnix (Quail) Gallus gallus (Chicken) Aquatic species Arbacia punctulata (Sea urchin) Aurelia aurita (Jellyfish) Fundulus heteroclitus (Killifish) Lytechinus pictus (Sea urchin) Opsanus tau (Toadfish) Oreochromis mossambicus (Cichlid fish) Oryzias latipes (Medaka fish) Rana catesbeiana (Bullfrog) Rana pipiens (Frog) Strongelocentrotus pupuratus (Sea urchin) Xenopus laevis (Frog) Xenopus laevis Daudin (South African toad) Xiphophorus helleri (Swordtail fish) 6
Some Organisms Studied in Space Bacteria Aeromonas proteolytica Bacillus mycoides Bacillus subtilis Bacillus thuringiensis Burkholderia cepacia Chaetomium globosum Deinococcus radiodurans Escherichia coli Nematospiroides dubius Rhodotorula rubra Salmonella typhimurium Trichophyton terrestre Invertebrates Acheta domesticus (Cricket) Araneus diadematus (Spider) Biomphalaria glabrata (Snail) Caenorhabditis elegans (Nematode) Cynops pyrrhogaster (Newt) Drosophila melanogaster (Fruit fly) Habrobracon juglandis (Wasp) Manduca sexta (Tobacco hornworm) Pelomyxa carolinensis (Amoeba) Pothetria dispar (Gypsy moth) Tribolium confusum (Beetle) Trigonoscelis gigas (Beetle) Plants Aesculus hippocastanum L. (Horse chestnut) Arabidopsis thaliana (Thale cress) Avena sativa (Oat) Brassica rapa (Field mustard) Capsicum annuum (Ornamental pepper) Ceratodon (Moss) Ceratopteris (Fern) Ceratophyllum demersum (Hornweed) Cucumis sativus (Cucumber) Dactylis glomerata L. (Orchard grass) Daucus carota (Carrot) Digitalis lanata (Foxglove) Digitalis purpurea L. (Foxglove) Elodea (Waterweed) Flammulina velutipes, Agaricales (Fungus) Glycine max (Soybean) Haplopappus gracilis (Haplopappus) Helianthus annuus L. (Sunflower) Hemerocallis (Daylily) Lepidium sativum (Garden cress) Linum usitatissimum (Flax) Lycoperscion esculentum (Tomato) Neurospora crassa (Fungus) Nicotiana tabacum (Tobacco) Oryza sativa (Rice) Physarum polycephalum (Slime mold) Pseudotsuga menziesii (Douglas fir) Pseudotsuga taeda (Loblolly pine) Saccharomyces cerevisiae (Yeast) Tradescantia (Spiderwort) Triticum aestivum (Wheat) Triticum vulgare (Wheat) Vigna radiata (Mung bean) Zea mays (Corn) Vertebrates Canis familiaris (Dog) Felix maniculata (Cat) Homo sapiens (Human) Macaca mulatta (Rhesus monkey) Macaca nemestrina (Pigtail macaque monkey) Mus musculus (Mouse) Oryctolagus cuniculus (Rabbit) Pan troglodytes (Chimpanzee) Perognathus longimembris (Pocket mouse) Rattus norvegicus (Rat) Saimiri sciureus (Squirrel monkey) Testudo horsfieldi Gray (Tortoise) Birds Coturnix coturnix (Quail) Gallus gallus (Chicken) Aquatic species Arbacia punctulata (Sea urchin) Aurelia aurita (Jellyfish) Fundulus heteroclitus (Killifish) Lytechinus pictus (Sea urchin) Opsanus tau (Toadfish) Oreochromis mossambicus (Cichlid fish) Oryzias latipes (Medaka fish) Rana catesbeiana (Bullfrog) Rana pipiens (Frog) Strongelocentrotus pupuratus (Sea urchin) Xenopus laevis (Frog) Xenopus laevis Daudin (South African toad) Xiphophorus helleri (Swordtail fish) 6
 Benefits of Studying Different Organisms Benefits to Space Exploration • Risk mitigation • Medical care • Life support Benefits to Life on Earth • Biology • Medicine • Technology • Education 7
Benefits of Studying Different Organisms Benefits to Space Exploration • Risk mitigation • Medical care • Life support Benefits to Life on Earth • Biology • Medicine • Technology • Education 7
 Human Studies in Space • Bone Deterioration • Muscle Atrophy • Cardiovascular Deconditioning • Immune Suppression • Sleep Disturbances • Balance Disorders 8
Human Studies in Space • Bone Deterioration • Muscle Atrophy • Cardiovascular Deconditioning • Immune Suppression • Sleep Disturbances • Balance Disorders 8
 Why Not Just Study Humans in Space? Primary Reasons: • Ethical • Practical • Biological • Medical • Logistical 9
Why Not Just Study Humans in Space? Primary Reasons: • Ethical • Practical • Biological • Medical • Logistical 9
 Why Study Microbes in Space? • Basic research • Bioregenerative life support • Nanotechnology 10
Why Study Microbes in Space? • Basic research • Bioregenerative life support • Nanotechnology 10
 Why Grow Plants in Space? • Basic research • Food source • Remove CO 2 • Produce O 2 & water vapor • Psychological benefits 11
Why Grow Plants in Space? • Basic research • Food source • Remove CO 2 • Produce O 2 & water vapor • Psychological benefits 11
 Current and Future Directions • Focus on cell and molecular biology • Understanding of underlying mechanisms • Use of “model” organisms • Reference studies 12
Current and Future Directions • Focus on cell and molecular biology • Understanding of underlying mechanisms • Use of “model” organisms • Reference studies 12
 Model Organisms • Well-characterized • Genetically sequenced • Appropriate for space research 13
Model Organisms • Well-characterized • Genetically sequenced • Appropriate for space research 13
 Escherichia coli (Bacteria) Bacteria (E. coli) 0. 5 -1. 5µm Growth Requirements Microfluidics Liquid culture likely for flight, can be grown on solid medium Sensors p. H, oxygen, carbon dioxide, temperature Temperature Will grow between 10 - 45°C; Optimum 37°C Salinity Tolerates low to moderate salinity Nutrients LB for bacteria: yeast extract, bacto-peptone, sodium chloride, water p. H Growth optimum between p. H 5. 5 - 8. 0 Doubling rate 20 minutes to several hours Light Not required Aeration E. coli is facultative anaerobe, i. e. does not require O 2 but grows better in its presence Wastes Gaseous (CO 2) and liquid (metabolites) 14
Escherichia coli (Bacteria) Bacteria (E. coli) 0. 5 -1. 5µm Growth Requirements Microfluidics Liquid culture likely for flight, can be grown on solid medium Sensors p. H, oxygen, carbon dioxide, temperature Temperature Will grow between 10 - 45°C; Optimum 37°C Salinity Tolerates low to moderate salinity Nutrients LB for bacteria: yeast extract, bacto-peptone, sodium chloride, water p. H Growth optimum between p. H 5. 5 - 8. 0 Doubling rate 20 minutes to several hours Light Not required Aeration E. coli is facultative anaerobe, i. e. does not require O 2 but grows better in its presence Wastes Gaseous (CO 2) and liquid (metabolites) 14
 Saccharomyces cerevisiae (Yeast) Yeast (S. cer. ) 5 -12µm Growth Requirements Microfluidics Liquid culture likely for flight, can be grown on solid medium Sensors p. H, oxygen, carbon dioxide, temperature, pressure Temperature Will grow at temperatures between 3 - 40°C; Optimum 28°C Salinity Yeast grows within a wide range of salt concentration Nutrients YPD : yeast extract, bacto-peptone, glucose, water p. H Growth optimum between 5. 0 - 6. 5 Doubling rate 1 -4 hours Light Not required Aeration Does not require O 2 for growth, but grows better in its presence Wastes Gaseous (CO 2) and liquid (metabolites) 15
Saccharomyces cerevisiae (Yeast) Yeast (S. cer. ) 5 -12µm Growth Requirements Microfluidics Liquid culture likely for flight, can be grown on solid medium Sensors p. H, oxygen, carbon dioxide, temperature, pressure Temperature Will grow at temperatures between 3 - 40°C; Optimum 28°C Salinity Yeast grows within a wide range of salt concentration Nutrients YPD : yeast extract, bacto-peptone, glucose, water p. H Growth optimum between 5. 0 - 6. 5 Doubling rate 1 -4 hours Light Not required Aeration Does not require O 2 for growth, but grows better in its presence Wastes Gaseous (CO 2) and liquid (metabolites) 15
 Caenorhabditis elegans (Nematode) Growth Requirements Microfluidics Sensors Temperature Salinity Nutrients p. H Doubling rate Light Aeration Wastes Liquid culture or solid culture. Organism is ~ 1 mm in length and grows in axenic defined liquid media or on solid media using bacteria as food carbon dioxide, oxygen, temperature 17°C optimum for growth; heat shock at 25°C; 30°C for > 20 hrs is lethal 0. 1 - 0. 5 molar simple inorganic salts (Na. Cl, KHPO 4), wide tolerance range Consumes dissolved nutrients in axenic liquid culture media, or on solid media consumes bacteria (E. coli). Optimum p. H 6. 0; tolerates p. H 3 - 9 3 -6 days to mature; temperature dependent Generally not required Chamber ventilation required for axenic liquid culture in gas-permeable opticell cartridges or for culture on solid media Gaseous (CO 2), liquid (metabolites), and debris (dead worms) 16
Caenorhabditis elegans (Nematode) Growth Requirements Microfluidics Sensors Temperature Salinity Nutrients p. H Doubling rate Light Aeration Wastes Liquid culture or solid culture. Organism is ~ 1 mm in length and grows in axenic defined liquid media or on solid media using bacteria as food carbon dioxide, oxygen, temperature 17°C optimum for growth; heat shock at 25°C; 30°C for > 20 hrs is lethal 0. 1 - 0. 5 molar simple inorganic salts (Na. Cl, KHPO 4), wide tolerance range Consumes dissolved nutrients in axenic liquid culture media, or on solid media consumes bacteria (E. coli). Optimum p. H 6. 0; tolerates p. H 3 - 9 3 -6 days to mature; temperature dependent Generally not required Chamber ventilation required for axenic liquid culture in gas-permeable opticell cartridges or for culture on solid media Gaseous (CO 2), liquid (metabolites), and debris (dead worms) 16
 Drosophila melanogaster (Fruit fly) Growth Requirements Microfluidics Sensors Temperature Nutrients p. H Doubling rate Light Aeration Wastes Solid medium p. H, oxygen, carbon dioxide, temperature 22 - 30°C with heat shock at 45°C. YPD : yeast extract, bacto-peptone, glucose, water ~ 6. 5 - 5. 0 optimum; range ~7. 2 – 4. 5 1 -4 hours Not required Active or passive aeration required Gaseous (CO 2) and liquid (metabolites) 17
Drosophila melanogaster (Fruit fly) Growth Requirements Microfluidics Sensors Temperature Nutrients p. H Doubling rate Light Aeration Wastes Solid medium p. H, oxygen, carbon dioxide, temperature 22 - 30°C with heat shock at 45°C. YPD : yeast extract, bacto-peptone, glucose, water ~ 6. 5 - 5. 0 optimum; range ~7. 2 – 4. 5 1 -4 hours Not required Active or passive aeration required Gaseous (CO 2) and liquid (metabolites) 17
 Arabidopsis thaliana (Brassica) Growth Requirements Microfluidics Sensors Temperature Humidity Nutrients/water Doubling rate Light Gas Composition Wastes Water/nutrient delivery system p. H, carbon dioxide, oxygen, ethylene, temperature, light 17 - 25°C; some protocols call for 15°C during dark cycle 65 - 100%; vegetative phase tolerant of a broad relative humidity range but above 855 can affect flowering and seed set Consistent with soil composition; well aerated soil required 4 -8 week growth cycle 16 hr light, 8 hr dark cycle; light intensity 250 u mol Typical air composition: 21% O 2, 78% N, 0. 05% CO 2 O 2, ethylene 18
Arabidopsis thaliana (Brassica) Growth Requirements Microfluidics Sensors Temperature Humidity Nutrients/water Doubling rate Light Gas Composition Wastes Water/nutrient delivery system p. H, carbon dioxide, oxygen, ethylene, temperature, light 17 - 25°C; some protocols call for 15°C during dark cycle 65 - 100%; vegetative phase tolerant of a broad relative humidity range but above 855 can affect flowering and seed set Consistent with soil composition; well aerated soil required 4 -8 week growth cycle 16 hr light, 8 hr dark cycle; light intensity 250 u mol Typical air composition: 21% O 2, 78% N, 0. 05% CO 2 O 2, ethylene 18
 Mammalian Cells Growth Requirements Microfluidics Sensors Temperature Humidity Salinity Nutrients p. H Doubling rate Light Aeration Wastes Liquid culture flowing above an adherent cell layer or cells growing in suspension in a liquid culture p. H, CO 2 , temperature, pressure, flow rate Tolerate only a very narrow temperature range, typically between 37°C - 42°C. Optimal temperature is 37°C ± 0. 5°C ~ 80% in incubators A variety of commercial buffered saline solutions used; most popular are Hank’s and Earle’s Different cell types require different media formulations, e. g. DMEM: glucose, Lglutamine, sodium pyruvate, phenol red. Between 5 -20% fetal bovine serum or horse serum is a common supplement. p. H range between 6. 8 - 7. 2 12 - 48 hours Not required Air, as oxygen source, must be added to culture in a way to avoid sheer stress to which mammalian cells are very sensitive. CO 2 levels must be kept at certain level; typically 5% 19 Gaseous (CO 2) and liquid (metabolites)
Mammalian Cells Growth Requirements Microfluidics Sensors Temperature Humidity Salinity Nutrients p. H Doubling rate Light Aeration Wastes Liquid culture flowing above an adherent cell layer or cells growing in suspension in a liquid culture p. H, CO 2 , temperature, pressure, flow rate Tolerate only a very narrow temperature range, typically between 37°C - 42°C. Optimal temperature is 37°C ± 0. 5°C ~ 80% in incubators A variety of commercial buffered saline solutions used; most popular are Hank’s and Earle’s Different cell types require different media formulations, e. g. DMEM: glucose, Lglutamine, sodium pyruvate, phenol red. Between 5 -20% fetal bovine serum or horse serum is a common supplement. p. H range between 6. 8 - 7. 2 12 - 48 hours Not required Air, as oxygen source, must be added to culture in a way to avoid sheer stress to which mammalian cells are very sensitive. CO 2 levels must be kept at certain level; typically 5% 19 Gaseous (CO 2) and liquid (metabolites)
 Rodents Housing & Husbandry Requirements Habitat Sensors Temperature Humidity Food/Water Population Density Light Ventilation Wastes Rodent cage: ventilated and kept free of contaminants from urine & feces O 2 , CO 2 , temperature, activity (video) 18°C - 26°C 30 - 70% Irradiated food bars (rodent chow) with long shelf-life / automatic watering manifolds or water bottles 6 rats or 10 mice per cage 8 -10 hours/day exposure during circadian cycle Control O 2 , CO 2 , particulate contaminants, animal odors Urine, feces must be contained 20
Rodents Housing & Husbandry Requirements Habitat Sensors Temperature Humidity Food/Water Population Density Light Ventilation Wastes Rodent cage: ventilated and kept free of contaminants from urine & feces O 2 , CO 2 , temperature, activity (video) 18°C - 26°C 30 - 70% Irradiated food bars (rodent chow) with long shelf-life / automatic watering manifolds or water bottles 6 rats or 10 mice per cage 8 -10 hours/day exposure during circadian cycle Control O 2 , CO 2 , particulate contaminants, animal odors Urine, feces must be contained 20
 Ethical Use of Animals at NASA Bioethical Principles • Respect for life • Societal benefit • Nonmalificence 21
Ethical Use of Animals at NASA Bioethical Principles • Respect for life • Societal benefit • Nonmalificence 21
 Regulations and Oversight • IACUC and federal regulations • Scientific standards • Agency oversight • Public scrutiny 22
Regulations and Oversight • IACUC and federal regulations • Scientific standards • Agency oversight • Public scrutiny 22
 References Medical Operations of Space Flight I Dr Arthur Arnold, Jr Kennedy Space Center Life into Space Life Sciences Experiments 1991 -1998 Eds. K Souza, G Etheridge & P. X. Callahan Model Organisms for Space Biology Research Dr Rita Briggs Lockheed Martin Fundamentals of Space Biology Eds M. Asashima & G. M. Malacinski (1990) Japan Scientific Societies Press & Springer-Verlag Early History of Space Biology and Medicine John P. Marbarger Acta Astronautica Vol. 43 No. 1 -2 pp 9 -12 1998 International Flight Experiments Database http: //www. mainsgate. com/IFE/index. html NASA Life Sciences Data Archive http: //www. lsda. jsc. nasa. gov 23
References Medical Operations of Space Flight I Dr Arthur Arnold, Jr Kennedy Space Center Life into Space Life Sciences Experiments 1991 -1998 Eds. K Souza, G Etheridge & P. X. Callahan Model Organisms for Space Biology Research Dr Rita Briggs Lockheed Martin Fundamentals of Space Biology Eds M. Asashima & G. M. Malacinski (1990) Japan Scientific Societies Press & Springer-Verlag Early History of Space Biology and Medicine John P. Marbarger Acta Astronautica Vol. 43 No. 1 -2 pp 9 -12 1998 International Flight Experiments Database http: //www. mainsgate. com/IFE/index. html NASA Life Sciences Data Archive http: //www. lsda. jsc. nasa. gov 23


