 ORGANIC COMPOUNDS
ORGANIC COMPOUNDS
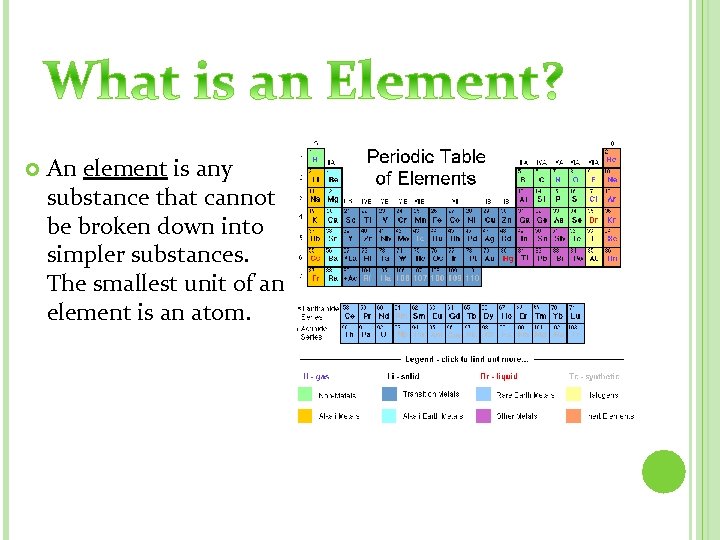 An element is any substance that cannot be broken down into simpler substances. The smallest unit of an element is an atom.
An element is any substance that cannot be broken down into simpler substances. The smallest unit of an element is an atom.
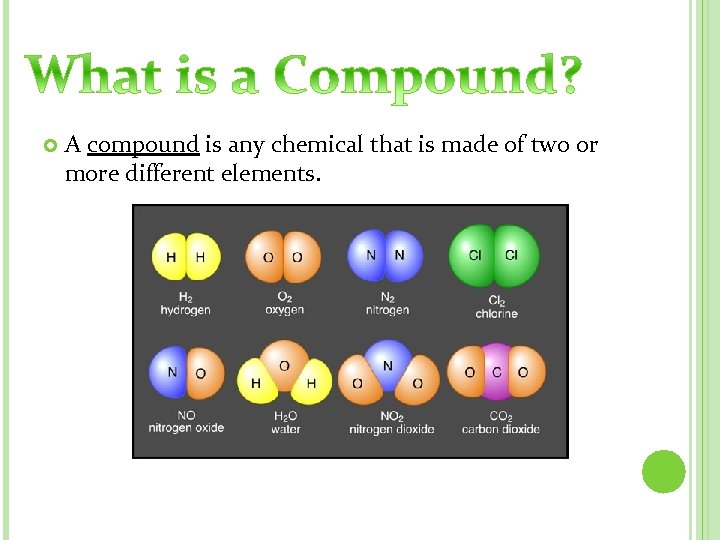 A compound is any chemical that is made of two or more different elements.
A compound is any chemical that is made of two or more different elements.
 Inorganic compounds do not contain carbon. Examples: Water, Salt, and acids like sulfuric acid
Inorganic compounds do not contain carbon. Examples: Water, Salt, and acids like sulfuric acid
 Organic Compounds contain carbon and hydrogen and are usually associated with living things.
Organic Compounds contain carbon and hydrogen and are usually associated with living things.
 CHONPS: C arbon Hydrogen Oxygen Nitrogen Phosphorus Sulfur
CHONPS: C arbon Hydrogen Oxygen Nitrogen Phosphorus Sulfur
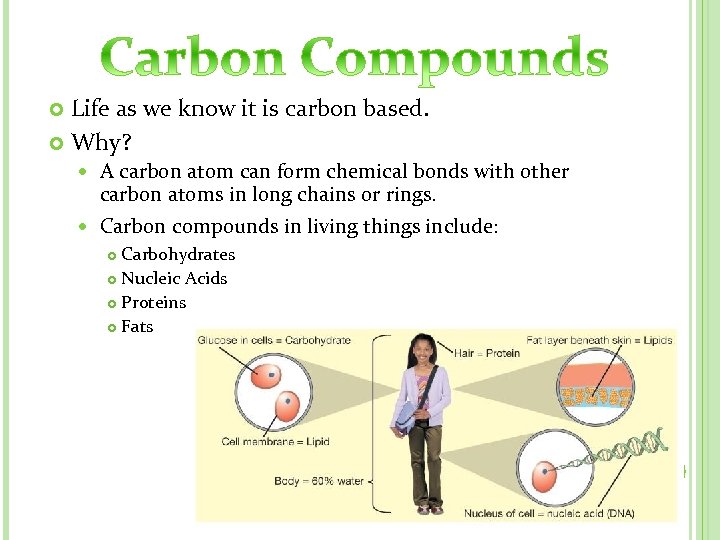 Life as we know it is carbon based. Why? A carbon atom can form chemical bonds with other carbon atoms in long chains or rings. Carbon compounds in living things include: Carbohydrates Nucleic Acids Proteins Fats
Life as we know it is carbon based. Why? A carbon atom can form chemical bonds with other carbon atoms in long chains or rings. Carbon compounds in living things include: Carbohydrates Nucleic Acids Proteins Fats
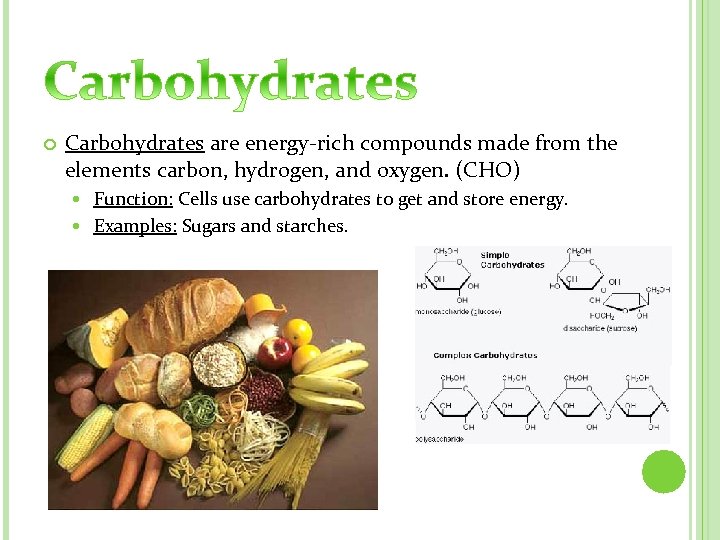 Carbohydrates are energy-rich compounds made from the elements carbon, hydrogen, and oxygen. (CHO) Function: Cells use carbohydrates to get and store energy. Examples: Sugars and starches.
Carbohydrates are energy-rich compounds made from the elements carbon, hydrogen, and oxygen. (CHO) Function: Cells use carbohydrates to get and store energy. Examples: Sugars and starches.
 Proteins are large molecules made of carbon, hydrogen, oxygen, nitrogen, and sulfur. (CHONS) Proteins are made of smaller molecules called amino acids. Function: Build organelles, build body parts, helps with body defense. Examples: Hair, nails, enzymes.
Proteins are large molecules made of carbon, hydrogen, oxygen, nitrogen, and sulfur. (CHONS) Proteins are made of smaller molecules called amino acids. Function: Build organelles, build body parts, helps with body defense. Examples: Hair, nails, enzymes.
 Lipids are made by cells to store energy for long periods of time. They are composed of the elements carbon, hydrogen, and oxygen. (CHO) Function: Store long term energy. Examples: Fats, oils, and waxes.
Lipids are made by cells to store energy for long periods of time. They are composed of the elements carbon, hydrogen, and oxygen. (CHO) Function: Store long term energy. Examples: Fats, oils, and waxes.
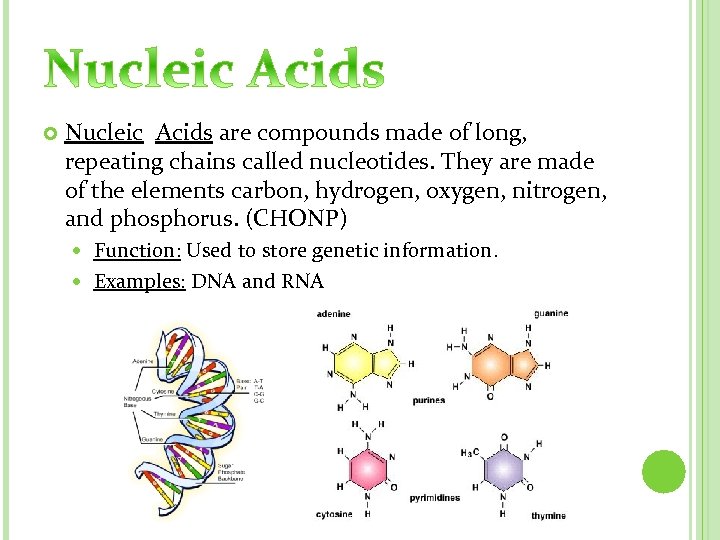 Nucleic Acids are compounds made of long, repeating chains called nucleotides. They are made of the elements carbon, hydrogen, oxygen, nitrogen, and phosphorus. (CHONP) Function: Used to store genetic information. Examples: DNA and RNA
Nucleic Acids are compounds made of long, repeating chains called nucleotides. They are made of the elements carbon, hydrogen, oxygen, nitrogen, and phosphorus. (CHONP) Function: Used to store genetic information. Examples: DNA and RNA